Potato Biofortification: A Systematic Literature Review on Biotechnological Innovations of Potato for Enhanced Nutrition
Abstract
1. Introduction
1.1. Biofortification: Technique of Nutrient Enhancement
1.2. Potato as the Most Suitable Crop Selected for Biofortification
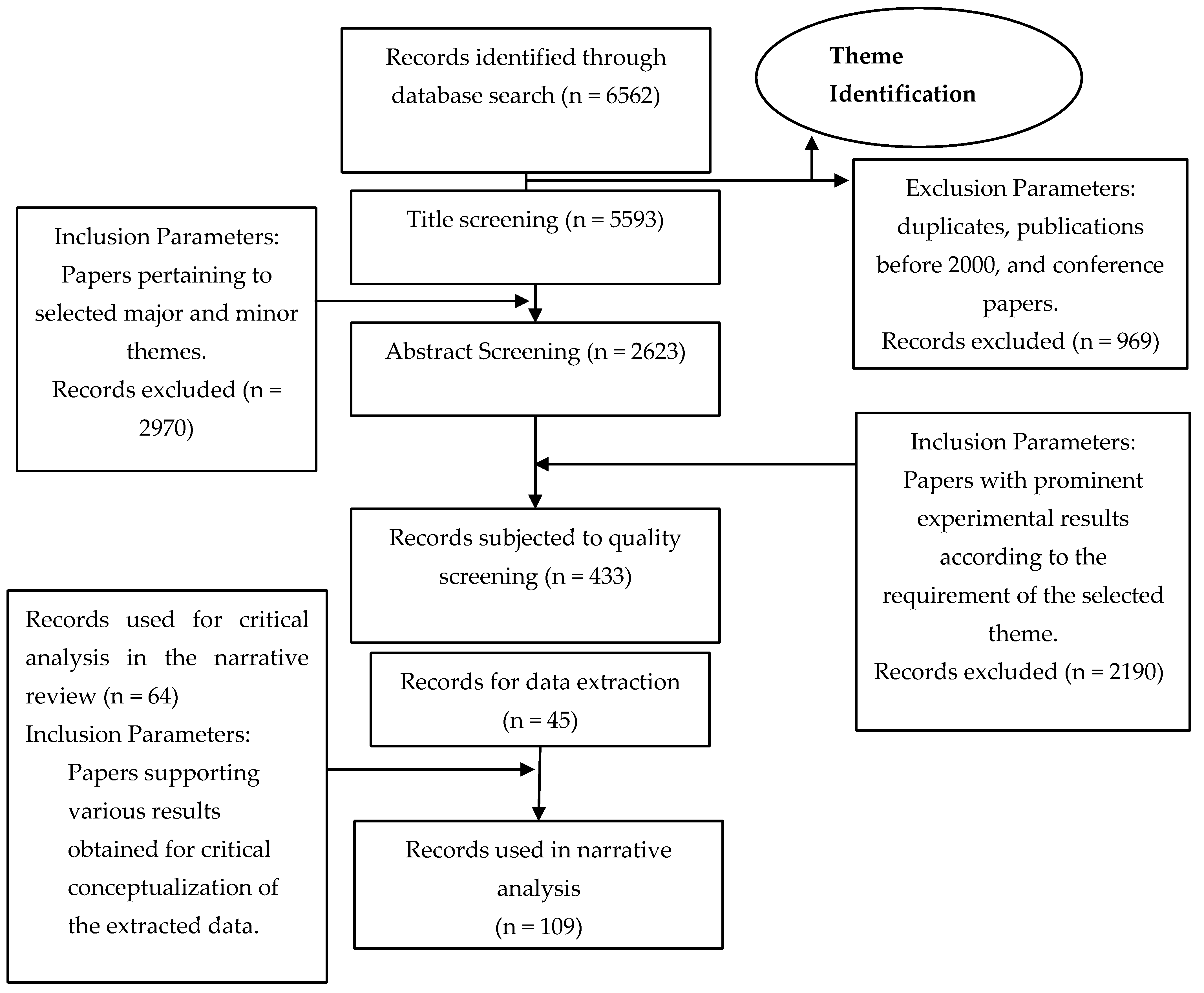
2. Methodology
3. Result
4. Discussion
4.1. Conventional Breeding
4.2. Agronomic Practices
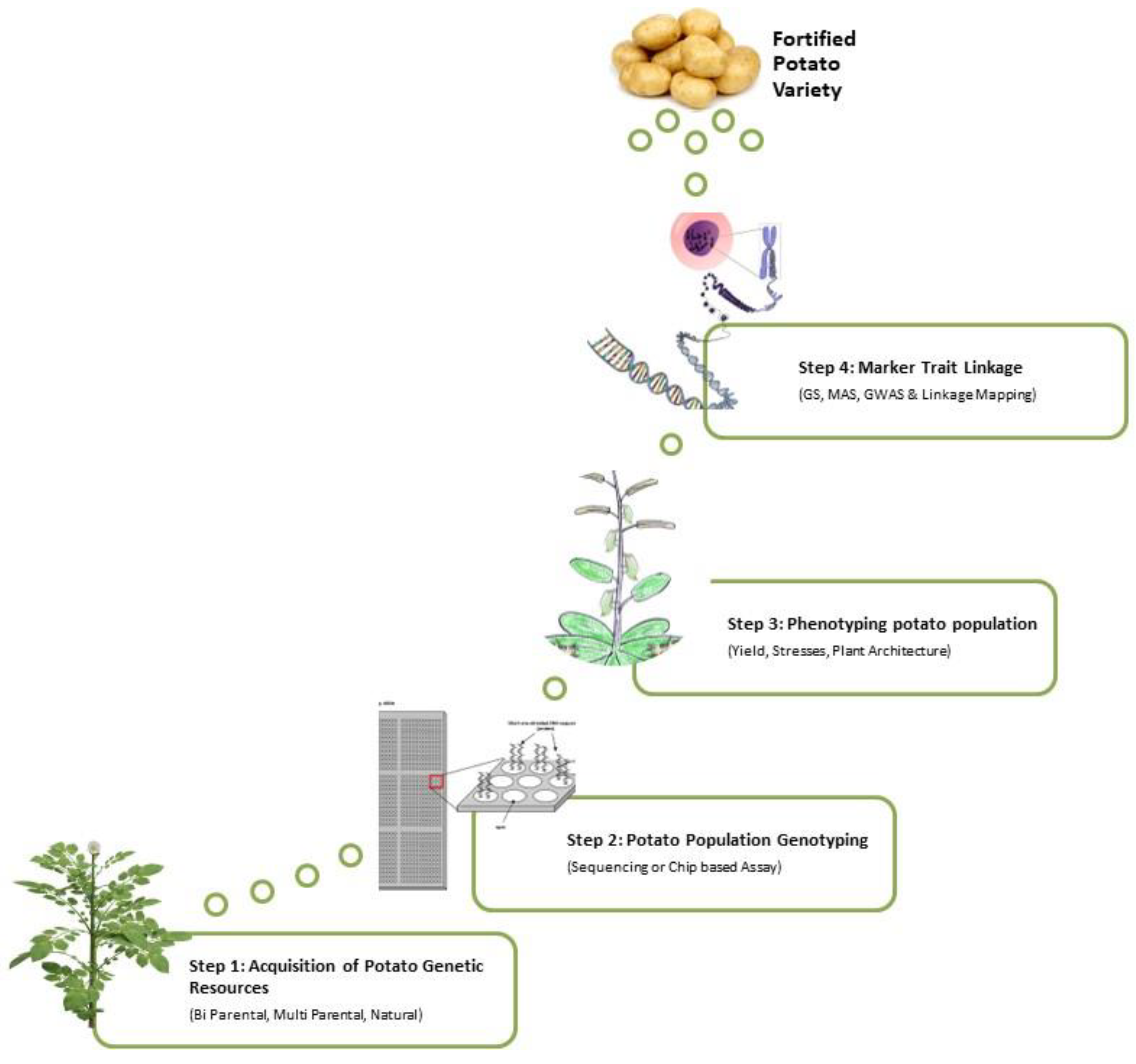
4.3. Molecular Approach
4.3.1. Transgenic Cultivars
4.3.2. Marker-Assisted Selection
Association Mapping and Quantitative Trait Loci (QTL)
4.3.3. Genomic Editing
Transcription Activator-Like Effector Nucleases (TALENs)
CRISPR-Cas Genome Editing Method
4.4. Future Prospective
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Bonierbale, M.W.; Amoros, W.R.; Salas, E.; De Jong, W. Potato breeding. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 163–217. [Google Scholar]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Tardy, A.L.; Ballesta, A.A.; Yilmaz, G.C.; Dan, M.; Ramirez, D.M.; Lam, H.Y.; Azais-Braesco, V.; Pouteau, E. Adult’s Dietary Intakes of Selected Vitamins & Minerals Essential for Energy Metabolism and Cognition: A Comparison Across Countries & Genders (FS10-04-19). Curr. Dev. Nutr. 2019, 3, 1501. [Google Scholar]
- FAO. Europe and Central Asia: Regional Overview of Food Security and Nutrition; Food and Agriculture Organization: Roma, Italy, 2018; ISBN 9789251311530. [Google Scholar]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Vlaic, R.A.; Mure¸San, C.C.; Muste, S.; Mure¸San, A.; Muresan, V.; Suharoschi, R.; Petru¸t, G.; Mihai, M. Food Fortification through Innovative Technologies. In Food Engineering; IntechOpen: London, UK, 2019. [Google Scholar]
- Sil, D.; Bhattacharjee, T.; Saha, S. Biofortification of Vegetable Crops. Vigyan Varta Int. E-Mag. Sci Enthus. 2023, 4, 137–140. [Google Scholar]
- Garrett, G.S. National mandated food fortification programs. In Food Fortification in a Globalized World; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2018; pp. 53–62. [Google Scholar]
- Mannar, M.G.V.; Hurrell, R.F. Chapter 1—Food fortification: Past experience, current status, and potential for globalization. In Food Fortification in a Globalized World; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2018; pp. 3–11. [Google Scholar]
- Somassè, Y.E.; Dramaix, M.; Traoré, B. The WHO recommendation of home fortification of foods with multiple-micronutrient powders in children under 2 years of age and its effectiveness on anaemia and weight: A pragmatic cluster-randomized controlled trial. Public Health Nutr. 2018, 21, 1350–1358. [Google Scholar] [CrossRef]
- Ledwożyw-Smoleń, I.; Smoleń, S.; Rożek, S.; Sady, W.; Strzetelski, P. Iodine Biofortification of Potato (Solanum tuberosum L.) Grown in Field. Agronomy 2020, 10, 1916. [Google Scholar] [CrossRef]
- Ofori, K.F.; Antoniello, S.; English, M.M.; Aryee, A.N.A. Improving nutrition through biofortification-A systematic review. Front. Nutr. 2022, 9, 1043655. [Google Scholar] [CrossRef] [PubMed]
- Habeych, E.; van Kogelenberg, V.; Sagalowicz, L. Strategies to limit colour changes when fortifying food products with iron. Food Res. Int. 2016, 88, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rojo, R.; Vaquero, M.P. Iron bioavailability from food fortification to precision nutrition. A review. Innov. Food Sci. Emerg. Technol. 2019, 51, 126–138. [Google Scholar] [CrossRef]
- Bouis, H.E. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc. Nutr. Soc. 2003, 62, 403–411. [Google Scholar] [CrossRef]
- Burgos, G.; Zum Felde, T.; Andre, C.; Kubow, S. The potato and its contribution to the human diet and health. In The Potato Crop; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 37–74. [Google Scholar]
