Abstract
Osteospermum ecklonis DC. NORL. is native to South Africa and is fully adapted to the Mediterranean climate. The aim of the study was to elucidate morphological and developmental changes in O. ecklonis plants associated with drought resistance in response to low doses of UV-C. Growth responses under three levels of drought stress (NW: normal watering, MD: moderate drought stress and SD: severe drought) were recorded. The results showed that 1 kJ m−2 UV-C significantly (p < 0.05) increased resistance to water stress without affecting growth and development or damaging photosystem II. Fresh weights of the upper parts and the root system of the irradiated plants were maintained at similar levels to those of the non-irradiated control plants. Fv/Fm values in the irradiated plants ranged from 0.73 to 0.82 depending on the stress level, while in the non-irradiated plants, the values ranged from 0.69 to 0.83. Differences between UV-C irradiated and non-irradiated plants were recorded in electrolyte leakage (EL), in malondialdehyde (MDA) and in relative water content (RWC) at all drought levels. The EL percentage of the non-irradiated plants at SD was 19.7%, while in the irradiated plants, it was 17.8%. RWC rates in the irradiated plants ranged between 60.6 and 76.4%, while in the non-irradiated plants, they ranged from 54.2 to 63.6%. Superoxide dismutase (SOD) and catalase (CAT) activities increased with UV-C irradiation, suggesting that antioxidant responses were induced and protected cell membranes from lipid peroxidation and damage. The results of the present study showed that UV-C irradiation at 1 kJ m−2 alleviated the drought symptoms of O. ecklonis by reducing oxidative stress and membrane lipid peroxidation.
1. Introduction
The “African daisy” [Osteospermum ecklonis (DC.) NORL.] is an ornamental perennial plant native to Southern and Eastern Africa and the Arabian Peninsula that belongs to the Asteraceae family. It is a tender plant that comes in different varieties, producing large numbers of flowers in different colours [1]. It is completely adapted to the Mediterranean climate and used extensively in garden designs as a ground cover. O. ecklonis is medium susceptible to drought stress, and at water deficit regimes of −3 to −10 kPa, flowering performance and growth decrease significantly [2]. In extreme water droughts, stomatal conductivity is reduced, and signs of leaf wilting are visibly apparent.
Drought stress may adversely affect plant growth, productivity, photosynthetic activity and flowering performance of ornamental plants [3,4,5,6,7]. One main response to water stress is the cessation of growth, associated with shoot and root elongation [6]. Such stress induces a series of physiological, biochemical and molecular responses, leading to the accumulation of reactive oxygen species (ROS) in chloroplasts [8]. ROS production and action then increase lipid peroxidation of the membranes and, consequently, membrane dysfunction that leads to cell death [9]. The level of peroxidation is usually measured by the electrolyte leakage (EL) developed in cells and the malondialdehyde (MDA) content as a marker of the oxidative damage. Furthermore, a decrease in the relative water content (RWC) of tissues in response to drought stress is always associated with differentiations in growth and development in various ornamental plants [4,7,10]. For example, when Impatiens walleriana plants were subjected to mild water stress, significant changes in root growth elongation, plant height and the number of flowers were recorded [10]. Oraee and Tehranifar [7] showed that prolonged drought stress during the cultivation of Viola x wittrokiana cv “Swiss Giants Rhinegold”, dramatically decreased RWC, chlorophyll content and antioxidant activity, while it increased the accumulation of H2O2 and MDA. The adaptation to water stress for Photinia fraseri cv. Red Robin and Eugenia uniflora cv “Etna Fire” was different throughout a 30-day period, although MDA content increased significantly in both species [4]. The stress response in both plants was associated with changes in stomatal conductance (gs), but significant reductions in the Fv/Fm ratio revealed extensive damage to photosystem II.
The ultraviolet-C (UV-C; 250–280 nm) is a highly energetic electromagnetic radiation that does not reach Earth’s surface, although it has been extensively used in agricultural practices. Brief pulses of UV-C were used pre-harvest to utilise physiological responses in ornamental plants associated with growth and flowering [11,12,13]. UV-C irradiation may affect the differentiation of physicochemical parameters and enzymatic activity [14,15] and significantly reduce pest (e.g., Macrosyphum rosae [16] and Tetranychus urticae [17]) and pathogen infections (e.g., Botrytis cinerea) [18,19]. It affects the photomorphogenic reactions in geranium (Pelargonium x hortorum) and impatiens (Impatiens hawkeri) plants and aid the reduction of plant height, the increase of lateral branching and the number of inflorescences [11,12]. Such responses, initiated by the UV-C, were found to be similar to those induced by plant growth regulators (PGRs) [13]. Plant responses to UV irradiation include changes in leaf area, the promotion of leaf thickness and stomatal density, which may lead to altered transpiration rates and increased water-use efficiencies. Therefore, UV light exhibits adaptive advantages under severe conditions associated with high light and temperature environments and water stress [20]. As a result, the application of low doses of UV-C during cultivation positively affect stomatal conductance and water loss from tissues and therefore provide adaptation advantages to ornamental plants. Robson et al. [21] showed that the negative effects of water deficits on birch (Betula pendula) were partially ameliorated by solar UV radiation. However, the latter was not confirmed for well-watered plants. It was suggested that adaptation to water stress is induced by UV-C.
