Assessment of Balkan Pepper (Capsicum annuum L.) Accessions for Agronomic, Fruit Quality, and Pest Resistance Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Germplasm Selection and Evaluation
2.2. Experimental Design and Field Evaluation
2.3. Trait Characterization
2.4. Fruit Compositional Quality
2.5. Screening for Insect Resistance under Natural Conditions
2.6. Statistical Analysis
3. Results and Discussion
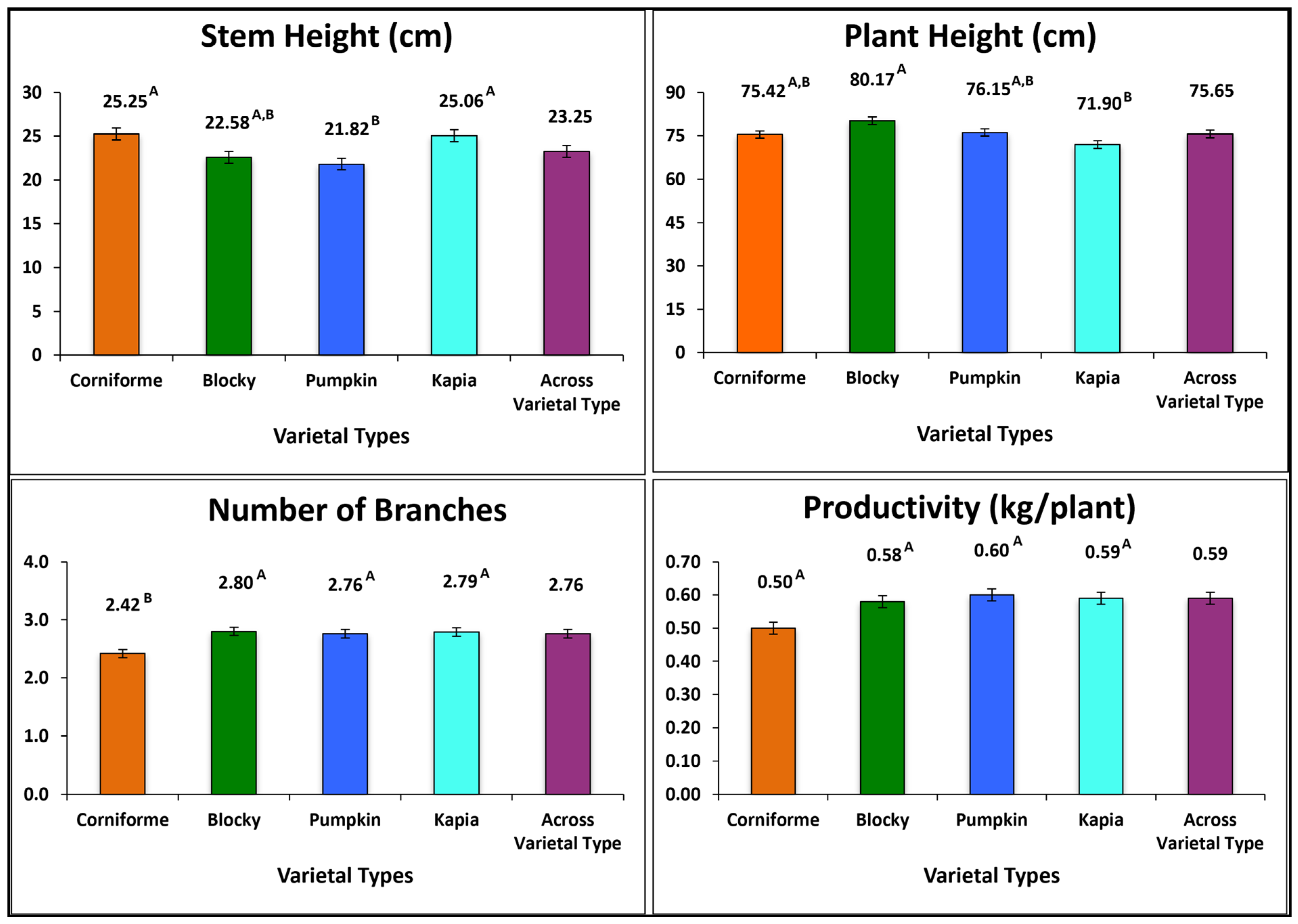
3.1. Inter-Varietal Grouping Variation
3.2. Intra-Varietal Grouping Variation
3.2.1. Plant Traits
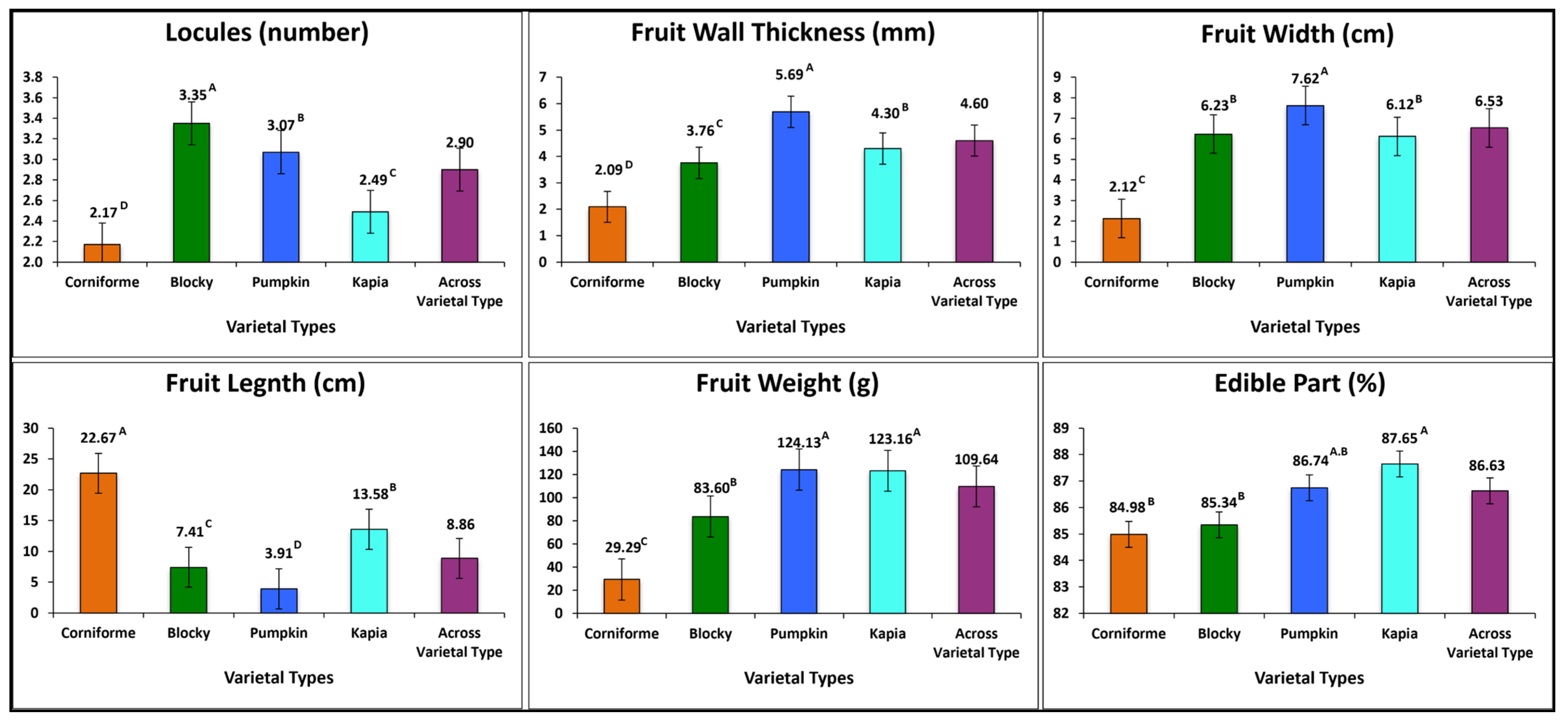
3.2.2. Fruit Morphology Traits
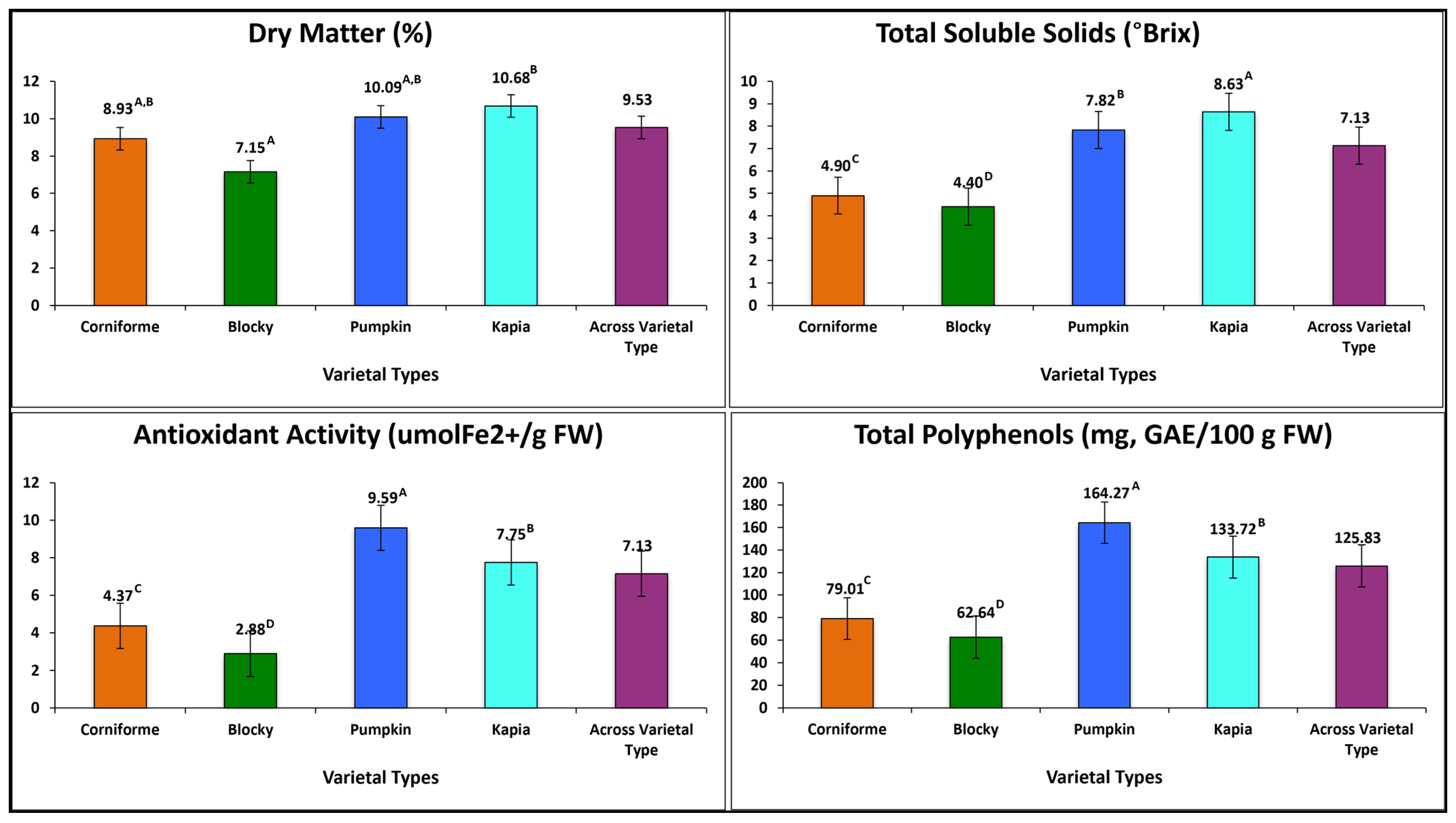
3.2.3. Fruit Quality Traits
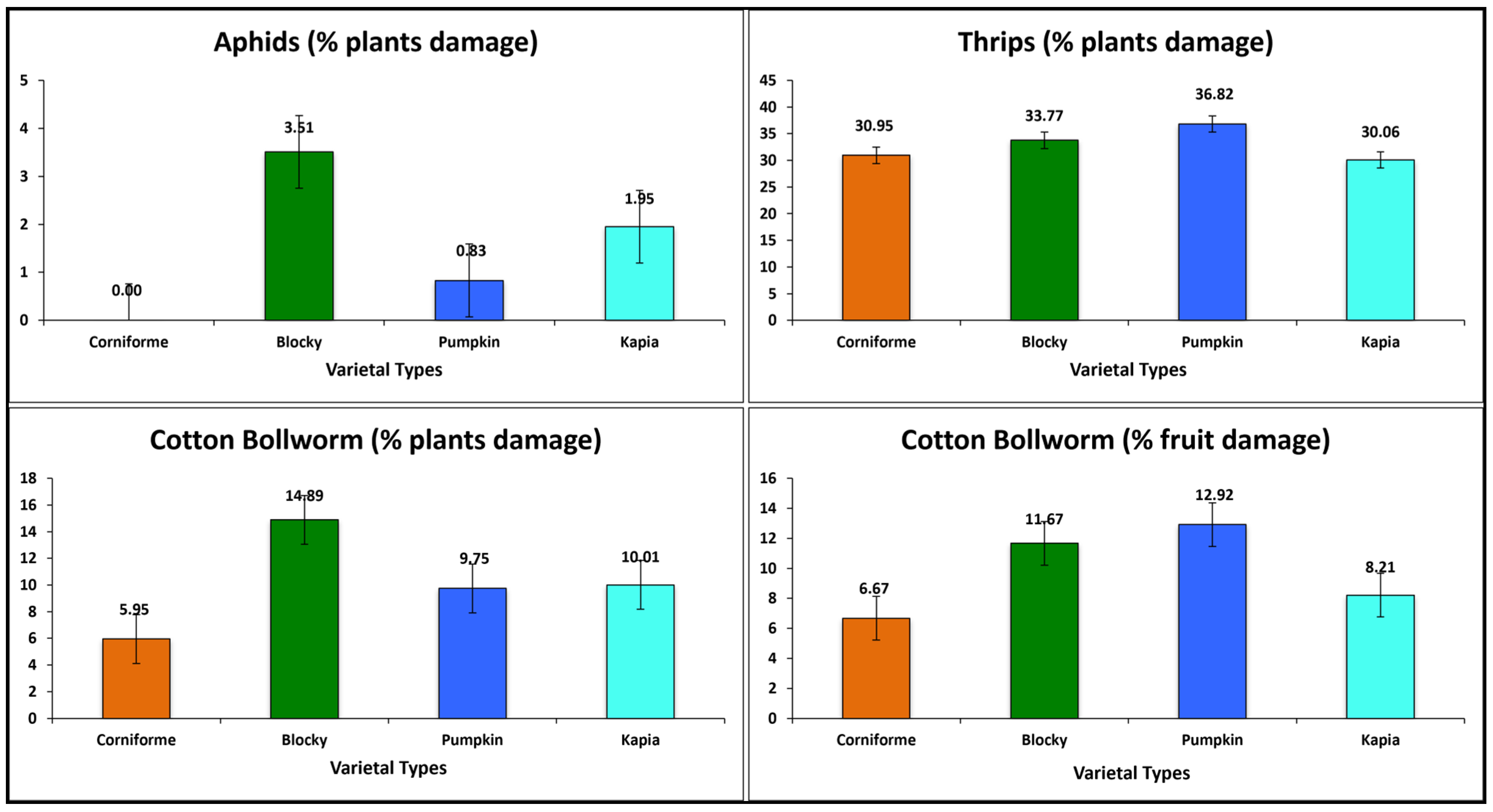
3.2.4. Insect and Pest Resistance
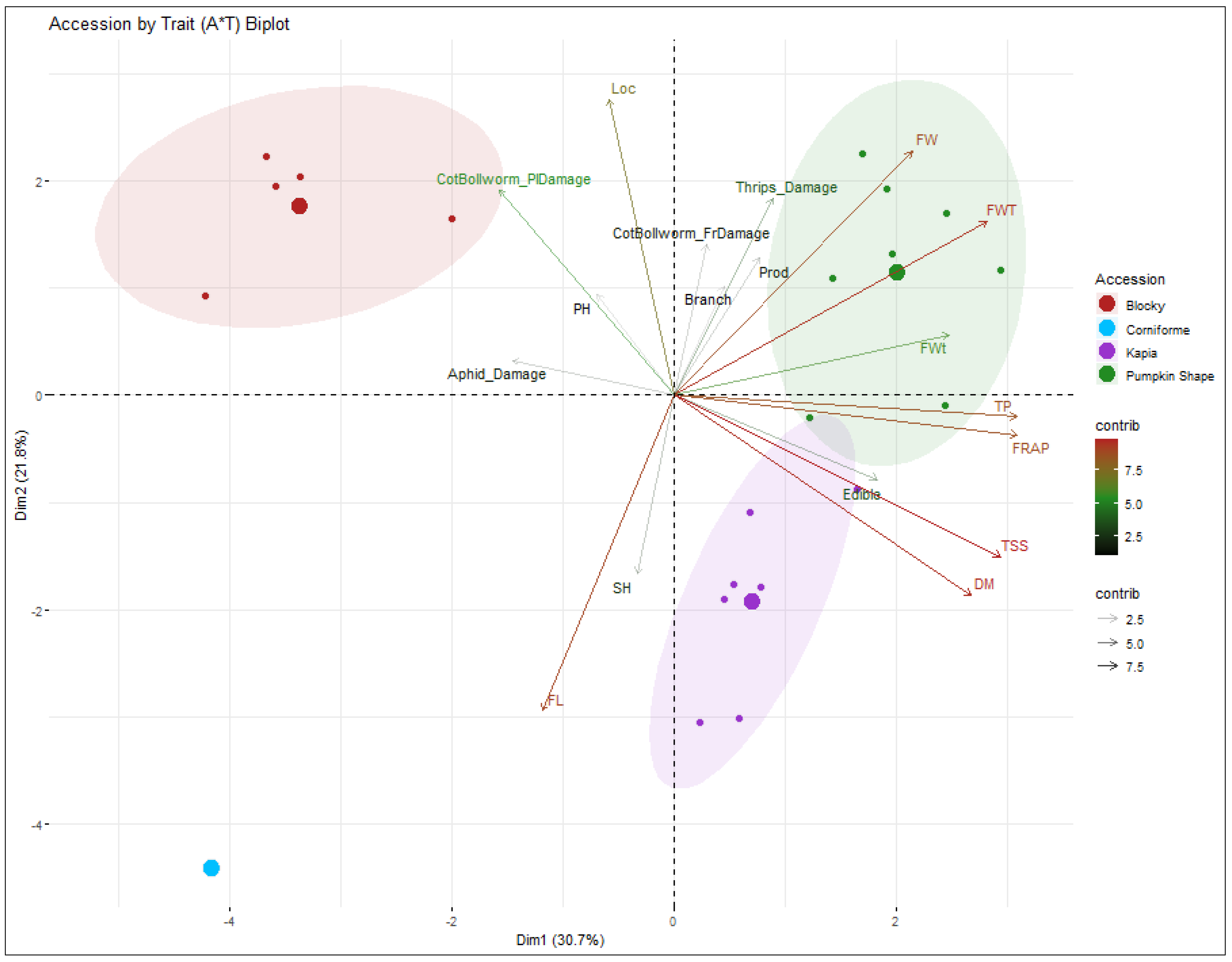
