Fungal and Fungal-like Diseases of Halophytes in the Mediterranean Basin: A State-of-the-Art Review
Abstract
1. Halophytes for Saline Agriculture
2. Bibliographic Research and Resources Accessed
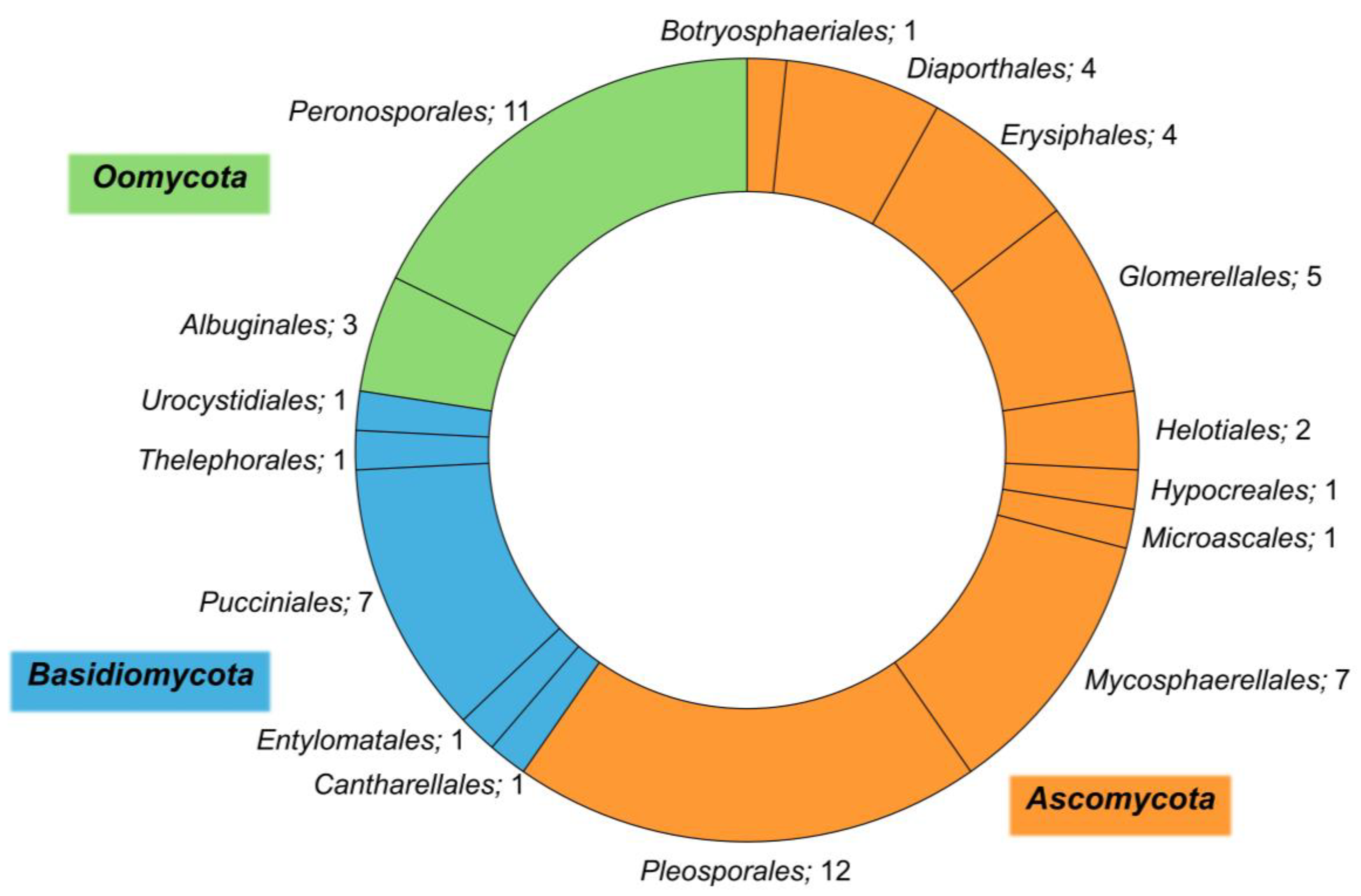
3. Plant Pathogens Associated with Halophytes
4. Diversity of Plant Pathogens among Halophytes of the Mediterranean Basin
4.1. Atriplex spp.
4.2. Beta vulgaris subsp. maritima
4.3. Cakile maritima
4.4. Crithmum maritimum
4.5. Eryngium maritimum
4.6. Lepidium spp.
4.7. Portulaca oleracea
4.8. Salicornia spp.
4.9. Salsola spp.
4.10. Soda inermis
4.11. Suaeda spp.
5. Host Range of Pathogens of the Mediterranean Halophytes
6. How Fungi and Fungal-like Organisms Deal with Osmotic Stress
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Alvarado, J.; Farris, E. Ecology and Evolution of Plants in the Mediterranean Basin: Perspectives and Challenges. Plants 2022, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, S.; Somot, S.; Li, L.; Artale, V.; Adani, M.; Bellucci, A.; Braun, A.; Calmanti, S.; Carillo, A.; Dell’Aquila, A.; et al. The CIRCE Simulations: Regional Climate Change Projections with Realistic Representation of the Mediterranean Sea. Bull. Am. Meteorol. Soc. 2013, 94, 65–81. [Google Scholar] [CrossRef]
- Luterbacher, J.; Xoplaki, E.; Casty, C.; Wanner, H.; Pauling, A.; Küttel, M.; Rutishauser, T.; Brönnimann, S.; Fischer, E.; Fleitmann, D.; et al. Chapter 1 Mediterranean Climate Variability over the Last Centuries: A Review. In Developments in Earth and Environmental Sciences; Lionello, P., Malanotte-Rizzoli, P., Boscolo, R., Eds.; Mediterranean; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 27–148. [Google Scholar]
- Rizzo, A.; Vandelli, V.; Gauci, C.; Buhagiar, G.; Micallef, A.S.; Soldati, M. Potential Sea Level Rise Inundation in the Mediterranean: From Susceptibility Assessment to Risk Scenarios for Policy Action. Water 2022, 14, 416. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil Degradation in the European Mediterranean Region: Processes, Status and Consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Rudi Hessel, R. Soil Threats in Europe; EUR 27607 EN; EU Publications: Luxembourg, 2015. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of Salt Tolerance in Halophytes: Current Understanding and Recent Advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Feng, Z.T.; Deng, Y.Q.; Liang, X.; Yuan, F.; Hao, J.L.; Zhang, J.C.; Sun, F.S.; Wang, B.S. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 2015, 238, 286–296. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Shiri, M.; Rabhi, M.; El Amrani, A.; Abdelly, C. Cross-tolerance to Abiotic Stresses in Halophytes: Application for Phytoremediation of Organic Pollutants. Acta Physiol. Plant. 2015, 37, 209. [Google Scholar] [CrossRef]
- Duarte, B.; Caçador, I. Iberian Halophytes as Agroecological Solutions for Degraded Lands and Biosaline Agriculture. Sustainability 2021, 13, 1005. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Lombardi, T.; Ventura, I.; Bertacchi, A. Floristic Inventory of Ethnobotanically Important Halophytes of North-Western Mediterranean Coastal Brackish Areas, Tuscany, Italy. Agronomy 2023, 13, 615. [Google Scholar] [CrossRef]
- Strinati, M. Salicornia, the Resilient Sea Asparagus. 2024. Available online: https://www.greatitalianfoodtrade.it/en/markets/salicornia-lasparagus-resilient-sea-fish/ (accessed on 14 March 2024).
