Transcriptome Analysis of the Preservation Effect of Three Essential Oil Microcapsules on Okra
Abstract
1. Introduction
2. Materials and Methods
2.1. Okra
2.2. Treatment of Okra
2.3. Preparation of Essential Oil Microcapsules
2.4. Sensory Evaluation
2.5. Respiratory Intensity, Total Plate Count, Firmness, and Weight Loss Rate
2.6. RNA Extraction, RNA-Seq Library Construction, and Data Analysis
2.7. Statistical Analysis of the Results
3. Results and Discussion
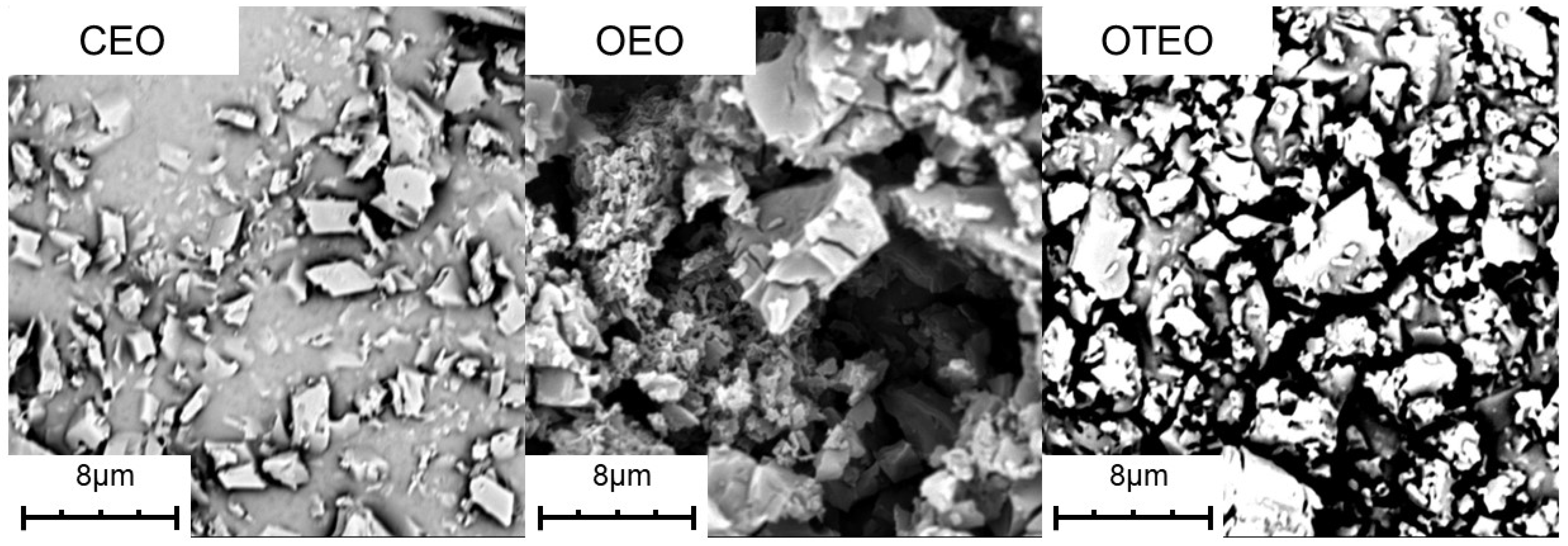
3.1. Scanning Electron Microscope Observation of Microencapsulation of Three Essential Oils
3.2. Effects of Microencapsulation of Different Essential Oils on Sensory Evaluation Quality of Okra
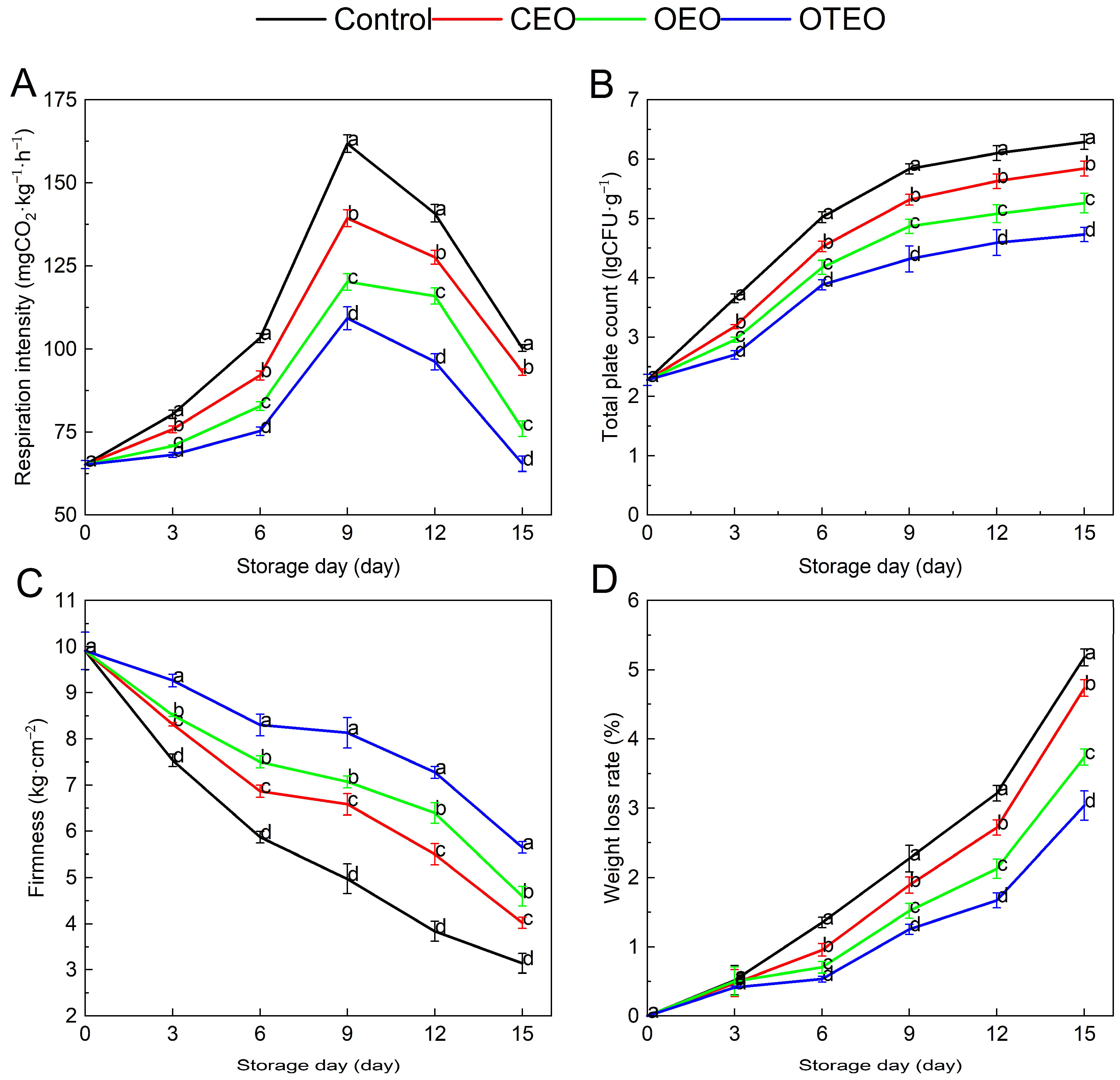
3.3. Effects of Different Essential Oil Microcapsules on the Respiration Intensity, Total Plate Count, Firmness, and Weight Loss Rate of Okra
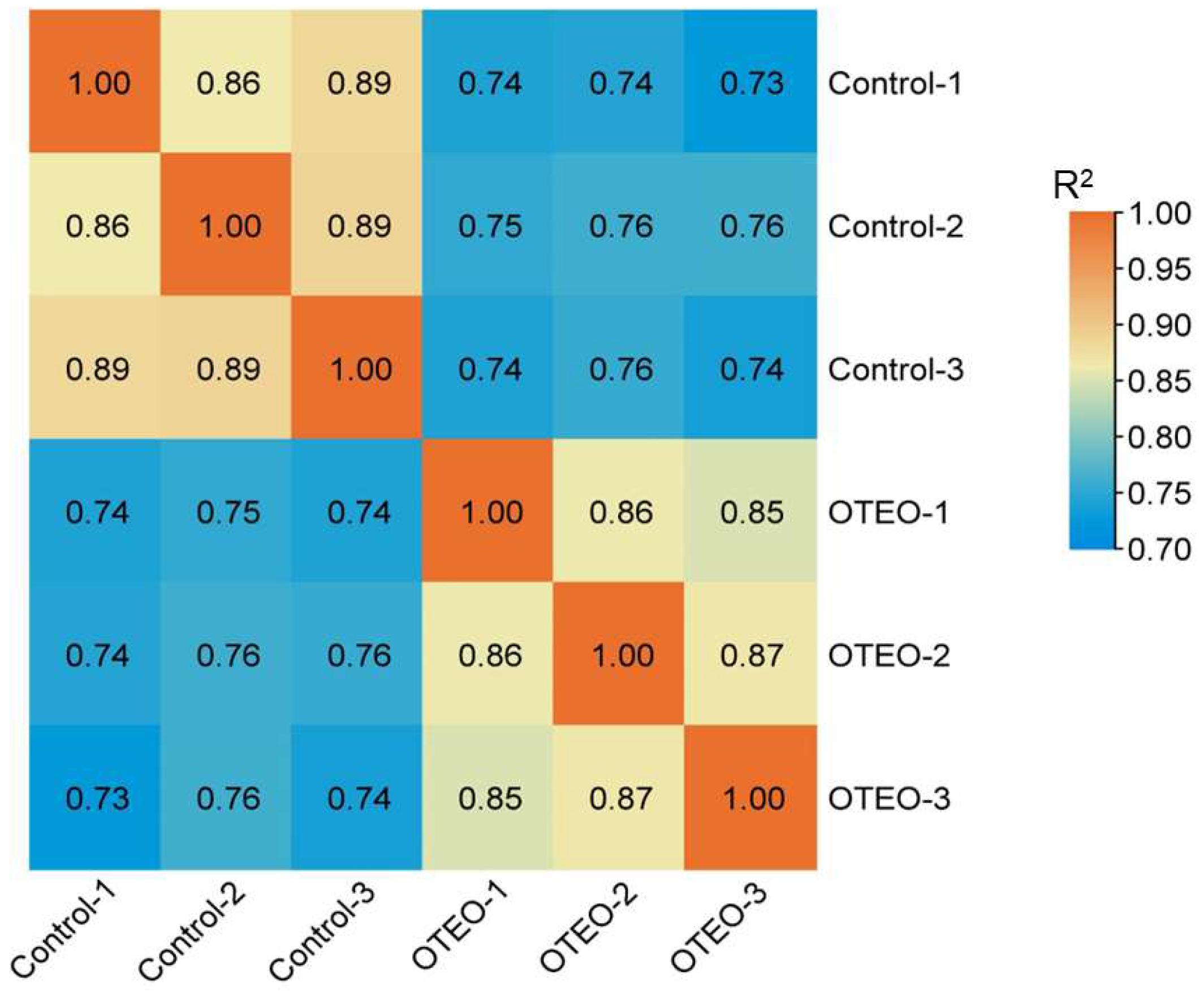
3.4. RNA Sequencing Results
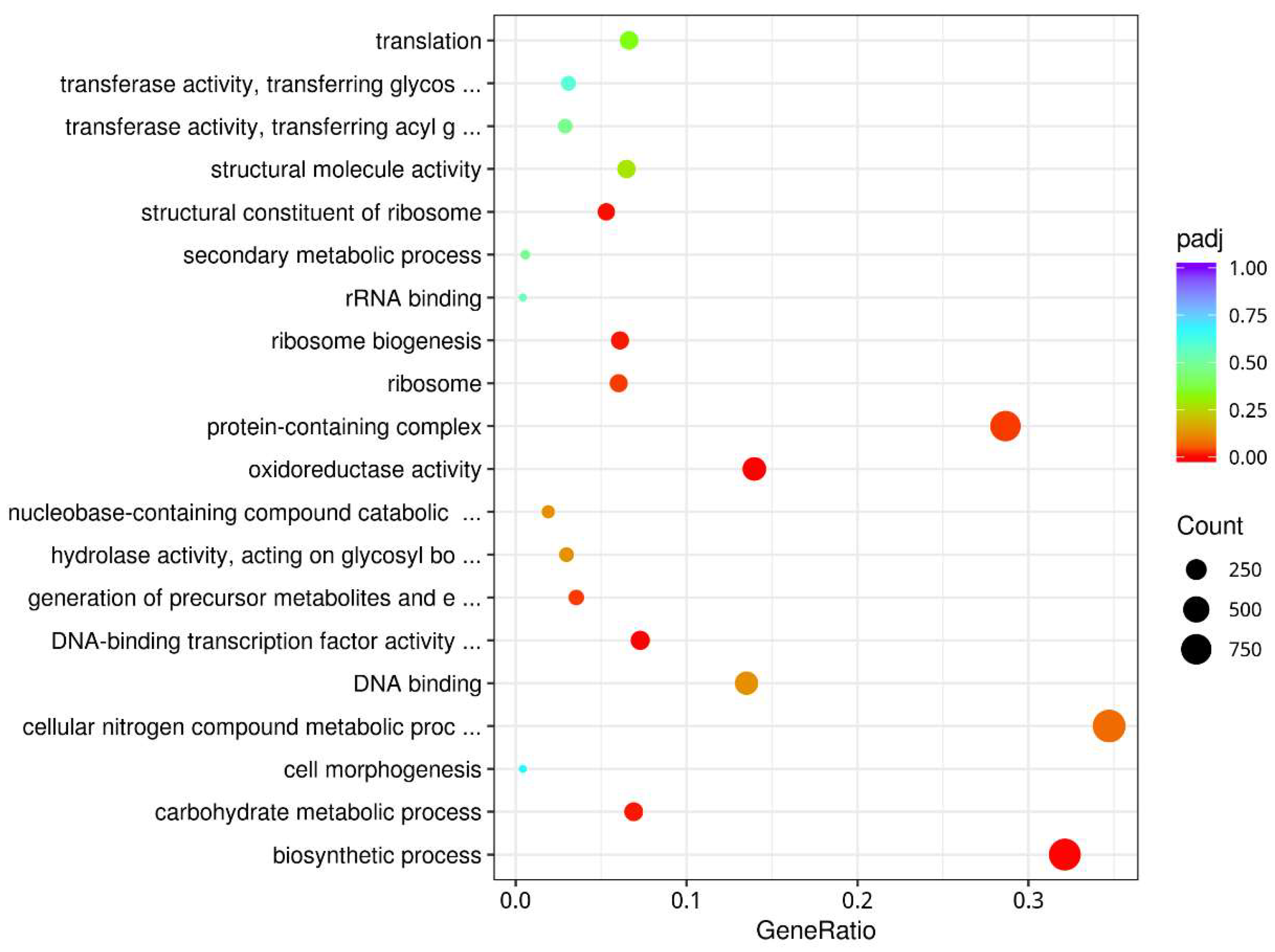
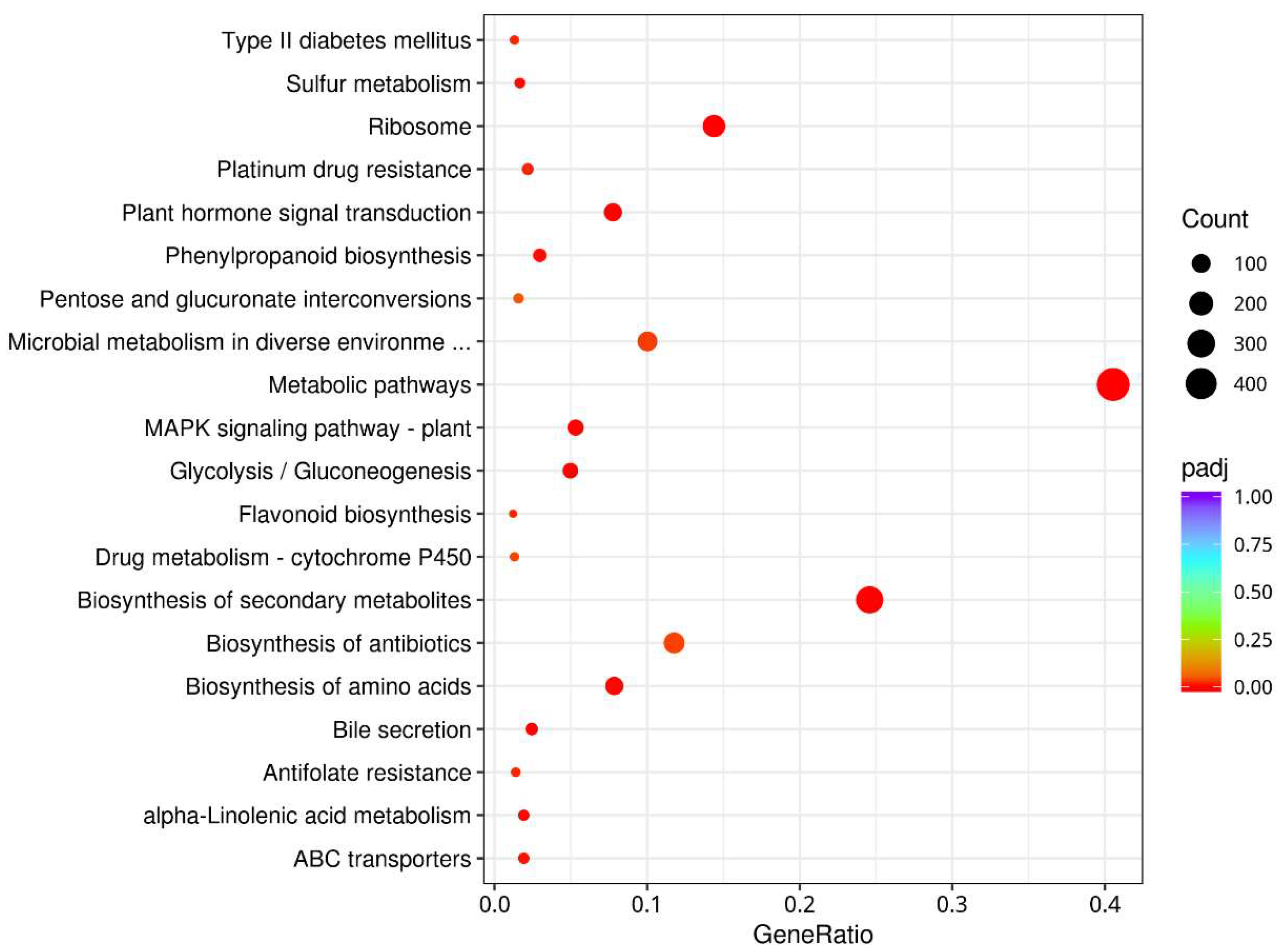
3.5. Annotation of Gene Function
3.6. Gene Expression
3.7. Differential Gene Expression Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, X.-R.; Li, H.-Y.; Du, G.; Lin, S.; Hu, R.; Li, H.-Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.-T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Saleh, M.; El-Gizawy, A.; El-Bassiouny, R.; Ali, H. Effects of anti-coloring agents on blackening inhibition and maintaining physical and chemical quality of fresh-cut okra during storage. Ann. Agric. Sci. 2013, 58, 239–245. [Google Scholar] [CrossRef]