3.3. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Kang, W.H.; Kang, B.C. Basic Information on Pepper. In Genetics, Genomics and Breeding of Peppers and Eggplants; Kang, B.-C., Kole, C., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2013; p. 1. [Google Scholar]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in Phytochemical and Antioxidant Activity of Selected Pepper Cultivars (Capsicum Species) As Influenced by Maturity. J. Agric. Food Chem. 2000, 48, 1713. [Google Scholar] [CrossRef] [PubMed]
- Gnayfeed, M.H.; Daood, H.G.; Biacs, P.A.; Alcaraz, C.F. Content of bioactive compounds in pungent spice red pepper (paprika) as affected by ripening and genotype. J. Sci. Food Agr. 2001, 81, 1580. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Martinez-Guirado, C.; Rebolloso-Fuentes, M.; Carrique-Perez, A. Nutrient composition and antioxidant activity of 10 pepper (Capsicum annuum L.) varieties. Eur. Food Res. Technol. 2006, 224, 1–9. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Tikunov, Y.; de Vos, R.C.H.; Pelgrom, K.T.B.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 2013, 9, 130. [Google Scholar] [CrossRef]
- Jürkenbeck, K.; Spiller, A.; Meyerding, S.G.H. Tomato attributes and consumer preferences—A consumer segmentation approach. Br. Food J. 2020, 122, 328–344. [Google Scholar] [CrossRef]
- Walsh, B.; Maltby, J.E.; Nolan, B.; Kay, I. Seasonal abundance of thrips (Thysanoptera) in Capsicum and chilli crops in south-east Queensland, Australia. Plant Prot. Q. 2012, 27, 19–22. Available online: https://search.informit.org/doi/10.3316/informit.528420674116304 (accessed on 20 October 2023).