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011; ISBN 978-92-79-20199-8. [Google Scholar] [CrossRef]
- Papanastasis, V.P.; Yiakoulaki, M.D.; Decandia, M.; Dini-Papanastasi, O. Integrating Woody Species into Livestock Feeding in the Mediterranean Areas of Europe. Anim. Feed. Sci. Technol. 2008, 140, 1–17. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant Properties, Phenolic Composition, Bioactive Compounds and Nutritive Value of Medicinal Halophytes Commonly Used as Herbal Teas. South Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Stanković, M.S.; Petrović, M.; Godjevac, D.; Stevanović, Z.D. Screening Inland Halophytes from the Central Balkan for Their Antioxidant Activity in Relation to Total Phenolic Compounds and Flavonoids: Are There Any Prospective Medicinal Plants? J. Arid. Environ. 2015, 120, 26–32. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Quah, E.P.L. Antioxidant Properties of Different Cultivars of Portulaca oleracea. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Wagenseil, N.B.; Buhmann, A.K.; Seal, C.E.; Wade, E.M.; Muscolo, A.; Papenbrock, J. Manipulating the Antioxidant Capacity of Halophytes to Increase Their Cultural and Economic Value through Saline Cultivation. AoB Plants 2014, 6, plu046. [Google Scholar] [CrossRef]
- Lopes, M.; Castilho, M.d.C.; Sanches-Silva, A.; Freitas, A.; Barbosa, J.; Gonçalves, M.J.; Cavaleiro, C.; Ramos, F. Evaluation of the Mycotoxins Content of Salicornia spp.: A Gourmet Plant Alternative to Salt. Food Addit. Contam. Part B 2020, 13, 162–170. [Google Scholar] [CrossRef]
- Gunning, D. Cultivating Salicornia Europaea (Marsh Samphire); BIM: Laoghaire, Ireland, 2016. [Google Scholar]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Kraouia, M.; Nartea, A.; Maoloni, A.; Osimani, A.; Garofalo, C.; Fanesi, B.; Ismaiel, L.; Aquilanti, L.; Pacetti, D. Sea Fennel (Crithmum maritimum L.) as an Emerging Crop for the Manufacturing of Innovative Foods and Nutraceuticals. Molecules 2023, 28, 4741. [Google Scholar] [CrossRef]
- Custódio, M.; Lillebø, A.I.; Calado, R.; Villasante, S. Halophytes as Novel Marine Products—A Consumers’ Perspective in Portugal and Policy Implications. Mar. Policy 2021, 133, 104731. [Google Scholar] [CrossRef]
- Maciel, E.; Domingues, P.; Domingues, M.R.M.; Calado, R.; Lillebø, A. Halophyte Plants from Sustainable Marine Aquaponics Are a Valuable Source of Omega-3 Polar Lipids. Food Chem. 2020, 320, 126560. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.; Maciel, E.; Domingues, M.R.; Calado, R.; Lillebø, A.I. Halophyte Plants Cultured in Aquaponics Hold the Same Potential for Valorization as Wild Conspecifics from Donor Sites. Appl. Sci. 2021, 11, 11586. [Google Scholar] [CrossRef]
- Oliveira, V.; Martins, P.; Marques, B.; Cleary, D.F.R.; Lillebø, A.I.; Calado, R. Aquaponics Using a Fish Farm Effluent Shifts Bacterial Communities Profile in Halophytes Rhizosphere and Endosphere. Sci. Rep. 2020, 10, 10023. [Google Scholar] [CrossRef] [PubMed]
- Spradlin, A.; Saha, S. Saline Aquaponics: A Review of Challenges, Opportunities, Components, and System Design. Aquaculture 2022, 555, 738173. [Google Scholar] [CrossRef]
- Stouvenakers, G.; Dapprich, P.; Massart, S.; Jijakli, M.H. Plant Pathogens and Control Strategies in Aquaponics. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 353–378. ISBN 978-3-030-15943-6. [Google Scholar]
- Calabon, M.S.; Jones, E.B.G.; Promputtha, I.; Hyde, K.D. Fungal Biodiversity in Salt Marsh Ecosystems. J. Fungi 2021, 7, 648. [Google Scholar] [CrossRef] [PubMed]
- Farr and Rossman Fungal Databases—Fungus-Host Distribution. Available online: https://fungi.ars.usda.gov/ (accessed on 14 January 2024).
- GBIF: The Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 9 January 2024).
- eHALOPH Halophyte Database v4.65. Available online: https://ehaloph.uc.pt/ (accessed on 9 January 2024).
- Index Fungorum Homepage. Available online: www.indexfungorum.org (accessed on 22 January 2024).
- Kirk, P.; Cannon, P.; Stalpers, J.; Minter, D.W. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Maciá-Vicente, J.G.; Ferraro, V.; Burruano, S.; Lopez-Llorca, L.V. Fungal Assemblages Associated with Roots of Halophytic and Non-Halophytic Plant Species Vary Differentially Along a Salinity Gradient. Microb. Ecol. 2012, 64, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Maciá-Vicente, J.G.; Jansson, H.-B.; Abdullah, S.K.; Descals, E.; Salinas, J.; Lopez-Llorca, L.V. Fungal Root Endophytes from Natural Vegetation in Mediterranean Environments with Special Reference to Fusarium spp. FEMS Microbiol. Ecol. 2008, 64, 90–105. [Google Scholar] [CrossRef]
- González-Menéndez, V.; Crespo, G.; de Pedro, N.; Diaz, C.; Martín, J.; Serrano, R.; Mackenzie, T.A.; Justicia, C.; González-Tejero, M.R.; Casares, M.; et al. Fungal Endophytes from Arid Areas of Andalusia: High Potential Sources for Antifungal and Antitumoral Agents. Sci. Rep. 2018, 8, 9729. [Google Scholar] [CrossRef]
- El-Morsy, E.-M. Fungi Isolated from the Endorhizosphere of Halophytic Plants from the Red Sea Coast of Egypt. Fungal Divers. 2000, 5, 43–54. [Google Scholar]
- Constaninescu, O. An Annotated List of Peronospora Names: Thunbergia 15. Mycologia 1992, 84, 953. [Google Scholar] [CrossRef]
- van der Aa, H.; van Kesteren, H.A. Some Pycnidial Fungi Occurring on Atriplex and Chenopodium. Persoonia Mol. Phylogeny Evol. Fungi 1979, 10, 267–276. [Google Scholar]
- Conners, I. An Annotated Index of Plant Diseases in Canada and Fungi Recorded on Plants in Alaska, Canada and Greenland. Aspen Bibliogr. 1967, 60, 222. [Google Scholar]
- Andrianova, T.V.; Minter, D.W. Stagonospora atriplicis. [Descriptions of Fungi and Bacteria]. Descr. Fungi Bact. 2005, Sheet 1630. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Klosterman, S.J.; Kummer, V.; Voglmayr, H.; Shin, H.-D.; Thines, M. Multi-Locus Tree and Species Tree Approaches toward Resolving a Complex Clade of Downy Mildews (Straminipila, Oomycota), Including Pathogens of Beet and Spinach. Mol. Phylogenetics Evol. 2015, 86, 24–34. [Google Scholar] [CrossRef][Green Version]
- Queiroz, J.; Santos, M.A.; Santos, A.M.J.; Michereff, S.; Freire, M. First Report of Diaporthe inconspicua Associated with Shoot Blight of Atriplex nummularia in Brazil. J. Plant Pathol. 2017, 99, 542. [Google Scholar] [CrossRef]
- Blagoveshchenskaya, E.Y. Peronosporales of Skadovsky Zvenigorod Biological Station (Moscow Region). Nov. Sist. Nizshikh Rastenii 2018, 52, 91–100. [Google Scholar] [CrossRef]
- Chater, A.O.; Woods, R.; Stringer, R.; Evans, D.; Smith, P. Downy Mildews (Peronosporaceae) and White Blister-Rusts (Albuginaceae) of Wales; A.O. Chater: Aberystwyth, UK, 2020. [Google Scholar]
- Müller, J.; Kokeš, P. Erweitertes Verzeichnis Der Falschen Mehltaupilze Mährens Und Tschechisch Schlesiens. Czech Mycol. 2008, 60, 91–104. [Google Scholar] [CrossRef]
- Francis, S.M.; Waterhouse, G.M. List of Peronosporaceae Reported from the British Isles. Trans. Br. Mycol. Soc. 1988, 91, 1–62. [Google Scholar] [CrossRef]
- Gustavsson, A. The Genus “Peronospora” in the Iberian Peninsula, Especially in Northern Spain and Andorra. An. Jardín Botánico Madr. 1991, 49, 3–38. [Google Scholar]
- Mułenko, W.; Majewski, T.; Ruszkiewicz-Michalska, M. A Preliminary Checklist of Micromycetes in Poland = Wstępna Lista Grzybów Mikroskopijnych Polski; Biodiversity of Poland; W. Szafer Institute of Botany Polish Academy of Sciences: Kraków, Poland, 2008; ISBN 978-83-89648-75-4. [Google Scholar]
- Pollack, F.G. An Annotated Compilation of Cercospora Names. Mycol. Mem. 1987, 12, 1–212. [Google Scholar]
