Chemical Composition of Healthy and Raspberry Leaf Blotch Emaravirus-Infected Red Raspberry ‘Willamette’ Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Sampling
2.2. Agrochemical Characteristics of Soil
2.3. RT-PCR Analysis
2.4. Extraction and Determination of Total Anthocyanins and Total Phenolics
2.5. HPLC-DAD Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. RLBV Detection
3.2. Chemical Properties of Raspberry Fruits
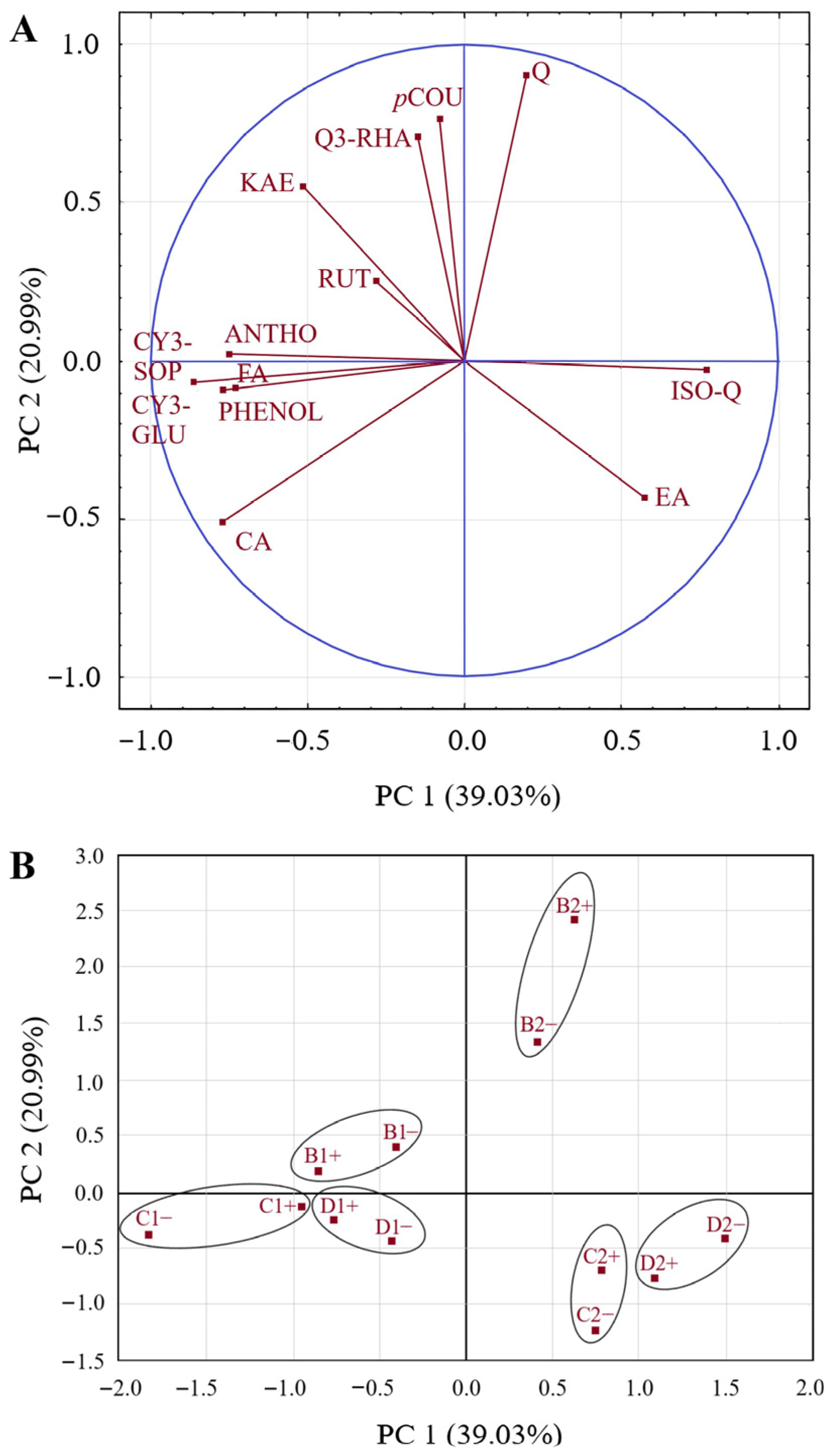
3.3. Influence of RLBV infection, Harvest Year, and Locality on Polyphenolic Profile of Raspberry Fruits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Chapter 29—Chemical composition of raspberry (Rubus spp.) cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 713–731. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 January 2023).