- Stewart, D.; Taylor, M. Potato–A Basis for Human Nutrition and Health Benefits; AHDB Potatoes: Kenilworth, UK, 2017; pp. 1–60. [Google Scholar]
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition—A review. Crit Rev Food Sci Nutr. 2016, 6, 711–721. [Google Scholar] [CrossRef]
- De Lepeleire, J.; Strobbe, S.; Verstraete, J.; Blancquaert, D.; Ambach, L.; Visser, R.G.F.; Stove, C.; Van Der Straeten, D. Folate Biofortification of Potato by Tuber-Specific Expression of Four Folate Biosynthesis Genes. Mol. Plant 2018, 11, 175–188. [Google Scholar] [CrossRef]
- Brown, C.R.; Edwards, C.G.; Yang, C.P.; Dean, B.B. Orange flesh trait in potato: Inheritance and carotenoid content. J. Am. Soc. Hortic. Sci. 2019, 118, 145–150. [Google Scholar] [CrossRef]
- Soare, R.; Dinu, M.; Babeanu, C.; Soare, M. Evaluation and comparison of antioxidant activity and biochemical compounds in some coloured potato cultivars. Plant Soil Environ. 2020, 66, 281–286. [Google Scholar] [CrossRef]
- Hellmann, H.; Goyer, A.; Navarre, D.A. Antioxidants in potatoes: A functional view on one of the major food crops worldwide. Molecules 2021, 26, 2446. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A. Potato peels as sources of functional compounds for the food industry: A review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Ercoli, S.; Parada, J.; Bustamante, L. Noticeable quantities of functional compounds and antioxidant activities remain after cooking of colored fleshed potatoes native from Southern Chile. Molecules 2021, 26, 314. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Goutam, U.; Kukreja, S.; Sharma, J.; Sood, S.; Bhardwaj, V. Potato biofortification: An effective way to fight global hidden hunger. Physiol. Mol. Biol. Plants 2021, 27, 2297–2313. [Google Scholar] [CrossRef]
- Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=76&contentid=11367-3 (accessed on 22 February 2024).
- Rasheed, H.; Ahmad, D.; Bao, J. Genetic diversity and health properties of polyphenols in potato. Antioxidants 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Ngobese, N.Z.; Workneh, T.S.; Alimi, B.A.; Tesfay, S. Nutrient composition and starch characteristics of eight European potato cultivars cultivated in South Africa. J. Food Compos. Anal. 2017, 55, 1–11. [Google Scholar] [CrossRef]
- Upadhyaya, C.P.; Bagri, D.S. Biotechnological approaches for nutritional improvement in potato (Solanum tuberosum L.). In Genome Engineering for Crop Improvement; Wiley: Hoboken, NJ, USA, 2021; pp. 253–280. [Google Scholar]
- Sahu, P.K.; Das, M.; Sarkar, B. Potato Production in India: A Critical Appraisal on Sustainability, Forecasting, Price and Export Behaviour. In Potato Research; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Dutt, S.; Pastal, P.M.; Das, H. Biotechnology for Nutritional and Associated Processing Quality Improvement in Potato. In Nutritional Quality Improvement in Plants; Jaiwal, P., Chhillar, A., Chaudhary, D., Jaiwal, R., Eds.; Concepts and Strategies in Plant Sciences; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Navarre, D.A.; Shakya, R.; Hellmann, H. Vitamins, phytonutrients, and minerals in potato. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: London, UK, 2016; pp. 117–166. [Google Scholar]
- Zeh, M.; Casazza, A.P.; Kreft, O.; Roessner, U.; Bieberich, K.; Willmitzer, L. Antisense inhibition of threonine synthase leads to high methionine content in transgenic potato plants. Plant Physiol. 2001, 127, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Romer, S.; Lubeck, J.; Kauder, F.; Steiger, S.; Adomat, C.; Sandmann, G. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab. Eng. 2002, 4, 263–272. [Google Scholar] [CrossRef]