- Dantas, T.L.; Buriti, F.C.A.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rafi, Z.; Baker, A.; Shoaib, A.; Alkhathami, A.G.; Asiri, M.; Alshahrani, M.Y.; Ahmad, I.; Alraey, Y.; Hakamy, A. Phytochemical screening, nutritional value, anti-diabetic, anti-cancer, and anti-bacterial assessment of aqueous extract from abelmoschus esculentus pods. Processes 2022, 10, 183. [Google Scholar] [CrossRef]
- Ngure, J.W.; Aguyoh, J.N.; Gaoquiong, L. Interactive effects of packaging and storage temperatures on the shelf-life of okra. ARPN J. Agric. Biol. Sci. 2009, 4, 44–48. [Google Scholar]
- Elkashif, M.E.; Elamin, O.M.; Alamri, K.M. Effect of cultivar, packaging treatments and temperature on post-harvest quality of okra. Gezira J. Agric. Sci. 2013, 11, 1–10. [Google Scholar]
- Jia, S.; Zhang, N.; Dong, C.; Zheng, P.; Ji, H.; Yu, J.; Yan, S.; Chen, C.; Liang, L. Effect of Cold Plasma Treatment on the Softening of Winter Jujubes (Ziziphus jujuba Mill. cv. Dongzao). Horticulturae 2023, 9, 986. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- De-Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Guerra-Hernandez, E.J.; Jiménez-Valera, M.; Garcia-Villanova, B.; Ruiz-Bravo, A.; Verardo, V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology 2021, 10, 1091. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.; Yin, C.; Khan, M.R.U.; Zhao, H.; Xu, Y.; Huang, L.; Zheng, D.; Qi, M. Preparation and characterization of β-cyclodextrin–oregano essential oil microcapsule and its effect on storage behavior of purple yam. J. Sci. Food Agric. 2020, 100, 4849–4857. [Google Scholar] [CrossRef] [PubMed]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different defense responses and brown rot control in two Prunus persica cultivars to essential oil vapours after storage. Postharvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Effects of Essential Oil Vapour Treatment on the Postharvest Disease Control and Different Defence Responses in Two Mango (Mangifera indica L.) Cultivars. Food Bioprocess Technol. 