Abstract
Understanding the diverse environmental influences on seedling growth is critical for maximizing yields. The need for a more comprehensive understanding of how various environmental factors affect seedling growth is required. Integrating sensor data and image processing techniques offers a promising approach to accurately detect stress symptoms and uncover hidden patterns, enhancing the comprehension of seedling responses to environmental factors. The objective of this study was to quantify environmental stress symptoms for six seedling varieties using image-extracted feature characteristics. Three sensors were used: an RGB camera for color, shape, and size information; a thermal camera for measuring canopy temperature; and a depth camera for providing seedling height from the image-extracted features. Six seedling varieties were grown under controlled conditions, with variations in temperature, light intensity, nutrients, and water supply, while daily automated imaging was conducted for two weeks. Key seedling features, including leaf area, leaf color, seedling height, and canopy temperature, were derived through image processing techniques. These features were then employed to quantify stress symptoms for each seedling type. The analysis of stress effects on the six seedling varieties revealed distinct responses to environmental stressors. Integration of color, size, and shape parameters established a visual hierarchy: pepper and pak choi seedlings showed a good response, cucumber seedlings showed a milder response, and lettuce and tomato seedlings displayed an intermediate response. Pepper and tomato seedlings exhibited a wide range of growth stress symptoms, at 13.00% to 83.33% and 2.96% to 70.01%, respectively, indicating considerable variability in their reactions to environmental stressors. The suggested classification approach provides valuable groundwork for advancing stress monitoring and enabling growers to optimize environmental conditions.
1. Introduction
By 2050, the world population is expected to reach 9.1 billion, a 34% increase from current levels, and to support this larger, more urbanized, and economically prosperous population, a 70% increase in food production is necessary [1]. Agricultural technology advancements provide a solution to meet rising global food demand amid challenges like population growth and climate change [2]. Vegetables play an important role in ensuring food and nutrition security [3]. Their production not only presents a viable economic opportunity but also aids in alleviating rural poverty and unemployment in developing nations, forming a vital aspect of farm diversification strategies [4]. As a cost-effective source of essential vitamins and minerals, vegetables contribute significantly to maintaining good health [4,5].
High-quality seedling production refers to the process of cultivating seedlings that meet defined levels of performance, such as survival and growth, on a particular planting site [6]. The controlled environment in the seedling production facility is designed for the precise regulation of factors such as temperature, humidity, lighting, and nutrient supply, ensuring consistent and efficient crop growth (Huang et al., 2020) [7]. This controlled setting not only enables the production of high-quality crops but also reduces reliance on pesticides and minimizes bacterial contamination [8]. High-quality seedlings are essential for successful vegetable and floriculture crop cultivation, improving early crop establishment, final quality, uniformity, and yield and reducing production time [9]. The growth and development of high-quality seedlings can be significantly affected by environmental factors such as temperature, light intensity, water, and nutrients, with vegetable production being especially susceptible to these abiotic environmental stresses [10,11,12]. Exposure to extreme temperature and light conditions can exacerbate these challenges, with susceptibility varying based on seedling conditioning and phenological stage [13]. Water loss consequences may extend over multiple growing seasons, impacting survival rates and growth [14].
Plant/seedling growth stress poses a significant threat to agricultural productivity, leading to a substantial decrease in crop yield and quality [15,16]. Early detection of seedling growth stress is crucial for enhancing crop production and ensuring agricultural sustainability. Stress can significantly impact the germination and early growth of seedlings. The major environmental factors that contribute to seedling stress include water availability (both drought and flooding), temperatures (heat and cold), light intensity (too much or too little), nutrient imbalances, and salinity [12]. These factors can hamper essential plant processes, leading to visible symptoms like wilting, discoloration, and stunted growth. Environmental stressors, such as temperature, light, water potential, and nutrient levels, can affect seed germination and pre-emergence seedling growth, leading to reduced crop establishment and yield [17]. Measuring seed vigor, which encompasses the ability of seeds to germinate and produce vigorous seedlings, is crucial for ensuring crop sustainability in changing climates [18]. Additionally, the genetic and physical quality of seedlings, influenced by early stress factors, plays a key role in determining the success of seedling production [19]. Quantifying and addressing early growth stress symptoms are essential for producing high-quality seedlings and improving crop establishment, uniformity, and yield [20]. Therefore, early stress quantification is necessary to identify and mitigate potential issues that could impact the overall success of seedling production.
Computer vision advancements have enabled the extensive application of image processing and machine learning in studying crop biotic and abiotic stress phenotypes [21]. Image analysis techniques, being non-invasive, offer significant potential for the automated detection of both types of stress in plants. This involves processing meticulously collected photographs to extract specific information [22,23]. Various imaging techniques, such as digital, fluorescence, thermography, LIDAR, multispectral, and hyperspectral imaging, can be used to effectively identify and assess stress in crops [15]. These images provide valuable data on plant physical attributes like canopy area, leaf color, texture, size, and shape [24]. Commonly used color spaces include RGB, LAB, YCBCR, and HSV [25]. Descriptive elements like image contrast, homogeneity, dissimilarity, energy, and entropy contribute to texture analysis [26]. Sun et al. [27] introduced a non-destructive method for diagnosing nutrient stress in paddy plants, specifically targeting nitrogen, phosphorus, and nitrogen, phosphorus, and potassium (NPK) deficiencies. Latte et al. [28] detected nutrient deficiencies in paddy crops by analyzing leaf color during mid-growth.
Visual sensors play a significant role in controlled plant production facilities, enabling comprehensive crop monitoring and automation through image analysis [29]. Integrating visual sensors allows farmers to make informed decisions, optimize resources, and improve crop yields, supporting sustainable vertical farming practices [30]. Image sensor fusion and image processing offer a powerful approach to understanding and managing seedling growth stress. By combining image sensor data from diverse imaging sources and advanced image analysis and offering a comprehensive perspective on the environmental conditions impacting seedling health, subtle changes in plant health can be detected early [31]. This allows for timely adjustments to lighting, nutrients, or water, ensuring optimal growing conditions. Studies utilizing RGB image sensors have effectively assessed plant stresses, identifying issues like biotic stress in wheat [31], nutrient deficiency in soybean [32] and black gram [33], and fungal blight in potato [34].
Machine learning algorithms establish optimal decision boundaries in high-dimensional feature spaces, forming the foundation for accessible image analysis systems [35]. Traditional approaches in machine learning, proven to be versatile and effective, analyze crop phenotypes related to stress conditions [32,36,37]. Feature extraction provides quantified parameters facilitating classification by assigning objects to specific classes [38]. Image segmentation, based on color and texture features in RGB and grayscale intensity spaces, improves representativeness and ease of analysis [39]. Karadag et al. [40] introduced a machine learning algorithm to differentiate healthy and fusarium-infected pepper plants. Support vector machine (SVM), which has been extensively applied in agriculture, proves effective for plant stress detection. SVM grades the severity of iron deficiency chlorosis in soybeans [41]. Prasad et al. [42] devised an imaging system for plant leaf disease identification, incorporating disease spot detection, Gabor-wavelet-transform-based feature extraction, and SVM-based disease classification.
The ability to accurately measure and analyze seedling growth stress helps us to identify environmental factors that may impede healthy growth and development. Understanding these stressors is essential for implementing timely interventions and optimizing plant growth conditions. The potential of sensor fusion and image processing techniques for quantifying seedling growth stress caused by various environmental factors is immense. Utilizing these technologies enables improved understanding of plant stress responses, optimizing growing conditions for enhanced crop yields, and sustainability in agriculture and controlled horticulture. The objective of this study was to quantify the seedling growth stress symptoms of six seedling varieties under controlled conditions, intentionally subjecting them to variations in light, temperature, water, and nutrient levels.
2. Materials and Methods
2.1. Experimental Site Preparation and Seedling Growth Conditions
The experiments took place in a plant factory situated on the premises of the College of Agriculture and Life Science at Chungnam National University in Daejeon, Republic of Korea. In this experiment, 6 seedling varieties were sown in 45-hole trays for tomato and pepper, 128-hole trays for lettuce and pak choi, and 40-hole trays for cucumber and watermelon using horticultural soils. Eight-day-old pepper (variety: Cheongyang), tomato (variety: Defness), lettuce (variety: Cheongchima), pak choi (variety: Modom), cucumber (variety: Begdadagi), and watermelon (variety: Jhob) seedlings were grown in five small chambers to observe the environmental stress effects. Each seedling cultivation chamber was 1500 × 1100 × 2500 mm (L × W × H). Within one chamber, a vertical frame with three beds accommodated three seedling trays on each bed. The overall size of the vertical frame was 980 × 600 × 2160 mm (L × W × H) and the growing bed was 900 × 600 × 150 mm (L × W × H). To maintain optimal conditions, each of the five cultivation rooms was equipped with air conditioners, heaters, humidifiers, dehumidifiers, and solenoid valves for temperature, humidity, and carbon dioxide control. Two axial fans per bed circulated air in the cultivation room. Fluorescent lights were used for adequate seedling lighting on the cultivation beds. Figure 1a,b illustrate the layout and configuration of the control room and cultivation chambers along with the actuator, fluorescent light, and seedling tray positions.