- Petrović, S.; Leposavić, A.; Jevremović, D. Raspberry—The Management, Processing and Marketing; Scientific Pomological Society of Serbia: Čačak, Serbia, 2017. [Google Scholar]
- Martin, R.R.; MacFarlane, S.; Sabanadzovic, S.; Quito, D.; Poudel, B.; Tzanetakis, I.E. Viruses and virus diseases of Rubus. Plant Dis. 2013, 97, 168–182. [Google Scholar] [CrossRef]
- Jevremović, D.; Leposavić, A.; Paunović, A.S. Genetic diversity of raspberry leaf blotch emaravirus in red raspberries from Serbia. Span. J. Agric. Res. 2019, 17, e1004. [Google Scholar] [CrossRef]
- McGavin, W.J.; Mitchell, C.; Cock, P.J.; Wright, K.M.; MacFarlane, S.A. Raspberry leaf blotch virus, a putative new member of the genus Emaravirus, encodes a novel genomic RNA. J. Gen. Virol. 2012, 93, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; McGavin, W.; Cock, P.J.A.; Schnettler, E.; Yan, F.; Chen, J.; MacFarlane, S. Newly identified RNAs of raspberry leaf blotch virus encoding a related group of proteins. J. Gen. Virol. 2015, 96, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Lemmetty, A.; Latvala, S.; Samuilova, O.; Valkonen, J.P.T. Occurrence and genetic diversity of Raspberry leaf blotch virus (RLBV) infecting cultivated and wild Rubus species in Finland. Ann. Appl. Biol. 2016, 168, 122–132. [Google Scholar] [CrossRef]
- Tan, J.L.; Trandem, N.; Fránová, J.; Hamborg, Z.; Blystad, D.R.; Zemek, R. Known and potential invertebrate vectors of raspberry viruses. Viruses 2022, 14, 571. [Google Scholar] [CrossRef] [PubMed]
- Jevremović, D.; Leposavić, A.; Miletić, N.; Vasilijević, B.; Popović, B.; Mitrović, O.; Milinković, M. Impact of raspberry leaf blotch emaravirus on red raspberry ‘Willamette’ fruits. Pestic. Fitomed. 2022, 37, 1–7. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Drobek, M.; Frac, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Will, F.; Krüger, E.; Kumar, K.; Patz, C.; Sønsteby, A. Effect of genotype and environment on the chemical composition of raspberry fruits. Acta Hortic. 2020, 1277, 321–328. [Google Scholar] [CrossRef]
- Kobori, R.; Yakami, S.; Kawasaki, T.; Saito, A. Changes in the polyphenol content of red raspberry fruits during ripening. Horticulturae 2021, 7, 569. [Google Scholar] [CrossRef]
- Malowicki, S.M.M.; Martin, R.; Qian, M.C. Comparison of sugar, acids, and volatile composition in raspberry bushy dwarf virus-resistant transgenic raspberries and the wild type ‘Meeker’ (Rubus idaeus L.). J. Agric. Food Chem. 2008, 56, 6648–6655. [Google Scholar] [CrossRef]
- Institute for Standardization of Serbia. Available online: https://iss.rs/en/project/show/iss:proj:71952 (accessed on 7 July 2022).
- International Organization for Standardization. Available online: https://www.iso.org/standard/51700.html (accessed on 8 July 2022).
