Microsatellite Sequence Polymorphisms Reveals Substantial Diversity in Caribbean Breadfruit [Artocarpus altilis (Parkinson) Fosberg] Germplasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Isolation and Purification
2.3. PCR Amplification and Sequencing
2.4. Data and Statistical Analysis
2.4.1. Sequence Editing and Alignment
2.4.2. Polymorphism and Diversity Analysis
2.4.3. Linkage Disequilibrium
2.4.4. Population Structure and Phylogenetic Analysis
3. Results
3.1. Sequence Analysis
3.2. Identity Analysis
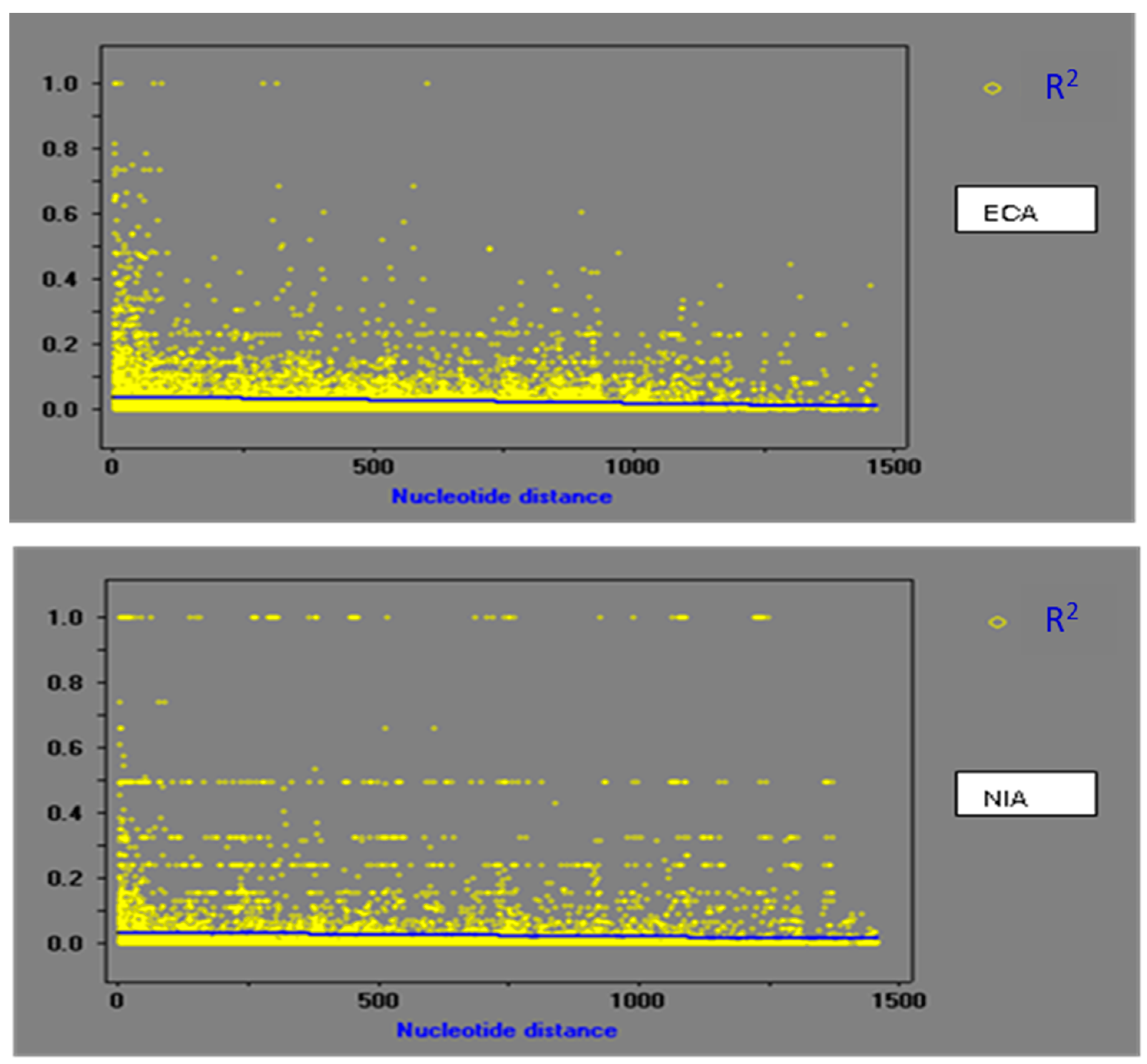
3.3. Population Recombination
3.4. Population Structure and Demographic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zerega, N.J.C.; Ragone, D.C.; Motley, T.J. Complex Origins of Breadfruit (Artocarpus altilis, Moraceae): Implications for Human Migrations in Oceania. Am. J. Bot. 2004, 91, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Zerega, N.J.C.; Ragone, D.C.; Motley, T.J. Breadfruit Origins, Diversity, and Human-Facilitated Distribution. In Darwin’s Harvest: New Approaches to Origins, Evolution, and Conservation of Crop Plants; Motley, T.J., Zerega, N.J.C., Ragone, D.C., Cross, H., Eds.; Columbia University Press: New York, NY, USA, 2006; pp. 213–238. [Google Scholar]
- Roberts-Nkrumah, L.B. An Overview of Breadfruit (Artocarpus altilis) in the Caribbean. Acta. Hort. 2007, 757, 51–60. [Google Scholar] [CrossRef]
- Powell, D. The Voyage of the Plant Nursery, H.M.S. Providence, 1791–1793. Econ. Bot. 1977, 31, 387–431. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Ragone, D.; Tavana, N.G.; Bernotas, D.W.; Murch, S.J. Beyond the Bounty: Breadfruit (Artocarpus altilis) for Food Security and Novel Foods in the 21st Century. Ethnobot. Res. Appl. 2011, 9, 129–149. [Google Scholar] [CrossRef]
- Roberts-Nkrumah, L.B. Enhancing Breadfruit Contribution to Food Security in the Caribbean Through Improved Supply. In Proceedings of the Caribbean Food Crop Society, Frigate Bay, Federation of St. Kitts and Nevis, 12–17 July 2009. [Google Scholar]
- Ragone, D. Breadfruit: Diversity, Conservation and Potential. Acta. Hort. 2007, 757, 19–30. [Google Scholar] [CrossRef]
- Zerega, N.J.C.; Ragone, D.C.; Motley, T.J. Systematics and Species Limits of Breadfruit (Artocarpus, Moraceae). Syst. Bot. 2005, 30, 603–615. [Google Scholar] [CrossRef]
- Ragone, D. Chromosome Numbers and Pollen Stainability of Three Species of Pacific Island Breadfruit (Artocarpus, Moraceae). Am. J. Bot. 2001, 88, 693–696. [Google Scholar] [CrossRef]
- Brantjes, N.B.M. Nectar and the Pollination of Breadfruit, Artocarpus altilis (Moraceae). Acta Bot. Neerl. 1981, 30, 345–352. [Google Scholar] [CrossRef]