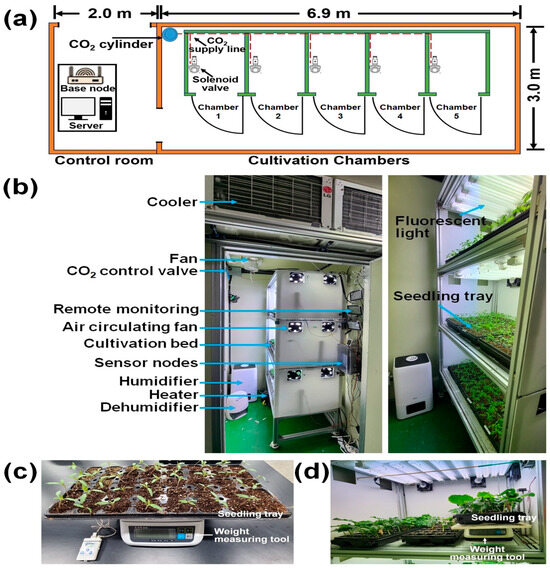
Figure 1.
Experimental chamber layout and continuous water content measurement during the experimental period using a weight balance. (a) Layout and configuration of the cultivation chambers; (b) image of the custom fabricated cultivation chamber showing the positions of the actuators, fluorescent lights, and seedling trays; (c) weight measurement using the seedling tray containing soil and plants; and (d) continuous water content measurement during the experiment.
The study focused on four specific environmental parameters: temperature, light intensity, nutrients, and water supply. To investigate the effect of environmental stress, the experiment maintained three levels of temperature, light intensity, nutrients, and water application. Table 1 shows the ambient environment parameters in all five controlled chambers. The standard growth conditions for the six seedling varieties were derived from the literature, as detailed for lettuce [43], tomato [44], pepper [45], watermelon [46], cucumber [47], and pak choi [48].

Table 1.
Ambient environment parameters for the experiments and control and stress conditions used in this study for six varieties of seedlings.
In a plant factory, effective water and nutrient application is vital for the healthy growth of seedlings. Table 1 highlights two distinct types of water and nutrient application. For plant chambers 1, 2, 4, and 5, 1 L/tray/day of water and nutrient solutions with an electrical conductivity (EC) of 1.0 dS m−1 and pH of 6.0 were provided. In chamber 3, it was aimed to investigate the impact of water and nutrient stress on seedlings. This involved experimenting with three different levels of water and nutrient supply. Water levels were adjusted to 1, 0.75, and 0.50 L/tray/day for water stress trials, while nutrient solution levels were 1, 3, and 6 dS m−1 for the nutrient stress trials. These variations enabled the examination of the effects of varying stress levels of water and nutrient availability. The selection of these supply levels was guided by established practices to ensure consistent nutrient delivery to the root zone, preventing both overwatering and underwatering of the seedlings. In this study, water mixed with commercial Hoagland nutrient solutions A and B (Daeyu Co., Ltd., Seoul, Republic of Korea) was applied to plant roots. All the essential and trace nutrient elements are combined in this commercial nutrient solution. The ingredients contained in each solution are shown in Table 2.

Table 2.
Ingredients contained in nutrient solutions A and B.
To achieve the desired nutrient level, 5 mL each of nutrient solutions A and B were mixed with 5 L of water, and 1 L of this mixture was provided daily to each bottom irrigation tray, positioned beneath the seedling growing tray. The targeted nutrient level was maintained by checking the EC and pH meter every day while watering the seedlings. The seedling soil absorbed water through the bottom irrigation tray, facilitating water uptake by plants through the osmosis process. Irrigation was carried out at rates of 0.75 L/tray/day and 0.50 L/tray/day, representing 75% and 50% of the standard irrigation application, while the control group received 100% of the standard irrigation. To measure water stress, we continuously weighed the water in the seedling trays at 10 min intervals using a CAS weighing balance (Total Weighing Solution, Yangju, Republic of Korea) positioned beneath the seedling trays, as illustrated in Figure 1c,d. Soil water content was calculated using Equation (1) based on weight basis method as follows:
where WC is the water content (%), WW is the weight of water (g), WM is the total weight of the materials (g), WS is the weight of the tray including soil (g), WD is the dry soil weight (g), WT is the weight of the empty tray under the soil tray (g), WP is the plant weight (g), and N is the number of plants in the tray.
Throughout the experiment, the remaining ambient environmental variables, such as humidity and CO2 levels, were maintained at constant levels. The optimum relative humidity and CO2 concentration were 60 ± 5% and 600 to 800 ppm, respectively. Table 1 also shows the control and stress conditions for the six seedling varieties used in this study.
2.2. Sensor Selection and Image Acquisition
The data collection process involved a multi-camera system, as shown in Figure 2. For top-view imaging of seedlings, an affordable, portable, and high-quality RGB camera (model: Raspberry Pi camera, Raspberry Pi Foundation, Cambridge, UK) was used. To capture thermal information, a thermal camera (Model: Seek Thermal Compact Pro, Seek Thermal Inc., Santa Barbara, CA, USA) was employed. Additionally, a depth camera (Model: Intel RealSense D435i, Intel Corporation, Santa Clara, CA, USA) was used for acquiring depth images. The specifications of these cameras are shown in Table 3. The cameras were strategically positioned vertically at a height of 0.36 m above the seedlings, ensuring optimal field-of-view (FOV) coverage (Figure 2). Throughout the image capture process for each seedling bed, consistent lighting conditions were maintained. The Raspberry Pi Camera Module V2 is a compact camera for Raspberry Pi single-board computers, capturing still images up to 8 megapixels (3280 × 2464 pixels). Utilizing the Sony IMX 219 sensor, it ensures high-quality images with excellent color reproduction and low-light performance. Small and lightweight, with a standard field of view of 62.2° × 48.8° and automatic image acquisition control, it was suitable for this study. The Intel RealSense D435i is an advanced depth camera designed for precise depth sensing, featuring a frame size of 1280 × 720 pixels and global shutter technology. With its stereoscopic depth sensing and integrated IMU, it offers a wide field of view of 87° × 58° and a depth range of 0.3 to 3.0 m. Its compact design and seamless integration make it an ideal choice for this applications. The Seek Compact Pro is a compact and potent thermal imaging camera with a frame size of 320 × 240 pixels, a field of view of 32° × 32°, and a temperature sensing range of −40 to 330 °C. Equipped with automatic control and calibration systems, its pocket-sized design makes it perfect for installation in seedling chambers to facilitate data acquisition.
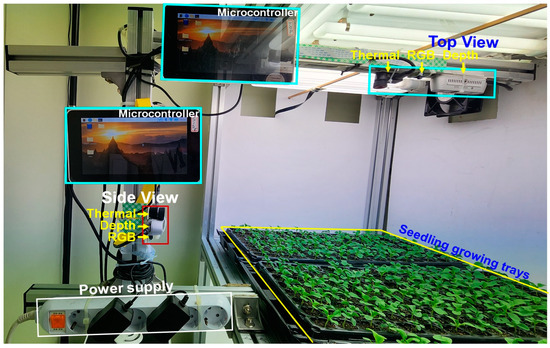
Figure 2.
Image acquisition tool using RGB, thermal, and depth cameras with microcontrollers to acquire top and side images of seedlings.

Table 3.
Specifications of RGB, thermal, and depth cameras used in this study.
Automation of the image capture process was achieved through the utilization of a microcontroller system (Model: Raspberry Pi 4B, Raspberry Pi Foundation, Cambridge, UK) equipped with an integrated display. This configuration facilitated seamless interfacing with the cameras, enabling the automated capture and storage of images. The system was designed for efficient remote monitoring and image acquisition tasks. For remote accessibility, a virtual network computing (VNC) viewer was implemented on the microcontroller system. This allowed for remote control through a graphical user interface (GUI) display, ensuring automatic initiation of the viewer upon device boot-up, as detailed by Islam et al. [49]. VNC, developed in the mid-1990s, is a remote desktop technology with open-source code licensed under the GNU General Public License. Commercial variations of VNC are also available. Images were saved in JPG format with a resolution of 3280 × 2464 pixels on a 128 GB micro SD memory card connected to the microcontroller. To mitigate the impact of camera jitter or potential unfocused images, three images were captured for each seedling bed. Subsequently, the average of these three images was computed and utilized for further analysis. Figure 3 shows sample images captured during the experiments for all six varieties of seedlings.
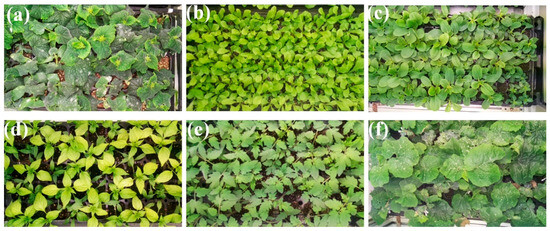
Figure 3.
RGB image acquisition for six seedling varieties using RGB camera from the top view: (a) cucumber seedlings, (b) pak choi seedlings, (c) lettuce seedlings, (d) pepper seedlings, (e) tomato seedlings, and (f) watermelon seedlings.