No previous work was found on the effects of UV-C irradiation on the enhancement of the drought resistance of ornamental plants. We hypothesise that UV-C irradiation applied during cultivation can induce physiological and biochemical changes in the tissues, enhance drought resistance in O. ecklonis and reduce oxidative stress. The objective of the study was to elucidate morphological and developmental changes in O. ecklonis plants associated with drought resistance in response to low doses of UV-C.
2. Materials and Methods
2.1. Plant Material, Drought Treatments and Experimental Lay-Out
Rooted O. ecklonis cuttings cv “Sunny Mary” were purchased from a local ornamental plant producer and were transplanted in individual 2.5 Lt plastic pots (6 cm height × 4 cm length) filled with peat (PLANTOBALT, Plantaflor, Vechta, Denmark, pH 5.8) and perlite (VIORYP Ltd., Greece, pH 7.0) at 3:1 (v:v).
The plants were cultivated inside a walk-in growth chamber (CMP SCA-2 series CDR CHRYSSAGIS HELLAS, Athens, Greece) running at 20–22 °C during the day and 18–20 °C at night. The relative humidity was maintained at 70–80% and the light at 100–120 μmol m−2 s−1 (fluorescence lamps, LED 20 W or 400 W, 2300 lux, OSRAM, Munich, Germany) with a photoperiod of 12 h light—12 h dark for a total of 7 weeks. The plants were grouped in three groups submitted to different drought stress levels. One group was watered every 5 days (normal watering—NW), the second group was watered every 10 days (moderate drought stress—MD) and the third group was watered every 15 days (severe drought stress—SD). In each watering, the plants received 250 mL water pot−1. No fertilization was applied. Plants in the drought treatment groups were either treated with UV-C or left untreated as controls. Plants inside the growth chamber were arranged in a randomised block design (RBD), and the experiment was replicated twice. In each experimental replication, 10 plants were used to a total of sixty plants per experiment.
2.2. UV-C Irradiation Treatments
UV-C irradiation was carried out in the walk-in growing chamber following the methodology described by Darras et al. [16]. UV-C dose rate was measured using a Multi-Sense optical radiometer fitted with a 254 nm UV-C light sensor (Steril Air, UV—Technologie, Gräfelfing, Germany). The UV-C irradiation dose was set at 1.0 kJ m−2, as calculated in sec at a 20-cm distance from surface of the plants [11,12]. The plants received a 50-s treatment. The plants received one irradiation per week for a total of 7 treatments. Control plants were not irradiated.
2.3. Growth and Development Assessments
Plant height (cm), plant diameter (cm) and number of flowers were measured every week for a total of 7 weeks. Plants’ fresh (FW) and dry weights (DW) (mg) were measured using a digital balance (Kern & Sohn Gmbh, Balingen, Germany). Briefly, at the end of the 7-week period, the plants were separated in upper (e.g., stems and leaves) and lower parts (e.g., roots), and the FW were recorded on a digital balance (Kern & Sohn Gmbh, Balingen, Germany). The samples were dried out in an oven (Daihan Labtech Co., Ltd., Gagok-ri, Republic of Korea) at 85 °C for 48 h, and their DWs were recorded.
2.4. Net CO2 Assimilation (As), Transpiration (E), Stomatal Conductance (gs) and Chlorophyll Fluorescence (Fv/Fm)
CO2 assimilation (As; μmol m−2 s−1), transpiration (E; mmol m−2 s−1) and stomatal conductance (gs; mmol m−2 s−1) were recorded during cultivation using an LCpro+ portable photosynthesis system (ADC Bioscientific Ltd. Great Amwell, Hertfordshire, UK). Data were recorded at the beginning of the experiments, at the midpoint and at the end in a total of 3 recordings. Recordings were taken on similarly sized, healthy young leaves between 09:00 and 11:00 a.m. Inside the leaf chamber, photosynthetic photon flux density (PPFD) was set at 1100 μmol m−2 s−1 and temperature at 22 °C. Growth chamber reference CO2 ranged between 495 and 545 ppm.
Minimum (F0), maximum (Fm) and relative chlorophyll fluorescence [Fv/Fm; (Fm − F0)/Fm)] were measured on the outside surface of three randomly selected leaves using a chlorophyll fluorimeter (Model: OS-30p, OptiSciences Inc., NH, USA). Before measurement, leaves received a 15 min dark adaptation using clips that exposed 5 mm2 of leaf surface to light.
2.5. Colour Parameters
Colour parameters’ degree of redness to greenness (a*), degree of yellowness to blueness (b*), lightness (L*) and chroma (C*) were recorded on weeks 1, 4 and 7 on designated spots on the surface of fully matured O. eclonis leaves using a Minolta colourimeter (Model CR-300, Minolta Co., Ltd., Osaka, Japan). Prior to measurements, the instrument was calibrated on a Minolta standard white reflector plate, and assessments were carried out by placing the colorimeter sensor (8 mm aperture) on the designated spots.
2.6. Relative Water Content (RWC)
The RWC was measured at the end of the 7-week period following the methodology by Toscano et al. [5] with slight modifications on the volume of the material used. Fully matured leaves were harvested between 10:00 and 12:00 a.m. and were immediately processed in the laboratory. Five 10 mm leaf discs per plant treatment were excised with a corkborer. A total of 50 discs were excised for each treatment and their fresh weights (FW) were measured on a digital balance (Kern & Sohn Gmbh, Balingen, Germany). The discs were then floated on distilled water inside 9 cm Petri dishes for 4 h to regain turgidity. The samples were blotted to remove excess water from their surface and were re-weighted (turgid weight—TW). Then, the samples were dried at 80 °C in an oven (Daihan Labtech Co., Ltd., Gagok-ri, Republic of Korea) for 24 h, and the dry weight (DW) was measured. The RWC was calculated using the following formula: RWC (%) = (FW − DW/TW − DW) × 100. Data are presented as % of water content in tissues and were subjected to ANOVA.