- Daley, O.O.; Roberts-Nkrumah, L.B.; Alleyne, A.T. Morphological diversity of breadfruit [Artocarpus altilis (Parkinson) Fosberg] in the Caribbean. Sci. Hort. 2020, 266, 109278. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Murch, S.J.; Wiseman, J.; Ragone, D.C. Morphological Diversity in Breadfruit (Artocarpus, Moraceae): Insights into Domestication, Conservation, and Cultivar Identification. Genet. Resour. Crop Evol. 2013, 60, 175–192. [Google Scholar] [CrossRef]
- Ragone, D.C. Description of Pacific Island Breadfruit Cultivars. Acta. Hort. 1995, 413, 93–98. [Google Scholar] [CrossRef]
- Daley, O.O.; Roberts-Nkrumah, L.B.; Alleyne, A.T.; Gloster, M.C. Folk nomenclature and traditional knowledge of breadfruit [Artocarpus altilis (Parkinson) Fosberg] diversity in four Anglophone Caribbean countries. J. Ethnobiol. Ethnomed. 2022, 18, 65. [Google Scholar] [CrossRef]
- Ragone, D.; Tavana, G.; Stevens, J.M.; Stewart, P.A.; Stone, R.; Cox, P.M.; Cox, P.A. Nomenclature of Breadfruit Cultivars in Samoa: Saliency, Ambiguity and Monomiality. J. Ethnobiol. 2004, 24, 33–49. [Google Scholar]
- Korir, N.K.; Han, J.; Shangguan, L.; Wang, C.; Kayesh, E.; Zhang, Y.; Fang, J. Plant Variety and Cultivar Identification: Advances and Prospects. Crit. Rev. Biotechnol. 2013, 33, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.K.; Xu, Q.; Mayer, C.; Deng, X. Genome Wide Characterization of Short Tandem Repeat Markers in Sweet Orange (Citrus sinensis). PLoS ONE 2014, 9, e104182. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.A.; Bravo, J.P.; Morelli, K.A.; Nobile, P.M. Microsatellites as Tools for Genetic Diversity Analysis. In Genetic Diversity in Microorganisms; Caliskan, M., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 149–170. [Google Scholar]
- Kanzaki, S.; Yonemori, K.; Sugiura, A.; Subhadrabandhu, S. Phylogenetic Relationships between the Jackfruit, the Breadfruit and Nine Other Artocarpus spp. from RFLP Analysis of an Amplified Region of cpDNA. Sci. Hort. 1997, 70, 57–66. [Google Scholar] [CrossRef]
- Sreekumar, V.B.; Binoy, A.M.; George, S.T. Genetic and Morphological Variation in Breadfruit (Artocarpus altilis (Park.) Fosberg) in the Western Ghats of India Using AFLP Markers. Genet. Resour. Crop Evol. 2007, 54, 1659–1665. [Google Scholar] [CrossRef]
- Witherup, C.; Ragone, D.; Wiesner-Hanks, T.; Irish, B.; Scheffler, B.; Simpson, S.; Zee, F.; Zuberi, M.I.; Zerega, N.J.C. Development of Microsatellite Loci in Artocarpus altilis (Moraceae) and Cross-Amplification in Congeneric Species. Appl. Plant Sci. 2013, 1, 1200423. [Google Scholar] [CrossRef] [PubMed]
- Zerega, N.J.C.; Wiesner-Hanks, T.; Ragone, D.; Irish, B.; Scheffler, B.; Simpson, S.; Zee, F. Diversity in the Breadfruit Complex (Artocarpus, Moraceae): Genetic Characterization of Critical Germplasm. Tree Genet. Genomes 2015, 11, 4. [Google Scholar] [CrossRef]
- Gardner, E.M.; Laricchia, K.M.; Murphy, M.; Ragone, D.; Scheffler, B.E.; Simpson, S.; Williams, E.W.; Zerega, N.J. Chloroplast Microsatellite Markers for Artocarpus (Moraceae) Developed from Transcriptome Sequences. Appl. Plant Sci. 2015, 3, 1500049. [Google Scholar] [CrossRef] [PubMed]