2017, 10, 1131–1141. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Comprehensive reviews in food science and food safety. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M. Introduction to the Special Issue: Application of Essential Oils in Food Systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.J.; Li, X.J.; Li, H.Z.; Cui, L.X.; He, D.L. Microcapsules biologically prepared using Perilla frutescens (L.) Britt. essential oil and their use for extension of fruit shelf life. J. Sci. Food Agric. 2018, 98, 1033–1041. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on physicochemical activity of mango. LWT-Food Sci. Technol. 2020, 131, 109700. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Piletti, R.; Bugiereck, A.; Pereira, A.; Gussati, E.; Magro, J.D.; Mello, J.; Dalcanton, F.; Ternus, R.; Soares, C.; Riella, H.; et al. Microencapsulation of eugenol molecules by β-cyclodextrine as a thermal protection method of antibacterial action. Mater. Sci. Eng. C 2017, 75, 259–271. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Simsek, O.; Donmez, D.; Kacar, Y.A. RNA-Seq analysis in fruit science: A review. Am. J. Plant Biol. 2017, 2, 1. [Google Scholar]
- Shen, J.; Xiao, Q.; Qiu, H.; Chen, C.; Chen, H. Integrative effect of drought and low temperature on litchi (Litchi chinensis Sonn.) floral initiation revealed by dynamic genome-wide transcriptome analysis. Sci. Rep. 2016, 6, 32005. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Khalil-Ur-Rehman, M.; Feng, J.; Tao, J. RNA-seq based transcriptomic analysis of CPPU treated grape berries and emission of volatile compounds. J. Plant Physiol. 2017, 218, 155–166. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Onik, J.C.; Xie, Y.; Duan, Y.; Hu, X.; Wang, Z.; Lin, Q. UV-C treatment promotes quality of early ripening apple fruit by regulating malate metabolizing genes during postharvest storage. PLoS ONE 2019, 14, e0215472. [Google Scholar] [CrossRef]
- Falade, K.O.; Omojola, B.S. Effect of Processing Methods on Physical, Chemical, Rheological, and Sensory Properties of Okra (Abelmoschus esculentus). Food Bioprocess Technol. 2010, 3, 387–394. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, N.; Ji, H.; Zhang, X.; Dong, C.; Yu, J.; Yan, S.; Chen, C.; Liang, L. Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato. Foods 2022, 11, 4088. [Google Scholar] [CrossRef]
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yang, X.-L.; Zhu, Z.-P.; Xu, Q.-Y.; Wu, K.-X.; Kang, Y.-J.; Wang, H.; Xiong, A.-S. Comparative transcriptome analysis provides insight into nitric oxide suppressing lignin accumulation of postharvest okra (Abelmoschus esculentus L.) during cold storage. Plant Physiol. Biochem. 2021, 167, 49–67. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157:H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013, 161, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Swaminathan, M.; Sivakumar, K.; Rajamohan, R. Preparation, characterization and molecular modeling studies of the inclusion complex of Caffeine with Beta-cyclodextrin. J. Mol. Struct. 2015, 1099, 616–624. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef]