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Loeppert, R.H.; Suarez, D.L. Carbonate and Gypsum. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnson, C.T., Sumner, M.E., Eds.; American Society of Agronomy & Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar] [CrossRef]
- Egnér, H.; Riehm, H.; Domingo, W.R. Chapter II Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. In Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung; Kungliga Lantbrukshögskolans Annaler; Lantbrukshögskolan: Uppsala, Sweden, 1960; Volume 26, pp. 199–215. [Google Scholar]
- Li, R.; Mock, R.; Huang, Q.; Abad, J.; Hartung, J.; Kinard, G. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J. Virol. Methods 2008, 154, 48–55. [Google Scholar] [CrossRef]
- Cervantes, L.; Martínez–Ferri, E.; Soria, C.; Ariza, M.T. Bioavailability of phenolic compounds in strawberry, raspberry and blueberry: Insights for breeding programs. Food Biosci. 2020, 37, 100680. [Google Scholar] [CrossRef]
- Miletić, N.; Popović, B.; Mitrović, O.; Kandić, M. Phenolic content and antioxidant capacity of fruits of plum cv. ‘Stanley’ (Prunus domestica L.) as influenced by maturity stage and on-tree ripening. Aust. J. Crop Sci. 2012, 6, 681–687. [Google Scholar]
- Miletić, N.; Jevremović, D.; Mitić, M.; Popović, B.; Petković, M. Influence of D and Rec strains of plum pox virus on phenolic profile and antioxidant capacity of fresh plum fruits of ‘Čačanska Lepotica’ cultivar. Span. J. Agric. Res. 2022, 20, e1005. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Lopez–Corona, A.V.; Valencia–Espinosa, I.; González–Sánchez, F.A.; Sánchez–López, A.L.; Garcia–Amezquita, L.E.; Garcia–Varela, R. Antioxidant, anti–inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Chemical fruit profiles of different raspberry cultivars grown in specific Norwegian agroclimatic conditions. Horticulturae 2022, 8, 765. [Google Scholar] [CrossRef]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci. Hortic. 2021, 285, 110204. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; He, F.; Wang, J.; Li, Z.; Pan, Q.H. Simple rain-shelter cultivation prolongs accumulation period of anthocyanins in wine grape berries. Molecules 2014, 19, 14843–14861. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Delgado, R.; Martín, P.; del Alamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Bogucka, B. The influence of nitrogen and potassium fertilisation on the content of polyphenolic compounds and antioxidant capacity of coloured potato. J. Food Comp. Anal. 2016, 47, 69–75. [Google Scholar] [CrossRef]
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs organic, on phytochemical composition of ‘Kweli’ and ‘Tulameen’ raspberries (Rubus idaeus L.). Food Chem. 2020, 328, 126833. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Garcìa, L.M.; Landi, M.; Guidi, L.; Massai, R.; Remorini, D. Influences of postharvest storage and processing techniques on antioxidant and nutraceutical properties of Rubus idaeus L.: A mini–review. Horticulturae 2020, 6, 105. [Google Scholar] [CrossRef]