The study investigated the impact of various environmental stress effects on six varieties of seedlings. A total of 540 images from the six seedling varieties were acquired for analysis. Among these, 288 images were related to stressed seedlings, while 252 images represented non-stressed seedlings, serving as a control group. Four different types of stresses (temperature, light intensity, nutrient, and water) were considered for this experiment. For each stress type, 72 images were captured, resulting in a balanced distribution of stress samples. The severity of the stresses applied to the seedlings in terms of temperature, light intensity, nutrients, and water ranged from low to high levels. To ensure accurate analysis, all images of stressed seedlings were appropriately labeled based on reference guidelines describing the growth conditions and characteristics specific to each stress class.
2.3. Data Processing and Analytical Procedures
Figure 4 shows the schematic diagram of the overall sensor fusion and image processing steps for stress quantification in this study. The schematic diagram illustrates the process of sensor fusion and image processing steps for stress quantification in seedling growth based on environmental factors.
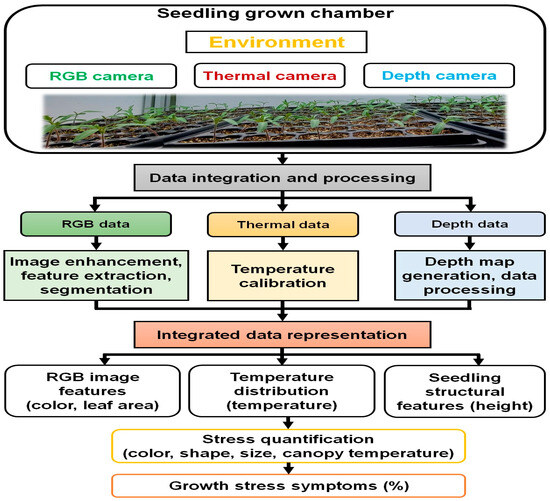
Figure 4.
The overall sensor fusion and image processing steps for the growth stress quantification in this study.
Beginning with the data acquisition from RGB, thermal, and depth sensors capturing various aspects of seedling growth and environmental conditions, the data then undergo integration and pre-processing to align and prepare them for analysis. This involves refining RGB imagery through image enhancement and feature extraction techniques, ensuring accurate representation of thermal data through temperature calibration, and generating depth maps for calculating seedling size during depth data processing. These integrated datasets are then represented visually for comprehensive analysis. The fusion data from different sensors was used to analyze seedling growth and environmental influences. Finally, stress quantification was obtained by analyzing the integrated data to identify color, shape, size, and canopy temperature patterns. Based on the pattern of healthy and stressed seedling data, seedling stress symptom levels (%) were calculated.
2.3.1. RGB Image Processing for Stress Symptom Features
Image quality is crucial in image analysis, influencing accuracy and effectiveness. Low image quality poses challenges in discerning details due to issues like overexposure, shadows, debris, and focusing issues. High-quality images enhance the processing efficiency, reducing complexity and improving feature extraction [50]. The schematic flowcharts for the RGB image processing and leaf area (LA) estimation are shown in Figure 5 and Figure 6 and illustrate the overall image processing and leaf area calculation steps. The initial step involved histogram equalization to address light source variation. Subsequent pre-processing reduced noise and enhanced contrast in the image, comprising the target plant and background. Direct binarization faced challenges due to insufficient contrast, artifacts, and distortions [51]. Contrast-limited adaptive histogram equalization (CLAHE) was employed to overcome these issues, enhancing the image contrast by limiting amplification and operating on small image areas called tiles [10]. Parameters like block size (BS) and clip limit (CL) governed improved image quality.
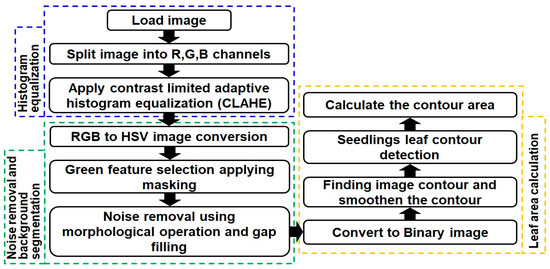
Figure 5.
Flow diagram of the overall RGB image processing and leaf area calculation.
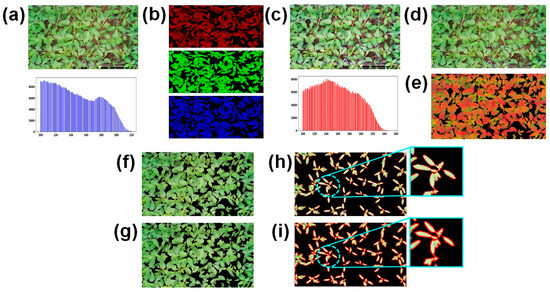
Figure 6.
RGB image preprocessing steps and leaf area calculation: (a) original image with histogram; (b) R, G, and B channel images; (c) enhanced image with histogram; (d) CLAHE enhanced image; (e) converted HSV image; (f) masking applied to select green features; (g) segmented image; (h) broken contours; and (i) smooth contours.
CLAHE, applied separately to red (R), green (G), and blue (B) channels with a CL limit of 0.5, enhanced local contrast and improved background segmentation. Gaussian blur further reduced noise. To extract foreground objects, the blurred image in HSV format set upper and lower bounds for the green color range, creating a mask. Morphological operations removed noise and filled gaps, enhancing segmentation. For leaf area calculation, contour detection was employed to capture seedling shapes from a binary image. However, image quality issues and noise resulted in broken contours, complicating seedling detection and area calculation. To address this, contour smoothing was applied using the total arc length of each polygonal curve, simplifying complex contours while preserving shape and structure. From the contour area, the leaf area was calculated. This quantifies the number of pixels within the leaf region as LA, which is the total one-sided leaf tissue area per unit ground or trunk surface area of a plant [52]. LA provides essential insights into leaf size and morphology and valuable information about seedling stress conditions and growth.
2.3.2. Depth Image Processing for Stress Symptom Features
For plant height estimation, using the Intel RealSense depth camera images involved several key processing steps. Figure 7 shows the flow diagram of the overall depth image processing to estimate plant height. Initially, the colorized depth image was loaded and the shortest and longest depth range for the camera were set to facilitate colorization. Subsequently, the depth map was restored using the colorized depth image, and the resulting map was saved at its original size [53]. The restored depth map was then displayed, providing a visual representation of the plant and its surroundings. Pixel coordinates (u, v) were specified to extract depth values at specific points of interest on the image. The image metadata, including focal length (fx, fy) and principal point (ppx, ppy), were input to facilitate accurate depth extraction. The sample pixel coordinates and their corresponding depth values were estimated based on the specified points. These pixel coordinates were then converted into real-world coordinates using the provided image metadata. By extracting this spatial information, the distance from the camera to the plant was calculated in three-dimensional space, providing a quantitative measure of the plant height [54]. Figure 8 shows the depth image processing results in different steps.
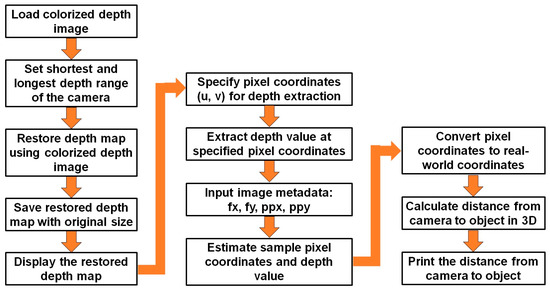
Figure 7.
Flow diagram of the overall depth image processing for plant height estimation.
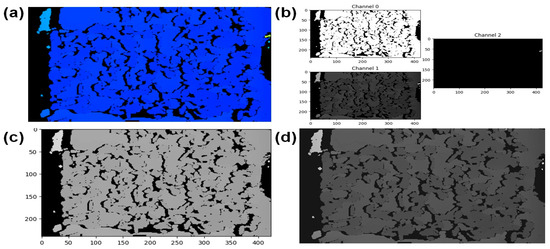
Figure 8.
Depth image processing for plant height estimation: (a) original color depth image, (b) raw depth data in three (R, G, B) channels, (c) combined depth map, and (d) restored depth map from the combined depth map and metadata.
2.3.3. Canopy Temperature Measurement
Canopy temperature detection from thermal leaf images is a multistep process that depends on converting gray values to temperature using a color bar as the reference [55,56]. The thermal image, captured from a fixed position, registers changes in gray levels as temperature fluctuations occur. Employing edge detection methods, the leaf area is isolated within the image. Within this defined leaf region, the gray pixel values represent the temperature of the leaves. By referencing the color bar and the established gray-to-temperature relationship, these gray values are subsequently converted into actual temperature measurements. This approach allows for the precise assessment of canopy temperature, making it a valuable tool for applications ranging from plant stress detection to microclimate analysis in agricultural and environmental studies. Figure 9a shows the steps of the canopy temperature extraction method, and Figure 9b–e show the thermal image processing steps and temperature profiling using the image processing method.