- Zerega, N.J.C.; Ragone, D. Toward a Global View of Breadfruit Genetic Diversity. Trop. Agr. 2016, 93, 77–91. [Google Scholar]
- De Bellis, F.; Malapa, R.; Kagy, V.; Lebegin, S.; Billot, C.; Labouisse, J.-P. New Development and Validation of 50 SSR Markers in Breadfruit (Artocarpus altilis, Moraceae) by Next-Generation Sequencing. Appl. Plant Sci. 2016, 4, 1600021. [Google Scholar] [CrossRef]
- Audi, L.; Shallow, G.; Robertson, E.; Bobo, D.; Ragone, D.; Gardner, E.M.; Jhurree-Dussoruth, B.; Wajer, J.; Zerega, N.J.C. Linking Breadfruit Cultivar Names across the Globe Connects Histories after 230 Years of Separation. Curr. Biol. 2023, 33, 287–297. [Google Scholar] [CrossRef]
- Barthe, S.; Gugerli, F.; Barkley, N.A.; Maggia, L.; Cardi, C.; Scotti, I. Always Look on Both Sides: Phylogenetic Information Conveyed by Simple Sequence Repeat Allele Sequences. PLoS ONE 2012, 7, e40699. [Google Scholar] [CrossRef]
- Estoup, A.; Jarne, P.; Cornuet, J.-M. Homoplasy and Mutation Model at Microsatellite Loci and Their Consequences for Population Genetics Analysis. Mol. Ecol. 2002, 11, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Nkrumah, L.B. The Breadfruit Germplasm Collection at the University of the West Indies, St Augustine Campus; University of the West Indies Press: Kingston, Jamaica, 2018. [Google Scholar]
- Roberts-Nkrumah, L.B. A Review of the Potential of Breadfruit Cultivar ‘Ma’afala’ for Commercial Production in Trinidad and Tobago. Trop. Agr. 2014, 91, 284–299. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.R. A New Statistic for Detecting Genetic Differentiation. Genetics 2000, 155, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Remington, D.L.; Thornsberry, J.M.; Matsuoka, Y.; Wilson, L.M.; Whitt, S.R.; Doebley, J.; Kresovich, S.; Goodman, M.M.; Buckler, E.S.T. Structure of Linkage Disequilibrium and Phenotypic Associations in the Maize Genome. Proc. Natl. Acad. Sci. USA 2001, 98, 11479–11484. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation Software for the Fast Assembly of Multi-Gene Datasets with Character set and Codon Information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Corander, J.; Marttinen, P.; Sirén, J.; Tang, J. Enhanced Bayesian Modelling in BAPS Software for Learning Genetic Structures of Populations. BMC Bioinform. 2008, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Corander, J.; Marttinen, P.; Sirén, J.; Tang, J. Baps: Bayesian Analysis of Population Structure; Department of Mathematics and Statistics, University of Helsinki: Helsinki, Finland, 2009; pp. 1–27. [Google Scholar]
- Swofford, D.L. PAUP* Version 4.0 b10. Phylogenetic Analysis Using Parsimony (* and Other Methods); Sinauer: Sunderland, MA, USA, 2002. [Google Scholar]