- Ssemwogerere, C.; Ochwo-Ssemakula, M.K.N.; Kovach, J.; Kyamanywa, S.; Karungi, J. Species composition and occurrence of thrips on tomato and pepper as influenced by farmers’ management practices in Uganda. J. Plant Prot. Res. 2013, 53, 158–164. [Google Scholar] [CrossRef]
- Riley, D.; Shimat, J.; Srinivasan, R.; Diffie, S. Thrips vectors of tospoviruses. J. Integr. Pest Manag. 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Visschers, I.G.S.; Peters, J.L.; van de Vondervoort, J.A.H.; Hoogveld, R.H.M.; van Dam, N.M. Thrips Resistance Screening Is Coming of Age: Leaf Position and Ontogeny Are Important Determinants of Leaf-Based Resistance in Pepper. Front. Plant Sci. 2019, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Frantz, J.D.; Gardner, J.; Hoffmann, M.P.; Jahn, M.M. Greenhouse Screening of Capsicum Accessions for Resistance to Green Peach Aphid (Myzus persicae). Hort Sci. 2004, 39, 1332–1335. [Google Scholar] [CrossRef]
- Mdellel, L.; Ben Halima Kamel, M. Effects of different varieties of pepper on the biological parameters of the green peach aphid Myzus persicae Sulzer (Hemiptera, Aphididae) in Tunisia. Eur. J. Environ. Sci. 2014, 4, 102–105. [Google Scholar] [CrossRef]
- Sannino, L.; Espinosa, B.; Caponero, A. Helicoverpa armigera (Hübner) harmful to pepper crops in Italy. Inf. Fitopatol. 2004, 54, 23–25. [Google Scholar]
- Li, D.-G.; Shang, X.-Y.; Reitz, S.; Nauen, R.; Lei, Z.-R.; Lee, S.H.; Gao, Y.-L. Field resistance to spinosad in western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae). J. Integr. Agric. 2016, 15, 2803–2808. [Google Scholar] [CrossRef]
- Weintraub, P.G. Integrated control of pests in tropical and subtropical sweet pepper production. Pest Manag. Sci. 2007, 63, 753–760. [Google Scholar] [CrossRef]
- Mouden, S.; Sarmiento, K.F.; Klinkhamer, P.G.L.; Leissm, K.A. Integrated pest management in western flower thrips: Past, present and future. Pest Manag. Sci. 2017, 73, 813–822. [Google Scholar] [CrossRef]
- Todorov, Y.; Todorova, V. Results and perspectives in the breeding and research work with pepper /C annuum L./. In Proceedings of the First Symposium on Horticulturae, Ohrid, Macedonia, 16–20 October 2002; Faculty of Agricultura Skopje University st Cyril and Methodius: Skopje, Macedonia, 2002; pp. 214–218. [Google Scholar]
- Denev, P.; Todorova, V.; Ognyanov, M.; Georgiev, Y.; Yanakieva, I.; Tringovska, I.; Grozeva, S.; Kostova, D. Phytochemical composition and antioxidant activity of 63 Balkan pepper (Capsicum annuum L.) accessions. J. Food Meas. Charact. 2019, 13, 2510–2520. [Google Scholar] [CrossRef]
- Nankar, A.N.; Todorova, V.; Tringovska, I.; Pasev, G.; Radeva, V.; Ivanova, V.; Kostova, D. A step towards Balkan Capsicum annuum L. core collection: Phenotypic and biochemical characterization of 180 accessions for agronomic, fruit quality, and virus resistance traits. PLoS ONE 2020, 15, e0237741. [Google Scholar] [CrossRef]
- Todorova, V.; Boteva, H.; Masheva, S.; Cholakov, T.; Kostova, D.; Yankova, V.; Dincheva, T. Technologies for open field pepper production. In Technologies for Production of Vegetable Crops and Potatoes; Masheva, S., Mihov, M., Todorova, V., Nacheva, E., Yankova, V., Boteva, H., Eds.; Blakom: Plovdiv, Bulgaria, 2014; pp. 41–66. [Google Scholar]
- IPGRI; AVRDC; CATIE. Descriptors for Capsicum (Capsicum spp.); International Plant Genetic Resources Institute: Rome, Italy; The Asian Vegetable Research and Development Center: Taipei, Taiwan; The Centro Agronomico Tropical de Investigacion y Ensenanza: Turrialba, Costa Rica, 1995; p. 44. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic. Acis Reagent. Acis Reagent. Amer. J. Enol. Viticult. 1965, 6, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Leclant, F.; Remaudiere, G. Elements pour la prise en consideration des aphides integree en vergers de peshers. Entomophaga 1970, 15, 53–81. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Use R!); Springer: New York, NY, USA, 2010. [Google Scholar]
- Josse, J.; Husson, F. missMDA: A package for handling missing values in multivariate data analysis. J. Stat. Softw. 2016, 70, 1–31. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.5. 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 24 July 2023).
- Moon, S.; Ro, N.; Kim, J.; Ko, H.-C.; Lee, S.; Oh, H.; Kim, B.; Lee, H.-S.; Lee, G.-A. Characterization of diverse pepper (Capsicum spp.) germplasms based on agro-morphological traits and phytochemical contents. Agronomy 2023, 13, 2665. [Google Scholar] [CrossRef]
- Elizondo-Cabalceta, E.; Monge-Perez, J.E. Morphological characterization of 15 bell pepper (Capsicum annuum) genotypes grown under greenhouse conditions in Costa Rica. InterSedes 2017, 18, 129–154. [Google Scholar] [CrossRef][Green Version]
