Phyllanthus Lignans: A Review of Biological Activity and Elicitation
Abstract
1. Introduction
2. Phyllanthus for Medicinal Purposes
3. Lignans in Plants
4. Lignans from Phyllanthus
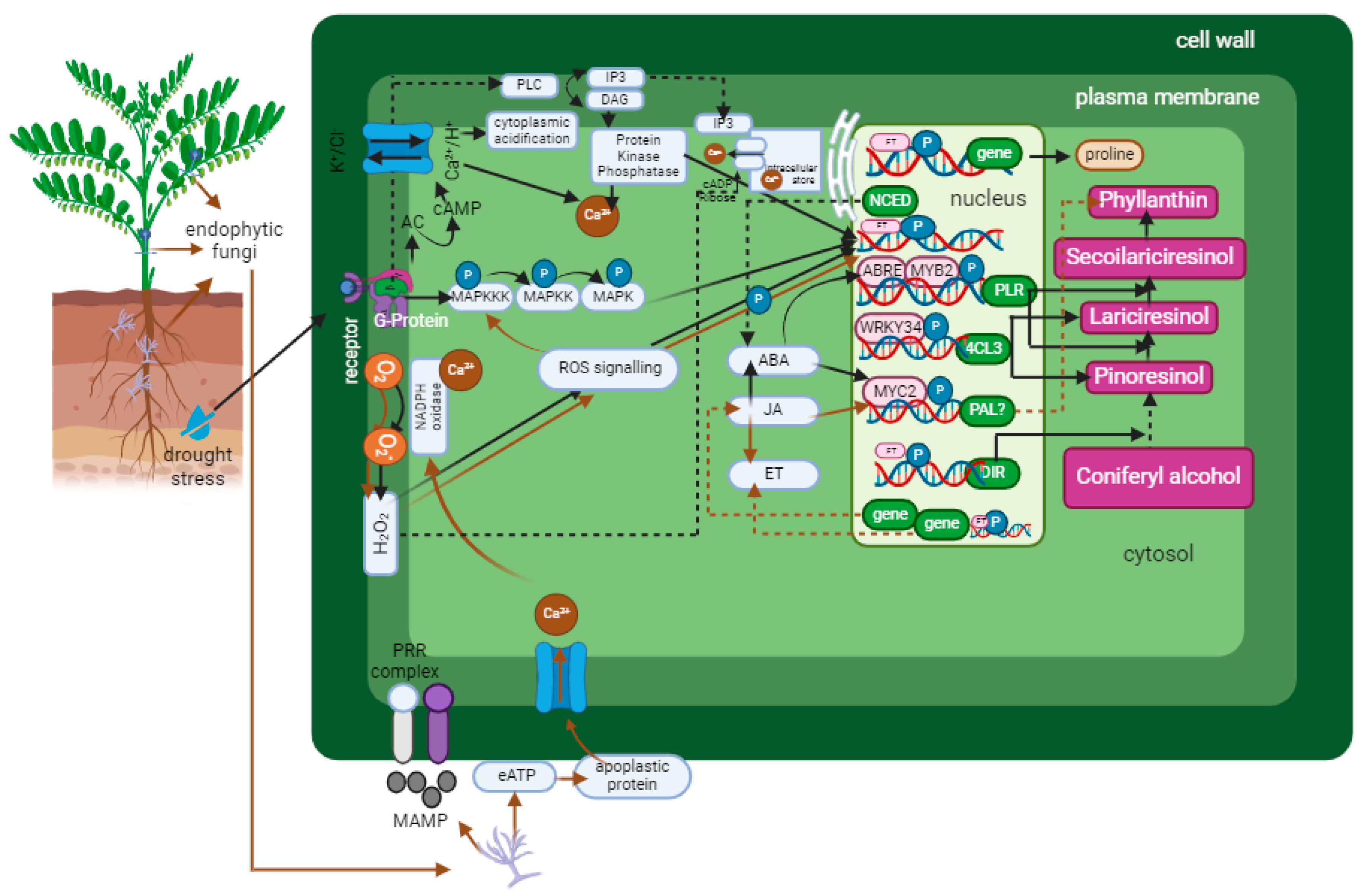
5. Endophytic Fungi and Lignan Biosynthesis
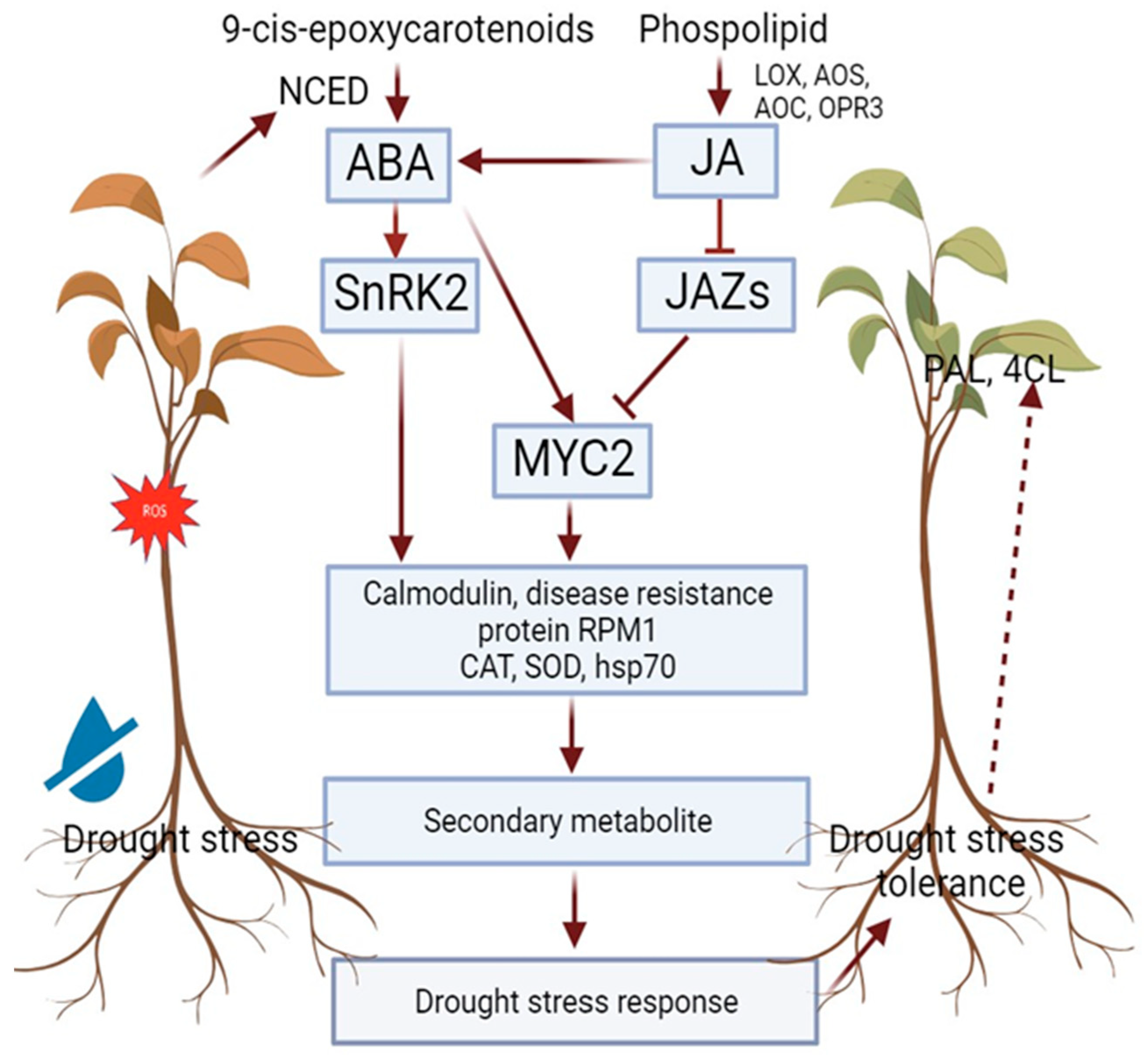
6. Drought Stress and Lignan Biosynthesis
7. Synergetic Treatment to Increase Lignan Production
8. Enhancing Lignan Production in Phyllanthus through Omics Approach
9. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- World Health Organization. National Policy on Traditional Medicine and Regulation of Herbal Medicines Report of a WHO Global Survey; WHO: Geneve, Switzerland, 2005. [Google Scholar]
- Khan, M.S.A.; Ahmad, I. Herbal Medicine: Current Trends and Future Prospects; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; WHO: Geneve, Switzerland, 2019. [Google Scholar]
- Mao, X.; Wu, L.F.; Guo, H.L.; Chen, W.J.; Cui, Y.P.; Qi, Q.; Li, S.; Liang, W.Y.; Yang, G.H.; Shao, Y.Y.; et al. The Genus Phyllanthus: An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evid. -Based Complement. Altern. Med. 2016, 2016, 7584952. [Google Scholar] [CrossRef] [PubMed]
- Gras, A.; Hidalgo, O.; D’ambrosio, U.; Parada, M.; Garnatje, T.; Vallès, J. The Role of Botanical Families in Medicinal Ethnobotany: A Phylogenetic Perspective. Plants 2021, 10, 163. [Google Scholar] [CrossRef]
- Azubuike Awomukwu, D. Identification of the Genus Phyllanthus (Family Phyllanthaceae) in Southern Nigeria Using Comparative Systematic Morphological and Anatomical Studies of the Vegetative Organs. J. Plant Sci. 2015, 3, 137. [Google Scholar] [CrossRef]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula. Nature 1936, 137, 1936. [Google Scholar]
- Calixto, J.B.; Santos, A.R.S.; Cechinel Filho, V.; Yunes, R.A. A Review of the Plants of the Genus Phyllanthus: Their Chemistry, Pharmacology, and Therapeutic Potential. Med. Res. Rev. 1998, 18, 225–258. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, B.; Sirhindi, G. Phytochemistry and Pharmacology of Phyllanthus niruri L.: A Review. Phyther. Res. 2017, 31, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent Advances in Research on Lignans and Neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Niculaes, C.; Morreel, K.; Kim, H.; Lu, F.; McKee, L.S.; Ivens, B.; Haustraete, J.; Vanholme, B.; De Rycke, R.; Hertzberg, M.; et al. Phenylcoumaran Benzylic Ether Reductase Prevents Accumulation of Compounds Formed under Oxidative Conditions in Poplar Xylem. Plant Cell 2014, 26, 3775–3791. [Google Scholar] [CrossRef] [PubMed]
- Tsopmo, A.; Awah, F.M.; Kuete, V. Lignans and Stilbenes from African Medicinal Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wang, C.Y.; Lee, S.S. Analysis and Identification of Lignans in Phyllanthus urinaria by HPLC-SPE-NMR. Phytochem. Anal. 2005, 16, 120–126. [Google Scholar] [CrossRef]
- Murugaiyah, V.; Chan, K.L. Determination of Four Lignans in Phyllanthus niruri L. by a Simple High-Performance Liquid Chromatography Method with Fluorescence Detection. J. Chromatogr. A 2007, 1154, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, L. Elicitor-like Effects of Low-Energy Ultrasound on Plant (Panax ginseng) Cells: Induction of Plant Defense Responses and Secondary Metabolite Production. Appl. Microbiol. Biotechnol. 2002, 59, 51–57. [Google Scholar] [PubMed]
- Dörnenburg, H.; Knorr, D. Strategies for the Improvement of Secondary Metabolite Production in Plant Cell Cultures. Enzyme Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Paithankar, V.V.; Raut, K.; Charde, R.; Vyas, J. Phyllanthus niruri: A Magic Herb. Res. Pharm. 2011, 1, 1–9. [Google Scholar]
- Narendra, K.; Swathi, J.; Sowjanya, K.; Satya Savithri, A. Phyllanthus niruri: A Review on Its Ethno Botanical, Phytochemical and Pharmacological Profile. J. Pharm. Res. 2012, 5, 4681–4691. [Google Scholar]
- Ervina, M.N.; Mulyono, Y. Etnobotani Meniran Hijau (Phyllanthus ninuri L) Sebagai Potensi Obat Kayap Ular (Herpes Zoster) Dalam Tradisi Suku Dayak Ngaju. J. Jejaring Mat. dan Sains 2019, 1, 30–38. [Google Scholar] [CrossRef]