- Moser, R.; Raffaelli, R.; Thilmany, D.D. Consumer preferences for fruit and vegetables with credence-based attributes: A review. Int. Food Agribus. Manag. Rev. 2011, 14, 121–142. [Google Scholar]
- Schifferstein, H.N.; Wehrle, T.; Carbon, C.-C. Consumer expectations for vegetables with typical and atypical colors: The case of carrots. Food Qual. Prefer. 2019, 72, 98–108. [Google Scholar] [CrossRef]
- Khare, C.; Nema, S.; Srivastava, J.; Yadav, V.; Sharma, N. Fungal Diseases of Okra (Abelmoschus esculentus L.) and Their Integrated Disease Management (IDM). In Crop Diseases and Their Management; CRC Press: Boca Raton, FL, USA, 2016; p. 169. [Google Scholar]
- Zhang, W.; Lin, M.; Feng, X.; Yao, Z.; Wang, T.; Xu, C. Effect of lemon essential oil-enriched coating on the postharvest storage quality of citrus fruits. Food Sci. Technol. 2022, 42, e125421. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Amraie, M.; Salehi, M.; Mohseni, M.; Aloui, H. Effect of chitosan-based coatings enriched with savory and/or tarragon essential oils on postharvest maintenance of kumquat (Fortunella sp.) fruit. Food Sci. Nutr. 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Greco, G.; Gargano, F.; Gaglio, R.; Settanni, L.; Inglese, P. Effect of Mucilage-Based Edible Coating Enriched with Oregano Essential Oil on Postharvest Quality and Sensorial Attributes of Fresh-Cut Loquat. Coatings 2023, 13, 1387. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Ghahfarrokhi, M.G.; Eş, I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.R.; Amiri, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2018, 42, e13441. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological spoilage of fruits and vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 135–183. [Google Scholar]