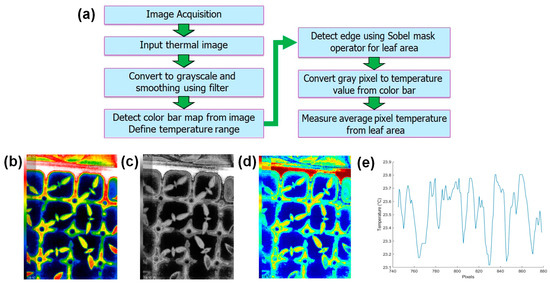
Figure 9.
Canopy temperature detection from the thermal images: (a) steps of thermal image processing, (b) original image, (c) grayscale image, (d) gray-pixel-to-temperature conversion, and (e) temperature profile.
2.4. Quantification of Stress Symptoms
A schematic diagram of the quantification of stress symptoms is shown in Figure 10. Seedling stress quantification through the extraction of leaf length, width, area, plant height, and canopy temperature from images is a multifaceted process involving advanced image processing and quantitative analysis. Initially, high-resolution images of plants were captured, and image processing techniques were applied to extract the features as explained in previous sections. The integration of infrared imaging technology aids in the extraction of canopy temperature data, providing additional insights into the plant’s physiological response to stress. The extracted numerical data, encompassing leaf dimensions, area, plant height, and canopy temperature, were meticulously organized into a structured dataset for further analysis. Stress quantification involves the labeling of plants as stressed or non-stressed based on domain knowledge, expert input, and additional horticultural data. Horticultural data encompass a wide range of information related to the cultivation and management of plants, particularly those cultivated for food, medicinal, ornamental, or landscaping purposes. These data are crucial for optimizing crop yields, ensuring plant health, and making informed decisions in horticultural practices.
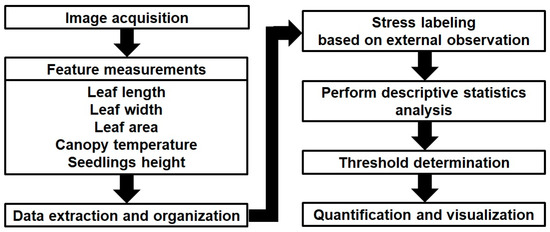
Figure 10.
Schematic diagram of the quantification of stress symptoms using feature measurements from the seedling images.
Descriptive statistical analyses (mean, standard deviation) were then applied to evaluate the significance of differences in extracted features between stressed and non-stressed groups. Subsequently, thresholds were determined to categorize stress levels, and the quantification of stress involved applying these thresholds to the dataset. The outputs were visualized through various graphical representations to facilitate a comprehensive understanding of the distribution of features across stressed and non-stressed seedlings. For the stress symptom quantification, we calculated all the features of seedlings such as leaf area, seedling height, and canopy temperature. Based on the calculated values from both the healthy and stressed seedlings, the stress symptoms (%) of seedlings were estimated and calculated using Equation (1) as follows:
where Ss is the calculated value (%) representing the degree of stress in the seedlings, Hv represents the feature values of the healthy seedlings, and Sv represents the feature values of the stress-induced seedlings.
3. Results
3.1. Stress Symptom Visualization Based on Seedling Color and Size
In visualizing stress effects on seedlings, a comprehensive approach, including color, size, and shape, provides a distinct representation of seedling health and response to stressors [57]. Vibrant and uniform colors signify health, while shifts towards yellows, oranges, and reds indicate increasing stress levels. Changes in plant size reflect growth patterns, with smaller sizes indicating potential stunted development under stress conditions. Altered plant morphology, such as variations in leaf shape, offers insights into stress responses [58]. By combining these elements, a visual hierarchy was created to depict the severity and nature of stress, enhancing the richness of the information. Interactive features and time-lapse visualizations further enabled dynamic exploration, allowing us to gain a deeper understanding of the stress effects on plants over time. Figure 11 shows the stress effect visualization based on color, size, and area changes for pepper, cucumber, tomato, watermelon, lettuce, and pak choi seedlings. Pepper and pak choi seedlings were more likely to be affected by stress than other seedlings and showed the most significant changes in color, size, and area under stress conditions. Watermelon seedlings were less likely to be affected by stress than other seedlings and showed the least significant changes in color, size, and area under stress conditions. Tomato seedlings and lettuce seedlings were moderately affected by stress. This was evident from the fact that the tomato and lettuce seedlings showed intermediate changes in color, size, and area under stress conditions. Lettuce seedlings were most affected by stress, which was evident from significant changes in color.
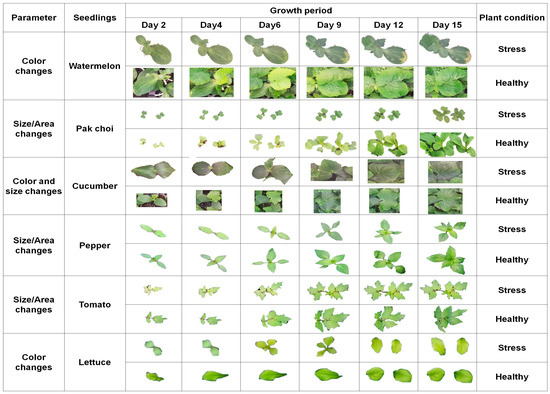
Figure 11.
Growth stress effects on different seedling varieties based on color, shape, and size.
3.2. Growth Features Based on Environmental Conditions and Growth Period
Leaf area serves as a valuable indicator for detecting stress in plants, with a reduction in leaf area being a common response to various stressors [59,60]. This is because plants are trying to conserve water and energy, and so they will reduce the amount of leaf surface area that is exposed to the environment [60,61]. For stress quantification, leaf area was measured for each type of seedling, as shown in Figure 12 and Table A1. For each environmental condition, 15 leaf samples were collected from the images for each day and each seedling type. Then, average values were calculated for each day. Pepper, tomato, lettuce, and pak choi seedlings showed significant differences in leaf area, indicating heightened sensitivity to these stressors. Pepper seedlings, for instance, experienced alterations in leaf area attributed to stressors related to both nutrition and light conditions. In contrast, the leaf area of the tomato seedlings was primarily influenced by variations in light exposure. Lettuce seedlings, however, demonstrated sensitivity to a broader range of stressors, with their leaf area being affected by light, nutrient levels, and temperature fluctuations. Meanwhile, pak choi seedlings displayed a unique pattern of sensitivity, with their leaf area being influenced by variations in light, nutrient availability, and water conditions. Watermelon and cucumber seedlings showed relatively lower significance in terms of changes in leaf area. This comprehensive examination emphasizes the intricate and varied reactions of these seedlings to distinct environmental stressors, providing insight into the complex nature of their growth dynamics.
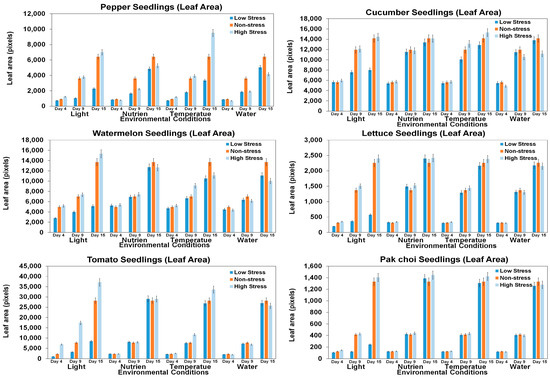
Figure 12.
Comparison of seedling leaf area of six seedling varieties under different environmental stress conditions in different growth periods.
Plant height is a key indicator for detecting stress in plants and vegetables. The stress tolerance index, which incorporates seedling height, serves as a crucial tool by which to assess seedling threshold potential against specific stress factors [23,62]. The influence of stress conditions, such as temperature, light, nutrients, and water supply, on the height of six seedling varieties was studied and the results are shown in Figure 13 and Table A2. The results show that the heights of the pepper and lettuce seedlings were influenced by stress related to light exposure and temperature variations, while the heights of the tomato and pak choi seedlings predominantly responded to changes in light conditions. Additionally, cucumber seedlings exhibited a subtle impact on their height in response to temperature stress, indicating a nuanced sensitivity compared to the other seedlings. However, the watermelon seedlings did not display a significant alteration in plant height under the observed stress conditions. The morphological and physiological responses of plants to different stress conditions, including light intensity and temperature variations, can impact their growth parameters. The results of this study highlight the varying sensitivities of different seedlings to different stress conditions. This suggests that the optimal growing conditions for each type of seedling will depend on its specific needs and tolerances.
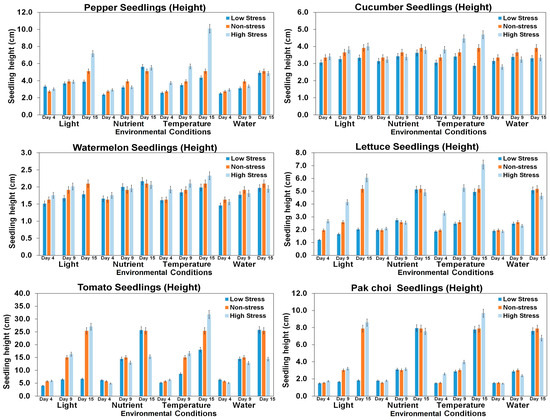
Figure 13.