- Janaki, M.; Venkata Ramana, C.; Naram Naidu, L.; Paratpara Rao, M. Performance of chilli (Capsicum annuum L.) genotypes for yield and yield attributing traits. Plant Arch. 2015, 15, 661–666. [Google Scholar]
- Hasanuzzaman, M.; Golam, F. Selection of traits for yield improvements in chilli (Capsicum annuum L.). J. Innov. Dev. Strategy 2011, 5, 78–87. [Google Scholar]
- Luitel, B.P.; Lee, T.J.; Kang, W.H. Variation for fruit yield and quality characteristics in sweet pepper (Capsicum annuum L.) germplasm collection. Kor. J. Breed Sci. 2011, 43, 139–144. [Google Scholar]
- Bogusz Junior, S.; Libardi, S.H.; Dias, F.F.G.; Coutinho, J.P.; Bochi, V.C.; Rodrigues, D.; Melo, A.M.T.; Godoy, H.T. Brazilian Capsicum peppers: Capsaicinoids content and antioxidant activity. J. Sci. Food Agric. 2018, 98, 217–224. [Google Scholar] [CrossRef]
- Constantino, L.V.; Suzuki Fukuji, A.Y.; Douglas, M.Z.; Baba, V.Y.; Corte, L.E.-D.; Giacomin, R.M.; Resende, J.T.V.; Gonçalves, L.S.A. Genetic variability in peppers accessions based on morphological, biochemical and molecular traits. Bragantia 2020, 79, 558–571. [Google Scholar] [CrossRef]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of Biotic Stresses in Pepper (Capsicum spp.): Sources of Genetic Resistance, Molecular Breeding and Genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef]
- Maharijaya, A.; Vosman, B.; Steenhuis-Broers, G.; Harpenas, A.; Purwito, A.; Visser, R.G.F.; Voorrips, R.E. Screening of pepper accessions for resistance against two thrips species (Frankliniella occidentalis and Thrips parvispinus). Euphytica 2011, 177, 401–410. [Google Scholar] [CrossRef]
- Maris, P.C.; Joosten, N.N.; Goldbach, R.W.; Peters, D. Restricted spread of tomato spotted wilt virus in thrips resistant pepper. Phytopathology 2003, 93, 1223–1227. [Google Scholar] [CrossRef]
- Maharijiva, A. Resistance to Thrips in Pepper. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2013; p. 109. [Google Scholar]
- Maharijaya, A.; Vosman, B.; Verstappen, F.; Steenhuis-Broers, G.; Mumm, R.; Purwito, A.; Visser, R.G.F.; Voorrips, R.E. Resistance factors in pepper inhibit larval development of thrips (Frankliniella occidentalis). Entomol. Exp. Et Appl. 2012, 145, 62–71. [Google Scholar] [CrossRef]
- La Rossa, F.R.; Vasicek, A.; López, M.C. Effects of pepper (Capsicum annuum) cultivars on the biology and life table parameters of Myzus persicae (Sulz.) (Hemiptera: Aphididae). Neotrop. Entomol. 2013, 42, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Fery, R.L.; Schalk, J.M. Resistance in pepper (Capsicum annuum L.) to western flower thrips [Frankliniella occidentalis (Pergande)]. HortScience 1991, 26, 1073–1074. [Google Scholar] [CrossRef]
- Tanpure, R.S.; Barbole, R.S.; Dawkar, V.V.; Waichal, Y.A.; Joshi, R.S.; Giri, A.P.; Gupta, V.S. Improved tolerance against Helicoverpa armigera in transgenic toma to over-expressing multi-domain proteinase inhibitor gene from Capsicum Annuum. Physiol. Mol. Biol. Plants 2017, 23, 597–604. [Google Scholar] [CrossRef]
- Dekebo, A. Major pests and pest management strategies in the sweet pepper (Capsicum annumm L.). In Capsicum—Current Trends and Perspectives; Yllano, O.B., Ed.; Intech: London, UK, 2022; pp. 82–134. [Google Scholar] [CrossRef]
- Tsonev, S.; Todorova, V.; Grozeva, S.; Popova, T.; Todorovska, E. Evaluation of diversity in Bulgarian pepper cultivars by agronomical traits and ISSR markers. Genetika 2017, 49, 647–662. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Mateos-Fernández, R.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. Phenotypic Divergence among Sweet Pepper Landraces Assessed by Agro-Morphological Characterization as a Biodiversity Source. Agronomy 2022, 12, 632. [Google Scholar] [CrossRef]
- Singh, P.; Jain, P.K.; Tiwari, A. Principal component analysis approach for yield attributing traits in chilli (Capsicum annum L.) genotypes. Chem. Sci. Rev. Lett. 2020, 9, 87–91. [Google Scholar] [CrossRef]
- Bianchi, P.A.; Dutra, I.P.; Moulin, M.M.; Santos, J.O.; Júnior, A.C.S. Morphological characterization and analysis of genetic variability among pepper accessions. Cienc. Rural 2016, 46, 1151–1157. [Google Scholar] [CrossRef]

| 2018 | 2019 | Across Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | Acces | Repl | A*R Inter | Acces | Repl | A*R Inter | Acces | Repl | Year | A*R Inter | A*Y Inter | R*Y Inter |
| DF | 2 | 2 | 4 | 2 | 2 | 4 | 2 | 2 | 1 | 4 | 2 | 2 |
| Morphological Traits: | ||||||||||||
| Plant Height | 3.20 ** | 0.01 | 46.95 *** | 4.41 *** | 0.79 | 7757 *** | 4.99 *** | 0.142 | 1.77 | 5.03 *** | 3.98 *** | 1.06 |
| Stem Height | 2.02 * | 0.20 | 4487 *** | 3.44 ** | 0.83 | 29.01 *** | 2.71 *** | 0.01 | 0.15 | 2.58 ** | 3.05 *** | 0.03 |