- Tapundu, A.S.; Anam, S. Studi Etnobotani Tumbuhan Obat Pada Suku Seko Di Desa Tanah Harapan, Kabupaten Sigi. Biocelebes 2015, 9, 66–86. [Google Scholar]
- Royyani, M.; Sihotang, V.; Agusta, A.; Efendy, O. Kajian Etnobotani Ramuan Pasca Melahirkan Pada Masyarakat Enggano. Ber. Biol. 2018, 17, 31–38. [Google Scholar] [CrossRef]
- Du, G.; Xiao, M.; Yu, S.; Wang, M.; Xie, Y.; Sang, S. Phyllanthus urinaria: A Potential Phytopharmacological Source of Natural Medicine. Int. J. Clin. Exp. Med. 2018, 11, 6509–6520. [Google Scholar]
- Ahmad, B.; Hafeez, N.; Rauf, A.; Bashir, S.; Linfang, H.; Rehman, M.-U.; Mubarak, M.S.; Uddin, S.; Bawazeer, S.; Shariati, M.A.; et al. Phyllanthus Emblica: A Comprehensive Review of Its Therapeutic Benefits. South African J. Bot. 2021, 138, 278–310. [Google Scholar] [CrossRef]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal Uses, Phytochemistry and Pharmacology: A Review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef]
- Widiadnyani, N.; Astawa, I.; Yasa, I.; Sukrama, I. Phytochemical Test and Identification of Active Compounds with LC-MS/MS in Green Meniran Leaf (Phyllanthus niruri Linn). Bali Med. J. 2021, 10, 132–136. [Google Scholar] [CrossRef]
- Sukmanadi, M.; Sabdoningrum, E.K.; Ananda, A.T. In Silico Study: Phyllanthus niruri L as Immunomodulator against Covid-19. Indian, J. Forensic Med. Toxicol. 2020, 14, 3156–3161. [Google Scholar]
- Acero, R.E.P.; Sapico, C.A.F.; Cui-Lim, K.M.R.; Amor, E.C. Bioprospecting of Philippine Plants from Northern Samar with Butyrylcholinesterase-Selective Inhibitory Activity. Philipp. J. Sci. 2022, 151, 1953–1962. [Google Scholar] [CrossRef]
- Husnunnisa, H.; Hartati, R.; Mauludin, R.; Insanu, M. A Review of the Phyllanthus Genus Plants: Their Phytochemistry, Traditional Uses, and Potential Inhibition of Xanthine Oxidase. Pharmacia 2022, 69, 681–687. [Google Scholar] [CrossRef]
- Hiremath, S.; Kumar, H.D.V.; Nandan, M.; Mantesh, M.; Shankarappa, K.S.; Venkataravanappa, V.; Basha, C.R.J.; Reddy, C.N.L. In Silico Docking Analysis Revealed the Potential of Phytochemicals Present in Phyllanthus amarus and Andrographis paniculata, Used in Ayurveda Medicine in Inhibiting SARS-CoV-2. 3 Biotech 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Markulin, L.; Corbin, C.; Renouard, S.; Drouet, S.; Gutierrez, L.; Mateljak, I.; Auguin, D.; Hano, C.; Fuss, E.; Lainé, E. Pinoresinol–Lariciresinol Reductases, Key to the Lignan Synthesis in Plants. Planta 2019, 249, 1695–1714. [Google Scholar] [CrossRef] [PubMed]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; de Lacour, J.L.; Leclerc, E.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef] [PubMed]
- Willfor, S.; Smeds, A.; Holmboma, B. Chromatographic Analysis of Lignans. J. Chromatogr. A 2006, 1112, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Bagniewska-Zadworna, A.; Barakat, A.; Łakomy, P.; Smoliński, D.J.; Zadworny, M. Lignin and Lignans in Plant Defence: Insight from Expression Profiling of Cinnamyl Alcohol Dehydrogenase Genes during Development and Following Fungal Infection in Populus. Plant Sci. 2014, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, N.; Sánchez-Bel, P.; Pastor, V.; Pastor-Fernández, J.; Mateu, D.; Pozo, M.J.; Cerezo, M.; Flors, V. Root-to-Shoot Signalling in Mycorrhizal Tomato Plants upon Botrytis cinerea Infection. Plant Sci. 2020, 298, 110595. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Pae, S.B.; Shim, K.B.; Baek, I.Y. Penicillium Sp. Mitigates Fusarium-Induced Biotic Stress in Sesame Plants. Biotechnol. Lett. 2013, 35, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant Activity of the Flaxseed Lignan Secoisolariciresinol Diglycoside and Its Mammalian Lignan Metabolites Enterodiol and Enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Toure, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefit. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Zuppolini, S.; Zarrelli, A. Isolation of Lignans as Seed Germination and Plant Growth Inhibitors from Mediterranean Plants and Chemical Synthesis of Some Analogues. Phytochem. Rev. 2013, 12, 717–731. [Google Scholar] [CrossRef]