- Adamska, I. Microscopic Fungus-like Organisms and Fungi of the Słowiński National Park. II. (NW PoIand). Acta Mycol. 2001, 36, 31–65. [Google Scholar] [CrossRef]
- Braun, U. Miscellaneous Notes on Phytopathogenic Hyphomycetes (II). Mycotaxon 1995, 55, 223–241. [Google Scholar]
- McKenzie, E.H.C. New Plant Disease Records in New Zealand: Miscellaneous Fungal Pathogens II. N. Z. J. Crop Hortic. Sci. 1990, 18, 65–73. [Google Scholar] [CrossRef]
- Shaw, C. Host Fungus Index for the Pacific Northwest-1. Hosts. Wash. State Univ. Agric. Exp. Stn. Bull. 1973, 765, 1–121. [Google Scholar]
- Priest, M.J. Fungi of Australia: Septoria; Australian Biological Resources Study/CSIRO Publishing: Clayton, Australia, 2006. [Google Scholar]
- Fraser, W.P. Cultures of Some Heteroecious Rusts. Mycologia 1911, 3, 67–74. [Google Scholar] [CrossRef]
- Piątek, M.; Wołczańska, A. Some Phytopathogenic Fungi Rare or New to Poland. Pol. Bot. J. 2004, 49, 67–72. [Google Scholar]
- Pirnia, M.; Zare, R.; Zamanizadeh, H.R.; Khodaparast, A. New Records of Cercosporoid Hyphomycetes from Iran. Mycotaxon 2012, 120, 157–169. [Google Scholar] [CrossRef]
- Amano, K. Host Range and Geographical Distribution of the Powdery Mildew Fungi., 2nd ed.; Japan Scientific Societies Press: Tokyo, Japan, 1986; ISBN 978-4-7622-9486-0. [Google Scholar]
- Gjærum, H.B. Rust Fungi from Madeira. Bol. Mus. Munic. Funchal 1982, 34, 5–22. [Google Scholar]
- Gjærum, H.B.; Sunding, P. Flora of Macaronesia. Checklist of rust fungi (Uredinales). Sommerfeltia 1986, 4, 1–46. [Google Scholar] [CrossRef]
- Oliver, E.J.; Thrall, P.H.; Burdon, J.J.; Ash, J.E. Vertical Disease Transmission in the Cakile-Alternaria Host-Pathogen Interaction. Aust. J. Bot. 2001, 49, 561–569. [Google Scholar] [CrossRef]
- Chalbi, A.; Sghaier-Hammami, B.; Meca, G.; Quiles, J.M.; Abdelly, C.; Marangi, C.; Logrieco, A.F.; Moretti, A.; Masiello, M. Characterization of Mycotoxigenic Alternaria Species Isolated from the Tunisian Halophyte Cakile maritima. Phytopathol. Mediterr. 2020, 59, 107–108. [Google Scholar] [CrossRef]
- Chalbi, A.; Sghaier-Hammami, B.; Baazaoui, N.; Hammami, S.B.M.; Ben-Jouira, H.; García-Caparrós, P.; Djébali, N.; Regaya, I.; Debez, A.; Jorrín-Novo, J.V.; et al. Comparative Study of the Effect of Salt Stress, Alternaria alternata Attack or Combined Stress on the Cakile maritima Growth and Physiological Performance. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12446. [Google Scholar] [CrossRef]
- Thrall, P.H.; Burdon, J.J.; Bock, C.H. Short-Term Epidemic Dynamics in the Cakile maritima–Alternaria brassicicola Host–Pathogen Association. J. Ecol. 2001, 89, 723–735. [Google Scholar] [CrossRef]
- Baka, Z.A.M. A Fine-structural Study of the Downy Mildew Fungus Peronospora parasitica in the New Host Cakile maritima. Arch. Phytopathol. Plant Prot. 1995, 30, 41–51. [Google Scholar] [CrossRef]
- Mckenzie, E.H.C.; Dingley, J.M. New Plant Disease Records in New Zealand: Miscellaneous Fungal Pathogens III. N. Z. J. Bot. 1996, 34, 263–272. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Baka, Z.A.M. Physiological and Biochemical Changes in Host Leaf Tissues Associated with the Growth of Two Biotrophic Fungi Growing in Egypt. Phyton 1991, 32, 129–142. [Google Scholar]
- García-Blázquez, G.; Constantinescu, O.; Telleria, M.; Martín, M. Preliminary Check List of Albuginales and Peronosporales (Chromista) Reported from the Iberian Peninsula and Balearic Islands. Mycotaxon 2006, 98, 185–188. [Google Scholar]
- Voytyuk, S.; Heluta, V.; Wasser, S.; Nevo, E.; Takamatsu, S. Biodiversity of the Powdery Mildew Fungi (Erysiphales, Ascomycota) of Israel (Biodiversity of Cyanoprocaryotes, Algae and Fungi of Israel); Ganter Verlag: Ruggell, Liechtenstein, 2009; ISBN 978-3-906166-74-2. [Google Scholar]
- Braun, U. The Powdery Mildews (Erysiphales) of Europe; Gustav Fischer Verlag: Jena, Germany, 1995; ISBN 978-3-334-60994-1. [Google Scholar]
- Pantidou, M.E. Fungus-Host Index for Greece; Benaki Phytopathological Institute: Kiphissia, Athens, 1973. [Google Scholar]
- Braun, U. A Monograph of Cercosporella, Ramularia and Allied Genera (Phytopathogenic Hyphomycetes); IHW-Verlag: Eching, Germany, 1995; Volume 1, ISBN 978-3-930167-11-1. [Google Scholar]
- Savchenko, K.G.; Carris, L.M.; Castlebury, L.A.; Heluta, V.P.; Wasser, S.P.; Nevo, E. Revision of Entyloma (Entylomatales, Exobasidiomycetes) on Eryngium. Mycologia 2014, 106, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants: An Identification Handbook; Richmond Publishing: London, UK, 1997. [Google Scholar]
- Unamuno, L.M. Enumeración y distribución geográfica de los Ascomicetos de la Península Ibérica y de las Islas Baleares. Mem. Real. Acad. Ci Exact. Madr. Ser. Ci Nat. 1941, 8, 1–403. [Google Scholar]
- Fotouhifar, K.-B.; Hedjaroude, G.-A.; Leuchtmann, A. ITS rDNA Phylogeny of Iranian Strains of Cytospora and Associated Teleomorphs. Mycologia 2010, 102, 1369–1382. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Thines, M.; Choi, I.-Y.; Shin, H.-D. Perofascia Is Not Monotypic: The Description of the Second Taxon Affecting the South American Crop Maca (Lepidium meyenii). Mycol. Prog. 2017, 16, 857–864. [Google Scholar] [CrossRef]
- Gannibal, P.; Gasich, E.L. Causal Agents of the Alternariosis of Cruciferous Plants in Russia: Species Composition, Geography and Ecology. Mikol. I Fitopatol. 2009, 43, 447–456. [Google Scholar]
- Bakhshi, M.; Arzanlou, M.; Babai-ahari, A.; Groenewald, J.Z.; Crous, P.W. Novel Primers Improve Species Delimitation in Cercospora. IMA Fungus 2018, 9, 299–332. [Google Scholar] [CrossRef]
- Gautam, A.; Avasthi, S. Diversity of Powdery Mildew Fungi from North Western Himalayan Region of Himachal Pradesh—A Checklist. Plant Pathol. Quar. 2018, 8, 78–99. [Google Scholar] [CrossRef]
- Kabaktepe, S.; Heluta, V.; Akata, I. Checklist of Powdery Mildews (Erysiphales) in Turkey. Biol. Divers. Conserv. 2015, 8, 128–146. [Google Scholar]
- Ramazanova, S.S.; Faizieva, F.K.; Sagdullaeva, M.S.; Kirgizbaeva, K.M.; Gaponenko, N.I. Fungal Flora of Uzbekistan, Rustfungi. Fan Publ. Task. 1986, 3, 1–229. [Google Scholar]
- Koike, S.T.; Sullivan, M.J.; Southwick, C.; Feng, C.; Correll, J.C. Characterization of White Rust of Perennial Pepperweed Caused by Albugo candida in California. Plant Dis. 2011, 95, 876. [Google Scholar] [CrossRef] [PubMed]
- Rector, B.G.; Wang, S.; Choi, Y.-J.; Thines, M. First Report of Albugo lepidii Causing White Rust on Broadleaved Pepperweed (Lepidium latifolium) in Nevada and California. Plant Dis. 2016, 100, 229. [Google Scholar] [CrossRef]
- Scholtz, H.; Scholtz, I. Die Brandpilze Deutschlands (Ustilaginales); Englera: La Jolla, CA, USA, 1988; Volume 8. [Google Scholar]
- Woolliams, G.E. Host Range and Symptomatology of Verticillium dahliae in Economic, Weed, and Native Plants in Interior British Columbia. Can. J. Plant Sci. 1966, 46, 661–669. [Google Scholar] [CrossRef]
- Ruszkiewicz-Michalska, M.; Michalski, M. Phytopathogenic Micromycetes in Central Poland. I. Peronosporales and Erysiphales. Acta Mycol. 2005, 40, 223–250. [Google Scholar] [CrossRef][Green Version]
- Simmonds, J.H. Host Index of Plant Diseases in Queensland; Queensland Department of Primary Industries: Brisbane, Australia, 1966.
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum Species with Curved Conidia from Herbaceous Hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Gayed, S.K. Host Range and Persistence of Thielaviopsis basicola in Tobacco Soil. Can. J. Plant Sci. 1972, 52, 869–873. [Google Scholar] [CrossRef]
- Alfieri, S.A., Jr.; Lanngdon, K.R.; Wehlburg, C.; Kimbrough, J.W. Index of Plant Diseases in Florida; Bulletin 11; Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1984.