Comparison of seedling heights of six seedling varieties under different environmental stress conditions in different growth periods.
Temperature stress is a major environmental stress that limits plant growth, metabolism, and productivity [63]. High-temperature stress is considered to be one of the major abiotic stresses for restricting crop production, while low-temperature stress can also affect plant growth [64]. The responses of plants to heat stress vary with the degree and duration of heat stress and the plant type. A detailed analysis of stress and canopy temperature effects on various seedlings has revealed distinct insights into the physiological responses of each plant type, as shown in Figure 14 and Table A3. Pepper seedlings exhibited noticeable changes in average canopy temperature when subjected to light and water stress, accompanied by an increased standard deviation. This heightened variability in response suggests a diverse reaction within the pepper seedlings. In contrast, tomato seedlings demonstrated a consistent response to stress conditions related to light, temperature, and water. However, a significant increase in standard deviation indicates amplified variability in their individual responses. Cucumber seedlings, while experiencing fluctuations in canopy temperature during light stress, displayed a comparatively stable response under other stress conditions. This implies a nuanced sensitivity of cucumber seedlings to specific environmental stressors. For the watermelon, lettuce, and pak choi seedlings, a distinct pattern was observed, showing insignificant changes in average canopy temperatures across all stress conditions. This unique trend suggests a potentially different stress response mechanism for these seedlings compared to their counterparts. The observed variations in average canopy temperatures and standard deviations indicate the diverse responses of different seedlings to distinct stressors, shedding light on the intricacies of their stress adaptation mechanisms.
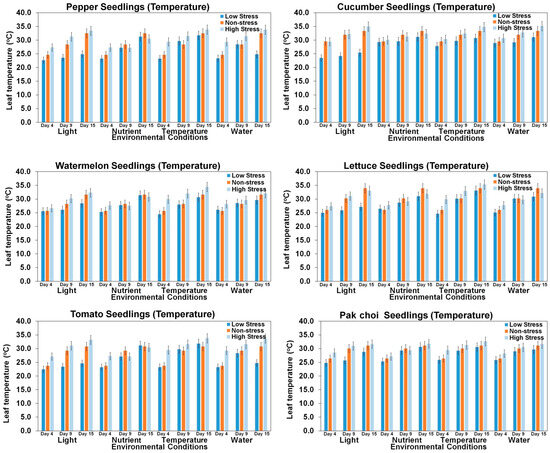
Figure 14.
Comparison of seedling leaf canopy temperatures of six seedling varieties under different environmental stress conditions in different growth periods.
3.3. Stress Quantification Based on Leaf Area Parameter
In this study, the stress responses of six different seedling varieties (pepper, cucumber, tomato, watermelon, lettuce, and pak choi) were quantified based on leaf area parameters under four environmental conditions (temperature, light, nutrients, and water supply). The stress factors considered include low and high light, nutrient levels, temperature, and water availability. Figure 15 shows the stress symptoms (%) for six varieties of seedlings based on the leaf area parameter, where the measurements were considered on days 4, 9, and 15 of the growth period to observe the seedlings’ stress responses over time.
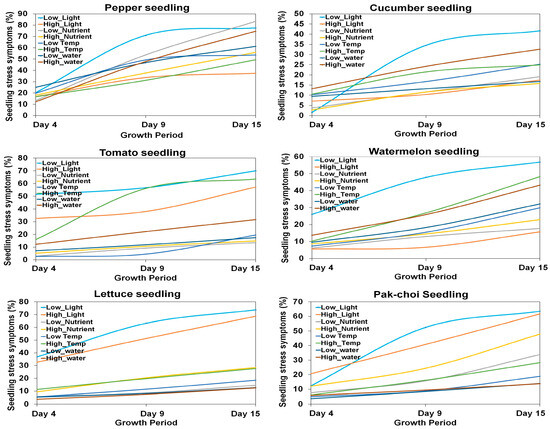
Figure 15.
Growth stress symptoms (%) for six varieties of seedlings based on the seedling leaf area parameter.
Across the observed time periods (days 4, 9, and 15), intriguing patterns emerged. Seedlings exhibited heightened sensitivity to high light, with watermelon and pak choi displaying the most significant increases in leaf area. High nutrient levels consistently led to larger leaf areas, particularly in lettuce and watermelon. Temperature variations also played a crucial role, with cucumber and pak choi responding prominently to high temperatures. The impact of water availability was complex, with watermelon and cucumber showing sensitivity to high water conditions, while lettuce and pak choi exhibited less pronounced responses. In the quantification of stress symptoms across the growth period from day 4 to day 15 for pepper, cucumber, tomato, watermelon, lettuce, and pak choi seedlings, a diverse range of stress (%) was observed. Pepper seedlings exhibited stress symptoms (%) from 13.00 to 83.33%, indicating considerable variability in their response to environmental stressors. Cucumber seedlings showed a stress symptom (%) range of 1.59 to 41.67%, suggesting a relatively milder response compared to pepper. Tomato seedlings displayed stress symptoms (%) ranging from 2.96 to 70.01%, showing a wide spectrum of sensitivity to the environmental factors under consideration. Watermelon seedlings exhibited stress symptoms (%) ranging from 5.63% to 56.88%, indicating a moderate to high degree of susceptibility to stressors. Lettuce seedlings showed a stress percentage range of 5.80 to 73.71%, suggesting a high range of stress responses over the observed period. Pak choi seedlings displayed stress symptoms ranging from 4.99 to 63.53%, indicating a moderate level of susceptibility to stressors. Figure 16 shows the average stress symptoms (%) induced in each type of seedling. These findings highlight the distinct stress response profiles of each seedling type, emphasizing the need for tailored management strategies to optimize their growth conditions and mitigate stress-induced symptoms.
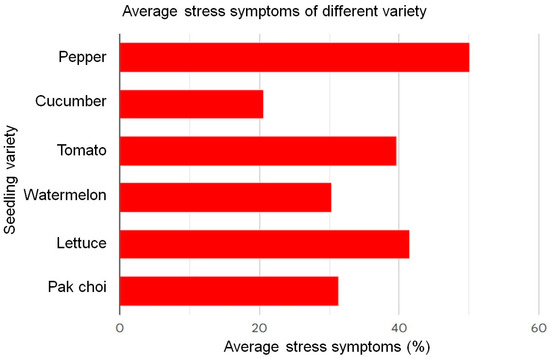
Figure 16.
Average growth stress symptoms (%) of different seedling varieties.
4. Discussion
This study centered on the integration of advanced methodologies, specifically sensor fusion and image processing techniques, to systematically evaluate the influence of various environmental factors on seedling stress within the context of a plant factory. Major stress-inducing conditions were investigated, including temperature fluctuations, light variations, nutrient deficiencies, and water supply irregularities. Six distinct varieties of seedlings were subjected to these stressors, and we aimed to precisely quantify the resulting stress symptoms.
The observed reduction in leaf area is a multifaceted response. As a consequence of stress-induced changes, smaller leaves are anticipated to maintain lower temperatures in light environments, effectively mitigating the risk of overheating [65]. This underscores the adaptive nature of plants in modulating their leaf morphology to cope with environmental challenges [66]. Moreover, this study supports earlier research indicating a direct correlation between growth rate and seedling leaf area, with individual leaf sizes influenced by daily temperature variations [67]. The study also aligns with previous research findings suggesting a correlation between decreasing leaf size and diminishing water availability [66]. This association underscores the intricate interplay between environmental factors and plant physiology, emphasizing the utility of leaf area as a measurable parameter for understanding and quantifying stress responses in diverse plant species. By employing techniques such as remote sensing or manual measurements, the assessment of leaf area variations provides valuable insights into the adaptive strategies employed by plants to contend with stress conditions.
When confronted with water scarcity, plants often demonstrate diminished growth marked by an overall reduction in plant height [68]. The stress induced by water scarcity triggers cell shrinkage, influencing cell elongation and consequently impacting the stature of the plant [69]. Insufficient availability of essential nutrients, particularly nitrogen, can lead to stunted growth and a decrease in overall plant height [70]. Extreme temperatures, whether excessively high or low, can exert substantial influence on plant height [71]. Elevated temperatures may prompt wilting and a decline in cell turgor pressure, thereby affecting the structural integrity of the plant, and extreme cold conditions can impede growth, resulting in a reduction in plant height [71]. Studies have shown that genotype and temperature strongly influence seedling growth, particularly in respect of height [72]. Elevated temperatures, coupled with water scarcity, can induce high-temperature stress, impacting seedling growth rates. Optimal seedling growth occurs at around 20 °C, with temperatures beyond this range detrimentally affecting seedling height [73]. Light stress significantly impacts seedling height, influencing processes like plant food production, stem length, leaf color, and flowering [74]. Strong light combined with soil moisture constraints restricts seedling upward growth, affecting overall growth and survival [75]. While higher light intensity generally supports better seedling growth, excessive light can induce stress, exemplified by tip burn in lettuce seedlings [76]. Both insufficient and excessive light can result in detrimental light stress, adversely affecting plant growth and productivity [74]. Observing changes in plant height serves as a valuable indicator of stress impacts, aiding in the comprehensive assessment of plant health under diverse environmental conditions.