| Branches | 1.88 | 0.36 | 576.38 *** | 1.25 | 0.03 | 21.39 *** | 1.72* | 1.01 | 4.98 * | 1.58 | 1.92 * | 1.88 |
| Productivity | 2.45 ** | 0.01 | 71.99 *** | 1.83 | 0.95 | 1086 *** | 0.86 | 0.01 | 16.7 *** | 0.90 | 2.27 ** | 0.01 |
| Fruit Length | 73.94 *** | 0.26 | 11.08 *** | 106.12 *** | 0.02 | 3.77 *** | 97.67 *** | 0.10 | 0.03 | 71.15 *** | 64.82 *** | 0.42 |
| Fruit Width | 10.23 *** | 0.39 | 5.20 *** | 25.81 *** | 0.03 | 5.64 *** | 23.29 *** | 0.06 | 6.71 ** | 15.75 *** | 15.39 *** | 0.13 |
| Fruit Wall Thickness | 10.94 *** | 0.04 | 12.02 *** | 7.06 *** | 0.36 | 6.85 *** | 10.13 *** | 3.53 | 0.06 | 9.79 *** | 9.35 *** | 1.26 |
| Locules | 3.44 ** | 0.02 | 21.80 *** | 3.98 *** | 0.32 | 22.10 *** | 6.41 *** | 0.18 | 0.30 | 5.03 *** | 4.29 *** | 0.67 |
| Fruit Weight | 10.32 *** | 0.07 | 12.07 *** | 8.38 *** | 0.01 | 14.83 *** | 13.10 *** | 0.21 | 0.18 | 8.58 *** | 8.61 *** | 0.05 |
| Edible Part | 2.98 ** | 0.15 | 267.76 *** | 1.71 | 1.86 | 556.17 *** | 1.23 | 3.28 | 1.92 | 1.16 | 2.78 *** | 2.17 |
| Fruit Quality Traits: | ||||||||||||
| Dry Matter | 13.50 *** | 0.01 | 508.26 *** | 31.30 *** | 0.01 | 73.64 *** | 24.31 *** | 0.15 | 0.36 | 23.16 *** | 23.68 *** | 0.003 |
| TSSs | 19.82 *** | 0.14 | 42.51 *** | 32.08 *** | 0.03 | 25.74 *** | 37.28 *** | 0.03 | 0.96 | 30.58 *** | 33.17 *** | 0.28 |
| FRAP | 1.93 | 0.49 | 145.52 *** | 16.15 *** | 0.03 | 29.70 *** | 6.17 *** | 0.41 | 17.2 *** | 4.99 *** | 8.30 *** | 0.04 |
| Total Polyphenols | 7.65 *** | 0.79 | 105.50 *** | 37.14 *** | 0.01 | 36.41 *** | 11.36 *** | 0.62 | 002 | 9.39 *** | 25.99 *** | 0.11 |
| Code | Accession | Name | Origin | Population Type | Plant Height (cm) | Stem Height(cm) | Branches (n) | Productivity (kg/plant) |
|---|---|---|---|---|---|---|---|---|
| Corniform | ||||||||
| G1 | K696 | Chorbadzhiyski Sladak | Bulgaria | Local Form | 75.42 E–H ± 13.39 | 25.25 A–D ± 7.56 | 2.42 E ± 0.51 | 0.50 B–D ± 0.15 |
| Dolma (Blocky) | ||||||||
| G2 | K1086 | B2E0048 | Bulgaria | Local Form | 70.42 G–J ± 9.64 | 20.42 E,F ± 4.50 | 2.58 C–E ± 0.51 | 0.66 A–C ± 0.33 |
| G3 | K1098 | 89601135/25466 | Greece | Breeding Line | 74.17 E–I ± 17.9 | 22.08 C–F ± 7.82 | 2.92 A–C ± 0.51 | 0.61 A–D ± 0.34 |
| G4 | K1099 | 89601136/25467 | Greece | Breeding Line | 76.25 D–H ± 13.51 | 22.08 C–F ± 3.34 | 3.00 A,B ± 0.00 | 0.53 B–D ± 0.21 |
| G5 | K1100 | 89601137/25468 | Greece | Breeding Line | 92.08 A ± 12.33 | 24.58 A–E ± 7.82 | 3.00 A,B ± 0.60 | 0.64 A–D ± 0.23 |
| G6 | K1112 | B1E0372 | Albania | Local Form | 87.92 A,B ± 9.88 | 23.75 B–E ± 6.08 | 2.50 D,E ± 0.52 | 0.46 B–D ± 0.11 |
| Pumpkin | ||||||||
| G7 | K1053 | B1E0021 | Bulgaria | Local Form | 75.00 D–H ± 9.53 | 26.67 A–C ± 5.37 | 2.67 B–E ± 0.49 | 0.50 B–D ± 0.12 |
| G8 | K1055 | B1E0059 | Bulgaria | Local Form | 72.08 F–J ± 10.76 | 22.08 C–F ± 4.98 | 2.75 B–E ± 0.45 | 0.50 C,D ± 0.11 |
| G9 | K1056 | B1E0061 | Bulgaria | Local Form | 82.92 A–E ± 12.52 | 21.67 D–F ± 3.89 | 2.42 E ± 0.51 | 0.59 A–D ± 0.15 |
| G10 | K1057 | B1E0062 | Bulgaria | Local Form | 80.42 B–G ± 9.88 | 23.34 B–E ± 4.44 | 2.67 B–E ± 0.67 | 0.65 A–D ± 0.12 |
| G11 | K1083 | B2E0040 | Bulgaria | Local Form | 74.58 D–H ± 10.76 | 20.42 E,F ± 3.96 | 2.58 C–E ± 0.51 | 0.49 B–D ± 0.09 |
| G12 | K1103A | Ruminska Sipka | Unknown | Not Applicable | 68.33 H–J ± 13.87 | 18.33 F ± 5.77 | 2.83 B–D ± 0.58 | 0.67 A–C ± 0.12 |
| G13 | K1115 | B1E0405 | Albania | Local Form | 69.17 H–J ± 12.94 | 17.92 F ± 5.42 | 2.92 A–C ± 0.51 | 0.74 A ± 0.31 |
| G14 | K712 | Kambi S-34 | Bulgaria | Local Form | 86.67 A–C ± 11.35 | 24.17 A–E ± 4.69 | 3.25 A ± 0.45 | 0.64 A–D ± 0.17 |
| Kapia | ||||||||
| G15 | K1074 | B1E0250 | North Macedonia | Local Form | 77.08 C–H ± 11.37 | 27.92 A,B ± 4.50 | 3.00 A,B ± 0.43 | 0.56 A–D ± 0.20 |
| G16 | K1081 | B2E0034 | Bulgaria | Local Form | 64.17 I–K ± 9.25 | 23.75 B–E ± 7.72 | 2.67 B–E ± 0.49 | 0.70 A,B ± 0.33 |
| G17 | K1093 | B1E0504 | Bulgaria | Local Form | 75.00 D–H ± 12.61 | 28.75 A ± 5.28 | 2.83 B–D ± 0.39 | 0.55 A–D ± 0.12 |
| G18 | K1094 | B1E0525 | Bulgaria | Local Form | 84.58 A–D ± 13.73 | 26.25 A–D ± 4.33 | 2.92 A–C ± 0.51 | 0.67 A–C ± 0.12 |
| G19 | K1103B | Ruminska Sipka | Unknown | Not Applicable | 57.50 K ± 9.17 | 20.00 E,F ± 3.69 | 2.67 B–E ± 0.49 | 0.54 A–D ± 0.16 |
| G20 | K1114 | B1E0378 | Bulgaria | Local Form | 82.08 A–F ± 12.15 | 26.67 A–C ± 5.37 | 2.58 C–E ± 0.51 | 0.60 A–D ± 0.20 |
| G21 | K697 | Kapia Sladka S-11 | Bulgaria | Local Form | 62.92 J,K ± 6.89 | 22.08 C–F ± 3.96 | 2.83 B–D ± 0.39 | 0.48 B–D ± 0.11 |
| Code | Accession | Locules (n) | Fruit | Edible Part (%) | |||
|---|---|---|---|---|---|---|---|
| Length (cm) | Width (cm) | Thickness (mm) | Weight (g) | ||||
| Corniform | |||||||