- Shivas, R.G. Fungal and Bacterial Diseases of Plants in Western Australia. J. R Soc. West. Aust. 1989, 72, 1–62. [Google Scholar]
- Mcdonald, M.R.; Stricker, S.; Gossen, B.D. Weed Hosts and Winter Survival of Stemphylium vesicarium on Onion in Ontario, Canada. Can. J. Plant Pathol. 2023, 45, 134–139. [Google Scholar] [CrossRef]
- Heidari, K.; Farokhinejad, R.; Mehrabi-Koushki, M. Occurrence of Purslane Leaf Spot Caused by Dichotomophthora lutea in Iran. Australas. Plant Dis. Notes 2018, 13, 33. [Google Scholar] [CrossRef]
- de Hoog, G.S.; van Oorschot, C.A.N. Taxonomy of the Dactylaria Complex I: Notes on the Genus Dichotomophthora. Proc. Van De K. Ned. Akad. Van Wet. Sect. C 1983, 86, 55–61. [Google Scholar]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.J.; Akulov, A.; Carnegie, A.J.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.Z.; Guarnaccia, V.; Halleen, F.; et al. Genera of Phytopathogenic Fungi: GOPHY 2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef]
- Venturella, G. A checklist of Sicilian Fungi. Bocconea 1991, 2, 5–221. [Google Scholar]
- Kalu, N.N.; Sutton, J.C.; Vaartaja, O. Pythium spp. Associated with Root Dieback of Carrot in Ontario. Can. J. Plant Sci. 1976, 56, 555–561. [Google Scholar] [CrossRef]
- Eken, C. Dichotomophthora portulacae on Portulaca oleracea in Turkey. Mycotaxon 2003, 87, 153–156. [Google Scholar]
- Mehrlich, F.P.; Fitzpatrick, H.M. Dichotomophthora portulacae, A Pathogen of Portulaca oleracea. Mycologia 1935, 27, 543–550. [Google Scholar] [CrossRef]
- Abdulla, Z.K. Dichotomophthora portulacae, a New Record from Iraq. Mycotaxon 2021, 136, 215–218. [Google Scholar] [CrossRef]
- Mitchell, J.K. Dichotomophthora portulacae Causing Black Stem Rot on Common Purslane in Texas. Plant Dis. 1986, 70, 603b. [Google Scholar] [CrossRef]
- Klisiewicz, J.M. Black Stem: A Fungal Disease of Common Purslane in California. Plant Dis. 1983, 67, 1162. [Google Scholar] [CrossRef]
- Rader, W.E. Helminthosporium portulacae a New Pathogen of Portulaca oleracea L. Mycologia 1948, 40, 342–346. [Google Scholar] [CrossRef]
- Savile, D.B.O.; Parmelee, J.A. Some Fungal Parasites of Portulacaceae. Mycologia 1956, 48, 573–590. [Google Scholar] [CrossRef]
- El-Buni, A.M.; Rattan, S.S. Check List of Libyan Fungi; Flora of Libya; Al Faateh University Faculty of Science Department of Botany: Tripoli, Libya, 1981. [Google Scholar]
- Riethmüller, A.; Voglmayr, H.; Göker, M.; Weiß, M.; Oberwinkler, F. Phylogenetic Relationships of the Downy Mildews (Peronosporales) and Related Groups Based on Nuclear Large Subunit Ribosomal DNA Sequences. Mycologia 2002, 94, 834–849. [Google Scholar] [CrossRef]
- Vrandecic, K.; Jerković, D.; Cosic, J.; Poštić, J.; Bijelić, Z. White Blister Species (Albuginaceae) on Weeds. Poljoprivreda 2011, 17, 47–51. [Google Scholar]
- Mahmoudi, E.; Ahmadi, A. White Rust Disease of Portulaca oleracea Caused by Wilsoniana portulacae, First Report in Isfahan. In Proceedings of the 1st International Conference on New Ideas in Agriculture, Islamic Azad University Khorasgan Branch, Isfahan, Iran, 26–27 January 2014. [Google Scholar]
- Heller, A.; Thines, M. Evidence for the Importance of Enzymatic Digestion of Epidermal Walls during Subepidermal Sporulation and Pustule Opening in White Blister Rusts (Albuginaceae). Mycol. Res. 2009, 113, 657–667. [Google Scholar] [CrossRef]
- Tóth, T.; Kövics, G. White Rust Species (Chromista, Peronosporomycetes, Albuginales, Albuginaceae) on Common Weeds in Hungary. Acta Agrar. Debreceniensis 2015, 66, 30–33. [Google Scholar] [CrossRef]
- Man in ’t Veld, W.A.; Rosendahl, K.C.H.M.; van Rijswick, P.C.J.; Meffert, J.P.; Boer, E.; Westenberg, M.; van der Heide, T.; Govers, L.L. Multiple Halophytophthora spp. and Phytophthora spp. Including P. gemini, P. inundata and P. chesapeakensis sp. nov. Isolated from the Seagrass Zostera marina in the Northern Hemisphere. Eur. J. Plant Pathol. 2019, 153, 341–357. [Google Scholar] [CrossRef]
- Braun, U. Die Rostpilze (Uredinales) der Deutschen Demokratischen Republik. Feddes Repert. 1982, 93, 213–333. [Google Scholar] [CrossRef]
- Henderson, D.M. A Checklist of the Rust Fungi of the British Isles; British Mycological Society: Manchester, UK, 2000; ISBN 978-0-9527704-4-2. [Google Scholar]
- Woods, R.; Stringer, R.; Evans, D.; Chater, A. Rust Fungus Red Data List and Census Catalogue for Wales; A.O. Chater: Aberystwyth, UK, 2015. [Google Scholar]
- Agustí-Brisach, C.; Gramaje, D.; León, M.; García-Jiménez, J.; Armengol, J. Evaluation of Vineyard Weeds as Potential Hosts of Black-Foot and Petri Disease Pathogens. Plant Dis. 2011, 95, 803–810. [Google Scholar] [CrossRef]
- Khodaparast, S.A.; Takamatsu, S.; Hedjaroude, G.-A. Phylogenetic Structure of the Genus Leveillula (Erysiphales: Erysiphaceae) Inferred from the Nucleotide Sequences of the rDNA ITS Region with Special Reference to the L. taurica Species Complex. Mycol. Res. 2001, 105, 909–918. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; McHenry, J. Check List and Host Index for Arizona Rust Fungi. Bull. Univ. Ariz. Agric. Exp. Stn. 1969, 186, 1–40. [Google Scholar]
- Jørstad, I. Uredinales of the Canary Islands. Skr. Utg. Av. Det. Nor. Vidensk. Akad. I Oslo. 1958, 2, 1–182. [Google Scholar]
- Hasan, S.; Sobhian, R.; Hérard, F. Biology, Impact and Preliminary Host-Specificity Testing of the Rust Fungus, Uromyces salsolae, a Potential Biological Control Agent for Salsola kali in the USA. Biocontrol Sci. Technol. 2001, 11, 677–689. [Google Scholar] [CrossRef]
- Richiteanu, A. Uredinales from North Algeria. Rev. Roum. Biol. 1960, 35, 33–44. [Google Scholar]
- Schwarczinger, I.; Vajna, L.; Bruckart, W.L. First Report of Colletotrichum gloeosporioides on Russian-Thistle. Plant Dis. 1998, 82, 1405. [Google Scholar] [CrossRef] [PubMed]
- Berner, D.K.; Cavin, C.A.; McMahon, M.B.; Loumbourdis, I. First Report of Anthracnose of Salsola tragus Caused by Colletotrichum gloeosporioides in Greece. Plant Dis. 2006, 90, 971. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, T.; Skatenok, O.; Alexandrova, A.; Mukhina, Z.; Matveeva, T.; Bogomaz, D.; Berner, D.K.; Cavin, C.A. First Report of Anthracnose of Salsola tragus Caused by Colletotrichum gloeosporioides in Russia. Plant Dis. 2008, 92, 1366. [Google Scholar] [CrossRef] [PubMed]
- Berner, D.; Lagopodi, A.L.; Kashefi, J.; Mukhina, Z.; Kolomiets, T.; Pankratova, L.; Kassanelly, D.; Cavin, C.; Smallwood, E. Field Assessment, in Greece and Russia, of the Facultative Saprophytic Fungus, Colletotrichum salsolae, for Biological Control of Russian Thistle (Salsola tragus). Biol. Control 2014, 76, 114–123. [Google Scholar] [CrossRef]
- Kolomiets, T.; Mukhina, Z.; Matveeva, T.; Bogomaz, D.; Berner, D.K.; Cavin, C.A.; Castlebury, L.A. First Report of Stem Canker of Salsola tragus Caused by Diaporthe eres in Russia. Plant Dis. 2009, 93, 110. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, N. The Rust Flora of Japan; Tsukuba Shuppankai: Tsukuba, Japan, 1992; ISBN 978-4-924753-11-2. [Google Scholar]
- Oner, M.; Dizbay, M.; Ekmekci, S. An Investigation of Some Leaf Rust, Smuts, Powdery Mildews and Leaf Spots Occurring of the Natural Flora of Southern Egean Region. Bitki Derg. 1974, 1, 426–431. [Google Scholar]
- Gjærum, H.B. Two Rust Species from the Salvage Islands. Bocagiana Mus. Munic. Funchal 1975, 41, 1–3. [Google Scholar]
- Dias, M.R.d.S.; Lucas, M.T. Fungi of Madeira and Selvagem Islands. Bol. Soc. Broteriana 1980, 53, 469–473. [Google Scholar]
- Le Houérou, H.N. The Role of Saltbushes (Atriplex spp.) in Arid Land Rehabilitation in the Mediterranean Basin: A Review. Agroforest. Syst. 1992, 18, 107–148. [Google Scholar] [CrossRef]
- Fries, E.M. Epicrisis Systematis Mycologici; E Typographia Academica, Upsaliae: Uppsala, Sweden, 1836. [Google Scholar]
- Fries, E.M. Summa Vegetabilium Scandinaviae; E Typographia Academica, Upsaliae: Uppsala, Sweden, 1845. [Google Scholar]
- Gäumann, E. Zur Kenntnis Der Chenopodiaceen Bewohnenden Peronospora-Arten. Mitteilungen Naturforschenden Ges. Bern. 1918, 45–66. [Google Scholar] [CrossRef]
- Săvulescu, T.; Rayss, T. Contribution a La Connaissance Des Peronsporacees de Roumanie. Ann. Mycol. 1932, 28, 297–320. [Google Scholar]