- Ducreux, L.J.M.; Morris, W.L.; Hedley, P.E.; Shepherd, T.; Davies, H.V.; Millam, S. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J. Exp. Bot. 2004, 56, 81–89. [Google Scholar] [CrossRef]
- Park, S.; Kang, T.S.; Kim, C.K.; Han, J.S.; Kim, S.; Smith, R.H.; Pike, L.M.; Hirschi, K.D. Genetic manipulation for enhancing calcium content in potato tuber. J. Agric. Food Chem. 2005, 53, 5598–5603. [Google Scholar] [CrossRef]
- Kim, C.K.; Han, J.S.; Lee, H.S.; Oh, J.Y.; Shigaki, T.; Park, S.H.; Hirschi, K. Expression of an Arabidopsis CAX2 variant in potato tubers increases calcium levels with no accumulation of manganese. Plant Cell Rep. 2006, 25, 1226–1232. [Google Scholar] [CrossRef]
- Diretto, G.; Tavazza, R.; Welsch, R.; Pizzichini, D.; Mourgues, F.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biol. 2006, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Van, E.J.; Zhou, X. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 2007, 18, 3594–3605. [Google Scholar] [CrossRef]
- Stiller, I.; Dancs, G.; Hesse, H.; Hoefgen, R.; Banfalvi, Z. Improving the nutritive value of tubers: Elevation of cysteine and glutathione contents in the potato cultivar White Lady by marker-free transformation. J. Biotechnol. 2007, 128, 335–343. [Google Scholar] [CrossRef]
- VanEck, J.; Conlin, B.; Garvin, D.F.; Mason, H.; Navarre, D.A.; Brown, C.R. Enhancing beta-carotene content in potato by rnai-mediated silencing of the beta-carotene hydroxylase gene. Am. J. Potato Res. 2007, 84, 331–342. [Google Scholar] [CrossRef]
- Lopez, A.B.; VanEck, J.; Conlin, B.J.; Paolillo, D.J.; O’Neill, J.; Li, L. Effect of the cauliflower or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J. Exp. Bot. 2008, 59, 213–223. [Google Scholar] [CrossRef]
- Dancs, G.; Kondrak, M.; Banfalvi, Z. The effects of enhanced methionine synthesis on amino acid and anthocyanin content of potato tubers. BMC Plant Biol. 2008, 8, 65. [Google Scholar] [CrossRef]
- Upadhyaya, C.P.; Young, K.E.; Akula, N.; SoonKim, H.; Heung, J.J.; Oh, O. Over-expression of strawberry d-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci. 2009, 177, 659–667. [Google Scholar] [CrossRef]
- Upadhyaya, C.P.; Akula, N.; Young, K.E.; Chun, S.C.; Kim, D.H.; Park, S.W. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol. Lett. 2010, 32, 321–330. [Google Scholar] [CrossRef]
- Qin, A.; Shi, Q.; Yu, X. Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol. Biol. Rep. 2011, 38, 1557–1566. [Google Scholar] [CrossRef]
- Hemavathi, H.; Upadhyaya, C.P.; Nookaraju, A. Biochemical analysis of enhanced tolerance in transgenic potato plants overexpressing D-galacturonic acid reductase gene in response to various abiotic stresses. Mol. Breed. 2011, 28, 105–115. [Google Scholar] [CrossRef]
- Du, H.H.; Yang, T.; Ma, C.Y.; Feng, D.; Zhang, N.; Si, H.J.; Wang, D. Effects of RNAi silencing of SSIII gene on phosphorus content and characteristics of starch in potato tubers. J. Integr. Agric 2012, 11, 1985–1992. [Google Scholar] [CrossRef]
- Jonik, C.; Sonnewald, U.; Hajirezaei, M.R.; Flügge, U.I.; Ludewig, F. Simultaneous boosting of source and sink capacities doubles tuber starch yield of potato plants. Plant Biotechnol. J. 2012, 10, 1088–1098. [Google Scholar] [CrossRef]
- Goo, Y.M.; Kim, T.W.; Lee, M.K.; Lee, S.W. Accumulation of PrLeg, a Perilla legumin protein in potato tuber results in enhanced level of Sulphur-containing amino acids. Comptes Rendus Biol. 2013, 336, 433–439. [Google Scholar] [CrossRef]
- Kostyn, K.; Szatkowski, M.; Kulma, A.; Kosieradzka, I.; Szopa, J. Transgenic potato plants with overexpression of dihydroflavonol reductase can serve as efficient nutrition sources. J. Agric. Food Chem. 2013, 61, 6743. [Google Scholar] [CrossRef]
- Huang, T.; Joshi, V.; Jander, G. The catabolic enzyme methionine gamma-lyase limits methionine accumulation in potato tubers. Plant Biotechnol. J. 2014, 12, 883–893. [Google Scholar] [CrossRef]