- Yamauchi, S.; Ichikawa, H.; Nishiwaki, H.; Shuto, Y. Evaluation of Plant Growth Regulatory Activity of Furofuran Lignan Bearing a 7,9′:7′,9-Diepoxy Structure Using Optically Pure (+)- and (-)-Enantiomers. J. Agric. Food Chem. 2015, 63, 5224–5228. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, J.; Li, Q.; Zhou, Y.; Bu, Q.; Zhou, J.; Tan, H.; Yang, Y.; Zhang, L.; Chen, W. IiWRKY34 Positively Regulates Yield, Lignan Biosynthesis and Stress Tolerance in Isatis indigotica. Acta Pharm. Sin. B 2020, 10, 2417–2432. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Puri, S.; Habbu, P.; Kulkarni, P.; Kulkarni, V. Hepatoprotective Potential of Endophytic Aspergillus niger Strain A6 Fractions Isolated from Phyllanthus Amarus Schum and Thonn. J. Pharm. Sci. 2021, 11, 21–31. [Google Scholar]
- Kandavel, D.; Sekar, S. Endophytic Fungi from Phyllanthus Amarus Schum. & Thonn. Capable of Producing Phyllanthin, Hypophyllanthin and/or Related Compounds. Int. J. Pharm. Pharm. Sci. 2015, 7, 253–257. [Google Scholar]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-Ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The Endophytic Fungus Trametes Hirsuta as a Novel Alternative Source of Podophyllotoxin and Related Aryl Tetralin Lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Puri, S.; Habbu, P.; Kulkarni, P.; Joshi, A.; Kulkarni, V.; Dixit, S. Hepatoprotective Activity and Constituents of Nigrospora Sp. CMH2_13: An Endophytic Fungus Isolated from Leaves of Phyllanthus amarus Schum. and Thonn. Ann. Phytomedicine An Int. J. 2020, 9, 239–246. [Google Scholar] [CrossRef]
- Huang, R.L.; Huang, Y.L.; Ou, J.C.; Chen, C.C.; Hsu, F.L.; Chang, C. Screening of 25 Compounds Isolated from Phyllanthus Species for Anti-Human Hepatitis B Virus in Vitro. Phyther. Res. 2003, 17, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Lin, M.T.; Lee, S.S.; Liu, K.C.S.C.; Hsu, F.L.; Lin, J.Y. Differential Inhibition of Reverse Transcriptase and Cellular DNA Polymerase-α Activities by Lignans Isolated from Chinese Herbs, Phyllanthus myrtifolius Moon, and Tannins from Lonicera japonica Thunb and Castanopsis hystrix. Antiviral Res. 1995, 27, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Sojan, J.; Krishna Radhika, N.; Asha, V.V. Anti-HBV Activity of the Different Extracts from Phyllanthus rheedei Wight in Cell Culture Based Assay Systems. J. Ethnopharmacol. 2014, 156, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.A.M.; Cunha, A.; Dantas, F.T.N.C.; Kaiser, C.R. NMR Characterization of Bioactive Lignans from Phyllanthus amarus Schum & Thonn. J. Magn. Reson. Imaging 2007, 6, 76–82. [Google Scholar]
- Nikule, H.A.; Nitnaware, K.M.; Chambhare, M.R.; Kadam, N.S.; Borde, M.Y.; Nikam, T.D. In-Vitro Propagation, Callus Culture and Bioactive Lignan Production in Phyllanthus tenellus Roxb: A New Source of Phyllanthin, Hypophyllanthin and Phyltetralin. Sci. Rep. 2020, 10, 10668. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Ding, S.T. A Review of the Phytochemistry and Pharmacology of Phyllanthus urinaria L. Front. Pharmacol. 2018, 9, 1109. [Google Scholar] [CrossRef] [PubMed]
- Syamasundar, K.V.; Singh, B.; Singh Thakur, R.; Husain, A.; Yoshinobu, K.; Hiroshi, H. Antihepatotoxic Principles of Phyllanthus niruri Herbs. J. Ethnopharmacol. 1985, 14, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Formularies; Health Policy Brief Series; Project HOPE: Washington, DC, USA, 2017. [Google Scholar]
- Thakur, I.; Nehru, J.; Hospital, C.; Bigoniya, P. Radioprotective and Free Radicals Scavenging Activity of Phyllanthin: Isolated from Phyllanthus. Int. J. Pharm. Biol. Sci. 2014, 4, 1–10. [Google Scholar]
- Liu, S.; Wei, W.; Shi, K.; Cao, X.; Zhou, M.; Liu, Z. In Vitro and in Vivo Anti-Hepatitis B Virus Activities of the Lignan Niranthin Isolated from Phyllanthus niruri L. J. Ethnopharmacol. 2014, 155, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lien, Y.C.; Liu, K.C.S.C.; Lee, S.S. Lignans from Phyllanthus urinaria. Phytochemistry 2003, 63, 825–833. [Google Scholar] [CrossRef]
- Patel, J.; Nagar, P.S.; Pal, K.; Singh, R.; Dhanani, T.; Patel, V.; Srivastava, S.; Kumar, S. Comparative Profiling of Four Lignans (Phyllanthin, Hypophyllanthin, Nirtetralin, and Niranthin) in Nine Phyllanthus Species from India Using a Validated Reversed Phase HPLC-PDA Detection Method. J. AOAC Int. 2021, 104, 485–497. [Google Scholar] [CrossRef]