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S.; Doskočil, I. Phytochemical composition of the fruits and leaves of raspberries (Rubus idaeus L.)—Conventional vs. organic and those wild grown. Appl. Sci. 2022, 12, 11783. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Stampar, F.; Marn, M.V. Effect of plum pox virus on chemical composition and fruit quality of plum. J. Agric. Food Chem. 2015, 63, 51–60. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant–defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–532. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. 17—Role of phenols and polyphenolics in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites—Applications and Immunization for Plant Growth and Protection; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2021; pp. 419–441. [Google Scholar] [CrossRef]
- Mikulic Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical profile of black currant fruit modified by different degree of infection with black currant leaf spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
- Moerschbacher, B.M.; Noll, U.; Gorrichon, L.; Reisener, H.J. Specific Inhibition of lignification breaks hypersensitive resistance of wheat to stem rust. Plant Physiol. 1990, 93, 465–470. [Google Scholar] [CrossRef]
- Schafer, J.F. Tolerance to plant disease. Annu. Rev. Phytopathol. 1971, 9, 235–252. [Google Scholar] [CrossRef]
- Jeger, M.J. Tolerance of plant virus disease: Its genetic, physiological, and epidemiological significance. Food Energy Secur. 2023, 12, e440. [Google Scholar] [CrossRef]

| Location | Harvest Year | |||
|---|---|---|---|---|
| 2019 | 2020 | |||
| RLBV + | RLBV − | RLBV + | RLBV − | |
| Bedina Varoš | B1+ | B1− | B2+ | B2− |
| Devići | D1+ | D1− | D2+ | D2− |
| Cerova | C1+ | C1− | C2+ | C2− |
| Location | Average Air Temperature (°C) | Average Precipitation (mm) | |||
|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | ||
| April | Bedina Varoš | 11.7 | 10.5 | 166.8 | 70.0 |
| Devići | 7.7 | 6.9 | 57.9 | 42.1 | |
| Cerova | 11.7 | 10.1 | 101.9 | 22.0 | |
| May | Bedina Varoš | 13.1 | 14.1 | 110.2 | 197.2 |
| Devići | 9.6 | 11.3 | 84.4 | 67.9 | |
| Cerova | 13.2 | 14.4 | 176.3 | 99.0 | |
| June | Bedina Varoš | 20.4 | 17.7 | 328.4 | 231.0 |
| Devići | 17.2 | 14.3 | 126.8 | 112.9 | |
| Cerova | 20.7 | 18.3 | 110.5 | 137.3 | |
| July | Bedina Varoš | 20.1 | 19.4 | 107.2 | 17.4 |
| Devići | 16.8 | 16.5 | 89.1 | 74.4 | |
| Cerova | 20.3 | 20.0 | 80.8 | 84.8 | |
| August | Bedina Varoš | 21.3 | 20.6 | 71.2 | 46.6 |
| Devići | 17.8 | 16.9 | 9.3 | 265.0 | |
| Cerova | 21.2 | 20.5 | 68.0 | 154.4 | |
| September | Bedina Varoš | 16.5 | 17.2 | 2.4 | 49.6 |