2.7. Antioxidant Enzyme Activity
At the 7th week of the experiment, fresh leaf discs of approx. 0.5 g were homogenized in 5 mL of ice-cold 50 mM sodium phosphate buffer at pH 7.8 containing 2% (w/v) polyvinylpyrrolidone and 1.0 mM EDTA using a mortar and pestle. The resulting pellet was centrifuged for 15 min at 12,000× g at 4 °C, and the supernatant was collected and stored at −81 °C [22]. This supernatant was later used for assessing catalase (CAT) and superoxide dismutase (SOD) enzyme activities. SOD activity was assessed by monitoring the inhibition of the photochemical reduction (initiated by light illumination) of nitro blue tetrazolium (NBT) according to the method by Giannopolitis and Ries [23]. One unit of NBT reduction at 560 nm represented the amount of enzyme required to 50% reduction of NBT. This reduction was expressed as units mg−1 protein. CAT activity was measured following the method by Aebi [24]. CAT activity was expressed as mmol min−1 mg−1 protein, as recorded by the decrease in absorbance at 240 nm for 1–2 min at 20 °C.
2.8. Electrolyte Leakage (EL) and Malondialdehyde (MDA)
Leaf discs of 8 mm diameter were removed with a corkborer and were placed in a 50 mL conical flask containing 5 mL 0.4 M mannitol and shaken gently on an automated shaker for 3 h at 20 °C [25]. After incubation, the electrical conductivity (EC) of the solution was measured (EC1) using a conductance meter (Jenway meter, Fisher Scientific, Leichestershire, UK). The samples were then autoclaved for 10 min at 120 °C to disrupt the cells, and a second conductance (EC2) measurement was recorded at 20 °C. The % of electrolyte leakage (EL) for each treated plant was determined using the formula EL (%) = EC1/EC2 × 100.
Lipid peroxidation in leaves after 7 weeks was determined with the thiobarbituric acid (TBA) test, measured as MDA following the method by Velikova et al. [26] with slight modifications. Briefly, 0.5 gr leaf material was homogenized in 5 mL 0.1% (w/v) TCA solution. The homogenate was centrifuged at 10,000× g for 10 min, and 0.5 mL of the supernatant was added to 1 mL 0.5% (w/v) TBA in 20% TCA. The mixture was incubated in boiling water for 30 min, and the reaction stopped by placing the tubes in an ice bath. The samples were centrifuged at 4000× g for 5 min, and 1 mL of the supernatant was placed in 1 mL plastic cuvettes. The absorbance at 532 nm was measured in a Hitachi U-2001 UV-Vis/Fluor—spectophotometer (Triad Scientific, Manasquan, NJ, USA). The value for non-specific absorption at 600 nm was subtracted and the amount of MDA–TBA complex (red pigment) was calculated using the extinction coefficient of 155 mM cm−1.
2.9. Statistical Analysis
Experiments were factorial with drought level and UV-C irradiation as the two factors. Data were subjected to two-way ANOVA using SPSS v. 21 (SPSS Inc., Chicago, IL, USA), and comparisons between treatment means were carried out using the Duncan’s multiple range test at p = 0.05.
3. Results
UV-C irradiation at 1 kJ m−2 positively affected O. ecklonis plants under all drought stress levels after the 7-week period of the experiments (Figure 1 and Figure 2). It was obvious that UV-C changed the structure of the canopy of the plants by altering their length and width. Additionally, the thickness of the leaves was increased. Leaves were smaller on the surface and were adjusted to an almost vertical position. These morphological changes were recorded as a result of the responses to the high-intensity irradiation. Non-irradiated control plants were severely damaged after the 7-week experimental period under the MD and SD treatments, while the UV-C treated plants showed a better adaptation (Figure 1).
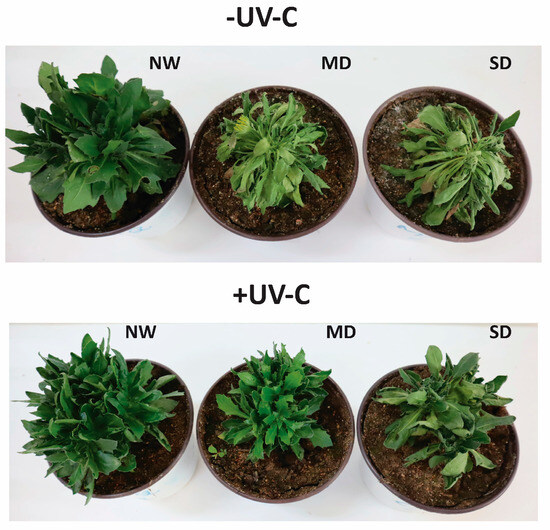
Figure 1.
Effects of UV-C irradiation at 1 kJ m−2 (±UV-C) and drought stress (NW: normal watering, MD: moderate drought, SD: severe drought) on O. ecklonis plants. Pictures were taken at the end of the 7-week period.

Figure 2.
Comparison between of UV-C irradiated at 1 kJ m−2 and non-irradiated O. ecklonis plants grown at three drought stress levels (NW: normal watering, MD: moderate drought, SD: severe drought). Pictures were taken at the end of the 7-week period.