- Yerkes, W.D.; Shaw, C.G. Taxonomy of the Peronospora Species on Cruciferae and Chenopodiaceae. Phytopathology 1959, 49, 499–507. [Google Scholar]
- Voglmayr, H. Phylogenetic Relationships of Peronospora and Related Genera Based on Nuclear Ribosomal ITS Sequences. Mycol. Res. 2003, 107, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Hong, S.-B.; Shin, H.-D. Re-Consideration of Peronospora farinosa Infecting Spinacia oleracea as Distinct Species, Peronospora effusa. Mycol. Res. 2007, 111, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Denchev, C.M.; Shin, H.-D. Morphological and Molecular Analyses Support the Existence of Host-Specific Peronospora Species Infecting Chenopodium. Mycopathologia 2008, 165, 155–164. [Google Scholar] [CrossRef]
- Thines, M.; Choi, Y.-J. Evolution, Diversity, and Taxonomy of the Peronosporaceae, with Focus on the Genus Peronospora. Phytopathology 2016, 106, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Danielsen, S.; Lübeck, M.; Hong, S.-B.; Delhey, R.; Shin, H.-D. Morphological and Molecular Characterization of the Causal Agent of Downy Mildew on Quinoa (Chenopodium quinoa). Mycopathologia 2010, 169, 403–412. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Thines, M. (2288) Proposal to Reject the Name Botrytis farinosa (Peronospora farinosa) (Peronosporaceae: Oomycetes). Taxon 2014, 63, 675–676. [Google Scholar] [CrossRef]
- May, T.W. Report of the Nomenclature Committee for Fungi—20. IMA Fungus 2017, 8, 189–203. [Google Scholar] [CrossRef]
- Wilson, K.L. Report of the General Committee: 18. Taxon 2017, 66, 742–744. [Google Scholar] [CrossRef]
- Kempenaar, C.; Horsten, P.J.F.M.; Scheepens, P.C. Spore Germination and Disease Development after Application of Pycnidiospores of Ascochyta caulina to Chenopodium album Plants. Eur. J. Plant Pathol. 1996, 102, 143–153. [Google Scholar] [CrossRef]
- van der Aa, H.; Vanev, S. A Revision of the Species Described in Phyllosticta; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002. [Google Scholar]
- de Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Redisposition of Phoma-like Anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Wanasinghe, D.N.; Hyde, K.D.; Jeewon, R.; Crous, P.W.; Wijayawardene, N.N.; Jones, E.B.G.; Bhat, D.J.; Phillips, A.J.L.; Groenewald, J.Z.; Dayarathne, M.C.; et al. Phylogenetic Revision of Camarosporium (Pleosporineae, Dothideomycetes) and Allied Genera. Stud. Mycol. 2017, 87, 207–256. [Google Scholar] [CrossRef]
- Weiland, J.; Koch, G. Sugarbeet Leaf Spot Disease (Cercospora beticola Sacc.). Mol. Plant Pathol. 2004, 5, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Palti, J. The Leveillula Mildews. Bot. Rev. 1988, 54, 423–535. [Google Scholar] [CrossRef]
- Heluta, V.P.; Minter, D.W. Leveillula cylindrospora. [Descriptions of Fungi and Bacteria]. Descr. Fungi Bact. 1998, Sheet 1373. [Google Scholar] [CrossRef]
- Takamatsu, S. Origin and Evolution of the Powdery Mildews (Ascomycota, Erysiphales). Mycoscience 2012, 54, 75–86. [Google Scholar] [CrossRef]
- Davy, A.J.; Scott, R.; Cordazzo, C.V. Biological Flora of the British Isles: Cakile maritima Scop. J. Ecol. 2006, 94, 695–711. [Google Scholar] [CrossRef]
- Bock, C.H.; Thrall, P.H.; Burdon, J.J. Genetic Structure of Populations of Alternaria brassicicola Suggests the Occurrence of Sexual Recombination. Mycol. Res. 2005, 109, 227–236. [Google Scholar] [CrossRef]
- Pinto, V.E.F.; Patriarca, A. Alternaria Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 13–32. ISBN 978-1-4939-6707-0. [Google Scholar]
- Holub, E.B. Phenotypic and Genotypic Variation in the Interaction between Arabidopsis thaliana and Albugo candida. Mol. Plant-Microbe Interact. MPMI 1995, 8, 916. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Hong, S.-B.; Shin, H.-D. Genetic Diversity within the Albugo candida Complex (Peronosporales, Oomycota) Inferred from Phylogenetic Analysis of ITS rDNA and COX2 mtDNA Sequences. Mol. Phylogenet. Evol. 2006, 40, 400–409. [Google Scholar] [CrossRef]
- Voglmayr, H.; Riethmüller, A. Phylogenetic Relationships of Albugo Species (White Blister Rusts) Based on LSU rDNA Sequence and Oospore Data. Mycol. Res. 2006, 110, 75–85. [Google Scholar] [CrossRef]
- Constantinescu, O.; Fatehi, J. Peronospora-like Fungi (Chromista, Peronosporales) Parasitic on Brassicaceae and Related Hosts. Nova Hedwig. 2002, 74, 291–338. [Google Scholar] [CrossRef]
- Abdallah, A.; Zouhaier, B.; Rabhi, M.; Abdelly, C.; Smaoui, A. Environmental Eco-Physiology and Economical Potential of the Halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plant Res. 2011, 5, 3564–3571. [Google Scholar]
- Renna, M. Reviewing the Prospects of Sea Fennel (Crithmum maritimum L.) as Emerging Vegetable Crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef]
- Vánky, K. Taxonomic Studies on Ustilaginomycetes—29. Mycotaxon 2009, 110, 289–324. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A Genus of Endophytic, Saprobic and Plant Pathogenic Fungi. Persoonia Mol. Phylogeny Evol. Fungi 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Farr, D.F.; Rossman, A.Y.; Jaklitsch, W. Diaporthe angelicae comb. nov., a Modern Description and Placement of Diaporthopsis in Diaporthe. Mycoscience 2003, 44, 203–208. [Google Scholar] [CrossRef]
- Ellis, J.B.; Kellerman, W.A. New Kansas Fungi. J. Mycol. 1887, 3, 102–105. [Google Scholar] [CrossRef]
- Young, J.A.; Palmquist, D.E.; Blank, R.R. The Ecology and Control of Perennial Pepperweed (Lepidium latifolium L.). Weed Technol. 1998, 12, 402–405. [Google Scholar] [CrossRef]
- Kaur, T.; Hussain, K.; Koul, S.; Vishwakarma, R.; Vyas, D. Evaluation of Nutritional and Antioxidant Status of Lepidium latifolium Linn.: A Novel Phytofood from Ladakh. PLoS ONE 2013, 8, e69112. [Google Scholar] [CrossRef]
- Conde-Rioll, M.; Gajate, C.; Fernández, J.J.; Villa-Pulgarin, J.A.; Napolitano, J.G.; Norte, M.; Mollinedo, F. Antitumor Activity of Lepidium latifolium and Identification of the Epithionitrile 1-Cyano-2,3-Epithiopropane as Its Major Active Component. Mol. Carcinog. 2018, 57, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Pane, C.; Manganiello, G.; Nicastro, N.; Cardi, T.; Carotenuto, F. Powdery Mildew Caused by Erysiphe cruciferarum on Wild Rocket (Diplotaxis tenuifolia): Hyperspectral Imaging and Machine Learning Modeling for Non-Destructive Disease Detection. Agriculture 2021, 11, 337. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Seed Germination Ecology of Portulaca oleracea L.: An Important Weed of Rice and Upland Crops. Ann. Appl. Biol. 2009, 155, 61–69. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical Composition and Yield of Six Genotypes of Common Purslane (Portulaca oleracea L.): An Alternative Source of Omega-3 Fatty Acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef]
- Thines, M.; Spring, O. A Revision of Albugo (Chromista, Peronosporomycetes). Mycotaxon 2005, 92, 443–458. [Google Scholar]
- Tan, Y.P.; Madrid, H.; Crous, P.W.; Shivas, R.G. Johnalcornia gen. et. comb. nov., and Nine New Combinations in Curvularia Based on Molecular Phylogenetic Analysis. Australas. Plant Pathol. 2014, 43, 589–603. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest Categorisation of Colletotrichum fructicola. EFSA J. 2021, 19, e06803. [Google Scholar] [CrossRef] [PubMed]
- Nel, W.J.; Duong, T.A.; Wingfield, B.D.; Wingfield, M.J.; de Beer, Z.W. A New Genus and Species for the Globally Important, Multihost Root Pathogen Thielaviopsis basicola. Plant Pathol. 2018, 67, 871–882. [Google Scholar] [CrossRef]
- French-Monar, R.D.; Jones, J.B.; Roberts, P.D. Characterization of Phytophthora capsici Associated with Roots of Weeds on Florida Vegetable Farms. Plant Dis. 2006, 90, 345–350. [Google Scholar] [CrossRef][Green Version]
- Barboza, E.A.; Cabral, C.S.; Rossato, M.; Martins, F.H.S.R.; Reis, A. Pythium and Phytopythium Species Associated with Weeds Collected in Vegetable Production Fields in Brazil. Lett. Appl. Microbiol. 2022, 74, 796–808. [Google Scholar] [CrossRef]