- Tilocca, M.G.; Serratrice, G.; Oggiano, M.A.; Mancuso, M.R.; Mascia, I.; Marongiu, E. Monitoring the presence of genetically modified potato EH92-527-1 (BPS-25271-9) in commercial processed food. Ital. J. Food Saf. 2014, 3, 57–59. [Google Scholar] [CrossRef]
- Kolachevskaya, O.O.; Alekseeva, V.V.; Sergeeva, L.I.; Rukavtsova, E.B.; Getman, I.A.; Vreugdenhil, D.; Buryanov, Y.I.; Romanov, G.A. Expression of auxin synthesis gene tms1 under control of tuber-specific promoter enhances potato tuberization in vitro. J. Integr. Plant Biol. 2015, 57, 734–744. [Google Scholar] [CrossRef]
- Goo, Y.M.; Han, E.H.; Jeong, J.C. Overexpression of the sweet potato IbOr gene results in the increased accumulation of carotenoid and confers tolerance to environmental stresses in transgenic potato. Comptes Rendus Biol. 2015, 338, 12–20. [Google Scholar] [CrossRef]
- Kusano, H.; Onodera, H.; Kihira, M.; Aoki, H.; Matsuzaki, H.; Shimada, H. A simple Gateway-assisted construction system of TALEN genes for plant genome editing. Sci. Rep. 2016, 6, 30234. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhu, W.J.; Tang, R.M.; Cai, J.H.; Chen, M.; Yang, Q. Over-expression of StLCYb increases β-carotene accumulation in potato tubers. Plant Biotechnol. Rep. 2016, 10, 95–104. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, A.; Lee, S.S. Overexpression of Golgi protein CYP21-4s improves crop productivity in potato and rice by increasing the abundance of Mannosidic glycoproteins. Front. Plant Sci. 2017, 20, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Kromann, P.; Valverde, F.; Alvarado, S. Can Andean potatoes be agronomically biofortified with iron and zinc fertilizers? Plant Soil. 2017, 411, 121–138. [Google Scholar] [CrossRef]
- Ma, J.; Xiang, H.; Donnelly, D.J.; Meng, F.R.; Xu, H.; Durnford, D.; Li, X.Q. Genome editing in potato plants by Agrobacterium-mediated transient expression of transcription activator-like effector nucleases. Plant Biotechnol. Rep. 2017, 11, 249–258. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Q.; Akbar, S. Genetic enhancement of oil content in potato tuber (Solanum tuberosum L.) through an integrated metabolic engineering strategy. Plant Biotechnol. J. 2017, 15, 56–67. [Google Scholar] [CrossRef]
- Kumar, P.; Jander, G. Concurrent overexpression of Arabidopsis thaliana cystathionine γ-synthase and silencing of endogenous methionine γ-Lyase enhance tuber methionine content in Solanum tuberosum. J. Agric. Food Chem. 2017, 65, 2737–2742. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Nicolia, A.; Fält, A.S.; Samuelsson, M.; Hofvander, P. Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep. 2017, 36, 117–128. [Google Scholar] [CrossRef]
- Bali, S.; Robinson, B.R.; Sathuvalli, V.; Bamberg, J.; Goyer, A. Single nucleotide polymorphism (SNP) markers associated with high folate content in wild potato species. PLoS ONE 2018, 13, e0193415. [Google Scholar] [CrossRef]
- Bagri, D.S.; Upadhyaya, D.C.; Kumar, A.; Upadhyaya, C.P. Overexpression of PDX-II gene in potato (Solanum tuberosum L.) leads to the enhanced accumulation of vitamin B6 in tuber tissues and tolerance to abiotic stresses. Plant Sci. 2018, 272, 267–275. [Google Scholar] [CrossRef]
- Vergara, C.V.M.; CecílioFilho, A.B.; de Almeida, H.J.; Gratão, P.L. Fortification and bioavailability of zinc in potato. J. Sci. Food Agric. 2019, 99, 3525–3529. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Wadas, W.; Kalinowski, K. Possibility of increasing micronutrient contents in potato tubers with foliar application of titanium. Appl. Ecol. Environ. Res. 2019, 17, 3633–3643. [Google Scholar] [CrossRef]