- Hudson, R.R.; Slatkin, M.; Maddison, W.P. Estimation of Levels of Gene Flow from DNA Sequence Data. Genetics 1992, 132, 583. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.R. Estimating the Recombination Parameter of a Finite Population Model without Selection. Genet. Res. 1987, 50, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bligh, W. The Log of H. M. S. Providence 1791–1793; Genesis Publications Limited: Guildford, UK, 1976. [Google Scholar]
- Ragone, D.C. Collection, Establishment, and Evaluation of a Germplasm Collection of Pacific Island Breadfruit. Ph.D. Thesis, University of Hawaiʻi at Manoa, Honolulu, HI, USA, 1991. [Google Scholar]
- Chen, H.; Morrell, P.L.; Cruz, M.d.l.; Clegg, M.T. Nucleotide Diversity and Linkage Disequilibrium in Wild Avocado (Persea americana Mill.). J. Hered. 2008, 99, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsson, P.K. Nucleotide Polymorphism and Linkage Disequilibrium within and among Natural Populations of European Aspen (Populus tremula L., Salicaceae). Genetics 2005, 169, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Creste, S.; Tulmann Neto, A.; Vencovsky, R.; de Oliveira Silva, S.; Figueira, A. Genetic Diversity of Musa Diploid and Triploid Accessions from the Brazilian Banana Breeding Program Estimated by Microsatellite Markers. Genet. Resour. Crop Evol. 2004, 51, 723–733. [Google Scholar] [CrossRef]
- Hedrick, P.W. Genetics of Populations, 4th ed.; Jones and Bartlett Publishers: Sadbury, MA, USA, 2011. [Google Scholar]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 4th ed.; Sinaer Associates, Inc. Publishers: Sunderland, MA, USA, 2007. [Google Scholar]
- Garris, A.J.; McCouch, S.R.; Kresovich, S. Population Structure and its Effect on Haplotype Diversity and Linkage Disequilibrium Surrounding the xa5 Locus of Rice (Oryza sativa L.). Genetics 2003, 165, 759. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Thornsberry, J.M.; Buckler, E.S., IV. Structure of Linkage Disequilibrium in Plants. Annu. Rev. Plant Biol. 2003, 54, 357–374. [Google Scholar] [CrossRef]
- Bamberg, J.B.; del Rio, A.H. Genetic Heterogeneity Estimated by RAPD Polymorphism of Four Tuber-Bearing Potato Species Differing by Breeding System. Am. J. Pot Res. 2004, 81, 377–383. [Google Scholar] [CrossRef]
- Simko, I.; Haynes, K.G.; Jones, R.W. Assessment of Linkage Disequilibrium in Potato Genome with Single Nucleotide Polymorphism Markers. Genetics 2006, 173, 2237–2245. [Google Scholar] [CrossRef]
- Nordborg, M. Linkage Disequilibrium, Gene Trees and Selfing: An Ancestral Recombination Graph with Partial Self-Fertilization. Genetics 2000, 154, 923–929. [Google Scholar] [CrossRef]