- Row, L.R.; Satyanarayana, P.; Rao, G.S.R.S. Crystalline Constituents of Euphorbiaceae—VI. Tetrahedron 1967, 23, 1915–1918. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, N.; Shanker, K.; Verma, R.K.; Gupta, A.K.; Gupta, M.M. Two New Lignans from Phyllanthus amarus. J. Asian Nat. Prod. Res. 2009, 11, 562–568. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Gomes, P.W.P.; Menegatto, M.B.d.S.; Lima, R.L.S.; Guimarães, P.H.; Reis, J.D.E.; Carvalho, A.R.V.; Pamplona, S.d.G.S.R.; Muribeca, A.d.J.B.; de Magalhães, J.C.; et al. Exploring the Antiviral Potential of Justicidin B and Four Glycosylated Lignans from Phyllanthus brasiliensis against Zika Virus: A Promising Pharmacological Approach. Phytomedicine 2024, 123, 6–14. [Google Scholar] [CrossRef]
- Ji, K.; Liu, W.; Yin, W.; Kong, X.; Xu, H.; Lai, Z.W.; Li, J.Y.; Yue, J.M. A New Class of Potent Liver Injury Protective Compounds: Structural Elucidation, Total Synthesis and Bioactivity Study. Acta Pharm. Sin. B 2023, 13, 3414–3424. [Google Scholar] [CrossRef] [PubMed]
- Matou, M.; Merciris, P.; Luz Sanchez-Villavicencio, M.; Herbette, G.; Neviere, R.; Haddad, P.; Marianne-Pepin, T.; Bercion, S. Polyphenolic Compounds of Phyllanthus Amarus Schum & Thonn. (1827) and Diabetes-Related Activity of an Aqueous Extract as Affected by in Vitro Gastrointestinal Digestion. J. Ethnopharmacol. 2023, 315, 116619. [Google Scholar] [PubMed]
- Chopade, A.R.; Somade, P.M.; Somade, P.P.; Mali, S.N. Identification of Anxiolytic Potential of Niranthin: In-Vivo and Computational Investigations. Nat. Products Bioprospect. 2021, 11, 223–233. [Google Scholar] [CrossRef]
- Shen, W.; Zhao, Y.U.; Chen, H.; Zhang, T.; Wu, S.; Liu, P. M3, a Natural Lignan Xyloside, Exhibits Potent Anticancer Activity in HCT116 Cells. Oncol. Lett. 2019, 17, 2117–2122. [Google Scholar] [CrossRef]
- Harikrishnan, H.; Jantan, I.; Haque, M.A.; Kumolosasi, E. Phyllanthin from Phyllanthus amarus Inhibits LPS-Induced Proinflammatory Responses in U937 Macrophages via Downregulation of NF-ΚB/MAPK/PI3K-Akt Signaling Pathways. Phyther. Res. 2018, 32, 2510–2519. [Google Scholar] [CrossRef]
- Wang, H.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Veeraraghavan, V.P.; Krishna Mohan, S.; Hussain, S.; Ramamoorthy, K.; Rengarajan, T. Phyllanthin inhibits MOLT-4 leukemic cancer cell growth and induces apoptosis through the inhibition of AKT and JNK signaling pathway. J Biochem Mol Toxicol. 2021, 35, 1–10. [Google Scholar] [CrossRef]
- Pamplona, S.; Fernandes, L.; Barros, M.; Fontes-Júnior, E.; Maia, C.; Yoshioka e Silva, C.Y.; da Silva, M.N. Anti-Inflammatory and Antinociceptive Studies of Hydroalcoholic Extract from the Leaves of Phyllanthus brasiliensis (Aubl.) Poir. and Isolation of 5-O-β-D-Glucopyranosyljusticidin B and Six Other Lignans. Molecules 2018, 23, 941. [Google Scholar]
- Sparzak, B.; Krauze-Baranowska, M.; Kawiak, A.; Sowiński, P. Cytotoxic Lignan from the Non-Transformed Root Culture of Phyllanthus amarus. Molecules 2015, 20, 7915–7924. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, C.; Deng, Y.; Kanagasabai, R.; Ninh, T.N.; Tu, V.T.; Chai, H.B.; Soejarto, D.D.; Fuchs, J.R.; Yalowich, J.C.; et al. Cytotoxic and Natural Killer Cell Stimulatory Constituents of Phyllanthus songboiensis. Phytochemistry 2015, 111, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Q.; Wang, Y.M.; Zhu, H.T.; Wang, D.; Li, S.H.; Cheng, R.R.; Yang, C.R.; Wang, Y.F.; Xu, M.; Zhang, Y.J. Highly Oxygenated Limonoids and Lignans from Phyllanthus flexuosus. Nat. Products Bioprospect. 