Different studies have shown the relationships between canopy temperature, stress conditions, and other plant characteristics. Leaf size can regulate leaf temperature via the thickness of the leaf boundary layer, where heat transfer is slower relative to the more turbulent air beyond the leaf [66]. Plant nutrition is vital in alleviating abiotic stress, including heat stress, by activating mechanisms like increased photosynthetic activity and reduced transpiration rates, and causing increases in canopy temperature [77]. Thermal infrared remote sensing is a widely used and effective method for detecting vegetation stress, and using the canopy temperature to track water stress is considered reliable for monitoring plant water status [78]. However, retrieving canopy component temperatures involves thermal infrared remote sensing problems, and the relationships between leaf temperature and water levels are not clear [79]. The interconnected impact of temperature, nutrient, light, and water stress on the seedling canopy temperature can be assessed by monitoring it, providing insights into the levels of stress experienced by the seedlings. Moreover, the influence of temperature and water stress on seed germination and seedling growth highlights their pivotal role in determining optimal conditions for seedling development. While this study focused on six specific seedling varieties, the observed trends regarding temperature, light, water supply, and nutrient impacts on seedling responses are consistent with similar studies on different species, indicating potential generalizability within certain bounds. However, further research with a broader variety of plant species is needed to validate this predictability.
Environmental factors such as temperature, light, water, and nutrients directly impact plant growth and health. Sensor fusion plays a crucial role in monitoring and understanding these relationships. By collecting and combining data from various cameras (RGB, thermal, and depth), it was possible to gain a more complete picture of the environmental conditions that the seedlings were experiencing. This data, coupled with image processing techniques, allowed us to precisely measure plant responses like leaf area, height, and canopy temperature. Color differences, canopy temperature variation, and seedling size monitoring provided more detailed information about the seedling growth conditions. Analyzing these sensor-derived measurements reveals how specific environmental stressors influence plant physiology, giving us insights into plant adaptation strategies and helping optimize growing conditions in plant factories. In essence, sensor fusion provided the environmental context, while image processing provided detailed measurements of the seedlings responses. By combining these two approaches, the researchers were able to gain a deeper understanding of the relationships between environmental factors and seedling stress conditions.
While sensor fusion and image processing offer valuable insights into plant stress responses, limitations were observed. Light variations affect image quality and processing accuracy, while external radiation sources can interfere with thermal and depth cameras, introducing data errors. The precision of image processing techniques in feature extraction requires careful evaluation. This research involved image processing techniques to assess key physiological parameters of the seedlings, including leaf area, seedling height, and canopy temperature. Future research in precision plant monitoring for stress detection could refine and extend the current findings by implementing controlled environments to isolate specific stressors. Moreover, advanced image processing and deep learning methods integrating additional sensors and utilizing larger datasets would offer a multidimensional view of plant health. Finally, this could lead to real-time stress monitoring systems for optimized plant growth in commercial plant factories.
5. Conclusions
This study aimed to develop a seedling stress quantification model in six seedling varieties for early growth stages. Six varieties of seedlings, aged one week, were cultivated in controlled chambers with varying conditions: temperature (20, 25, and 30 °C), light intensity (50, 250, and 450 µmol m−2s−1), nutrients (1, 3, and 6 dS m−1), and water supply (1, 0.75, and 0.50 L/day). RGB, thermal, and depth camera sensors were used to capture canopy images from the top of the seedling beds automatically and daily for two weeks.
Stress quantification was performed with reference to major characteristics of the seedlings, such as leaf area, seedling height, and canopy temperature. The comprehensive visualization and quantification of stress effects on the six different seedlings revealed distinctive responses to various environmental stressors. The integration of color, size, and shape parameters provided slight differences, enabling us to establish a visual hierarchy. Pepper and pak choi seedlings exhibited high sensitivity to stress, while cucumber seedlings demonstrated a milder response, and lettuce and tomato seedlings displayed intermediate sensitivity. Pepper and tomato seedlings displayed a wide range of stress symptoms (%), at 13.00% to 83.33% and 2.96% to 70.01%, respectively, indicating considerable variability in their response to environmental stressors.
Sensor fusion and image processing provided valuable tools for plant stress analysis, but limitations like light variability and external radiation interference must be addressed and minimized. Future research should focus on controlled environments, advanced image processing techniques, integration of diverse sensors. This study on seedling stress quantification in seedling growth facilities through sensor fusion and image processing represents a significant step towards understanding and improving the efficiency of controlled environment agriculture. The integration of diverse data sources provides a more distinct and accurate assessment of seedling health, paving the way for informed decision making and sustainable agricultural practices.
Author Contributions
Conceptualization, S.I. and S.-O.C.; methodology, S.I. and S.-O.C.; software, S.I. and M.N.R.; validation, S.I., M.N.R., S.A., S., Y.J.C. and D.H.N.; formal analysis, S.A., S., Y.J.C. and D.H.N.; investigation, D.H.N. and S.-O.C.; resources, S.-O.C.; data curation, S.I., M.N.R., S.A. and S.; writing—original draft preparation, S.I.; writing—review and editing, M.N.R., D.H.N. and S.-O.C.; visualization, S.I., S.A., S. and Y.J.C.; supervision, S.-O.C.; project administration, S.-O.C.; funding acquisition, S.-O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Smart Farm Innovation Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Project No. 421035-04), Republic of Korea.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Comparison of leaf area using RGB image sensor of six seedling varieties with different stress conditions.