- Dong, T.; Cao, Y.; Jiang, C.Z. Cysteine protease inhibitors reduce enzymatic browning of potato by lowering the accumulation of free amino acids. J. Agric. Food Chem. 2020, 68, 2467–2476. [Google Scholar] [CrossRef]
- Xu, X.Y.; Akbar, S.; Shrestha, P. A synergistic genetic engineering strategy induced triacylglycerol accumulation in potato (Solanum tuberosum) leaf. Front. Plant Sci. 2020, 11, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ierna, A.; Pellegrino, A.; Mauro, R.P.; Leonardi, C. Micronutrient foliar fertilization for the biofortification of raw and minimally processed early potatoes. Agronomy 2020, 10, 1744. [Google Scholar] [CrossRef]
- Dobosy, P.; Endrédi, A.; Sandil, S.; Vetési, V.; Rékási, M.; Takács, T.; Záray, G.Y. Biofortification of Potato and Carrot with Iodine by Applying Different Soils and Irrigation with Iodine-Containing Water. Front. Plant Sci. 2020, 11, 593047. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.D. Nutrient biofortification of food crops. Annu. Rev. Nutr. 2009, 29, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M.; Graham, R.D. A new paradigm for world agriculture: Meeting human needs-productive, sustainable, nutritious. Field Crop. Res. 1999, 60, 1–10. [Google Scholar] [CrossRef]
- Zhu, C.; Naqvi, S.; Gomez-Galera, S.; Pelacho, A.M.; Capell, T.; Christou, P. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 2007, 12, 548–555. [Google Scholar] [CrossRef]
- Hameed, A.; Zaidi, S.S.; Shakir, S.; Mansoor, S. Applications of New Breeding Technologies for Potato Improvement. Front. Plant Sci. 2018, 9, 925. [Google Scholar] [CrossRef]
- Ahmad, D.; Zhang, Z.; Rasheed, H.; Xu, X.; Bao, J. Recent Advances in Molecular Improvement for Potato Tuber Traits. Int. J. Mol. Sci. 2022, 23, 9982. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Morote, M.; Stangoulis, J.; Bonierbale, M. Fe and Zn concentration of native Andean potato cultivars from a human nutrition perspective. J. Food Sci. Agric . 2007, 87, 668–675. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- de Haan, S.; Burgos, G.; Liria, R. The nutritional contribution of potato varietal diversity in andean food systems: A case study. Am. J. Potato Res. 2019, 96, 151–163. [Google Scholar] [CrossRef]
- Hefferon, K.L. Can biofortified crops help attain food security? Curr. Mol. Biol. Rep. 2016, 2, 180–185. [Google Scholar] [CrossRef][Green Version]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 69, 172–180. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. Soil and foliar zinc biofortification in field pea (Pisum sativum L.): Grain accumulation and bioavailability in raw and cooked grains. Food Chem. 2016, 212, 427–433. [Google Scholar] [CrossRef]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Sec. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Pérez-Massot, E.; Banakar, R.; Gómez-Galera, S. The contribution of transgenic plants to better health through improved nutrition: Opportunities and constraints. Genes Nutr. 2013, 8, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chakraborty, N.; Agrawal, L.; Ghosh, S.; Narula, K.; Shekhar, S. Next-generation protein-rich potato expressing the seed protein gene AmA1 is a result of proteome rebalancing in transgenic tuber. Proc. Natl. Acad. Sci. USA 2010, 107, 17533–17538. [Google Scholar] [CrossRef]
- Bradshaw, J.E. Improving the nutritional value of potatoes by conventional breeding and genetic modification. In Quality Breeding in Field Crops; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 41–84. [Google Scholar]
- Dolničar, P. Importance of potato as a crop and practical approaches to potato breeding. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 3–20. [Google Scholar]