| Devići | 13.2 | 13.6 | 54.7 | 52.8 | |
| Cerova | 15.9 | 16.6 | 22.6 | 9.9 | |
| Bedina Varoš | 12.4 | 11.8 | 5.4 | 152.6 | |
| October | Devići | 8.6 | 8.4 | 24.8 | 80.6 |
| Cerova | 11.3 | 11.1 | 32.5 | 64.4 | |
| Compounds/ Class of Compounds | Bedina Varoš | Devići | Cerova | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |||||||
| RLBV + | RLBV − | RLBV + | RLBV − | RLBV + | RLBV − | RLBV + | RLBV − | RLBV + | RLBV − | RLBV + | RLBV − | |
| CA | 9.2 ± 1.6 | 5.8 ± 1.0 | 3.8 ± 0.3 | 4.6 ± 0.6 | 8.6 ± 0.7 | 10.2 ± 0.7 | 5.9 ± 0.7 | 5.2 ± 0.8 | 11.9 ± 0.7 | 11.9 ± 0.2 | 7.7 ± 0.1 | 7.9 ± 0.3 |
| bc | de | e | e | bc | ab | de | e | a | a | cd | cd | |
| pCOU | 4.1 ± 1.1 | 1.7 ± 0.5 | 26.3 ± 1.5 | 7.9 ± 0.2 | 6.4 ± 0.4 | 6.9 ± 0.3 | 4.1 ± 0.2 | 4.3 ± 0.2 | 12.6 ± 1.8 | 8.6 ± 1.5 | 1.8 ± 0.3 | 2.3 ± 0.3 |
| ef | f | a | c | cde | cd | ef | def | b | c | f | f | |
| FA | 10.7 ± 1.6 | 6.4 ± 0.7 | 8.3 ± 0.9 | 4.9 ± 1.0 | 12.1 ± 0.6 | 11.1 ± 0.8 | 6.0 ± 0.3 | 6.1 ± 0.3 | 5.6 ± 0.6 | 17.5 ± 0.6 | 5.8 ± 0.8 | 6.6 ± 0.6 |
| b | cd | c | d | b | B | d | cd | d | a | d | cd | |
| EA | 194.2 ± 10.0 | 118.6 ± 6.3 | 212.8 ± 8.0 | 162.3 ± 7.3 | 130.1 ± 4.0 | 106.3 ± 5.9 | 248.3 ± 10.5 | 358.2 ± 8.4 | 222.3 ± 15.5 | 198.3 ± 6.5 | 502.4 ± 10.9 | 434.0 ± 6.6 |
| f | h | ef | g | h | h | d | c | e | ef | a | b | |
| RUT | 145.9 ± 5.6 | 92.9 ± 6.1 | 142.7 ± 6.4 | 33.4 ± 1.9 | 71.4 ± 3.2 | 69.5 ± 3.9 | 54.6 ± 3.8 | 67.7 ± 5.7 | 124.2 ± 7.8 | 91.5 ± 6.3 | 116.5 ± 5.3 | 83.1 ± 5.9 |
| a | c | a | f | d | de | e | de | b | c | b | cd | |
| ISO-Q | 50.7 ± 3.0 | 35.6 ± 2.5 | 57.5 ± 2.3 | 32.4 ± 1.9 | 37.1 ± 1.5 | 29.6 ± 1.5 | 54.3 ± 2.6 | 78.0 ± 2.1 | 36.6 ± 0.9 | 17.1 ± 1.0 | 72.6 ± 2.0 | 42.5 ± 1.7 |
| c | e | b | ef | de | f | bc | a | e | g | a | d | |
| Q3-RHA | 63.2 ± 0.9 | 64.9 ± 1.0 | 82.8 ± 2.1 | 79.8 ± 0.8 | 39.7 ± 1.4 | 35.3 ± 2.7 | 22.6 ± 1.3 | 57.2 ± 1.1 | 58.4 ± 1.5 | 70.2 ± 1.6 | 72.2 ± 1.3 | 27.9 ± 1.5 |
| c | c | a | a | e | e | g | d | d | b | b | f | |
| Q | 6.6 ± 0.5 | 11.6 ± 1.3 | 46.0 ± 1.7 | 47.3 ± 1.3 | 10.3 ± 0.7 | 5.2 ± 0.5 | 5.5 ± 0.4 | 5.3 ± 0.4 | 6.2 ± 0.4 | 5.8 ± 0.5 | 5.0 ± 0.3 | 5.3 ± 0.2 |
| c | b | a | a | b | c | c | c | c | c | c | c | |
| KAE | 18.1 ± 1.7 | 16.0 ± 0.5 | 12.5 ± 0.6 | 11.9 ± 1.1 | 12.6 ± 0.7 | 12.2 ± 0.6 | 6.9 ± 0.4 | 5.9 ± 0.6 | 10.1 ± 0.8 | 8.3 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.2 |
| CY3-GLU | 132.8 ± 7.6 | 69.9 ± 6.8 | 15.1 ± 1.7 | 56.5 ± 3.6 | 62.4 ± 4.5 | 51.4 ± 4.4 | 20.5 ± 1.1 | 22.3 ± 0.8 | 105.8 ± 5.5 | 107.6 ± 3.1 | 35.1 ± 1.3 | 36.3 ± 1.3 |
| a | c | f | d | cd | d | f | f | b | b | e | e | |
| CY3-SOP | 594.4 ± 6.2 | 304.0 ± 6.3 | 95.2 ± 4.4 | 186.1 ± 5.3 | 304.7 ± 6.0 | 238.3 ± 6.9 | 87.3 ± 2.4 | 123.7 ± 4.8 | 503.0 ± 8.6 | 460.8 ± 5.0 | 124.0 ± 4.5 | 167.3 ± 5.4 |
| a | d | I | f | d | e | i | h | b | c | h | g | |