Table A1.
Comparison of leaf area using RGB image sensor of six seedling varieties with different stress conditions.
| Pepper seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 963.1 | 1477.0 | 2901.8 | 1003.3 | 4467.9 | 7488.1 | 1873.4 | 4702.0 | 8322.5 |
| Min | 602.2 | 861.2 | 1678.2 | 822.4 | 3082.7 | 5823.5 | 952.7 | 3182.8 | 6125.8 |
| Avg | 731.4 | 1048.5 | 2279.3 | 915.9 | 3607.6 | 6425.5 | 1226.6 | 3772.7 | 6997.3 |
| STD | 118.1 | 196.3 | 429.2 | 60.5 | 448.6 | 539.7 | 301.9 | 446.7 | 756.0 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 1083.2 | 2464.6 | 5539.8 | 1003.3 | 4467.9 | 7488.1 | 966.8 | 2633.1 | 6208.7 |
| Min | 617.6 | 1148.7 | 4463.3 | 822.4 | 3082.7 | 5823.5 | 676.4 | 1843.8 | 3993.5 |
| Avg | 846.5 | 1651.6 | 4859.1 | 915.9 | 3607.6 | 6425.5 | 770.6 | 2230.5 | 5242.5 |
| STD | 145.3 | 397.9 | 403.9 | 60.5 | 448.6 | 539.7 | 96.1 | 276.4 | 668.7 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 843.0 | 1970.9 | 4033.0 | 1003.3 | 4467.9 | 7488.1 | 1470.0 | 4888.0 | 12,138.0 |
| Min | 615.5 | 1486.6 | 2498.4 | 822.4 | 3082.7 | 5823.5 | 877.0 | 3009.0 | 7254.0 |
| Avg | 732.1 | 1811.5 | 3340.3 | 915.9 | 3607.6 | 6425.5 | 1183.6 | 3919.7 | 9512.7 |
| STD | 84.5 | 173.4 | 439.4 | 60.5 | 448.6 | 539.7 | 172.3 | 620.2 | 1701.2 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 1075.7 | 2050.3 | 5478.3 | 1003.3 | 4467.9 | 7488.1 | 946.9 | 2259.9 | 5081.2 |
| Min | 689.3 | 1665.9 | 4298.7 | 822.4 | 3082.7 | 5823.5 | 609.2 | 1512.2 | 3546.9 |
| Avg | 868.7 | 1855.3 | 5042.2 | 915.9 | 3607.6 | 6425.5 | 700.3 | 1906.3 | 4143.4 |
| STD | 118.5 | 127.4 | 384.3 | 60.5 | 448.6 | 539.7 | 104.9 | 268.6 | 464.0 |
| Cucumber seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 6905.9 | 12,921.8 | 12,362.5 | 6842.3 | 15,435.3 | 18,722.0 | 6842.3 | 13,469.5 | 16,853.2 |
| Min | 4322.2 | 2688.3 | 5618.2 | 4290.0 | 9263.5 | 9786.6 | 5236.6 | 10,456.4 | 11,854.9 |
| Avg | 5641.4 | 7560.8 | 7999.5 | 5609.6 | 11,944.6 | 14,173.0 | 5953.2 | 12,115.3 | 14,447.9 |
| STD | 794.9 | 4011.0 | 2148.5 | 790.6 | 1936.1 | 2736.8 | 627.2 | 1020.8 | 1610.4 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 6342.1 | 14,935.1 | 16,874.5 | 6842.3 | 15,435.3 | 18,722.0 | 6539.4 | 15,133.4 | 18,427.9 |
| Min | 4736.4 | 9452.3 | 9286.4 | 4290.0 | 9263.5 | 9786.6 | 5163.5 | 8960.6 | 11,362.5 |
| Avg | 5407.4 | 11,542.8 | 13,387.9 | 5609.6 | 11,944.6 | 14,173.0 | 5737.0 | 11,760.5 | 14,129.3 |
| STD | 697.7 | 1810.8 | 2466.1 | 790.6 | 1936.1 | 2736.8 | 494.9 | 1834.2 | 2374.8 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 6325.6 | 12,962.3 | 16,727.8 | 6842.3 | 15,435.3 | 18,722.0 | 6312.2 | 15,194.7 | 18,847.8 |
| Min | 4569.3 | 6060.4 | 9613.7 | 4290.0 | 9263.5 | 9786.6 | 5095.1 | 10,951.0 | 12,615.3 |
| Avg | 5437.1 | 10,079.2 | 12,859.0 | 5609.6 | 11,944.6 | 14,173.0 | 5714.1 | 13,107.5 | 15,290.8 |
| STD | 540.3 | 2180.1 | 2657.1 | 790.6 | 1936.1 | 2736.8 | 385.6 | 1783.3 | 1720.0 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 6442.9 | 13,648.3 | 18,341.6 | 6842.3 | 15,435.3 | 18,722.0 | 6041.6 | 12,162.8 | 12,779.0 |
| Min | 4836.2 | 9562.1 | 9785.6 | 4290.0 | 9263.5 | 9786.6 | 3896.3 | 8462.8 | 8986.9 |
| Avg | 5458.3 | 11,478.0 | 13,826.9 | 5609.6 | 11,944.6 | 14,173.0 | 4867.0 | 10,518.3 | 11,172.3 |
| STD | 583.2 | 1431.9 | 2663.0 | 790.6 | 1936.1 | 2736.8 | 701.3 | 1323.3 | 1157.3 |
| Tomato seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 1506.0 | 4062.0 | 9653.0 | 2980.0 | 10,057.0 | 29,595.0 | 7785.0 | 21,365.0 | 42,369.0 |
| Min | 733.0 | 2586.0 | 7062.0 | 1610.0 | 4969.0 | 25,147.0 | 5628.0 | 12,635.0 | 28,963.0 |
| Avg | 1035.0 | 3220.6 | 8445.6 | 2245.3 | 7701.6 | 28,183.4 | 6836.1 | 17,392.1 | 37,076.1 |
| STD | 245.2 | 499.6 | 922.9 | 422.4 | 1742.5 | 1575.6 | 673.0 | 3033.6 | 4493.5 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 2980.0 | 10,057.0 | 29,595.0 | 2980.0 | 10,057.0 | 29,595.0 | 2980.0 | 10,057.0 | 30,159.0 |
| Min | 1610.0 | 5863.0 | 28,436.0 | 1610.0 | 4969.0 | 25,147.0 | 1843.0 | 4969.0 | 27,456.0 |
| Avg | 2312.0 | 8102.9 | 29,104.7 | 2245.3 | 7701.6 | 28,183.4 | 2334.7 | 8012.0 | 29,032.6 |
| STD | 440.3 | 1308.0 | 441.0 | 422.4 | 1742.5 | 1575.6 | 335.8 | 1536.6 | 827.1 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 2502.0 | 9746.0 | 28,763.0 | 2980.0 | 10,057.0 | 29,595.0 | 3265.0 | 14,365.0 | 38,752.0 |
| Min | 1610.0 | 4969.0 | 25,147.0 | 1610.0 | 4969.0 | 25,147.0 | 1610.0 | 7895.0 | 29,568.0 |
| Avg | 2170.4 | 7546.6 | 26,910.4 | 2245.3 | 7701.6 | 28,183.4 | 2567.3 | 11,633.4 | 33,582.7 |
| STD | 319.4 | 1593.6 | 1334.0 | 422.4 | 1742.5 | 1575.6 | 526.6 | 2052.6 | 3082.0 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 2258.0 | 8742.0 | 29,595.0 | 2980.0 | 10,057.0 | 29,595.0 | 2245.0 | 8452.0 | 29,595.0 |
| Min | 1610.0 | 4969.0 | 24,695.0 | 1610.0 | 4969.0 | 25,147.0 | 1610.0 | 4969.0 | 22,459.0 |
| Avg | 2054.7 | 7243.4 | 27,003.1 | 2245.3 | 7701.6 | 28,183.4 | 1924.9 | 6823.6 | 25,682.9 |
| STD | 221.5 | 1315.2 | 1779.7 | 422.4 | 1742.5 | 1575.6 | 205.1 | 1114.6 | 2359.5 |
| Watermelon seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 5631.0 | 5305.0 | 7476.0 | 6023.0 | 12,728.0 | 26,421.0 | 6016.0 | 12,701.0 | 26,389.0 |
| Min | 693.0 | 1994.0 | 4137.0 | 4216.0 | 4983.0 | 7190.0 | 4409.0 | 5496.0 | 9038.0 |
| Avg | 2786.6 | 3995.7 | 5126.1 | 4956.7 | 7000.4 | 13,730.3 | 5191.6 | 7355.7 | 15,333.9 |
| STD | 2008.5 | 1231.4 | 1050.9 | 697.0 | 2448.8 | 6268.0 | 547.4 | 2307.9 | 5718.7 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 6323.8 | 10,456.0 | 19,442.8 | 6023.0 | 12,728.0 | 26,421.0 | 6444.4 | 13,149.4 | 19,563.4 |
| Min | 4516.8 | 5283.8 | 9745.0 | 4216.0 | 4983.0 | 7190.0 | 4637.4 | 5404.4 | 7611.4 |
| Avg | 5257.5 | 6933.7 | 12,728.2 | 4956.7 | 7000.4 | 13,730.3 | 5377.9 | 7421.8 | 12,636.3 |
| STD | 697.0 | 1611.5 | 2966.9 | 697.0 | 2448.8 | 6268.0 | 697.1 | 2448.8 | 3821.5 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 5727.0 | 8965.0 | 12,683.0 | 6023.0 | 12,728.0 | 26,421.0 | 6542.0 | 13,147.0 | 12,986.0 |
| Min | 3865.2 | 5623.0 | 7185.0 | 4216.0 | 4983.0 | 7190.0 | 3452.0 | 4561.0 | 9563.0 |
| Avg | 4700.2 | 6676.9 | 10,526.7 | 4956.7 | 7000.4 | 13,730.3 | 5237.1 | 9097.1 | 11,102.6 |
| STD | 664.0 | 1094.9 | 1883.6 | 697.0 | 2448.8 | 6268.0 | 1007.4 | 2908.0 | 1107.0 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 5499.4 | 11,741.0 | 15,236.0 | 6023.0 | 12,728.0 | 26,421.0 | 5277.8 | 11,982.8 | 12,543.0 |