- Docimo, T.; Scotti, N.; Tamburino, R.; Villano, C.; Carputo, D.; D’Amelia, V. Potato Nutraceuticals: Genomics and Biotechnology for Bio-fortification. In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Rinder, J.; Casazza, A.P.; Hoefgen, R.; Hesse, H. Regulation of aspartate-derived amino acid homeostasis in potato plants (Solanum tuberosum L.) by expression of E. coli homoserine kinase. Amino Acids 2008, 34, 213–222. [Google Scholar] [CrossRef][Green Version]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Del Mar Martínez-Prada, M.; Curtin, S.J.; Gutiérrez-González, J.J. Potato improvement through genetic engineering. GM Crop. Food 2021, 12, 479–496. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Cortelezzi, J.I.; Fumagalli, M.; Capiati, D.A. Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol. Biol. 2018, 98, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, V.; Kempa, S.; Zeh, M. Engineering of cysteine and methionine biosynthesis in potato. Amino Acids 2002, 22, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Hardigan, M.A.; Bamberg, J.; Buell, C.R.; Douches, D.S. Taxonomy and genetic differentiation among wild and cultivated germplasm of Solanum sect. Petota. Plant Genome 2015, 8, plantgenome2014.06.0025. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Haltermanm, D.A.; Janskym, S.H. Potato germplasm enhancement enters the genomics era. Agronomy 2019, 9, 575. [Google Scholar] [CrossRef]
- Klaassen, M.T.; Bourke, P.M.; Maliepaard, C.; Trindade, L.M. Multi-allelic QTL analysis of protein content in a bi-parental population of cultivated tetraploid potato. Euphytica 2019, 215, 14. [Google Scholar] [CrossRef] [PubMed]
- Śliwka, J.; Sołtys-Kalina, D.; Szajko, K.; Wasilewicz-Flis, I.; Strzelczyk-Żyta, D.; Zimnoch-Guzowska, E.; Jakuczun, H.; Marczewski, W. Mapping of quantitative trait loci for tuber starch and leaf sucrose contents in diploid potato. Theor. Appl. Genet. 2016, 129, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Werij, J.S.; Furrer, H.; van Eck, H.J.; Visser, R.G.F.; Bachem, C.W.B. A limited set of starch related genes explain several interrelated traits in potato. Euphytica 2012, 186, 501–516. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, J.; Gong, S.; Li, M.; Yang, T.; Zhang, J. Physicochemical properties of potato starch fermented by amylolytic Lactobacillus plantarum. Int. J. Biol. Macromol. 2020, 158, 656–661. [Google Scholar] [CrossRef]
- Nicolia, A.; Proux-Wéra, E.; Åhman, I.; Onkokesung, N.; Andersson, M.; Andreasson, E.; Zhu, L.H. Targeted gene mutation in tetraploid potato through transient TALEN expression in protoplasts. J. Biotechnol. 2015, 204, 17–24. [Google Scholar] [CrossRef]
- Forsyth, A.; Weeks, T.; Richael, C.; Duan, H. Transcription activator-like effector nucleases (TALEN)-mediated targeted DNA insertion in potato plants. Front. Plant Sci. 2016, 7, 1572. [Google Scholar] [CrossRef]
- Butler, N.M.; Baltes, N.J.; Voytas, D.F.; Douches, D.S. Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases. Front Plant Sci. 2016, 7, 1045. [Google Scholar] [CrossRef]
- Nadakuduti, S.S.; Starker, C.G.; Voytas, D.F. Genome editing in potato with CRISPR/Cas9. In Plant Genome Editing with CRISPR Systems: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2019; pp. 183–201. [Google Scholar] [CrossRef]
- Bamberg, J.B.; Martin, M.W.; Abad, J. In vitro technology at the US Potato Genebank. Vitr. Cell Dev. Biol-Plant. 2016, 52, 213–225. [Google Scholar] [CrossRef]


| SL No | Name of Parameter | Values/100 gm | SL No | Name of Parameter | Values/100 gm |
|---|---|---|---|---|---|
| 1 | Protein | 2.57 g | 8 | Potassium | 411 mg |
| 2 | Energy | 59 kcal | 9 | Iron | 3.14 mg |
| 3 | Total dietary fibre | 2.1 g | 10 | Calcium | 30 mg |
| 4 | Vitamin C | 10.9 mg | 11 | Sodium | 12 mg |
| 5 | Vitamin B 6 | 0.237 mg | 12 | Zinc | 0.32 mg |