| PHENOL | 259.8 ± 6.0 | 303.5 ± 0.4 | 267.6 ± 3.2 | 286.9 ± 7.5 | 337.9 ± 15.2 | 296.9 ± 5.5 | 255.1 ± 8.0 | 233.6 ± 3.0 | 315.7 ± 6.9 | 340.9 ± 8.1 | 300.2 ± 1.4 | 273.8 ± 3.6 |
| e | bc | de | cd | a | bc | e | f | b | a | bc | de | |
| ANTHO | 68.9 ± 15.1 | 102.6 ± 7.2 | 87.2 ± 3.7 | 72.3 ± 1.1 | 103.3 ± 4.5 | 78.8 ± 16.3 | 65.4 ± 0.6 | 54.9 ± 0.4 | 98.3 ± 7.1 | 134.1 ± 6.4 | 94.4 ± 0.7 | 73.5 ± 1.2 |
| ef | b | bcde | def | b | cde | ef | f | bc | a | bcd | def | |
| Compounds/ Class of Compounds | A (Viral Status) | B (Harvest Year) | C (Locality) | [A × B] | [A × C] | [B × C] | [A × B × C] |
|---|---|---|---|---|---|---|---|
| CA | ns | *** | *** | ns | * | ns | *** |
| pCOU | *** | ** | *** | *** | *** | *** | *** |
| FA | * | *** | *** | *** | *** | *** | *** |
| EA | *** | *** | *** | *** | *** | *** | *** |
| RUT | *** | *** | *** | *** | *** | *** | *** |
| ISO-Q | *** | *** | *** | * | *** | *** | *** |
| Q3-RHA | ns | ** | *** | *** | *** | *** | *** |
| Q | ns | *** | *** | ns | *** | *** | *** |
| KAE | ** | *** | *** | ns | ns | ns | ns |
| CY3-GLU | ** | *** | *** | *** | ** | *** | *** |
| CY3-SOP | *** | *** | *** | *** | *** | *** | *** |
| PHENOL | ns | *** | *** | *** | *** | *** | *** |
| ANTHO | ns | *** | *** | *** | *** | *** | *** |
| Locality of Orchard | pH (in 1 M KCl) | CaCO3 (%) | Humic Substances (%) | Total Nitrogen (%) | Accessible Phosphorus (mg P2O5/100 g) | Accessible Potassium (mg K2O/100 g) |
|---|---|---|---|---|---|---|
| Bedina Varoš | 4.08 | 0.5 | 6.04 | 0.30 | 40.45 | 61.4 |
| Devići | 4.15 | 0.5 | 6.07 | 0.30 | 1.46 | 26.2 |
| Cerova | 5.02 | 0.0 | 3.33 | 0.17 | 7.28 | 16.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miletić, N.; Mitić, M.; Popović, B.; Petković, M.; Vasilijević, B.; Katanić, V.; Jevremović, D. Chemical Composition of Healthy and Raspberry Leaf Blotch Emaravirus-Infected Red Raspberry ‘Willamette’ Fruits. Horticulturae 2024, 10, 187. https://doi.org/10.3390/horticulturae10020187
Miletić N, Mitić M, Popović B, Petković M, Vasilijević B, Katanić V, Jevremović D. Chemical Composition of Healthy and Raspberry Leaf Blotch Emaravirus-Infected Red Raspberry ‘Willamette’ Fruits. Horticulturae. 2024; 10(2):187. https://doi.org/10.3390/horticulturae10020187
Chicago/Turabian StyleMiletić, Nemanja, Milan Mitić, Branko Popović, Marko Petković, Bojana Vasilijević, Vera Katanić, and Darko Jevremović. 2024. "Chemical Composition of Healthy and Raspberry Leaf Blotch Emaravirus-Infected Red Raspberry ‘Willamette’ Fruits" Horticulturae 10, no. 2: 187. https://doi.org/10.3390/horticulturae10020187
APA StyleMiletić, N., Mitić, M., Popović, B., Petković, M., Vasilijević, B., Katanić, V., & Jevremović, D. (2024). Chemical Composition of Healthy and Raspberry Leaf Blotch Emaravirus-Infected Red Raspberry ‘Willamette’ Fruits. Horticulturae, 10(2), 187. https://doi.org/10.3390/horticulturae10020187