2014, 4, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lantvit, D.D.; Deng, Y.; Kanagasabai, R.; Gallucci, J.C.; Ninh, T.N.; Chai, H.B.; Soejarto, D.D.; Fuchs, J.R.; Yalowich, J.C.; et al. Potent Cytotoxic Arylnaphthalene Lignan Lactones from Phyllanthus poilanei. J. Nat. Prod. 2014, 77, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, X.; Wang, K.; Zheng, Z.; Zhou, M. Lignans with Anti-Hepatitis B Virus Activities from Phyllanthus Niruri, L. Phyther. Res. 2012, 26, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mukherjee, T.; Mukhopadhyay, R.; Mukherjee, B.; Sengupta, S.; Chattopadhyay, S.; Jaisankar, P.; Roy, S.; Majumder, H.K. The Lignan Niranthin Poisons Leishmania Donovani Topoisomerase IB and Favours a Th1 Immune Response in Mice. EMBO Mol. Med. 2012, 4, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyah, V.; Chan, K. Mechanisms of Antihyperuricemic Effect of Phyllanthus niruri and Its Lignan Constituents. J. Ethnopharmacol. 2009, 124, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Patra, D.; Kundu, R. Lignan Enriched Fraction (LRF) of Phyllanthus amarus Promotes Apoptotic Cell Death in Human Cervical Cancer Cells in Vitro. Sci. Rep. 2019, 9, 14950. [Google Scholar] [CrossRef] [PubMed]
- Sparzak-Stefanowska, B.; Krauze-Baranowska, M. Phyllanthus amarus Shoot Cultures as a Source of Biologically Active Lignans: The Influence of Selected Plant Growth Regulators. Sci. Rep. 2022, 12, 11505. [Google Scholar] [CrossRef] [PubMed]
- Benavides, K.; Sánchez-Kopper, A.; Jiménez-Quesada, K.; Perez, R.; Garro-Monge, G. Evaluation of Salicylic Acid and Methyl Jasmonate as Elicitors in Phyllanthus acuminatus Hairy Roots by Non-Targeted Analysis Using High-Resolution Mass Spectrometry. Molecules 2023, 29, 80. [Google Scholar] [CrossRef]
- Kartini, K.; Wijayati, A.S.; Jayani, N.I.E.; Setiawan, F.; Budiono, R. Straightforward Thin-layer Chromatography-Densitometric Method for the Determination of Phyllanthin in Phyllanthus niruri from Different Phytogeographical Zones. J. Planar Chromatogr. 2023, 1–10. [Google Scholar] [CrossRef]
- Windayani, N.; Rukayadi, Y.; Syah, Y.M.; Cahyanto, T. Lignans from Phyllanthus niruri L. and Their Antifusarium Properties. J. Kim. Val. 2022, 8, 153–162. [Google Scholar] [CrossRef]
- de Oliveira, M.E.B.S.; Sartoratto, A.; Carlos Cardoso, J. In Vitro Calli Production Resulted in Different Profiles of Plant-Derived Medicinal Compounds in Phyllanthus amarus. Molecules 2020, 25, 5895. [Google Scholar] [CrossRef]
- Muthusamy, A.; Prasad, H.N.N.; Sanjay, E.R.; Rao, M.R.; Satyamoorthy, K. Impact of Precursors and Plant Growth Regulators on in Vitro Growth, Bioactive Lignans, and Antioxidant Content of Phyllanthus Species. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 598–607. [Google Scholar] [CrossRef]
- Nitnaware, K.M.; Naik, D.G.; Nikam, T.D. Thidiazuron-Induced Shoot Organogenesis and Production of Hepatoprotective Lignan Phyllanthin and Hypophyllanthin in Phyllanthus amarus. Plant Cell. Tissue Organ Cult. 2010, 104, 101–110. [Google Scholar] [CrossRef]
- Seenivasan, A.; Manikkam, R.; Kaari, M.; Sahu, A.K.; Said, M.; Dastager, S.G. 2,4-Di-Tert-Butylphenol (2,4-DTBP) Purified from Streptomyces Sp. KCA1 from Phyllanthus niruri: Isolation, Characterization, Antibacterial and Anticancer Properties. J. King Saud Univ.-Sci. 2022, 34, 102088. [Google Scholar] [CrossRef]
- Tashackori, H.; Sharifi, M.; Chashmi, N.A. Physiological, Biochemical, and Molecular Responses of Linum Album to Digested Cell Wall of Piriformospora Indica. Physiol. Mol. Biol. Plants 2021, 27, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, C.; Caarls, L.; Vos, I.A.; Pieterse, C.M.J.; Van Wees, S.C.M. Ethylene: Traffic Controller on Hormonal Crossroads to Defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant Adv. 2012, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Plant Growth-Promoting Microbial-Mediated Induced Systemic Resistance in Plants: Induction, Mechanism, and Expression. In Microbial-Mediated Induced Systemic Resistance in Plants; Choudhary, D., Varma, A., Eds.; Springer Science+Business Media: Singapore, 2016; pp. 213–226. [Google Scholar]
- Junaidi, A.R.; Bolhassan, M.H. Screening of Indole-3-Acetic Acid (IAA) Productions by Endophytic Fusarium oxysporum Isolated from Phyllanthus niruri. Borneo J. Resour. Sci. Technol. 2017, 7, 56–59. [Google Scholar] [CrossRef]
- Bhatia, N.; Gupta, T.; Sharma, B.; Sarethy, I.P. Endophytes from Phyllanthus niruri: Selection, Characterization and Metabolite Production. J. Mater. Sci. Eng. 2019, 6, 888–894. [Google Scholar]