- Hudson, R.R. Linkage Disequilibrium and Recombination. In Handbook of Statistical Genetics; Balding, D.J., Bishop, M., Cannings, C., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Liu, A.; Burke, J.M. Patterns of Nucleotide Diversity in Wild and Cultivated Sunflower. Genetics 2006, 173, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Song, Q.J.; Hyten, D.L.; Van Tassell, C.P.; Matukumalli, L.K.; Grimm, D.R.; Hyatt, S.M.; Fickus, E.W.; Young, N.D.; Cregan, P.B. Single-Nucleotide Polymorphisms in Soybean. Genetics 2003, 163, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.J.; Hamrick, J.L. Genetic Diversity in the Endangered Tropical Tree, Guaiacum sanctum (Zygophyllaceae). J. Hered. 2010, 101, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Nkrumah, L.B. Towards a Description of the Breadfruit Germplasm in St Vincent. Fruits 1997, 52, 27–35. [Google Scholar]



| No. | Sample ID | Cultivar Name | Taxon * | Ploidy * | Sample Collection Site | Accession Grouping |
|---|---|---|---|---|---|---|
| 1 | 12A | Huehue | Aa × Am | 3n | UWI | NIA |
| 2 | 13A | Hope Marble | Aa | 3n | UWI | ECA |
| 3 | 15A | Ulu’ea | Aa | 2n | UWI | NIA |
| 4 | 18A | Meitehid | Aa | 3n | UWI | NIA |
| 5 | 19A | White | Aa | 3n | UWI | ECA |
| 6 | 1A | Yellow | Aa | 3n | UWI | ECA |
| 7 | 41A | Macca | Aa | 3n | UWI | ECA |
| 8 | 42B | Yellow Heart | Aa | 3n | UWI | ECA |
| 9 | 43B | Yellow Heart | Aa | 3n | UWI | ECA |
| 10 | 44A | Aveloloa | Aa | 3n | UWI | NIA |
| 11 | 45A | Creole | Aa | 3n | UWI | ECA |
| 12 | 47A | White | Aa | 3n | UWI | ECA |
| 13 | 48B | White | Aa | 3n | UWI | ECA |
| 14 | 49B | White | Aa | 3n | UWI | ECA |
| 15 | 50B | Porohiti | Aa | 3n | UWI | NIA |
| 16 | 51A | Porohiti | Aa | 3n | UWI | NIA |
| 17 | 522B | Captain Bligh | Aa | 3n | SVG | ECA |
| 18 | 523B | Unidentfied 1 | Aa | 3n | SVG | ECA |
| 19 | 524A | Creole | Aa | 3n | SVG | ECA |
| 20 | 52B | Toneno | Aa | 3n | UWI | NIA |
| 21 | 532B | Sally Young | Aa | 3n | SVG | ECA |
| 22 | 534A | White | Aa | 3n | SVG | ECA |
| 23 | 537A | Hog Pen | Aa | 3n | SVG | ECA |
| 24 | 539B | Dessert | Aa | 3n | SVG | ECA |
| 25 | 53B | Roiha’a | Aa | 3n | UWI | NIA |
| 26 | 544B | Kashee Bread | Aa | 3n | SVG | ECA |
| 27 | 545A | Hope Marble | Aa | 3n | SVG | ECA |
| 28 | 546A | Lawyer Caine | Aa | 3n | SVG | ECA |
| 29 | 548A | Dessert | Aa | 3n | SVG | ECA |
| 30 | 54A | Tapeha’a | Aa | 3n | UWI | NIA |
| 31 | 552B | Sally Young | Aa | 3n | SVG | ECA |
| 32 | 553A | White | Aa | 3n | SVG | ECA |
| 33 | 555B | Waterloo/Cotton | Aa | 3n | SVG | ECA |
| 34 | 556A | Soursop | Aa | 3n | SVG | ECA |
| 35 | 558A | Liberal | Aa | 3n | SVG | ECA |
| 36 | 559A | Yellow Heart | Aa | 3n | JAM | ECA |
| 37 | 55A | Piipiia | Aa × Am | 3n | UWI | NIA |
| 38 | 561A | Timor | Aa | 3n | JAM | ECA |
| 38 | 562B | Yellow Heart | Aa | 3n | JAM | ECA |
| 40 | 563A | Couscous | Aa | 3n | JAM | ECA |
| 41 | 565A | Timor | Aa | 3n | JAM | ECA |
| 42 | 567B | Yellow Heart | Aa | 3n | JAM | ECA |
| 43 | 569B | Yellow Heart | Aa | 3n | JAM | ECA |
| 44 | 56A | Meinpadahk | Aa × Am | 3n | UWI | NIA |
| 45 | 571A | Timor | Aa | 3n | JAM | ECA |