- Sacristán, S.; García-Arenal, F. The evolution of virulence and pathogenicity in plant pathogen populations. Mol. Plant Pathol. 2008, 9, 369–384. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte Crop Cultivation: The Case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An Overview of the Emerging Trends of the Salicornia L. Genus as a Sustainable Crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Mosyakin, S.L. A Taxonomic Synopsis Of The Genus Salsola (Chenopodiaceae) In North America. Ann. Mo. Bot. Gard. 1996, 83, 387–395. [Google Scholar] [CrossRef]
- Altay, V.; Ozturk, M. The Genera Salsola and Suaeda (Amaranthaceae) and Their Value as Fodder. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 2333–2344. ISBN 978-3-030-57635-6. [Google Scholar]
- Bruckart, W.L.; Thomas, J.L.; Frederick, R.D.; Aime, M.C.; Abbasi, M. First Report of a Rust Disease Caused by Uromyces sp. on Suaeda californica in California. Plant Dis. 2019, 103, 1784. [Google Scholar] [CrossRef]
- Berner, D.K.; Bruckart, W.L.; Cavin, C.A.; Michael, J.L.; Carter, M.L.; Luster, D.G. Best Linear Unbiased Prediction of Host-Range of the Facultative Parasite Colletotrichum gloeosporioides f. sp. salsolae, a Potential Biological Control Agent of Russian Thistle. Biol. Control 2009, 51, 158–168. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides Species Complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Centofanti, T.; Bañuelos, G. Evaluation of the Halophyte Salsola soda as an Alternative Crop for Saline Soils High in Selenium and Boron. J. Environ. Manag. 2015, 157, 96–102. [Google Scholar] [CrossRef]
- Iannuzzi, A.M.; Moschini, R.; De Leo, M.; Pineschi, C.; Balestri, F.; Cappiello, M.; Braca, A.; Del-Corso, A. Chemical Profile and Nutraceutical Features of Salsola soda (Agretti): Anti-Inflammatory and Antidiabetic Potential of Its Flavonoids. Food Biosci. 2020, 37, 100713. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs Cultivated Halophytes: Nutritional and Functional Differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.; Saaghari, M.; Esfahan, E.Z.; Jaimand, K. Investigation on Potential of Suaeda fruticosa as a Source of Edible Oil. J. Bio Environ. Sci. 2013, 3, 101–107. [Google Scholar]
- Anderson, J.P.; Gleason, C.A.; Foley, R.C.; Thrall, P.H.; Burdon, J.B.; Singh, K.B. Plants versus pathogens: An evolutionary arms race. Funct. Plant Biol. 2010, 37, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Dikilitas, M.; Karakas, S. Chapter 8—Crop Plants under Saline-Adapted Fungal Pathogens: An Overview. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 173–192. ISBN 978-0-12-800875-1. [Google Scholar]
- Danti, S.; Broggio, M. Impact of Salinity Stress on the Biometric Responses of an Isolate of Verticillium dahliae Kleb. from Potato. In Proceedings of the Seventh International Verticillium Symposium, Cape Sounion, Athens, Greece, 6–10 October 1997; p. 53. [Google Scholar]
- Dikilitas, M. Effect of Salinity and Its Interactions with Verticillium albo-atrum on the Disease Develoment in Tomato (Lycopersicon esculentum Mill.) and Lucerne (Medicago sativa & M. Media) Plants. Ph.D. Thesis, University of Wales, Swansea, UK, 2003. [Google Scholar]
- Bhai, S.; Muthuswamy, A.; Srinivasan, V. Validation of Farmer’s Practice of Using Sodium Chloride for Controlling Foot Rot Disease of Black Pepper (Piper nigrum). Indian J. Agric. Sci. 2009, 79, 722–726. [Google Scholar]
- Hasan, H. Gibberellin and Auxin-Indole Production by Plant Root-Fungi and Their Biosynthesis under Salinity-Calcium Interaction. Acta Microbiol. Immunol. Hung. 2005, 49, 105–118. [Google Scholar] [CrossRef]
- Porter, D.M.; Adamsen, F.J. Effect of Sodic Water and Irrigation on Sodium Levels and the Development of Early Leaf Spot in Peanuts. Plant Dis. 1993, 77, 480. [Google Scholar] [CrossRef]
- Firdous, H.; Shahzad, S. Effect of Some Salts on in Vitro Growth of Fusarium solani. Pak. J. Bot. 2001, 33, 117–124. [Google Scholar]
- Ragazzi, A.; Vecchio, V.; Dellavalle, I.; Cucchi, A.; Mancini, F. Variations in the Pathogenicity of Fusarium oxysporum f. sp. vasinfectum in Relation to the Salinity of the Nutrient Medium. J. Plant Dis. Prot. 1994, 101, 263–266. [Google Scholar]
- El-Abyad, M.S.; Abu-Taleb, A.M.; Khalil, M.S. Impact of Salinity Stress on Soil-Borne Fungi of Sugarbeet. Plant Soil. 1992, 143, 75–83. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Masoudi, R.S.; Al-Habsi, N.; Al-Said, F.A.; Al-Rawahy, S.A.; Ahmed, M.; Deadman, M.L. Effect of Salinity on Pythium Damping-off of Cucumber and on the Tolerance of Pythium aphanidermatum. Plant Pathol. 2010, 59, 112–120. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Martín-García, B.; Verardo, V.; Romano, R.; Sacchi, R.; Masi, P. New Biotechnological Production of EPA by Pythium irregulare Using Alternative Sustainable Media Obtained from Food Industry By-Products and Waste. Sustainability 2023, 15, 1147. [Google Scholar] [CrossRef]
- Ambikapathy, J.; Marshall, J.S.; Hocart, C.H.; Hardham, A.R. The Role of Proline in Osmoregulation in Phytophthora nicotianae. Fungal Genet. Biol. 2002, 35, 287–299. [Google Scholar] [CrossRef]
- Luard, E.J. Growth and Accumulation of Solutes by Phytophthora cinnamomi and Other Lower Fungi in Response to Changes in External Osmotic Potential. Microbiology 1982, 128, 2583–2590. [Google Scholar] [CrossRef][Green Version]
- Lacalendola, N.; Tayagui, A.; Ting, M.; Malmstrom, J.; Nock, V.; Willmott, G.R.; Garrill, A. Biomechanical Responses of Encysted Zoospores of the Oomycete Achlya bisexualis to Hyperosmotic Stress Are Consistent with an Ability to Turgor Regulate. Fungal Genet. Biol. 2022, 159, 103676. [Google Scholar] [CrossRef]
- Gostinčar, C.; Lenassi, M.; Gunde-Cimerman, N.; Plemenitaš, A. Chapter 3—Fungal Adaptation to Extremely High Salt Concentrations. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: New York, NY, USA, 2011; Volume 77, pp. 71–96. [Google Scholar]
- Hohmann, S. Osmotic Stress Signaling and Osmoadaptation in Yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 300–372. [Google Scholar] [CrossRef]
- Fettich, M.; Lenassi, M.; Veranič, P.; Gunde-Cimerman, N.; Plemenitaš, A. Identification and Characterization of Putative Osmosensors, HwSho1A and HwSho1B, from the Extremely Halotolerant Black Yeast Hortaea werneckii. Fungal Genet. Biol. 2011, 48, 475–484. [Google Scholar] [CrossRef]
- Lenassi, M.; Plemenitaš, A. Novel Group VII Histidine Kinase HwHhk7B from the Halophilic Fungi Hortaea werneckii Has a Putative Role in Osmosensing. Curr. Genet. 2007, 51, 393–405. [Google Scholar] [CrossRef]
- Furukawa, K.; Hoshi, Y.; Maeda, T.; Nakajima, T.; Abe, K. Aspergillus nidulans HOG Pathway Is Activated Only by Two-Component Signaling Pathway in Response to Osmotic Stress. Mol. Microbiol. 2005, 56, 1246–1261. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Y.; Liu, Y.; Zhang, C.; Ma, Z. The Transmembrane Protein FgSho1 Regulates Fungal Development and Pathogenicity via the MAPK Module Ste50-Ste11-Ste7 in Fusarium graminearum. New Phytol. 2015, 206, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Zhou, L.; Yin, C.-M.; Zhang, D.-D.; Klosterman, S.J.; Wang, B.-L.; Song, J.; Wang, D.; Hu, X.-P.; Subbarao, K.V.; et al. The Verticillium dahliae Sho1-MAPK Pathway Regulates Melanin Biosynthesis and Is Required for Cotton Infection. Environ. Microbiol. 2019, 21, 4852–4874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.; Zhang, X.; Song, W.; Zhao, Q.; Dong, Y.; Guo, M.; Zheng, X.; Zhang, Z. A Two-Component Histidine Kinase, MoSLN1, Is Required for Cell Wall Integrity and Pathogenicity of the Rice Blast Fungus, Magnaporthe oryzae. Curr. Genet. 2010, 56, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, S.; Zhou, X.; Wang, C.; Xiang, P.; Zheng, Q.; Xu, J.-R. The FgHOG1 Pathway Regulates Hyphal Growth, Stress Responses, and Plant Infection in Fusarium graminearum. PLoS ONE 2012, 7, e49495. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.-L.; Chen, L.-H.; Chung, K.-R. How the Pathogenic Fungus Alternaria alternata Copes with Stress via the Response Regulators SSK1 and SHO1. PLoS ONE 2016, 11, e0149153. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, Y.; Yu, J.; Xiong, D.; Zhao, H.; Tian, C. The Mitogen-Activated Protein Kinase Kinase VdPbs2 of Verticillium dahliae Regulates Microsclerotia Formation, Stress Response, and Plant Infection. Front. Microbiol. 2016, 7, 1532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yin, D.; Yin, Y.; Cao, Y.; Ma, Z. The Response Regulator BcSkn7 Is Required for Vegetative Differentiation and Adaptation to Oxidative and Osmotic Stresses in Botrytis cinerea. Mol. Plant Pathol. 2015, 16, 276–287. [Google Scholar] [CrossRef]