- Joe, M.M.; Devaraj, S.; Benson, A.; Sa, T. Isolation of Phosphate Solubilizing Endophytic Bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of Plant Growth Promotion and Antioxidant Activity under Salt Stress. J. Appl. Res. Med. Aromat. Plants 2016, 3, 71–77. [Google Scholar]
- Kumar, V.; Sahai, V.; Bisaria, V.S. Effect of Piriformospora Indica on Enhanced Biosynthesis of Anticancer Drug, Podophyllotoxin, in Plant Cell Cultures of Linum album. In Soil Biology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 33, pp. 119–137. [Google Scholar]
- Bahabadi, S.E.; Sharifi, M.; Safaie, N.; Murata, J.; Yamagaki, T.; Satake, H. Increased Lignan Biosynthesis in the Suspension Cultures of Linum album by Fungal Extracts. Plant Biotechnol. Rep. 2011, 5, 367–373. [Google Scholar] [CrossRef]
- Filho, E.G.A.; Braga, L.N.; Silva, L.M.A.; Miranda, F.R.; Silva, E.O.; Canuto, K.M.; Miranda, M.R.; de Brito, E.S.; Zocolo, G.J. Physiological Changes for Drought Resistance in Different Species of Phyllanthus. Sci. Rep. 2018, 8, 15141. [Google Scholar] [CrossRef]
- Nawfetrias, W.; Nurhangga, E.; Reninta, R.; Chotimah, S.; Bidara, I.S.; Maretta, D.; Devy, L.; Esyanti, R.R.; Faizal, A. IOP Conference Series: Earth and Environmental Science Metabolite Profiling of the Medicinal Herb Phyllanthus niruri L. under Drought Stress Metabolite Profiling of the Medicinal Herb Phyllanthus niruri L. under Drought Stress. IOP Conf. Ser. Earth Environ. Sci. 2023, 1255, 012046. [Google Scholar] [CrossRef]
- Maheswary, V.; Khairun, H.N.; Vasanthi, S.; Qistina, O.N. Digital Gene Expression of Water-Stressed Phyllanthus urinaria (Dukung Anak). J. Trop. Agric. Food Sci. 2016, 44, 121–136. [Google Scholar]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Bhalothia, P.; Bansal, P.; Basantani, M.K.; Bharti, V.; Mehrotra, S. Abscisic Acid and Abiotic Stress Tolerance—Different Tiers of Regulation. J. Plant Physiol. 2014, 171, 486–496. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of Jasmonic Acid in Plants: The Molecular Point of View. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid Are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop. J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and Molecular Approaches Reveal Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Corbin, C.; Renouard, S.; Lopez, T.; Lamblin, F.; Lainé, E.; Hano, C. Identification and Characterization of Cis-Acting Elements Involved in the Regulation of ABA- and/or GA-Mediated LuPLR1 Gene Expression and Lignan Biosynthesis in Flax (Linum usitatissimum L.) Cell Cultures. J. Plant Physiol. 2013, 170, 516–522. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a Tool to Improve the Profiles of High-Value Secondary Metabolites and Pharmacological Properties of Hypericum perforatum. J. Pharm. Pharmacol. 2017, 71, 70–82. [Google Scholar] [CrossRef]
- Hano, C.; Martin, I.; Fliniaux, O.; Legrand, B.; Gutierrez, L.; Arroo, R.R.J.; Mesnard, F.; Lamblin, F.; Lainé, E. Pinoresinol-Lariciresinol Reductase Gene Expression and Secoisolariciresinol Diglucoside Accumulation in Developing Flax (Linum usitatissimum) Seeds. Planta 2006, 224, 1291–1301. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Król, A.; Luczkiewicz, M.; Ekiert, H. Improved Production of Dibenzocyclooctadiene Lignans in the Elicited Microshoot Cultures of Schisandra Chinensis (Chinese Magnolia Vine). Appl. Microbiol. Biotechnol. 2018, 102, 945–959. [Google Scholar] [CrossRef]
- Anjum, S.; Komal, A.; Drouet, S.; Kausar, H.; Hano, C.; Abbasi, B.H. Feasible Production of Lignans and Neolignans in Root-Derived in Vitro Cultures of Flax (Linum usitatissimum L.). Plants 2020, 9, 409. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Tsipinana, S.; Husseiny, S.; Alayande, K.A.; Raslan, M.; Amoo, S.; Adeleke, R. Contribution of Endophytes towards Improving Plant Bioactive Metabolites: A Rescue Option against Red-Taping of Medicinal Plants. Front. Plant Sci. 2023, 14, 1248319. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Akinola, S.A.; Adedayo, A.A.; Glick, B.R.; Babalola, O.O. Synergistic Relationship of Endophyte-Nanomaterials to Alleviate Abiotic Stress in Plants. Front. Environ. Sci. 2022, 10, 2398. [Google Scholar] [CrossRef]