| 46 | 572A | Couscous | Aa | 3n | JAM | ECA |
| 47 | 573B | White Heart | Aa | 3n | JAM | ECA |
| 48 | 574B | Macca | Aa | 3n | JAM | ECA |
| 49 | 575B | Brambram | Aa | 3n | JAM | ECA |
| 50 | 57B | Momolega | Aa | 2n | UWI | NIA |
| 51 | 581B | Yellow Heart | Aa | 3n | JAM | ECA |
| 52 | 58B | Unidentified 2 | Aa | 3n | UWI | NIA |
| 53 | 590A | White Heart | Aa | 3n | JAM | ECA |
| 54 | 591A | Monkey Breadfruit | Aa | 3n | JAM | ECA |
| 55 | 592A | Monkey Breadfruit | Aa | 3n | JAM | ECA |
| 56 | 596A | Banjam | Aa | 3n | JAM | ECA |
| 57 | 59A | Pua’a | Aa | 3n | UWI | NIA |
| 58 | 5B | Meitehid | Aa | 3n | UWI | NIA |
| 59 | 601A | Portland Breadfruit | Aa | 3n | JAM | ECA |
| 60 | 602A | Man Bread | Aa | 3n | JAM | ECA |
| 61 | 603A | Ma’afala | Aa | 2n | JAM | NIA |
| 62 | 60A | Unidentified 3 | Aa | - | UWI | - |
| 63 | 61B | Mahani | Aa | 3n | UWI | NIA |
| 64 | 62A | Afara | Aa | 3n | UWI | NIA |
| 65 | 63B | Fafai | Aa | 3n | UWI | NIA |
| 66 | 64B | Yellow | Aa | 3n | UWI | NIA |
| 67 | 65B | Otea | Aa | 3n | UWI | NIA |
| 68 | 66B | Puou | Aa | 2n | UWI | NIA |
| 69 | 69A | Breadnut/ Chataigne | Ac | 2n | UWI | ECA |
| 70 | 730A | Masunwa | Aa | 2n | TRI | NIA |
| 71 | 731A | Ma’afala | Aa | 2n | TRI | NIA |
| 72 | 741B | Yellow | Aa | 3n | TRI | ECA |
| 73 | 742A | Ma’afala | Aa | 2n | TRI | NIA |
| 74 | 743A | White | Aa | 3n | TRI | ECA |
| 75 | 764C | Unidentified 4 | Aa | 3n | SKN | ECA |
| 76 | 780C | Unidentified 5 | Aa | 3n | SKN | ECA |
| 77 | 782A | Unidentified 6 | Aa | 3n | TOB | ECA |
| 78 | 783B | Butter Breadfruit | Aa | 3n | TOB | ECA |
| 79 | 788B | Choufchouf | Aa | 3n | TOB | ECA |
| 80 | 790C | Unidentified 7 | Aa | 3n | TOB | ECA |
| 81 | 798A | Ma’afala | Aa | 2n | TRI | NIA |
| 82 | 7A | Timor | Aa | 3n | UWI | ECA |
| 83 | 800 B | White | Aa | 3n | TOB | ECA |
| 84 | 805 A | Pu’upu’u | Aa | 3n | TOB | NIA |
| 85 | 806 A | Meitehid | Aa | 3n | TOB | ECA |
| 86 | 808 A | Timor | Aa | 3n | TOB | ECA |
| 87 | 821 B | White | Aa | 3n | TOB | ECA |
| 88 | 827 B | Ma’afala | Aa | 2n | TOB | ECA |
| 89 | 828 A | Local Yellow | Aa | 3n | TOB | ECA |
| 90 | 833A | Local Yellow | Aa | 3n | TRI | ECA |
| 91 | 835A | Ma’afala | Aa | 2n | TRI | NIA |
| 92 | 9B | Cassava | Aa | 3n | UWI | ECA |
| 93 | BF12 | Unidentfied 8 | Aa | - | UWI | NIA |
| 94 | NO.17 B | Pu’upu’u | Aa | 3n | UWI | NIA |
| 95 | SV4A | Cocobread | Aa | 3n | UWI | ECA |
| Locus | Primer Sequence (5′--3′) | Repeat Motif a |
|---|---|---|
| MAA40 | F: AGCATTTCAGGTTGGTGAC R: TTGTTCTGTTTGCCTCATC | (TG)16 |
| MAA54a | F: AACCTCCAAACACTAGGACAAC R: AGCTACTTCCAAAACGTGACA | (CA)5,(AT)4 |
| MAA71 | F: TTCCTATTTCTTGCAGATTCTC R: AGTGGTGGTAAGATTCAAAGTG | (CT)11(CA)19 |
| MAA85 | F: TCAGGGTGTAGCGAAGACA R: AGGGCTCCTTTGATGGAA | (CA)11 |
| MAA96 | F: GGACCTCAAGGATGTGATCTC R: ACACGGTCTTCTTTGGATAGC | (CA)14(TA)7(TG)3(GT) |