- Martínez, K.; Ortiz, M.; Albis, A.; Castañeda, C.G.G.; Valencia, M.E.; Grande Tovar, C.D. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria × ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Huang, S.; Li, T.; Jiang, G.; Xie, W.; Chang, S.; Jiang, Y.; Duan, X. 1-Methylcyclopropene reduces chilling injury of harvested okra (Hibiscus esculentus L.) pods. Sci. Hortic. 2012, 141, 42–46. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, L.; Zhou, W.; Zhong, Z.; Yu, K.; Xu, J.; Zou, L.; Liu, W. Effect of modified atmosphere packaging combined with plant essential oils on preservation of fresh-cut lily bulbs. LWT 2022, 162, 113513. [Google Scholar] [CrossRef]
- Saltveit, M.E. Respiratory Metabolism. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–91. [Google Scholar]
- Chen, Q.; Zhang, W.; Cai, J.; Ni, Y.; Xiao, L.; Zhang, J. Transcriptome analysis in comparing carcass and meat quality traits of Jiaxing Black Pig and Duroc × Duroc × Berkshire × Jiaxing Black Pig crosses. Gene 2022, 808, 145978. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Asao, S.; Millar, A.H.; Atkin, O.K. Core principles which explain variation in respiration across biological scales. New Phytol. 2019, 222, 670–686. [Google Scholar] [CrossRef]
- Street, H.E.; Cockburn, W. Plant Metabolism; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Stitt, M.; Sulpice, R.; Keurentjes, J. Metabolic Networks: How to Identify Key Components in the Regulation of Metabolism and Growth. Plant Physiol. 