- Byregowda, R.; Prasad, S.R.; Oelmüller, R.; Nataraja, K.N.; Prasanna Kumar, M.K. Is Endophytic Colonization of Host Plants a Method of Alleviating Drought Stress? Conceptualizing the Hidden World of Endophytes. Int. J. Mol. Sci. 2022, 23, 9194. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Sun, M.C.; Chong, S.L.; Si, J.P.; Wu, L.S. Transcriptomic and Metabolomic Approaches Deepen Our Knowledge of Plant–Endophyte Interactions. Front. Plant Sci. 2022, 12, 700200. [Google Scholar] [CrossRef]
- Bajaj, R.; Huang, Y.; Gebrechristos, S.; Mikolajczyk, B.; Brown, H.; Prasad, R.; Varma, A.; Bushley, K.E. Transcriptional Responses of Soybean Roots to Colonization with the Root Endophytic Fungus Piriformospora indica Reveals Altered Phenylpropanoid and Secondary Metabolism. Sci. Rep. 2018, 8, 10227. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xiao, Y.; Lv, Z.; Tan, H.; Chen, R.; Li, Q.; Chen, J.; Wang, Y.; Yin, J.; Zhang, L.; et al. AP2/ERF Transcription Factor, Ii049, Positively Regulates Lignan Biosynthesis in Isatis indigotica through Activating Salicylic Acid Signaling and Lignan/Lignin Pathway Genes. Front. Plant Sci. 2017, 8, 1361. [Google Scholar] [CrossRef]
- Kumari, A.; Dogra, V.; Joshi, R.; Kumar, S. Stress-Responsive Cis-Regulatory Elements Underline Podophyllotoxin Biosynthesis and Better Performance of Sinopodophyllum hexandrum Under Water Deficit Conditions. Front. Plant Sci. 2022, 12, 751846. [Google Scholar] [CrossRef]
- Liang, Q.; Dun, B.; Li, L.; Ma, X.; Zhang, H.; Su, Y.; Wu, D. Metabolomic and Transcriptomic Responses of Adiantum (Adiantum nelumboides) Leaves under Drought, Half-Waterlogging, and Rewater Conditions. Front. Genet. 2023, 14, 1113470. [Google Scholar] [CrossRef]
- Sun, T.T.; Su, Z.H.; Wang, R.; Liu, R.; Yang, T.; Zuo, W.T.; Wen, S.S.; Wang, L.Q. Transcriptome and Metabolome Analysis Reveals the Molecular Mechanisms of Tamarix Taklamakanensis under Progressive Drought and Rehydration Treatments. Environ. Exp. Bot. 2022, 195, 104766. [Google Scholar] [CrossRef]
- Perez-Matas, E.; Garcia-Perez, P.; Bonfill, M.; Lucini, L.; Hidalgo-Martinez, D.; Palazon, J. Impact of Elicitation on Plant Antioxidants Production in Taxus Cell Cultures. Antioxidants 2023, 12, 887. [Google Scholar] [CrossRef]
- García-Pérez, P.; Miras-Moreno, B.; Lucini, L.; Gallego, P.P. The Metabolomics Reveals Intraspecies Variability of Bioactive Compounds in Elicited Suspension Cell Cultures of Three Bryophyllum Species. Ind. Crops Prod. 2021, 163, 113322. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Escobedo-Alvarez, D.E.; Mendoza-Sánchez, M.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Ramos-Gómez, M. Phytochemical Profile and Composition of Chickpea (Cicer arietinum L.): Varietal Differences and Effect of Germination under Elicited Conditions. Plants 2023, 12, 3093. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rodríguez, E.; Vera-Reyes, I.; Rodríguez-Hernández, A.A.; López-Laredo, A.R.; Ramos-Valdivia, A.C.; Trejo-Tapia, G. Mixed Elicitation with Salicylic Acid and Hydrogen Peroxide Modulates the Phenolic and Iridoid Pathways in Castilleja Tenuiflora Plants. Planta 2023, 258, 20. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.; Karimi, F.; Razavi, K. Phenylpropanoid Biosynthetic Gene Expression in Cell Suspension Culture of Haplophyllum virgatum Spach. under Chitin Treatment. Vitr. Cell. Dev. Biol. -Plant 2023, 59, 49–60. [Google Scholar] [CrossRef]
- Dougué Kentsop, R.A.; Consonni, R.; Alfieri, M.; Laura, M.; Ottolina, G.; Mascheretti, I.; Mattana, M. Linum lewisii Adventitious and Hairy-Roots Cultures as Lignan Plant Factories. Antioxidants 2022, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Elboutachfaiti, R.; Molini, R.; Mathiron, D.; Maillot, Y.; Fontaine, J.; Pilard, S.; Qu, A.; Dols-lafargue, M.; Delattre, C.; Petit, E. Secondary Metabolism Rearrangements in Linum sitatissimum L. after Biostimulation of Roots with COS Oligosaccharides from Fungal Cell Wall. Molecules 2022, 27, 2372. [Google Scholar] [CrossRef] [PubMed]

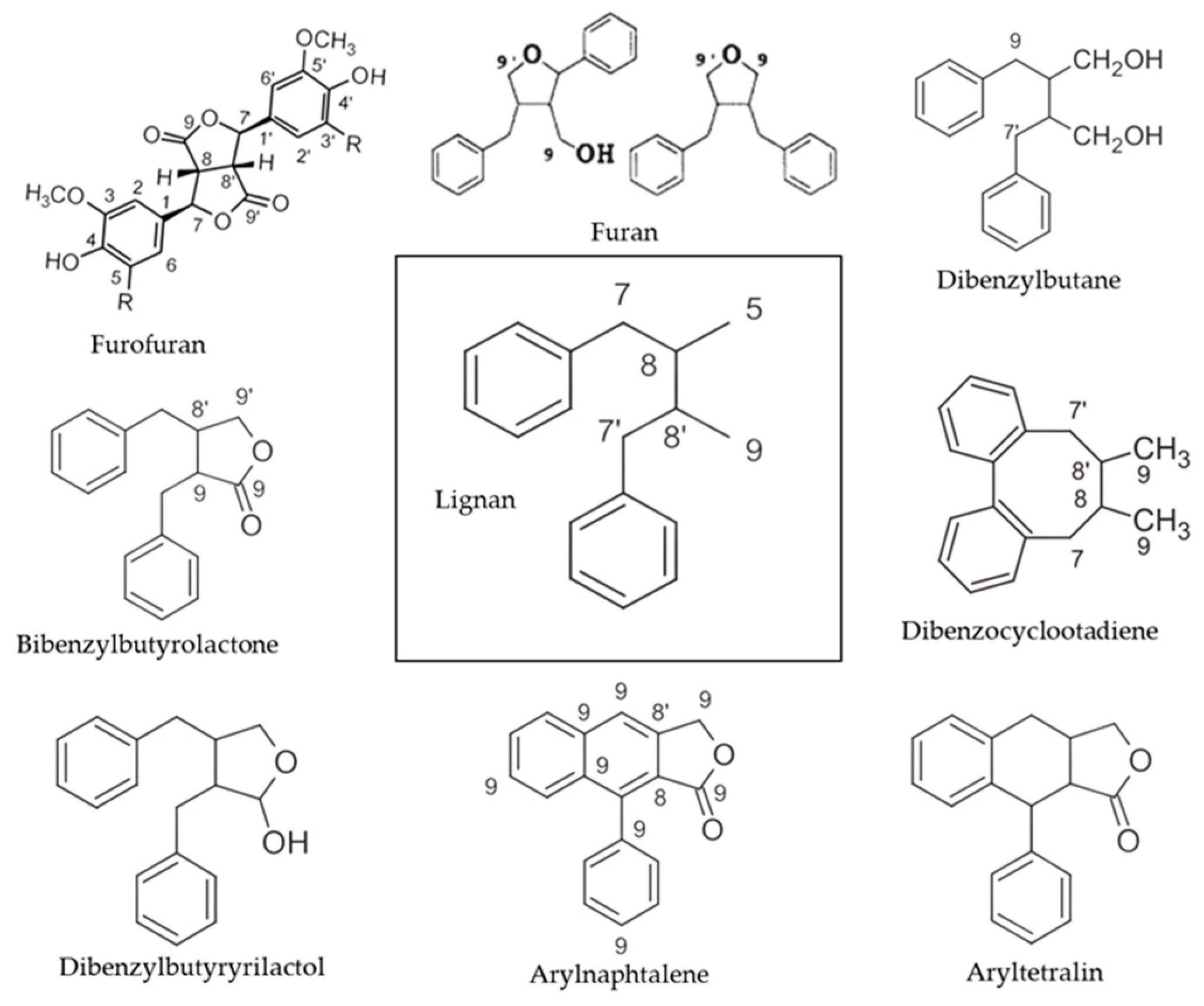

| Lignan | Structure | Species | Refs. |