| 6 | Riboflavin | 0.038 mg | 13 | Thiamin | 0.02 mg |
| 7 | Folate | 17 µg | 14 | Niacin | 1.03 mg |
| Name of Parameter | Values/100 gm | Name of Parameter | Values/100 gm |
|---|---|---|---|
| Protein | 2.11 g | Vitamin A | 3.8 IU |
| Carbohydrate | 25 g | Vitamin C | 9.5 mg |
| Energy | 107 kcal | Vitamin K | 2.6 mcg |
| Total lipid | 0.15 g | Vitamin E | 0.01 mg |
| Sugar | 1.1 g | Riboflavin | 0.02 mg |
| Total dietary fat | 2.3 g | Niacin | 1.6 mg |
| Carotene | 2.5 mcg | Thiamin | 0.1 mg |
| Pantothenic acid | 0.67 mg | Folate | 11.3 mcg |
| Lutein & Zeaxanthin | 11.3 mcg | Calcium | 10 mg |
| Sl No | Name of Authors with Year | Biofortification Strategies | Improved Nutrient Value | Outcome |
|---|---|---|---|---|
| 1 | [34] | “Antisense inhibition of threonine synthase” | Increasing methionine content (239-fold higher | Higher nutrient content |
| 2 | [35] | “Zeaxanthin epoxidase gene expressions in transgenic mode” | Zeaxanthin content | Higher zeaxanthin content |
| 3 | [36] | Incorporating PSY gene | Provitamin A and lutein | Higher nutrient content |
| 4 | [37] | “Expression of the Arabidopsis H+/Ca2+ transporter scax1” | Calcium content | Higher calcium content |
| 5 | [38] | “Expression of an Arabidopsis CAX2 variant” | Increases calcium levels | Higher calcium content |
| 6 | [39] | “Simultaneous incorporation of PSY, phytoene desaturase, and lycopene β-cyclase genes” | Carotenoids | Higher nutrient content |
| 7 | [39] | “Tuber-specific silencing of lycopene epsilon cyclise” | Carotenoid content | Higher carotenoid content |
| 8 | [40] | Transgenic approach of Orange (Or) gene from cauliflower in potato | Β-carotene accumulation | Higher β-carotene accumulation |
| 9 | [41] | Transgenic approach for cysE gene | cysteine and glutathione contents | Higher cysteine and glutathione contents |
| 10 | [42] | “RNAi silencing of beta-carotene hydroxylase gene” | Beta-carotene content | Higher Beta-carotene content |
| 11 | [43] | Incorporation of orange cauliflower mutant Or gene | Carotenoids, phytoene, phytofluene, and z-carotene content | Higher nutrient content |
| 12 | [44] | “Co-expression of cystathionine γ-synthase (CgSΔ90) and methionine-rich storage protein” | Methionine content | Higher methionine content |
| 13 | [45] | “Overexpression of strawberry GalUR” | Vitamin C content | Higher vitamin C content |
| 14 | [46] | Transgenic approach | ascorbic acid content | Higher ascorbic acid content |
| 15 | [46] | “Over-expression of L-gulono-γ-lactone oxidase gene” | L-Ascorbic acid content | Increases ability to withstand abiotic stresses |
| 16 | [47] | “Overexpression of two dehydroascorbate reductase genes” | Ascorbic acid contents | Higher Ascorbic acid content |
| 17 | [48] | “Overexpression of D-galacturonic acid reductase” | Tolerance in transgenic potato to abiotic stress | |
| 18 | [49] | RNAi silencing of SSIII gene | phosphorus and starch content | Higher phosphorus and starch content |
| 19 | [50] | Transgenic approach for PsGPT gene | starch yield | Higher starch yield |
| 20 | [51] | Expression of prleg polypeptide potato | Methionine content | Higher Methionine content |
| 21 | [52] | Over expression of dihydroflavonol reductase | Phenolic antioxidant content | Transgenic potato with efficient nutrient values |
| 22 | [53] | Silencing of stmgl1 | Higher methionine to isoleucine ratio | Higher methionine to isoleucine content |
| 23 | [54] | Genome-wide association studies | Starch of the amylopectin | Enhancing its industrial application |
| 24 | [55] | Expression of auxin synthesis gene tms1 | in vitrotuberization | Higher tuber yield |
| 25 | [56] | “Overexpression of the sweet potato ibor gene” | Carotenoid content | Elevates tolerance to environmental stresses |