| MAA140 | F: CCATCCCCCATCTTTCCT R: TCCTCGTTTGCCACAGTG | (CT)25 |
| * MAA178a | F: GATGGAGACACTTTGAACTAGC R: CACCAGGGTTTAAGATGAAAC | (GT)3,(GT)6,(GT)3,(GA)3,(GA)10 |
| * MAA178b | F: GATGGAGACACTTTGAACTAGC R: CACCAGGGTTTAAGATGAAAC | (GT)3,(GT)3,(GA)3,(GA)11 |
| MAA182 | F: TACTGGGTCTGAAAAGATGTCT R: CGTTTGCGTTTGGATAAAT | (CT)19 |
| MAA251 | F: ATCGTCTTTGTCACCACCAC R: ATAGCCGAGTAACTGGATGGA | (ATC)10 |
| Microsatellite Locus | Group | SNP (Parsimony Informative) | Nucleotide Diversity | Nucleotide Polymorphism ) | Tajima’s D Test | Fu’s F Test | Harpending Index |
|---|---|---|---|---|---|---|---|
| MAA40 | Total | 40 (9) | 0.334 | 0.16 | −1.709 | −2.338 | 0.004 |
| ECA | 50 (7) | 0.323 | 0.204 | −1.269 | −1.266 | 0.002 | |
| NIA | 61 (22) | 0.349 | 0.182 | −1.181 | −2.285 | 0.004 | |
| MAA54A | Total | 50 (7) | 0.328 | 0.154 | −0.536 | −2.468 | 0.046 |
| ECA | 51 (8) | 0.338 | 0.171 | −0.398 | −2.518 | 0.032 | |
| NIA | 56 (30) | 0.193 | 0.1 | −2.069 | −2.609 | 0.065 | |
| MAA71 | Total | 46 (8) | 0.276 | 0.141 | −0.643 | −2.815 | 0.010 |
| ECA | 70 (13) | 0.34 | 0.239 | 0.911 | −1.432 | 0.002 | |
| NIA | 37 (19) | 0.185 | 0.108 | −1.29 | −2.221 | 0.021 | |
| MAA85 | Total | 55 (1) | 0.303 | 0.253 | 1.48 | −0.451 | 0.001 |
| ECA | 60 (3) | 0.286 | 0.251 | −0.426 | −0.329 | 0.002 | |
| NIA | 72 (9) | 0.325 | 0.297 | 1.479 | 0.303 | 0.004 | |
| MAA96 | Total | 66 (13) | 0.305 | 0.171 | 0.021 | −1.969 | 0.002 |
| ECA | 71 (17) | 0.297 | 0.163 | −1.567 | −1.491 | 0.003 | |
| NIA | 90 (27) | 0.324 | 0.209 | 0.172 | −1.693 | 0.005 | |
| MAA140 | Total | 25 (10) | 0.301 | 0.087 | −2.236 | −3.685 | 0.019 |
| ECA | 33 (23) | 0.302 | 0.096 | −2.287 | −4.752 | 0.030 | |
| NIA | 54 (27) | 0.304 | 0.154 | −1.853 | −2.598 | 0.014 | |
| MAA178A | Total | 57 (12) | 0.33 | 0.181 | −1.498 | −1.545 | 0.003 |
| ECA | 62 (12) | 0.337 | 0.202 | −1.392 | −1.502 | 0.003 | |
| NIA | 63 (17) | 0.28 | 0.192 | −1.182 | −1.396 | 0.007 | |
| MAA178B | Total | 83 (18) | 0.259 | 0.176 | −1.081 | −1.874 | 0.001 |
| ECA | 86 (26) | 0.23 | 0.167 | −0.953 | −1.798 | 0.002 | |
| NIA | 106 (36) | 0.277 | 0.206 | −0.978 | −1.715 | 0.004 | |
| MAA182 | Total | 0 (0) | 0 | 0 | 0 | 0 | ND |
| ECA | 10 (0) | 0.383 | 0.247 | −1.084 | 0.167 | 0.082 | |
| NIA | 39 (1) | 0.333 | 0.348 | 0.164 | 0.075 | 0.006 | |
| MAA251 | Total | 64 (50 | 0.355 | 0.269 | −0.805 | −1.356 | 0.001 |
| ECA | 67 (8) | 0.284 | 0.233 | −0.625 | −0.974 | 0.001 | |
| NAC | 71 (14) | 0.397 | 0.3 | −0.927 | −1.222 | 0.004 | |
| Average | Total | 48.6 (8.3) | 0.159 | 0.279 | −0.701 | −1.850 | 0.010 |
| ECA | 56 (11.7) | 0.197 | 0.312 | −0.909 | −1.590 | 0.016 | |
| NIA | 64.9 (20.2) | 0.210 | 0.297 | −0.767 | −1.536 | 0.013 |
| Loci | Accession Group | No. of Unique Haplotypes | Haplotype Diversity (h) | RM | ρ | Walls’ B Statistic |
|---|---|---|---|---|---|---|
| MAA40 | ECA | 58 | 0.996 ± 0.004 | 10 | 0.225 | 0.000 |