3.1. Growth and Development
The height and the canopy diameter of the UV-C irradiated plants were significantly lower compared to the non-irradiated controls (Figure 3A,B). The number of inflorescences were significantly increased by UV-C treatments at the MD stress level (Figure 3C). UV-C irradiation did not affect growth and development under NW and SD.
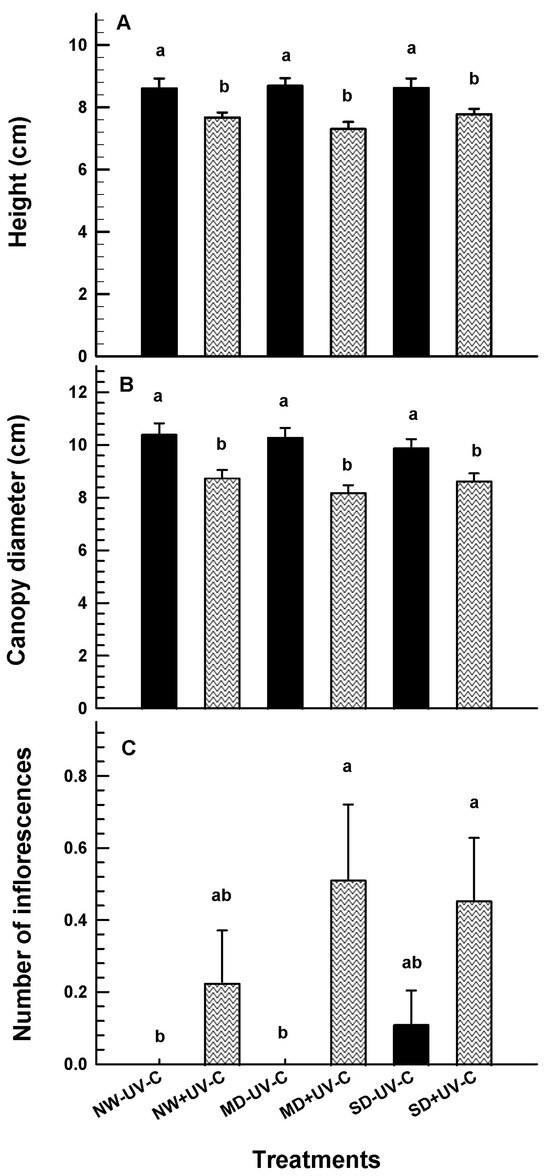
Figure 3.
Height ((A); cm), canopy diameter ((B); cm) and number of inflorescences (C) of O. ecklonis plants treated with UV-C irradiation at 1 kJ m−2 (+UV-C) or left non-irradiated (-UV-C) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded after the 7-week experimental period. Vertical bars are standard error of means, and different letters indicate the significant differences according to Duncan’s multiple range test at p = 0.05.
At the end of the 7-week evaluation period, the fresh and dry weight of the upper and the root part was recorded (Figure 4). The FW of the upper parts was significantly reduced at MD and SD for the irradiated and the non-irradiated plants (Figure 4A), although the irradiated plants maintained significantly higher weight at the MD treatment. On the contrary, Figure 4B shows that the dry weight of the upper part of the non-irradiated plants was significantly higher. The fresh and dry weight of the roots was at similar levels for the irradiated and the non-irradiated plants, although the highest value was recorded for the non-irradiated NW plants (Figure 4C,D). RWC maintained significantly higher in the UV-C irradiated plants compared to the non-irradiated controls at all levels of drought (Figure 5).
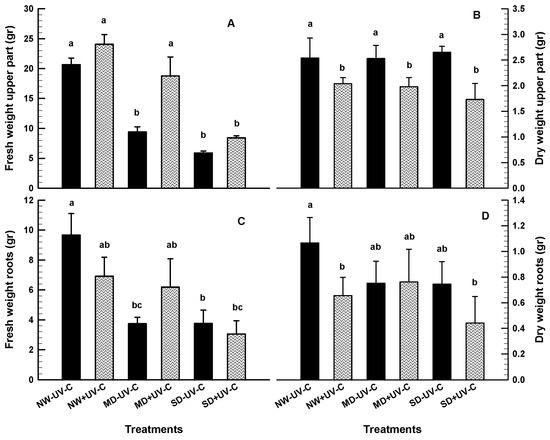
Figure 4.
Fresh weight upper part ((A); gr), dry weight upper part ((B); gr), fresh weight roots ((C); gr) and dry weight roots ((D); gr) of O. ecklonis plants treated with UV-C irradiation at 1 kJ m−2 or left non-irradiated (controls) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded after the 7-week experimental period. Vertical bars are standard error of means, and different letters indicate the significant differences according to Duncan’s multiple range test at p = 0.05.
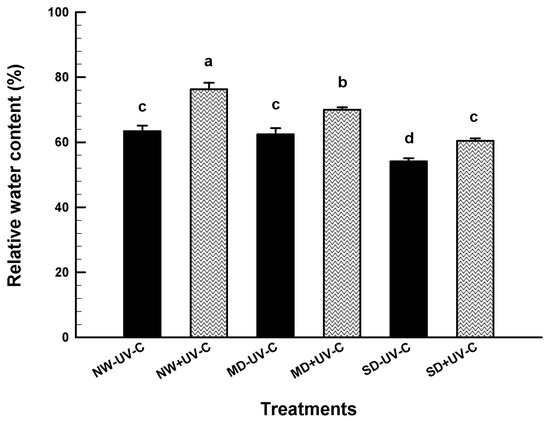
Figure 5.