|---|---|---|---|
| Phyllanthin C24H34O6 | 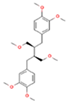 | P. niruri P. amarus P. tenellus P. urinaria | [49,52,53,54] |
| Hypophyllanthin C24H30O7 | 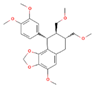 | P. niruri P. amarus P. tenellus P. urinaria | |
| Phylltetralin C24H32O6 |  | P. niruri P. tenellus P. urinaria | |
| Niranthin C24H32O7 | 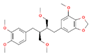 | P. niruri P. amarus P. tenellus P. urinaria | |
| Isolintetralin C23H28O6 |  | P. amarus P. urinaria | |
| Nirtetralin C24H30O7 | 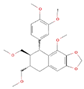 | P. urinaria | |
| Lintetralin C23H28O6 | 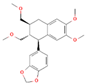 | P. urinaria | |
| Heliobuphthalmin lactone C20H18O6 | 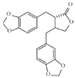 | P. urinaria | |
| Virgatusin C23H28O7 | 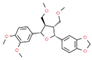 | P. urinaria | |
| Urinatetralin C22H24O6 |  | P. urinaria | |
| Dextrobursehernin C21H22O6 |  | P. urinaria | |
| Urinaligran C22H24O7 |  | P. urinaria |
| Species | Lignan | Potency | Refs. |
|---|---|---|---|
| P. brasiliensis |
|
| [63] |
| P. franchetianus |
|
| [64] |
| P. amarus |
|
| [65] |
| Phyllanthus |
|
| [66] |
| P. taxodiifolius |
|
| [67] |
| P. amarus |
|
| [68] |
| Phyllanthus |
|
| [69] |
| P. brasiliensis |
|
| [70] |
| P. amarus |
|
| [71] |
| P. songboiensis |
|
| [72] |
| P. niruri |
|
| [58] |
| P. flexuosus |
|
| [73] |
| P. poilanei |
|
| [74] |
| P. niruri |
|
| [75] |
| P. amarus |
|
| [76] |
| P. niruri |
|
| [77] |
| Species | Part of Plant | Method/Assay/Determination | Metabolite | Refs. |
|---|---|---|---|---|
| P. amarus | whole plants |
|
| [78] |
| P. amarus | shoot culture |
|
| [79] |
| P. tenellus | leaf derived callus |
|
| [53] |
| P. acuminatus | hairy roots |
|
| [80] |
| P. niruri | aerial part |
|
| [81] |
| P. niruri | leaves |
|
| [82] |
| P. amarus | aerial part |
|
| [83] |
| P. amarus | callus |
|
| [84] |
| P. amarus | callus from shoot |
|
| [85] |
| Species | Elicitation Treatment | Lignan Enhancement | Omics Approach | Refs. |
|---|---|---|---|---|
| Glycine max | Co-cultivation with endophyte Piriformospora indica |
| Trancriptomics | [115] |
| Isatis indigotica | Hairy root culture under salt and drought stresses |
| Trancriptomics | [43] |
| Isatis indigotica | Hairy root culture with salicylic acid |
| Genomics | [116] |
| Sinopodophyllum hexandrum | Water-deficit treatment |
| Genomics | [117] |
| Adianum nelumboides | Drought stress treatment |
| Metabolomics Transcriptomics | [118] |
| Tamarix taklamakanensis | Drought stress treatment |
| Metabolomics Transcriptomics | [119] |
| Taxus baccata | Salicylic acid |
| Metabolomics | [120] |
| Bryophyllum sp. | Methyl jasmonate Salicylic acid |
| Metabolomics | [121] |
| Phyllanthus acuminatus | Methyl jasmonate Salicylic acid |
| Metabolomics | [80] |
| Cicer arietinum | Salicylic acid Chitosan Hydrogen peroxide |
| Metabolomics | [122] |
| Castilleja tenuiflora | Salicylic acid Hydrogen peroxide |
| Metabolomics | [123] |
| Haplophyllum virgatum Spach. | chitin treatment |
| Genomics Metabolomics | [124] |
| Linum lewisii | Methyl jasmonate |
| Genomics Metabolomics | [125] |
| Linum usitatissimum L. | Chitosan oligosaccharides |
| Metabolomics | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawfetrias, W.; Devy, L.; Esyanti, R.R.; Faizal, A. Phyllanthus Lignans: A Review of Biological Activity and Elicitation. Horticulturae 2024, 10, 195. https://doi.org/10.3390/horticulturae10020195
Nawfetrias W, Devy L, Esyanti RR, Faizal A. Phyllanthus Lignans: A Review of Biological Activity and Elicitation. Horticulturae. 2024; 10(2):195. https://doi.org/10.3390/horticulturae10020195
Chicago/Turabian StyleNawfetrias, Winda, Lukita Devy, Rizkita Rachmi Esyanti, and Ahmad Faizal. 2024. "Phyllanthus Lignans: A Review of Biological Activity and Elicitation" Horticulturae 10, no. 2: 195. https://doi.org/10.3390/horticulturae10020195
APA StyleNawfetrias, W., Devy, L., Esyanti, R. R., & Faizal, A. (2024). Phyllanthus Lignans: A Review of Biological Activity and Elicitation. Horticulturae, 10(2), 195. https://doi.org/10.3390/horticulturae10020195