2010, 152, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, L.; Jiang, D.; Wang, M.; Yu, H.; Yao, W. Combined metabolome and transcriptome analysis reveal the mechanism of eugenol inhibition of Aspergillus carbonarius growth in table grapes (Vitis vinifera L.). Food Res. Int. 2023, 170, 112934. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, H.; Yan, H.; Wang, Q.; Yan, B.; Jian, H.; Tang, K.; Qiu, X. RcTGA1 and glucosinolate biosynthesis pathway involvement in the defence of rose against the necrotrophic fungus Botrytis cinerea. BMC Plant Biol. 2021, 21, 223. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. Effect of preharvest application of tea tree oil on strawberry fruit quality parameters and possible disease resistance mechanisms. Sci. Hortic. 2018, 241, 18–28. [Google Scholar] [CrossRef]


| Treatment | Amount of Essential Oil Microcapsules Added (Based on the Weight of the Okra) |
|---|---|
| Control | Microcapsules without essential oil |
| CEO | 3% (Cinnamon essential oil microcapsules) 1 |
| OEO | 3% (Oregano essential oil microcapsules) 1 |
| OTEO | 3% (Oregano–thyme essential oil microcapsules) 1 |
| Essential Oil Microcapsules | Emulsifier Content (%) | Solid Content (%) | Proportion of Wall Core Material | Embedding Rate (%) | Emulsion Stability (%) |
|---|---|---|---|---|---|
| Cinnamon essential oil microcapsules | 3.0 ± 0.17 | 21.0 ± 2.34 | 3.10:1 | 66.92 ± 2.59 | 54.72 ± 2.17 |
| Oregano essential oil microcapsules | 3.1 ± 0.15 | 21.0 ± 3.05 | 4.40:1 | 75.86 ± 3.57 | 65.66 ± 2.53 |
| Oregano–thyme essential oil microcapsules | 2.9 ± 0.12 | 19.9 ± 1.57 | 4.50:1 | 63.20 ± 4.05 | 66.68 ± 2.34 |
| Project | Scoring Criteria | Score |
|---|---|---|
| Color (10 pts) | Bright green | 10 |
| Green | 8 | |
| Slightly faded | 6 | |
| Severe fading | 4 | |
| Yellow | 2 | |
| Rot (15 pts) | No decay | 15 |