Relative water content (%) of O. ecklonis plants treated with UV-C irradiation at 1 kJ m−2 or left non-irradiated (controls) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded after the 7-week experimental period. Vertical bars are standard error of means, and different letters indicate the significant differences according to Duncan’s multiple range test at p = 0.05.
3.2. Net CO2 Assimilation (As), Transpiration (E), Stomatal Conductance (gs) and Chlorophyll Fluorescence (Fv/Fm)
Net CO2 assimilation was similar for UV-C irradiated and non-irradiated plants except for those cultivated at the MD level only (Figure 6A). The stomatal conductance was similar for UV-C irradiated and non-irradiated plants except for the plants cultivated at NW only. Those UV-C irradiated plants had significantly lower stomatal conductance (Figure 6B). Transpiration was similar for both the irradiated and non-irradiated plants (Figure 6C). Significant differences were recorded in the Fv/Fm ratio between the irradiated and the non-irradiated plants (Figure 6D). At MD and SD, the non-irradiated plants had a significantly lower Fv/Fm ratio compared to the irradiated, with values below the 0.7 threshold suggesting serious damage to photosystem II (Figure 6D).
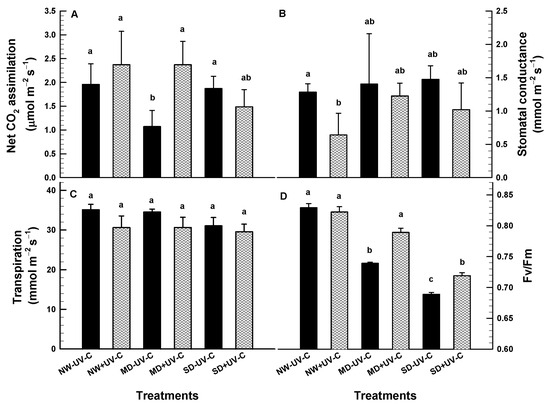
Figure 6.
Net CO2 assimilation ((A); μmol m−2 s−1), stomatal conductance ((B); mmol m−2 s−1) transpiration ((C); mmol m−2 s−1) and chlorophyll fluorescence ((D); Fv/Fm) of O. ecklonis plants treated with UV-C irradiation at 1 kJ m−2 or left non-irradiated (controls) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded on the 4th week of the experiment. Vertical bars are standard error of means, and different letters indicate the significant differences according to Duncan’s multiple range test at p = 0.05.
3.3. Colour Parameters
Colour values changed over the 7-week evaluation period (Figure 7). All plants, irrespective of the irradiation or the drought treatments, showed a gradual increase in a* values, suggesting changes from green to red-magenta hues (Figure 7A). Furthermore, the b* values were reduced irrespective of irradiation or drought treatments, suggesting changes from yellow to blue hues (Figure 7B). Lightness was either maintained at similar levels (e.g., non-irradiated plants) or were reduced (e.g., NW irradiated plants) over the 7-week period (Figure 7C). Chroma values were slightly reduced irrespective of the irradiation or the drought conditions (Figure 7D).
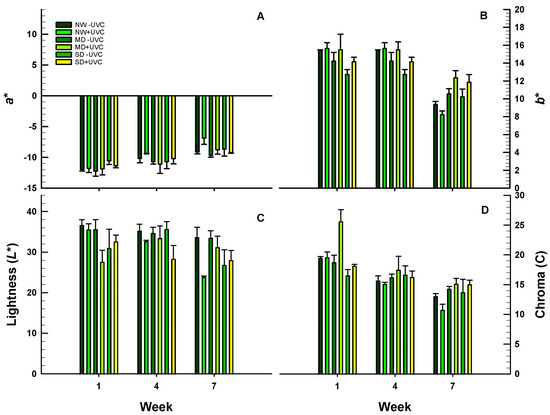
Figure 7.
Colour parameters of a* (A), b* (B), lightness (L*; C) and chroma (C; D) of O. ecklonis leaves treated with UV-C irradiation at 1 kJ m−2 or left non-irradiated (controls) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded on the 1st, 4th and 7th week. Vertical bars are standard error of means.
3.4. Electrolyte Leakage (EL) and MDA
UV-C irradiation provided protection against membrane structural damage, as shown by the MDA and EL values (Figure 8). Plants irradiated with 1 kJ m−2 had significantly lower MDA values compared to the non-irradiated plants both at MD and at SD (Figure 8A). Likewise, EL maintained significantly lower in the UV-C irradiated plants at MD and SD conditions, suggesting an increased level of protection to the cell membranes (Figure 8B).
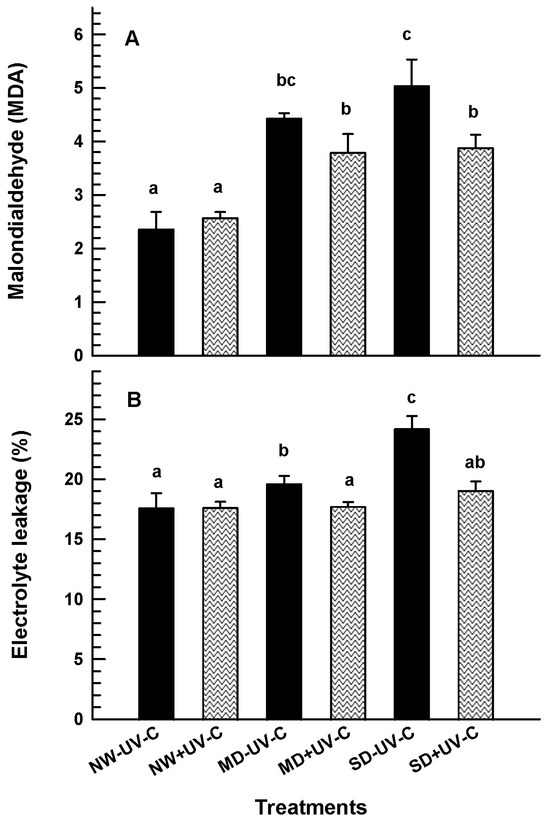
Figure 8.