| G1 | K696 | 2.17 H ± 0.39 | 22.67 A ± 2.72 | 2.12 G ± 0.29 | 2.09 J ± 0.46 | 29.29 J ± 8.47 | 84.98 D–G ± 6.11 |
| Blocky | |||||||
| G2 | K1086 | 3.25 A,B ± 0.45 | 7.65 D ± 1.47 | 6.03 F ± 1.09 | 4.42 E–G ± 1.05 | 87.06 H,I ± 32.23 | 88.41 A–C ± 3.60 |
| G3 | K1098 | 3.58 A ± 0.51 | 7.53 D ± 0.67 | 6.20 E,F ± 0.38 | 3.48 I ± 0.54 | 76.38 I ± 14.59 | 85.29 C–F ± 2.37 |
| G4 | K1099 | 3.58 A ± 0.67 | 7.63 D ± 0.79 | 6.64 E,F ± 0.80 | 3.35 I ± 0.69 | 98.69 G,H ± 19.85 | 84.58 E–G ± 3.96 |
| G5 | K1100 | 3.25 A,B ± 0.62 | 7.44 D ± 0.70 | 6.23 E,F ± 0.57 | 3.81 G–I ± 1.14 | 81.27 H,I ± 15.04 | 83.89 G–F ± 2.60 |
| G6 | K1112 | 3.08 B–D ± 0.51 | 6.78 D ± 0.89 | 6.06 F ± 0.64 | 3.76 G–I ± 0.48 | 74.62 I ± 16.56 | 84.52 E–G ± 6.04 |
| Pumpkin | |||||||
| G7 | K1053 | 3.08 B–D ± 0.51 | 3.42 E,F ± 0.94 | 7.61 B,C ± 0.79 | 5.14 D,E ± 0.87 | 124.64 D,E ± 13.32 | 86.94 A–F ± 3.45 |
| G8 | K1055 | 3.25 A,B ± 0.45 | 4.48 E ± 0.94 | 8.58 A ± 0.89 | 5.96 A,B ± 1.07 | 151.82 B ± 45.14 | 86.84 A–F ± 2.90 |
| G9 | K1056 | 3.17 B,C ± 0.39 | 4.06 E,F ± 1.06 | 8.08 A,B ± 1.16 | 5.71 B,C ± 1.10 | 148.11 B,C ± 31.29 | 87.24 A–F ± 7.37 |
| G10 | K1057 | 3.08 B–D ± 0.67 | 4.35 E ± 0.92 | 7.59 B,C ± 0.80 | 6.66 A ± 1.26 | 129.59 C–E ± 13.72 | 85.23 C–F ± 3.04 |
| G11 | K1083 | 2.83 C–F ± 0.72 | 4.13 E ± 0.74 | 7.54 B–D ± 0.88 | 5.83 B,C ± 0.87 | 128.85 C–E ± 48.21 | 88.98 A,B ± 3.70 |
| G12 | K1103A | 2.75 D–F ± 0.45 | 2.87 F ± 0.68 | 6.18 E,F ± 0.38 | 5.28 B–D ± 1.05 | 68.86 I ± 11.09 | 83.44 G ± 5.02 |
| G13 | K1115 | 3.17 B,C ± 0.39 | 4.08 E,F ± 0.92 | 7.68 B,C ± 0.79 | 5.65 B,C ± 0.97 | 120.16 D–F ± 17.86 | 87.39 A–E ± 3.50 |
| G14 | K712 | 3.25 A,B ± 0.45 | 3.91 E,F ± 0.98 | 7.69 B,C ± 0.72 | 5.31 B–D ± 1.00 | 121.01 D,E ± 24.19 | 87.89 A–E ± 4.86 |
| Kapia | |||||||
| G15 | K1074 | 2.33 H–G ± 0.49 | 12.83 C ± 1.34 | 6.88 D,E ± 1.00 | 4.71 D–F ± 1.01 | 131.69 B–D ± 27.69 | 89.11 A,B ± 2.91 |
| G16 | K1081 | 2.17 H ± 0.39 | 12.93 C ± 1.39 | 6.14 F ± 1.33 | 4.37 E–H ± 1.07 | 122.53 D,E ± 28.44 | 88.10 A–D ± 2.88 |
| G17 | K1093 | 2.58 F,G ± 0.51 | 13.26 C ± 0.90 | 6.26 E,F ± 0.72 | 4.07 F–I ± 1.28 | 138.60 B–D ± 21.49 | 89.57 A ± 2.07 |
| G18 | K1094 | 2.33 H–G ± 0.49 | 13.57 C ± 1.59 | 7.37 C,D ± 0.80 | 4.66 D–F ± 0.31 | 178.48 A ± 25.41 | 88.10 A–D ± 2.73 |
| G19 | K1103B | 2.67 E–G ± 0.78 | 12.88 C ± 1.76 | 6.16 F ± 1.16 | 4.63 D–F ± 1.20 | 111.02 E–G ± 28.76 | 86.00 B–F ± 3.50 |
| G20 | K1114 | 2.33 H–G ± 0.49 | 16.27 B ± 3.13 | 5.11 G ± 0.44 | 4.02 F–I ± 1.02 | 99.67 F–H ± 25.77 | 86.29 A–F ± 6.07 |
| G21 | K697 | 3.00 B–E ± 0.00 | 13.33 C ± 1.09 | 4.92 G ± 0.40 | 3.65 I,H ± 0.49 | 80.16 H,I ± 9.47 | 86.34 A–F ± 2.36 |
| Code | Accession | Harvesting | Dry Matter (%) | TSSs (Brix) | FRAP (µmol Fe2+/g FW) | Total Polyphenols (mg GAE/100 g FW) |
|---|---|---|---|---|---|---|
| Corniform | ||||||
| G1 | K696 | before # | 8.93 G ± 0.53 | 4.90 G ± 0.55 | 4.37 F,G ± 1.34 | 79.01 J ± 25.92 |
| Blocky | ||||||
| G2 | K1086 | before | 6.64 I ± 0.47 | 4.45 G,H ± 0.28 | 3.72 G ± 0.67 | 67.64 J,K ± 17.43 |
| G3 | K1098 | before | 7.40 H ± 0.56 | 4.43 G,H ± 0.73 | 3.12 G ± 0.78 | 67.81 J,K ± 20.95 |
| G4 | K1099 | before | 6.65 I ± 0.56 | 4.13 H ± 0.34 | 2.20 G ± 0.54 | 54.66 K ± 10.21 |
| G5 | K1100 | before | 7.55 H ± 0.24 | 4.50 G,H ± 0.47 | 2.81 G ± 0.38 | 61.63 J,K ± 8.97 |
| G6 | K1112 | before | 7.53 H ± 0.69 | 4.47 G,H ± 0.45 | 2.56 G ± 0.51 | 61.47 J,K ± 19.10 |
| Pumpkin | ||||||
| G7 | K1053 | at maturity ## | 10.02 D–F ± 1.35 | 8.07 C–E ± 1.47 | 8.40 B–E ± 3.87 | 138.58 F–H ± 26.64 |
| G8 | K1055 | at maturity | 9.97 D–F ± 0.58 | 7.87 D,E ± 0.55 | 11.48 A ± 4.65 | 161.82 C–E ± 34.04 |
| G9 | K1056 | at maturity | 9.64 E,F ± 0.21 | 7.53 E,F ± 0.43 | 10.17 A–C ± 3.11 | 150.48 D–F ± 42.70 |
| G10 | K1057 | at maturity | 10.02 D–F ± 0.77 | 7.58 E,F ± 0.77 | 8.67 B–E ± 4.74 | 154.54 C–F ± 55.86 |
| G11 | K1083 | at maturity | 10.52 C,D ± 1.01 | 8.50 B,C ± 1.01 | 8.46 B–E ± 2.82 | 140.16 E–H ± 20.96 |
| G12 | K1103A | at maturity | 10.23 C–E ± 0.70 | 7.80 D–F ± 0.73 | 11.49 A ± 5.39 | 189.55 A ± 43.79 |
| G13 | K1115 | at maturity | 9.56 F,G ± 0.55 | 7.22 F ± 0.62 | 8.08 C–E ± 2.99 | 175.59 B,C ± 53.50 |
| G14 | K712 | at maturity | 10.73 B,C ± 0.53 | 7.97 C–E ± 0.73 | 9.99 A–D ± 3.98 | 203.44 A ± 46.64 |
| Kapia | ||||||
| G15 | K1074 | at maturity | 10.57 C,D ± 0.74 | 8.78 A,B ± 0.56 | 6.47 E,F ± 1.26 | 114.51 I ± 20.86 |
| G16 | K1081 | at maturity | 10.52 C,D ± 0.68 | 8.42 B–D ± 0.66 | 6.70 E ± 1.18 | 122.90 H,I ± 27.68 |
| G17 | K1093 | at maturity | 10.46 C,D ± 0.40 | 8.73 B ± 0.51 | 7.05 E ± 1.89 | 123.31 G–I ± 23.88 |
| G18 | K1094 | at maturity | 9.95 D–F ± 0.71 | 8.37 B–D ± 0.66 | 7.79 D,E ± 1.93 | 125.78 G–I ± 30.29 |
| G19 | K1103B | at maturity | 10.33 C,D ± 0.96 | 7.95 C–E ± 0.15 | 8.22 C–E ± 1.64 | 138.55 F–H ± 27.99 |