- Kogej, T.; Ramos, J.; Plemenitaš, A.; Gunde-Cimerman, N. The Halophilic Fungus Hortaea werneckii and the Halotolerant Fungus Aureobasidium pullulans Maintain Low Intracellular Cation Concentrations in Hypersaline Environments. Appl. Environ. Microbiol. 2005, 71, 6600–6605. [Google Scholar] [CrossRef]
- Gorjan, A.; Plemenitaš, A. Identification and Characterization of ENA ATPases HwENA1 and HwENA2 from the Halophilic Black Yeast Hortaea werneckii. FEMS Microbiol. Lett. 2006, 265, 41–50. [Google Scholar] [CrossRef][Green Version]
- Vaupotič, T.; Plemenitaš, A. Differential Gene Expression and Hog1 Interaction with Osmoresponsive Genes in the Extremely Halotolerant Black Yeast Hortaea werneckii. BMC Genom. 2007, 8, 280. [Google Scholar] [CrossRef]
- Lenassi, M.; Zajc, J.; Gostinčar, C.; Gorjan, A.; Gunde-Cimerman, N.; Plemenitaš, A. Adaptation of the Glycerol-3-Phosphate Dehydrogenase Gpd1 to High Salinities in the Extremely Halotolerant Hortaea werneckii and Halophilic Wallemia ichthyophaga. Fungal Biol. 2011, 115, 959–970. [Google Scholar] [CrossRef]
- Petrovič, U.; Gunde-Cimerman, N.; Plemenitaš, A. Cellular Responses to Environmental Salinity in the Halophilic Black Yeast Hortaea werneckii. Mol. Microbiol. 2002, 45, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; van Voorst, F.; Martins, A.; Neves, L.; Oliveira, R.; Kielland-Brandt, M.C.; Lucas, C.; Brandt, A. A Member of the Sugar Transporter Family, Stl1p Is the Glycerol/H+ Symporter in Saccharomyces cerevisiae. Mol. Biol. Cell 2005, 16, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, S.; Antelo, L.; Grünewald, C.; Yemelin, A.; Andresen, K.; Jacob, S. Rapid Adaptation of Signaling Networks in the Fungal Pathogen Magnaporthe oryzae. BMC Genom. 2019, 20, 763. [Google Scholar] [CrossRef] [PubMed]
- Kogej, T.; Wheeler, M.H.; Lanišnik Rižner, T.; Gunde-Cimerman, N. Evidence for 1,8-Dihydroxynaphthalene Melanin in Three Halophilic Black Yeasts Grown under Saline and Non-Saline Conditions. FEMS Microbiol. Lett. 2004, 232, 203–209. [Google Scholar] [CrossRef]
- Turk, M.; Méjanelle, L.; Šentjurc, M.; Grimalt, J.O.; Gunde-Cimerman, N.; Plemenitaš, A. Salt-Induced Changes in Lipid Composition and Membrane Fluidity of Halophilic Yeast-like Melanized Fungi. Extremophiles 2004, 8, 53–61. [Google Scholar] [CrossRef]
- Turk, M.; Abramović, Z.; Plemenitaš, A.; Gunde-Cimerman, N. Salt Stress and Plasma-Membrane Fluidity in Selected Extremophilic Yeasts and Yeast-like Fungi. FEMS Yeast Res. 2007, 7, 550–557. [Google Scholar] [CrossRef]


| Botanical Name | Family | Common Name |
|---|---|---|
| Atriplex hortensis L. | Amaranthaceae | Garden orache |
| Atriplex littoralis L. | Amaranthaceae | Grassleaf orache |
| Atriplex nummularia Lindl. | Amaranthaceae | Oldman saltbush |
| Atriplex patula L. | Amaranthaceae | Common orache |
| Atriplex prostrata Boucher ex DC (=Syn.: Atriplex hastata L. var. prostrata (Boucher ex DC.) Lange) | Amaranthaceae | Hastate orache |
| Beta vulgaris L. subsp. maritima (L.) Arcang. | Amaranthaceae | Sea beet |
| Cakile maritima Scop. subsp. maritima | Brassicaceae | Searocket |
| Crithmum maritimum L. | Apiaceae | Sea fennel |
| Eryngium maritimum L. | Apiaceae | Sea holly |
| Lepidium latifolium L. | Brassicaceae | Broadleaved pepperweed |
| Lepidium perfoliatum L. | Brassicaceae | Clasping pepperweed |
| Lepidium ruderale L. | Brassicaceae | Stinking pepperweed |
| Lepidium subulatum L. | Brassicaceae | - |
| Portulaca oleracea L. | Portulacaceae | Common purslane |
| Salicornia perennans Willd. subsp. perennans (=Syn.: Salicornia europaea L.) | Amaranthaceae | Glasswort |
| Salicornia perennis Mill. (=Syn.: Salicornia perennis (Mill.) A.J. Scott subsp. perennis) | Amaranthaceae | Perennial glasswort |
| Salicornia ramosissima (Hook.f.) J. Woods ex W.A. Clarke & E.S. Marshall | Amaranthaceae | Twiggy glasswort |
| Salsola kali L. (=Syn.: Kali turgidum (Dumort.) Gutermann) | Amaranthaceae | Common saltwort |
| Salsola tragus L. (=Syn.: Kali tragus subsp. tragus (L.) Scoop.) | Amaranthaceae | Russian thistle |
| Salsola soda L. (=Syn.: Soda inermis Fourr.) | Amaranthaceae | Monk’s beard |
| Suaeda maritima (L.) Dumort. (=Syn.: Chenopodium maritimum L.) | Amaranthaceae | Sea-blite |
| Suaeda vera J.F. Gmel (=Syn.: Suaeda fruticosa (L.) Forssk. subsp. vera (J.F. Gmel.) Maire & Weiller) | Amaranthaceae | Shrubby sea-blite |
| Plant Genus | Plant Species | Disease | Pathogenic Species | Order | Origin | Reference(s) |
|---|---|---|---|---|---|---|
| Atriplex | A. hortensis | Downy mildew | Peronospora atriplicis-hortensis | Peronosporales | Herbarium specimen(s) | [43] |
| Leaf spot | Ascochyta caulina | Pleosporales | Herbarium specimen(s) | [44] | ||
| Passalora spegazzinii | Mycosphaerellales | Wild | [45] | |||
| Stagonospora atriplicis | Pleosporales | Herbarium specimen(s) | [46] | |||
| A. littoralis | Downy mildew | Peronospora litoralis | Peronosporales | Herbarium specimen(s) | [43,47] | |
| Leaf spot | Ascochyta caulina | Pleosporales | Herbarium specimen(s) | [44] | ||
| Stagonospora atriplicis | Pleosporales | Herbarium specimen(s) | [46] | |||
| A. nummularia | Shoot blight | Diaporthe inconspicua | Diaporthales | Wild | [48] | |
| A. patula | Downy mildew | Peronospora minor. | Peronosporales | Herbarium specimen(s) | [43,47] | |
| Wild | [49,50,51,52,53] | |||||
| Leaf spot | Ascochyta caulina | Pleosporales | Herbarium specimen(s) | [44] | ||
| Passalora dubia | Mycosphaerellales | - | [54,55] | |||
| Wild | [56,57,58] | |||||
| Passalora spegazzinii | Mycosphaerellales | - | [55,59] | |||
| Stagonospora atriplicis | Pleosporales | Herbarium specimen(s) | [46,60] | |||
| Rust | Uromyces peckianus | Pucciniales | Wild | [61] | ||
| A. prostrata (=Syn.: A. hastata) | Downy mildew | Peronospora atriplicis-hastatae | Peronosporales | Herbarium specimen(s) | [43,47] | |
| Leaf spot | Ascochyta atriplicis | Pleosporales | - | [54] | ||
| Herbarium specimen(s) | [62] | |||||
| Ascochyta caulina | Pleosporales | Herbarium specimen(s) | [44] | |||
| Stagonospora atriplicis | Pleosporales | Herbarium specimen(s) | [46] | |||
| Beta | B. vulgaris subsp. maritima | Downy mildew | Peronospora schachtii | Peronosporales | Wild | [53] |
| Leaf blotch | Septoria betae | Mycosphaerellales | - | [60] | ||
| Leaf spot | Cercospora beticola | Mycosphaerellales | Wild | [63] | ||
| Powdery mildew | Leveillula taurica | Erysiphales | - | [64] | ||
| Rust | Uromyces betae | Pucciniales | Wild | [65,66] | ||
| Cakile | C. maritima | Black leaf spot | Alternaria alternata | Pleosporales | Wild | [67] |
| Wild and pot (artificial inoculation on detached leaves) | [68] | |||||
| Pot (artificial inoculation in salinity-NaCl 200 mM) | [69] | |||||
| Alternaria arborescens | Pleosporales | Wild and pot (artificial inoculation on detached leaves) | [68] | |||
| Alternaria brassicicola | Pleosporales | Wild | [67,70] | |||
| Alternaria mali | Pleosporales | Wild and pot (artificial inoculation on detached leaves) | [68] | |||
| Downy mildew | Hyaloperonospora parasitica | Peronosporales | Wild | [71] | ||
| Powdery mildew | Erysiphe cruciferarum | Erysiphales | - | [64] | ||
| Ring spot | Mycosphaerella brassicicola | Mycosphaerellales | Herbarium specimen(s) | [72] | ||
| White rust | Albugo candida | Albuginales | Wild | [50,73,74] | ||
| Crithmum | C. maritimum | Powdery mildew | Leveillula taurica | Erysiphales | - | [64,75] |
| Wild | [76] | |||||
| Leveillula lanuginosa | Erysiphales | - | [75] | |||
| Eryngium | E. maritimum | Leaf spot | Cylindrosporium eryngii | Helotiales | - | [77] |
| Pseudocercosporella eryngii | Mycosphaerellales | - | [54] | |||
| Wild and herbarium specimen(s) | [78] | |||||