Malondialdehyde (A) and electrolyte leakage ((B); %) of O. ecklonis plants treated with UV-C irradiation at 1 kJ m−2 or left non-irradiated (controls) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded after the 7-week experimental period. Vertical bars are standard error of means, and different letters indicate the significant differences according to Duncan’s multiple range test at p = 0.05.
3.5. Antioxidant Enzyme Activity—SOD and CAT
Significant antioxidant enzyme activity was recorded in plants irradiated with 1 kJ m−2 (Figure 9). SOD and CAT activity was induced by UV-C at all levels of drought. CAT activity in the NW plants was increased from 16.76 to 23.1 units mg−1 protein after UV-C irradiation (Figure 9A). Drought stress did not increase the production of CAT in the irradiated and the non-irradiated plants. Likewise, at NW, UV-C irradiation boosted SOD production by 3-fold (Figure 9B). SOD activity was induced at the MD conditions in the non-irradiated plants, while the UV-C treated plants showed a gradual reduction of SOD values.
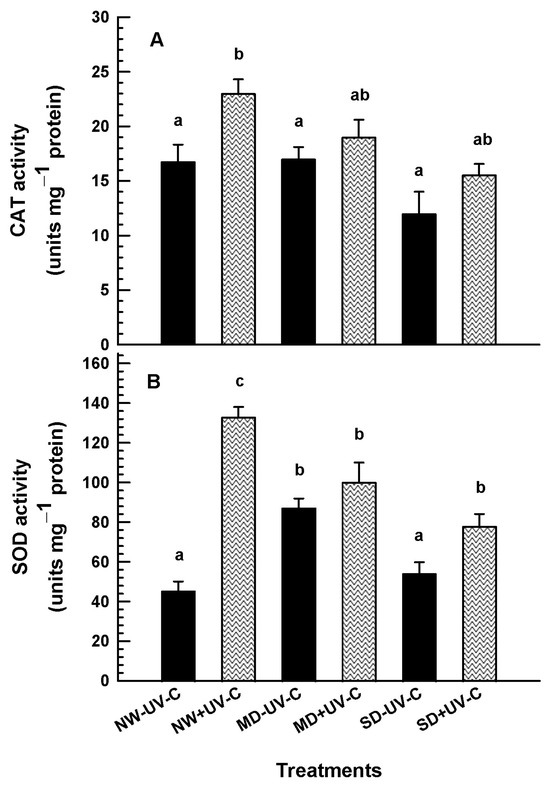
Figure 9.
CAT ((A); units mg−1 protein) and SOD ((B); units mg−1 protein) activity of O. ecklonis plants treated with UV-C irradiation at 1 kJ m−2 or left non-irradiated (controls) and cultivated at three different drought levels (NW: normal watering, MD: moderate drought, SD: severe drought). Data were recorded at the 4th week of the experimental period. Vertical bars are standard error of means, and different letters indicate the significant differences according to Duncan’s multiple range test at p = 0.05.
4. Discussion
Drought stress induces alterations in ornamental plants associated with decreases in shoot fresh and dry biomass and increases in the root-to-shoot ratio. In long-term drought conditions, ornamental plants may respond to the consequent oxidation and ROS production with the induction of antioxidant enzymes that act protectively [14]. In the present study, severe drought dramatically affected the growth and development of O. ecklonis plants. After 7 weeks, significant reductions in the fresh and dry weight of the upper and root biomass were recorded. EL was increased by 32% and MDA by up to 2-fold, indicating a significant damage to the cell membranes. Fv/Fm ratio was dropped below the 0.70 threshold, indicating serious damage to PSII. CAT activity on the MD and SD plants did not increase, although significant increases in SOD activity in MD plants were recorded. Likewise, CAT and SOD activities of water stressed Eugenia uniflora and Photinia x fraseri plants increased after 5–14 days of the water-deficit treatments [5]. Numerous ornamental plants adapt to drought conditions and can tolerate drought stress and promote adaptation mechanisms to overcome various morphological, physiological and biochemical alteration. For example, Gaillardia aristata and Leucanthemum x superbum were able to regulate shoot water loss effectively by displaying drought avoidance mechanisms. They both showed wilting symptoms when water was limited and showed new growth after they were watered [3,4]. However, when drought stress was present for longer periods, decreases in net CO2 assimilation occurred as part of the stomatal function, and plants were permanently damaged [7,27]. Furthermore, at extended drought periods, oxidation took place, and damage on the cell membranes and components was recorded [5,7].