| G20 | K1114 | at maturity | 11.66 A ± 0.60 | 9.38 A ± 0.58 | 7.41 E ± 1.97 | 144.97 D–G ± 12.77 |
| G21 | K697 | at maturity | 11.28 A,B ± 0.74 | 8.78 A,B ± 0.74 | 10.60 A,B ± 1.92 | 166.04 C,D ± 19.42 |
| Code | Accession | Green Peach Aphid | Thrips | Cotton Bollworm | |||
|---|---|---|---|---|---|---|---|
| Damaged Plants (%) | Degree of Infestation | Damaged Plants (%) | Degree of Infestation | Damaged Plants (%) | Damaged Fruit (%) | ||
| Corniform | |||||||
| G1 | K696 | 0.00 | 0.00 | 30.95 | 1.42 | 5.95 | 6.67 |
| Blocky | |||||||
| G2 | K1086 | 0.00 | 0.00 | 39.05 | 1.17 | 12.15 | 11.67 |
| G3 | K1098 | 4.78 | 0.67 | 32.88 | 1.25 | 14.32 | 16.67 |
| G4 | K1099 | 0.00 | 0.00 | 30.00 | 1.42 | 15.84 | 10.00 |
| G5 | K1100 | 3.13 | 0.50 | 34.59 | 0.92 | 18.96 | 8.34 |
| G6 | K1112 | 9.67 | 0.50 | 32.34 | 1.08 | 13.17 | 11.67 |
| Pumpkin | |||||||
| G7 | K1053 | 0.84 | 0.09 | 28.89 | 0.92 | 8.52 | 10.84 |
| G8 | K1055 | 2.50 | 0.17 | 41.55 | 1.09 | 12.15 | 11.67 |
| G9 | K1056 | 0.00 | 0.00 | 33.34 | 1.00 | 10.56 | 15.84 |
| G10 | K1057 | 0.00 | 0.00 | 31.10 | 1.09 | 10.27 | 32.50 |
| G11 | K1083 | 0.84 | 0.00 | 40.84 | 1.00 | 5.00 | 5.00 |
| G12 | K1103A | 0.00 | 0.00 | 40.71 | 1.00 | 12.69 | 7.50 |
| G13 | K1115 | 2.50 | 0.09 | 38.26 | 1.17 | 13.26 | 8.34 |
| G14 | K712 | 0.00 | 0.00 | 39.93 | 1.00 | 5.61 | 11.67 |
| Kapia | |||||||
| G15 | K1074 | 2.69 | 0.59 | 30.93 | 0.92 | 11.21 | 5.00 |
| G16 | K1081 | 2.50 | 0.09 | 39.65 | 1.25 | 13.93 | 9.17 |
| G17 | K1093 | 2.50 | 0.17 | 27.98 | 1.67 | 13.57 | 10.84 |
| G18 | K1094 | 0.84 | 0.09 | 34.01 | 1.50 | 8.23 | 10.84 |
| G19 | K1103B | 1.79 | 0.50 | 29.65 | 1.42 | 8.57 | 1.67 |
| G20 | K1114 | 3.34 | 0.17 | 28.24 | 0.92 | 7.87 | 11.67 |
| G21 | K697 | 0.00 | 0.00 | 20.00 | 0.75 | 6.67 | 8.34 |
| Trait | Features | Corr. Coeff. (R2) | Eigenvector | Eigenvalue | Variance (%) | Cumulative Variance (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | 1 | 2 | ||||
| Plant Height | 0.73 | 1.90 | −0.072 | −0.05 | −0.072 | −0.045 | 5.530 | 30.74 | 30.74 |
| Stem Height | 0.16 | 5.95 | −0.07 | 0.29 | −0.072 | 0.290 | 3.910 | 21.77 | 52.52 |
| Branches | 0.31 | 2.23 | 0.01 | −0.10 | 0.011 | −0.098 | 2.060 | 11.46 | 63.98 |
| Productivity | 0.91 | 3.55 | −0.19 | 0.37 | −0.200 | 0.367 | 1.470 | 8.19 | 72.18 |
| Fruit Length | 2.14 | 18.66 | 0.29 | −0.26 | 0.291 | −0.257 | 1.180 | 6.56 | 78.75 |
| Fruit Width | 7.00 | 11.17 | 0.39 | −0.17 | 0.398 | −0.168 | 1.080 | 6.00 | 84.75 |
| Fruit Wall Thickness | 12.12 | 5.64 | −0.06 | −0.42 | −0.061 | −0.419 | 0.890 | 4.99 | 89.75 |
| Locules | 0.52 | 16.41 | 0.29 | 0.03 | 0.296 | 0.030 | 0.770 | 4.28 | 94.03 |
| Fruit Weight | 9.38 | 0.69 | 0.22 | 0.16 | 0.218 | 0.155 | 0.370 | 2.08 | 96.11 |
| Edible Part | 5.12 | 1.34 | 0.07 | −0.16 | 0.067 | −0.158 | 0.220 | 1.26 | 97.38 |
| Dry Matter | 10.95 | 7.48 | 0.28 | 0.33 | 0.279 | 0.330 | 0.180 | 1.02 | 98.40 |
| Total Soluble Solids | 13.20 | 4.88 | 0.26 | 0.39 | 0.259 | 0.389 | 0.130 | 0.72 | 99.13 |
| FRAP | 14.58 | 0.29 | 0.40 | −0.02 | 0.402 | −0.017 | 0.060 | 0.34 | 99.47 |
| Total Polyphenols | 14.54 | 0.08 | 0.39 | −0.02 | 0.392 | −0.024 | 0.040 | 0.23 | 99.70 |
| Aphid Damage | 3.21 | 0.23 | −0.15 | 0.08 | −0.150 | 0.076 | 0.020 | 0.15 | 99.86 |
| Thrips-Damage | 1.20 | 7.29 | 0.15 | −0.29 | 0.150 | −0.298 | 0.010 | 0.09 | 99.95 |
| Cotton Ballworm Plant Damage | 3.80 | 7.91 | −0.24 | −0.23 | −0.240 | −0.230 | 0.004 | 0.02 | 99.98 |
| Fruit Damage | 0.14 | 4.29 | −0.01 | −0.20 | −0.001 | −0.204 | 0.002 | 0.01 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, V.; Nankar, A.N.; Yankova, V.; Tringovska, I.; Markova, D. Assessment of Balkan Pepper (Capsicum annuum L.) Accessions for Agronomic, Fruit Quality, and Pest Resistance Traits. Horticulturae 2024, 10, 389. https://doi.org/10.3390/horticulturae10040389
Todorova V, Nankar AN, Yankova V, Tringovska I, Markova D. Assessment of Balkan Pepper (Capsicum annuum L.) Accessions for Agronomic, Fruit Quality, and Pest Resistance Traits. Horticulturae. 2024; 10(4):389. https://doi.org/10.3390/horticulturae10040389
Chicago/Turabian StyleTodorova, Velichka, Amol N. Nankar, Vinelina Yankova, Ivanka Tringovska, and Dima Markova. 2024. "Assessment of Balkan Pepper (Capsicum annuum L.) Accessions for Agronomic, Fruit Quality, and Pest Resistance Traits" Horticulturae 10, no. 4: 389. https://doi.org/10.3390/horticulturae10040389
APA StyleTodorova, V., Nankar, A. N., Yankova, V., Tringovska, I., & Markova, D. (2024). Assessment of Balkan Pepper (Capsicum annuum L.) Accessions for Agronomic, Fruit Quality, and Pest Resistance Traits. Horticulturae, 10(4), 389. https://doi.org/10.3390/horticulturae10040389