| Rotten area < 1 cm2 | 12 | |
| Decay area < 1/10 of the tender pod | 9 | |
| Decay area < 1/5 of tender pod | 6 | |
| Decay area > 1/10 of the tender pod | 3 | |
| Brittleness (15 pts) | Brittle and hard | 15 |
| Folded < 90°, fracture | 12 | |
| 90° < folded < 180, fracture | 9 | |
| Fracture after folding in half | 6 | |
| Folded in half without fracture and very soft | 3 | |
| Rusty stains (10 pts) | Norusty stains | 10 |
| There are a few tiny brown spots on the surface | 8 | |
| More tiny brown spots, mild depression | 6 | |
| Large spots, obvious depression | 4 | |
| The rusty stains are continuous, and the maximum longitudinal diameter is >1.5 cm | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Zhang, H.; Qi, Q.; Yan, S.; Chen, C.; Liang, L. Transcriptome Analysis of the Preservation Effect of Three Essential Oil Microcapsules on Okra. Horticulturae 2024, 10, 193. https://doi.org/10.3390/horticulturae10020193
Jia S, Zhang H, Qi Q, Yan S, Chen C, Liang L. Transcriptome Analysis of the Preservation Effect of Three Essential Oil Microcapsules on Okra. Horticulturae. 2024; 10(2):193. https://doi.org/10.3390/horticulturae10020193
Chicago/Turabian StyleJia, Sitong, Hongyan Zhang, Qiushuang Qi, Shijie Yan, Cunkun Chen, and Liya Liang. 2024. "Transcriptome Analysis of the Preservation Effect of Three Essential Oil Microcapsules on Okra" Horticulturae 10, no. 2: 193. https://doi.org/10.3390/horticulturae10020193
APA StyleJia, S., Zhang, H., Qi, Q., Yan, S., Chen, C., & Liang, L. (2024). Transcriptome Analysis of the Preservation Effect of Three Essential Oil Microcapsules on Okra. Horticulturae, 10(2), 193. https://doi.org/10.3390/horticulturae10020193