In search of new methods to increase the tolerance of ornamental plants to drought, we evaluated the effects of low doses of UV-C irradiation. We know from previous studies that high levels of UV-C irradiation (e.g., >8 kJ m−2) may damage the DNA of living organisms by disrupting the integrity and functioning of chloroplasts and mitochondria [28]. On the contrary, low doses may affect changes in leaf area, leaf thickness, stomatal density, photosynthetic pigment production and altered stem elongation and branching patterns [20]. Such responses may lead to improved transpiration rates and water-use efficiencies. Therefore, the application of UV-C in the field may, more likely, serve as a photomorphogenic signal rather than as an environmental stressor that triggers cell damage [11,14,20,29]. It has been shown in previous studies that low doses of UV-C may induce the antioxidant response and protect from subsequent abiotic and biotic stress factors, such as pathogens, pests and drought [15,16,17,18,19,20]. This was the first attempt to apply UV-C irradiation as a photomorphogenic signal to increase the drought tolerance of O. ecklonis plants. The application of 1 kJ m−2 UV-C increased the antioxidant activity, resulting in better adaptation to severe drought. CAT and SOD activities were increased by 26% and by approx. 2.5-fold, respectively. This response facilitated lower levels of EL and MDA in the UV-treated plants compared to that in the non-irradiated controls. Furthermore, plants that received the UV-C treatments maintained higher RWC and a more functional PSII. UV-C treated plants showed a generally better response to moderate and severe drought, although net CO2 assimilation, transpiration and stomatal conductance were not affected by the treatments. Likewise, differences in the fresh and dry weights of the upper parts of the plants or the root systems were scarce and did not show a clear pattern. UV-C irradiation resulted in an increase in fresh weights of the upper parts and the root system in the MD-treated plants only.
UV-C irradiation induces similar defense responses to those induced via the octadecanoid pathway that leads to the production of jasmonic acid [30]. When methyl jasmonate, the hormone-like compound derived after the methylation of jasmonic acid, was applied to plants, it mitigated oxidative stress responses induced by drought [31]. MeJA activated the antioxidant systems to scavenge ROS in the stressed plants and alleviated the oxidation process. The application of MeJA on Impatiens walleriana plants increased certain biochemical markers and resistance in severe drought conditions [32]. Apart from the octadecanoid pathway, the shikimic pathway regulated by phenylalanine (also called phenylpropanoid pathway) is induced during stress conditions [33]. Plants grown under drought stress boost a biochemical cascade to produce secondary metabolites that act as protective agents or as signals to the induction of defense responses. For example, the plant growth regulator salicylic acid (SA), a derivative of benzoic acid via the phenylpropanoid pathway, may confer protection from drought stress in different ornamental plant species [34]. On recent experiments, the foliar application of 2 mM SA on I. walleriana plants exposed to drought prevented wilting, maintained RWC, increased proline accumulation, modulated antioxidant enzymes and significantly reduced wilting [35]. The exogenous application of SA at 0.724 mM on Helianthus annus L. plants significantly improved the head diameter, number of achenes, 1000-achene weight, achene yield and oil yield under water stress conditions [36]. The application of 1 mM SA on Ocimum basilicum L. plants alleviated the drought effects through a partial recovery of chlorophyll levels and photosynthetic activity [37]. SA induced antioxidant enzyme activities that significantly reduced EL, MDA and H2O2, suggesting a protective response to membrane lipid peroxidation.
UV-C irradiation can strongly induce the phenylpropanoid pathway and the production of various derivatives from this pathway [14]. We suggest that low doses of UV-C induced biochemical reactions in O. ecklonis plants that led to the production of protective compounds or molecular signals that activated defenses against drought. UV-C protected O. ecklonis plants from oxidative stress, and on the cellular level, it protected membrane lipid peroxidation and wilting. Additionally, UV-C treatments may increase the production of lignin and suberin, the main phenylpropanoid components of the cuticle, and aid the development of resistance to water loss, thus exhibiting adaptive advantages under severe conditions associated with high light and temperature environments and water stress [38].
5. Conclusions
The right doses of UV-C irradiation induce photomorphogenic and biochemical reactions in ornamental plants associated with defense and increase the resistance against various biotic and abiotic stressors. In the present study, UV-C irradiation applied at 1 kJ m−2 alleviated the drought symptoms of O. ecklonis by reducing oxidative stress and membrane lipid peroxidation. These promising results can help us find new approaches of stress regulation in ornamental species.