| 26 | [57] | TALEN genes GBSS for genome editing | Starch quality | Better Starch quality |
| 27 | [58] | Expression of lycopene β-cyclase [stlcyb] | Beta-carotene content | Higher Beta-carotene content |
| 28 | [59] | “Over expression of AtCYP21-4 and OsCYP21-4 genes” | Mannosidic-glycoproteins content | 20% increase in mannosidic-glycoproteins |
| 29 | [60] | Agronomic biofortification | iron and zinc Content | Higher iron and zinc content |
| 30 | [61] | “Transient expression of transcription activator-like effector nucleases (TALEN) Genome editing of StvacINV2” | cold-induced sweetening (CIS) or reducing sugar content | Regulation of CIS |
| 31 | [62] | Integrated metabolic engineering strategy | Oil content | Higher oil content |
| 32 | [63] | “Overexpression of cystathionine γ-synthase and silencing of endogenous methionine γ-Lyase” | Methionine content | Higher methionine content |
| 33 | [64] | “Multiallelic mutagenesis in tetraploid potato through CRISPR-Cas9 expression at GBSS gene” | Starch quality | Better Starch quality |
| 34 | [65] | “Transgenic approach with Single nucleotide polymorphism markers” | Folate content | High folate content |
| 35 | [66] | “Overexpression of PDX-II gene” | “Vitamin B6 content” | Higher vitamin B6 content |
| 36 | [20] | “Tuber-specific expression of four folate biosynthesis genes HPPK/DHPS and/or FPGS in mitochondrial folate biosynthesis” | Folate biofortification | “Augmentation of folates to satisfactory levels (12-fold) with stability” |
| 37 | [67] | “Agronomic biofortification (Tuber priming)” | zinc Content | Higher zinc content |
| 38 | [68] | Agronomic biofortification (foliar application) | Selenium Content | Higher Selenium content |
| 39 | [69] | Agronomic biofortification (titanium foliar application) | Fe, Zn, Mn, Ti content | Higher nutrient content |
| 40 | [70] | “Inhibition of cysteine StPI 143 and StPI 146” | reduction in protease activities | Regulate free amino acid contents |
| 41 | [71] | “Wrinkled1, Diacylglycerol acyl transferase 1 and oleosin” | “30-fold increase in triacylglycerols” | Higher triacylglycerols content |
| 42 | [72] | “Application of foliar microelement-containing solutions” | “Enhanced micronutrients content (B, Cu, Fe, Mn, Mo and Zn)” | Fortified micronutrients content |
| 43 | [12] | “Foliar spraying with KIO3 in a dose of 2.0 kg I ha−1”. | Iodine content | “Potatoes biofortified with iodine can be a source of i in a daily diet” |
| 44 | [73] | “Using irrigationWater containing iodine at concentrations of 0.1 and 0.5 mg/L” | Iodine content | Higher iodine content |
| 45 | [26] | “Marker-assisted selection, speed breeding and transgenic approaches” | Iron content | Higher iron content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, S.; Kumar, A.; Gupta, Y.; Trivedi, A. Potato Biofortification: A Systematic Literature Review on Biotechnological Innovations of Potato for Enhanced Nutrition. Horticulturae 2024, 10, 292. https://doi.org/10.3390/horticulturae10030292
Agrawal S, Kumar A, Gupta Y, Trivedi A. Potato Biofortification: A Systematic Literature Review on Biotechnological Innovations of Potato for Enhanced Nutrition. Horticulturae. 2024; 10(3):292. https://doi.org/10.3390/horticulturae10030292
Chicago/Turabian StyleAgrawal, Smita, Amit Kumar, Yash Gupta, and Ayushi Trivedi. 2024. "Potato Biofortification: A Systematic Literature Review on Biotechnological Innovations of Potato for Enhanced Nutrition" Horticulturae 10, no. 3: 292. https://doi.org/10.3390/horticulturae10030292
APA StyleAgrawal, S., Kumar, A., Gupta, Y., & Trivedi, A. (2024). Potato Biofortification: A Systematic Literature Review on Biotechnological Innovations of Potato for Enhanced Nutrition. Horticulturae, 10(3), 292. https://doi.org/10.3390/horticulturae10030292