| Leaf smut | Entyloma eryngii-maritimi | Entylomatales | Herbarium specimen(s) | [79] | ||
| Stem necrosis | Diaporthe angelicae | Diaporthales | - | [80] | ||
| Wild | [81] | |||||
| Lepidium | L. latifolium | Canker and dieback | Cytospora ribis | Diaporthales | Wild | [82] |
| Downy mildew | Perofascia lepidii | Peronosporales | Herbarium specimen(s) | [83] | ||
| Leaf spot | Alternaria brassicae | Pleosporales | Herbarium specimen(s) | [84] | ||
| Cercospora bizzozeriana | Mycosphaerellales | Herbarium specimen(s) | [85] | |||
| Powdery mildew | Erysiphe cruciferarum | Erysiphales | - | [54,64] | ||
| Wild | [62,76,86,87] | |||||
| Leveillula taurica | Erysiphales | - | [64] | |||
| Rust | Puccinia isiacae | Pucciniales | - | [88] | ||
| White rust | Albugo candida | Albuginales | Wild | [74] | ||
| Wild and pot (artificial inoculation) | [89] | |||||
| Albugo lepidii | Albuginales | Wild and growth chamber (artificial inoculation) | [90] | |||
| L. perfoliatum | Downy mildew | Hyaloperonospora lepidii-perfoliati | Peronosporales | Herbarium specimen(s) | [43] | |
| Leaf smut | Urocystis coralloides | Urocystidiales | - | [91] | ||
| Powdery mildew | Erysiphe cruciferarum | Erysiphales | - | [64] | ||
| Wild | [76] | |||||
| Rust | Puccinia aristidae | Pucciniales | - | [59] | ||
| Stunting | Verticillium dahliae | Glomerellales | Greenhouses, gardens, fields, and orchards | [92] | ||
| L. ruderale | Downy mildew | Hyaloperonospora parasitica | Peronosporales | Herbarium specimen(s) | [51] | |
| Perofascia lepidii | Peronosporales | Herbarium specimen(s) | [51,83] | |||
| Leaf smut | Urocystis coralloides | Urocystidiales | - | [91] | ||
| Powdery mildew | Erysiphe cruciferarum | Erysiphales | - | [54,64] | ||
| Wild | [76,93] | |||||
| White rust | Albugo candida | Albuginales | - | [54] | ||
| L. subulatum | Downy mildew | Perofascia lepidii | Peronosporales | Wild | [53] | |
| Portulaca | P. oleracea | Anthracnose | Colletotrichum fructicola | Glomerellales | - | [94] |
| Colletotrichum gleosporioides species complex | Glomerellales | - | [94] | |||
| Colletotrichum spinaciae | Glomerellales | Herbarium specimen(s) | [95] | |||
| Black root rot | Berkeleyomyces basicola | Microascales | Tobacco field | [96] | ||
| Basal stem rot | Rhizoctonia solani | Cantharellales | Wild | [97] | ||
| Sclerotium rolfsii | Thelephorales | - | [94] | |||
| Crown rot | Sclerotinia sclerotiorum | Helotiales | Wild | [98] | ||
| Leaf blight | Stemphylium vesicarium | Pleosporales | Onion field and growth chamber (artificial inoculation) | [99] | ||
| Leaf spot | Dichotomophthora lutea | Pleosporales | Field and pot (artificial inoculation) | [100] | ||
| Herbarium specimen(s) | [101,102] | |||||
| Phyllosticta portulacae | Botryosphaeriales | Wild | [103] | |||
| Root dieback | Globisporangium intermedium | Peronosporales | Carrot field | [104] | ||
| Globisporangium irregulare | Peronosporales | Carrot field | [104] | |||
| Stem rot | Dichotomophthora portulacae | Pleosporales | Cultivated (not specified) and pot in glasshouse (artificial inoculation) | [105] | ||
| Wild | [106,107] | |||||
| Wild and greenhouse (artificial inoculation) | [108] | |||||
| Wild and pots in growth chamber (artificial inoculation) | [109] | |||||
| Curvularia portulacae | Pleosporales | Field and greenhouse | [110] | |||
| Not specified | [111] | |||||
| Stunting | Verticillium dahliae | Glomerellales | Greenhouses, gardens, fields, and orchards | [92] | ||
| White rust | Wilsoniana portulacae | Albuginales | - | [51,77,112] | ||
| Herbarium specimen(s) | [113] | |||||
| Maize, soybean, sugar beet, and sunflower fields | [114] | |||||
| Wild | [74,98,103,115,116] | |||||
| Wild and maize and sunflower fields | [117] | |||||
| Salicornia | S. perennans (=Syn.: S. europaea) | Black lesions on leaves | Phytophthora inundata | Peronosporales | Wild | [118] |
| Rust | Uromyces salicorniae | Pucciniales | - | [54,77] | ||
| Wild | [119,120,121] | |||||
| S. perennis | Rust | Uromyces salicorniae | Pucciniales | Wild | [121] | |
| S. ramosissima | Rust | Uromyces salicorniae | Pucciniales | Wild | [121] | |
| Salsola | S. kali | Black foot | Dactylonectria macrodidyma | Hypocreales | Grapevine field | [122] |
| Powdery mildew | Leveillula cylindrospora | Erysiphales | Wild | [123] | ||
| Leveillula taurica | Erysiphales | - | [64] | |||
| Rust | Puccinia aristidae | Pucciniales | Herbarium specimen(s) | [124] | ||
| Uromyces chenopodii | Pucciniales | Wild | [66,98,125] | |||
| Uromyces salsolae | Pucciniales | Pots under greenhouse | [126] | |||
| Wild | [127] | |||||
| Stunting | Verticillium dahliae | Glomerellales | Greenhouses, gardens, fields, and orchards | [92] | ||
| S. tragus | Anthracnose | Colletotrichum salsolae | Glomerellales | Wild and greenhouse (artificial inoculation) | [128,129,130] | |
| Field | [131] | |||||
| S. tragus | Stem canker | Diaporthe eres | Diaporthales | Wild and greenhouse (artificial inoculation) | [132] | |
| Soda | S. inermis (=Syn.: Salsola soda) | Powdery mildew | Leveillula cylindrospora | Erysiphales | Wild | [76] |
| Rust | Uromyces salsolae | Pucciniales | - | [133] | ||
| Suaeda | S. maritima | Rust | Uromyces chenopodii | Pucciniales | - | [134] |
| Wild | [103,135] | |||||
| S. vera | Rust | Uromyces chenopodii | Pucciniales | - | [77,136] | |
| Wild | [66,120] |
| Pathogenic Species | Host Family | Host Species |
|---|---|---|
| Albugo candida | Brassicaceae | Cakile maritima |
| Lepidium latifolium | ||
| Lepidium ruderale | ||
| Ascochyta caulina | Amaranthaceae | Atriplex hortensis |
| Atriplex littoralis | ||
| Atriplex patula | ||
| Atriplex prostrata | ||
| Erysiphe cruciferarum | Brassicaceae | Cakile maritima |
| Lepidium latifolium | ||
| Lepidium ruderale | ||
| Lepidium perfoliatum | ||
| Hyaloperonospora parasitica | Brassicaceae | Cakile maritima |
| Lepidium ruderale | ||
| Leveillula cylindrospora | Amaranthaceae | Salsola kali |
| Soda inermis | ||
| Leveillula taurica | Amaranthaceae | Beta vulgaris subsp. maritima |
| Salsola kali | ||
| Brassicaceae | Lepidium latifolium | |
| Apiaceae | Crithmum maritimum | |
| Passalora spegazzinii | Amaranthaceae | Atriplex patula |
| Atriplex hortensis | ||
| Perofascia lepidii | Brassicaceae | Lepidium latifolium |
| Lepidium ruderale | ||
| Lepidium subulatum | ||
| Puccinia aristidae | Amaranthaceae | Salsola kali |
| Brassicaceae | Lepidium perfoliatum | |
| Stagonospora atriplicis | Amaranthaceae | Atriplex hortensis |
| Atriplex littoralis | ||
| Atriplex patula | ||
| Atriplex prostrata | ||
| Urocystis coralloides | Brassicaceae | Lepidium perfoliatum |
| Lepidium ruderale | ||
| Uromyces chenopodii | Amaranthaceae | Salsola kali |
| Suaeda maritima | ||
| Suaeda vera | ||
| Uromyces salicorniae | Amaranthaceae | Salicornia perennans |
| Salicornia perennis | ||
| Salicornia ramosissima | ||
| Uromyces salsolae | Amaranthaceae | Salsola tragus |
| Salsola kali | ||
| Soda inermis | ||
| Verticillium dahliae | Amaranthaceae | Salsola kali |
| Brassicaceae | Lepidium perfoliatum | |
| Portulacaceae | Portulaca oleracea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delli Compagni, E.; Pardossi, A.; Pecchia, S. Fungal and Fungal-like Diseases of Halophytes in the Mediterranean Basin: A State-of-the-Art Review. Horticulturae 2024, 10, 313. https://doi.org/10.3390/horticulturae10040313
Delli Compagni E, Pardossi A, Pecchia S. Fungal and Fungal-like Diseases of Halophytes in the Mediterranean Basin: A State-of-the-Art Review. Horticulturae. 2024; 10(4):313. https://doi.org/10.3390/horticulturae10040313
Chicago/Turabian StyleDelli Compagni, Emiliano, Alberto Pardossi, and Susanna Pecchia. 2024. "Fungal and Fungal-like Diseases of Halophytes in the Mediterranean Basin: A State-of-the-Art Review" Horticulturae 10, no. 4: 313. https://doi.org/10.3390/horticulturae10040313
APA StyleDelli Compagni, E., Pardossi, A., & Pecchia, S. (2024). Fungal and Fungal-like Diseases of Halophytes in the Mediterranean Basin: A State-of-the-Art Review. Horticulturae, 10(4), 313. https://doi.org/10.3390/horticulturae10040313