Author Contributions
Conceptualization, A.I.D.; methodology, A.I.D.; validation, A.K., K.D., M.A., I.M. and V.K.; formal analysis, A.I.D. and V.K.; investigation, A.K., K.D., M.A., I.M. and V.K.; data curation, A.K., K.D. and E.K.; writing—original draft preparation, A.I.D.; writing—review and editing, A.I.D.; supervision, A.I.D. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dole, J.M.; Wilkins, H.F. Floriculture: Principles and Species; Prentice-Hall Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Treder, J.; Nowak, J. The effect of irrigation frequency on growth, flowering and stomatal conductance of osteospermum ‘Denebola’ and New Guinea impatiens’ Timor’ grown on ebb· and-flow benches. Acta Agrobot. 2001, 54, 59–68. [Google Scholar] [CrossRef]
- Zollinger, N.; Kjelgren, R.; Cerny-Koenig, T.; Kopp, K.; Koenig, R. Drought responses of six ornamental herbaceous perennials. Sci. Hortic. 2006, 109, 267–274. [Google Scholar] [CrossRef]
- Cirillo, C.; Rouphael, Y.; Caputo, R.; Raimondi, G.; De Pascale, S. The influence of deficit irrigation on growth, ornamental quality, and water use efficiency of three potted Bougainvillea genotypes grown in two shapes. HortScience 2014, 49, 1284–1291. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A. Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Sci. Hortic. 2020, 265, 109225. [Google Scholar] [CrossRef]
- Pang, C.-H.; Wang, B.-S. Oxidative stress and salt tolerance in plants. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2008; pp. 231–245. [Google Scholar]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Darras, A.I.; Demopoulos, V.; Bali, I.; Tiniakou, C. Photomorphogenic reactions in geranium (Pelargonium × hortorum) plants stimulated by brief exposures of ultraviolet-C irradiation. Plant Growth Regul. 2012, 68, 343–350. [Google Scholar] [CrossRef]
- Darras, A.I.; Demopoulos, V.; Bali, I.; Katsiloulis, O.; Kratimenou, E. Brief exposures of ultraviolet-C (UV-C) irradiation improves flowering of ornamental plants. Acta Hortic. 2013, 1002, 95–102. [Google Scholar] [CrossRef]
- Bridgen, M. Using ultraviolet-C light as plant growth regulator©. Acta Hortic. 2015, 1085, 167–169. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Charles, M.T.; Fan, J.; Charlebois, D.; Khanizadeh, S.; Rolland, D.; Rousel, D.; Deschenes, M.; Dube, C. Effects of preharvest ultraviolet-C irradiation on fruit phytochemical profiles and antioxidant capacity in three strawberry (Fragaria × ananassa Duch.) cultivars. J. Sci. Food Agric. 2015, 95, 2996–3002. [Google Scholar] [CrossRef]
- Darras, A.I.; Skouras, P.J.; Assimomitis, P.; Labropoulou, C.; Stathas, G.J. Application of UV-C irradiation to Rosa × hybrida plants as a tool to minimise Macrosiphum rosae populations. Agronomy 2021, 11, 702. [Google Scholar] [CrossRef]
- Short, B.D.; Janisiewicz, W.; Takeda, F.; Leskey, T.C. UV-C irradiation as a management tool for Tetranychus urticae on strawberries. Pest Manag. Sci. 2018, 74, 2419–2423. [Google Scholar] [CrossRef]
- Darras, A.I.; Bali, I.; Argyropoulou, E. Disease resistance and growth responses in Pelargonium × hortorum plants to brief pulses of UV-C irradiation. Sci. Hortic. 2015, 181, 95–101. [Google Scholar] [CrossRef]
- Forges, M.; Vasquez, H.; Charles, F.; Chabane Sari, D.; Urban, L.; Lizzi, Y.; Bardin, M.; Aarrouf, J. Impact of UV-C radiation on the sensitivity of three strawberry plant cultivars (Fragaria × ananassa) against Botrytis cinerea. Sci. Hortic. 2018, 240, 603–613. [Google Scholar] [CrossRef]
- Gitz, D.C.; Liu-Gitz, L. How do UV photomorphogenic responses confer water stress tolerance? Photochem. Photobiol. 2003, 78, 529–534. [Google Scholar] [CrossRef]
- Robson, T.M.; Hartikainen, S.M.; Aphalo, P.J. How does solar ultraviolet-B radiation improve drought tolerance of silver birch (Betula pendula Roth.) seedlings? Plant Cell Environ. 2015, 38, 953–967. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–130. [Google Scholar]
- Karabal, E.; Yucel, M.; Oktem, H.A. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 2003, 164, 925–933. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A.J.P.S. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, J. Changes in growth rate, root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit. Sci. Hortic. 2013, 156, 54–62. [Google Scholar] [CrossRef]
- Saxena, S.C.; Joshi, P.K.; Grimm, B.; Arora, S. Alleviation of ultraviolet-C-induced oxidative damage through overexpression of cytosolic ascorbate peroxidase. Biologia 2011, 66, 1052–1059. [Google Scholar] [CrossRef]
- Loconsole, D.; Santamaria, P. UV lighting in horticulture: A sustainable tool for improving production quality and food safety. Horticulturae 2021, 7, 9. [Google Scholar] [CrossRef]
- Kondo, S.; Fiebig, A.; Okawa, K.; Ohara, H.; Kowitcharoen, L.; Nimitkeatkai, H.; Kittikorn, M.; Kim, M. Jasmonic acid, polyamine, and antioxidant levels in apple seedlings as affected by Ultraviolet-C irradiation. Plant Growth Regul. 2011, 64, 83–89. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef]
- Đuric, M.; Subotic, A.; Prokic, L.; Trifunivic-Momcilov, M.; Milocevic, S. Foliar application of methyl jasmonate affects impatiens walleriana growth and leaf physiology under drought stress. Plant Signal. Behav. 2023, 18, 2219936. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 2nd ed.; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 1998. [Google Scholar]
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: A case study in field-grown Salvia officinalis L. plants. Environ. Exp. Bot. 2008, 64, 105–112. [Google Scholar] [CrossRef]
- Antonic, D.A.; Subotic, A.R.; Dragicevic, M.B.; Pantelic, D.; Milocevic, S.M.; Simonovic, A.D.; Momcilovic, I. Effects of exogenous salicylic acid on drought response and characterization of dehydrins in Impatiens walleriana. Plants 2020, 9, 1589. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Finnegan, P.M.; Younis, A.; Nafees, M.; Zorrig, W.; Hamed, K.B. Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Zheng, X.; Wang, Q.; Silvy, E.A.; Li, Y.; Han, Y.; Bi, Y.; Prusky, D. UV-C irradiation accelerated the deposition of suberin and lignin at wounds by activating phenylpropanoid metabolism in potato tubers. Sci. Hortic. 2023, 309, 111634. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