| NIA | 31 | 0.998 ± 0.008 | 7 | 0.404 | 0.000 | |
| MAA54A | ECA | 28 | 0.836 ± 0.039 | 7 | 0.623 | 0.000 |
| NIA | 13 | 0.843 ± 0.043 | 5 | 1.196 | 0.000 | |
| MAA71 | ECA | 62 | 1.000 ± 0.003 | 9 | 0.164 | 0.125 |
| NIA | 20 | 0.950 ± 0.024 | 8 | 0.559 | 0.071 | |
| MAA85 | ECA | 61 | 0.999 ± 0.003 | 17 | 0.129 | 0.000 |
| NIA | 32 | 1.000 ± 0.008 | 23 | 0.138 | 0.024 | |
| MAA96 | ECA | 56 | 0.992 ± 0.007 | 10 | 0.458 | 0.129 |
| NIA | 31 | 0.998 ± 0.008 | 11 | 0.286 | 0.075 | |
| MAA140 | ECA | 29 | 0.914 ± 0.024 | 1 | 1.173 | 0.375 |
| NIA | 25 | 0.956 ± 0.029 | 5 | 0.534 | 0.207 | |
| MAA178A | ECA | 59 | 0.998 ± 0.003 | 7 | 0.343 | 0.174 |
| NIA | 32 | 1.000 ± 0.008 | 13 | 0.172 | 0.091 | |
| MAA178B | ECA | 62 | 1.000 ± 0.003 | 17 | 0.241 | 0.125 |
| NIA | 32 | 1.000 ± 0.008 | 16 | 0.154 | 0.127 | |
| MAA182 | ECA | 28 | 0.955 ± 0.011 | 1 | 0.171 | 0.000 |
| NIA | 31 | 0.998 ± 0.008 | 15 | 0.087 | 0.044 | |
| MAA251 | ECA | 60 | 0.999 ± 0.003 | 15 | 0.332 | 0.000 |
| NIA | 32 | 1.000 ± 0.008 | 8 | 0.156 | 0.044 | |
| Average | ECA | 50.3 ± 5.268 | 0.969 ± 0.055 | 9.400 ± 5.778 | 0.386 ± 0.315 | 0.093 ± 0.121 |
| NIA | 27.900 ± 6.574 | 0.974 ± 0.050 | 11.100 ± 5.724 | 0.369 ± 0.336 | 0.068 ± 0.063 |
| Locus | All Accessions (n = 94) | |
|---|---|---|
| FST a | Snn b | |
| MAA40 | −0.0002 | 0.6084 |
| MAA54A | 0.0105 | 0.5533 |
| MAA71 | 0.0147 | 0.6008 |
| MAA85 | 0.0178 | 0.7018 *** |
| MAA96 | 0.0332 | 0.6939 *** |
| MAA140 | 0.0513 | 0.6024 ** |
| MAA178A | 0.0035 | 0.5829 |
| MAA178B | 0.0358 | 0.7128 ** |
| MAA182 | - | - |
| MAA251 | 0.1095 | 0.6720 ** |
| Average (n = 9) | 0.0306 | 0.6365 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daley, O.O.; Alleyne, A.T.; Roberts-Nkrumah, L.B.; Motilal, L.A. Microsatellite Sequence Polymorphisms Reveals Substantial Diversity in Caribbean Breadfruit [Artocarpus altilis (Parkinson) Fosberg] Germplasm. Horticulturae 2024, 10, 253. https://doi.org/10.3390/horticulturae10030253
Daley OO, Alleyne AT, Roberts-Nkrumah LB, Motilal LA. Microsatellite Sequence Polymorphisms Reveals Substantial Diversity in Caribbean Breadfruit [Artocarpus altilis (Parkinson) Fosberg] Germplasm. Horticulturae. 2024; 10(3):253. https://doi.org/10.3390/horticulturae10030253
Chicago/Turabian StyleDaley, Oral O., Angela T. Alleyne, Laura B. Roberts-Nkrumah, and Lambert A. Motilal. 2024. "Microsatellite Sequence Polymorphisms Reveals Substantial Diversity in Caribbean Breadfruit [Artocarpus altilis (Parkinson) Fosberg] Germplasm" Horticulturae 10, no. 3: 253. https://doi.org/10.3390/horticulturae10030253
APA StyleDaley, O. O., Alleyne, A. T., Roberts-Nkrumah, L. B., & Motilal, L. A. (2024). Microsatellite Sequence Polymorphisms Reveals Substantial Diversity in Caribbean Breadfruit [Artocarpus altilis (Parkinson) Fosberg] Germplasm. Horticulturae, 10(3), 253. https://doi.org/10.3390/horticulturae10030253