| Min | 3692.4 | 4459.4 | 6666.4 | 4216.0 | 4983.0 | 7190.0 | 3470.8 | 4237.8 | 6544.8 |
| Avg | 4484.0 | 6366.4 | 11,099.0 | 4956.7 | 7000.4 | 13,730.3 | 4366.7 | 6155.9 | 10,018.3 |
| STD | 668.7 | 2290.4 | 2913.9 | 697.0 | 2448.8 | 6268.0 | 642.2 | 2466.0 | 2133.7 |
| Lettuce seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 275.0 | 592.0 | 1025.0 | 412.0 | 2191.0 | 2469.0 | 475.3 | 2187.0 | 3264.0 |
| Min | 102.0 | 145.0 | 343.0 | 256.0 | 1045.0 | 1986.0 | 246.0 | 1037.0 | 1863.0 |
| Avg | 195.9 | 365.7 | 573.6 | 314.4 | 1371.6 | 2259.7 | 351.4 | 1507.0 | 2397.4 |
| STD | 54.7 | 156.8 | 248.6 | 55.5 | 361.7 | 161.8 | 69.2 | 436.0 | 517.0 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 414.0 | 2193.0 | 2946.0 | 412.0 | 2191.0 | 2469.0 | 414.3 | 1963.0 | 2947.0 |
| Min | 264.0 | 1047.0 | 2115.0 | 256.0 | 1045.0 | 1986.0 | 270.3 | 1164.3 | 2209.3 |
| Avg | 330.8 | 1493.4 | 2398.6 | 314.4 | 1371.6 | 2259.7 | 340.7 | 1519.0 | 2419.9 |
| STD | 51.4 | 449.0 | 252.3 | 55.5 | 361.7 | 161.8 | 44.4 | 235.8 | 233.5 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 376.5 | 1652.3 | 2428.8 | 412.0 | 2191.0 | 2469.0 | 418.4 | 2197.4 | 2654.0 |
| Min | 252.8 | 1041.8 | 1982.8 | 256.0 | 1045.0 | 1986.0 | 274.4 | 1051.4 | 2214.4 |
| Avg | 305.1 | 1291.8 | 2167.0 | 314.4 | 1371.6 | 2259.7 | 338.7 | 1440.9 | 2380.0 |
| STD | 43.9 | 201.5 | 134.6 | 55.5 | 361.7 | 161.8 | 47.1 | 382.7 | 144.7 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 405.0 | 1845.0 | 2462.0 | 412.0 | 2191.0 | 2469.0 | 370.1 | 1796.0 | 2464.1 |
| Min | 255.0 | 1038.0 | 1846.0 | 256.0 | 1045.0 | 1986.0 | 251.1 | 1040.1 | 1879.0 |
| Avg | 308.7 | 1316.2 | 2180.0 | 314.4 | 1371.6 | 2259.7 | 301.1 | 1304.5 | 2155.8 |
| STD | 46.1 | 256.0 | 207.4 | 55.5 | 361.7 | 161.8 | 43.1 | 236.5 | 176.3 |
| Pak choi seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 126.0 | 135.0 | 312.0 | 156.0 | 581.0 | 1638.0 | 201.0 | 522.0 | 1554.0 |
| Min | 86.0 | 106.0 | 163.0 | 89.0 | 306.0 | 664.0 | 68.0 | 351.0 | 1232.0 |
| Avg | 106.1 | 120.1 | 242.9 | 122.9 | 415.3 | 1331.6 | 143.7 | 425.3 | 1404.1 |
| STD | 13.1 | 8.7 | 48.8 | 27.2 | 85.6 | 342.3 | 45.4 | 49.1 | 104.3 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 159.2 | 489.0 | 1641.2 | 156.0 | 581.0 | 1638.0 | 159.2 | 489.0 | 1641.2 |
| Min | 100.2 | 309.2 | 1123.0 | 89.0 | 306.0 | 664.0 | 105.0 | 352.2 | 1189.2 |
| Avg | 123.2 | 423.9 | 1388.6 | 122.9 | 415.3 | 1331.6 | 127.5 | 438.7 | 1445.3 |
| STD | 22.6 | 62.4 | 196.5 | 27.2 | 85.6 | 342.3 | 19.8 | 42.6 | 166.7 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 159.2 | 484.0 | 1637.2 | 156.0 | 581.0 | 1638.0 | 155.0 | 489.0 | 1641.2 |
| Min | 92.2 | 309.2 | 667.2 | 89.0 | 306.0 | 664.0 | 101.5 | 326.0 | 1086.2 |
| Avg | 120.9 | 408.7 | 1307.8 | 122.9 | 415.3 | 1331.6 | 127.2 | 432.4 | 1420.2 |
| STD | 23.6 | 65.8 | 324.0 | 27.2 | 85.6 | 342.3 | 22.6 | 47.3 | 216.5 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 151.3 | 576.3 | 1633.3 | 156.0 | 581.0 | 1638.0 | 151.3 | 479.0 | 1633.3 |
| Min | 84.3 | 330.0 | 659.3 | 89.0 | 306.0 | 664.0 | 84.3 | 301.3 | 640.0 |
| Avg | 119.3 | 406.8 | 1259.0 | 122.9 | 415.3 | 1331.6 | 115.3 | 396.7 | 1277.0 |
| STD | 27.1 | 79.7 | 322.1 | 27.2 | 85.6 | 342.3 | 24.4 | 62.2 | 324.7 |
Number of sample, n = 15; Unit = pixels.

Table A2.
Comparison of plant height using depth image sensor of six seedling varieties with different stress conditions.
Table A2.
Comparison of plant height using depth image sensor of six seedling varieties with different stress conditions.
| Pepper seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 3.4 | 4.1 | 4.1 | 3.1 | 4.2 | 5.9 | 3.3 | 4.2 | 8.3 |
| Min | 3.2 | 3.2 | 3.6 | 2.3 | 3.4 | 2.6 | 2.7 | 3.5 | 6.0 |
| Avg | 3.3 | 3.7 | 3.9 | 2.7 | 3.9 | 5.1 | 3.0 | 3.9 | 7.2 |
| STD | 0.1 | 0.3 | 0.2 | 0.2 | 0.2 | 1.1 | 0.2 | 0.2 | 0.7 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 2.7 | 3.6 | 6.2 | 3.1 | 4.2 | 5.9 | 3.2 | 3.9 | 6.1 |
| Min | 1.8 | 2.8 | 5.2 | 2.3 | 3.4 | 2.6 | 2.6 | 2.9 | 5.1 |
| Avg | 2.4 | 3.2 | 5.6 | 2.7 | 3.9 | 5.1 | 2.9 | 3.2 | 5.5 |
| STD | 0.3 | 0.3 | 0.4 | 0.2 | 0.2 | 1.1 | 0.2 | 0.4 | 0.3 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 2.8 | 3.8 | 4.7 | 3.1 | 4.2 | 5.9 | 4.9 | 6.1 | 11.6 |
| Min | 2.3 | 2.9 | 4.1 | 2.3 | 3.4 | 2.6 | 2.7 | 5.4 | 8.4 |
| Avg | 2.6 | 3.5 | 4.3 | 2.7 | 3.9 | 5.1 | 3.7 | 5.7 | 10.1 |
| STD | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 1.1 | 0.6 | 0.2 | 1.0 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 2.8 | 3.6 | 5.2 | 3.1 | 4.2 | 5.9 | 3.2 | 3.8 | 5.3 |
| Min | 1.9 | 2.8 | 4.6 | 2.3 | 3.4 | 2.6 | 2.5 | 2.9 | 4.2 |
| Avg | 2.5 | 3.1 | 4.9 | 2.7 | 3.9 | 5.1 | 2.9 | 3.3 | 4.8 |
| STD | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 1.1 | 0.2 | 0.3 | 0.4 |
| Cucumber seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 3.5 | 3.8 | 4.1 | 4.0 | 4.3 | 4.3 | 3.6 | 4.5 | 4.4 |
| Min | 2.6 | 3.0 | 2.6 | 2.6 | 3.0 | 3.5 | 3.2 | 3.4 | 3.5 |
| Avg | 3.1 | 3.3 | 3.3 | 3.4 | 3.7 | 3.9 | 3.4 | 3.8 | 4.0 |
| STD | 0.3 | 0.2 | 0.4 | 0.4 | 0.5 | 0.3 | 0.2 | 0.3 | 0.3 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 3.5 | 4.4 | 4.1 | 4.0 | 4.3 | 4.3 | 4.1 | 3.8 | 4.4 |
| Min | 2.8 | 2.8 | 2.9 | 2.6 | 3.0 | 3.5 | 2.3 | 3.1 | 3.2 |
| Avg | 3.1 | 3.4 | 3.6 | 3.4 | 3.7 | 3.9 | 3.2 | 3.4 | 3.8 |
| STD | 0.2 | 0.5 | 0.4 | 0.4 | 0.5 | 0.3 | 0.5 | 0.3 | 0.4 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 3.6 | 4.2 | 3.9 | 4.0 | 4.3 | 4.3 | 4.6 | 5.5 | 5.4 |
| Min | 2.4 | 2.8 | 1.9 | 2.6 | 3.0 | 3.5 | 3.0 | 3.6 | 3.6 |
| Avg | 3.1 | 3.4 | 2.9 | 3.4 | 3.7 | 3.9 | 3.8 | 4.5 | 4.7 |
| STD | 0.4 | 0.4 | 0.6 | 0.4 | 0.5 | 0.3 | 0.5 | 0.5 | 0.6 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 4.1 | 4.7 | 4.0 | 4.0 | 4.3 | 4.3 | 3.3 | 3.5 | 3.6 |
| Min | 2.3 | 2.9 | 2.6 | 2.6 | 3.0 | 3.5 | 2.2 | 3.1 | 2.9 |
| Avg | 3.2 | 3.4 | 3.3 | 3.4 | 3.7 | 3.9 | 2.8 | 3.2 | 3.3 |
| STD | 0.5 | 0.6 | 0.4 | 0.4 | 0.5 | 0.3 | 0.4 | 0.1 | 0.2 |
| Tomato seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 5.3 | 7.5 | 7.4 | 8.6 | 16.8 | 33.4 | 6.4 | 19.3 | 31.4 |
| Min | 2.9 | 5.8 | 5.5 | 4.5 | 13.5 | 18.4 | 5.3 | 13.1 | 21.8 |
| Avg | 4.0 | 6.4 | 6.7 | 5.7 | 15.0 | 25.4 | 5.9 | 16.3 | 27.0 |
| STD | 0.8 | 0.5 | 0.6 | 1.3 | 1.0 | 4.8 | 0.4 | 2.1 | 3.3 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 7.1 | 15.8 | 29.3 | 8.6 | 16.8 | 33.4 | 5.4 | 15.6 | 18.7 |
| Min | 5.4 | 12.8 | 22.4 | 4.5 | 13.5 | 18.4 | 4.1 | 9.0 | 12.6 |
| Avg | 6.1 | 14.5 | 25.7 | 5.7 | 15.0 | 25.4 | 4.9 | 13.0 | 15.3 |
| STD | 0.7 | 1.1 | 2.2 | 1.3 | 1.0 | 4.8 | 0.4 | 2.5 | 2.2 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 6.5 | 11.7 | 23.2 | 8.6 | 16.8 | 33.4 | 7.2 | 22.2 | 33.6 |
| Min | 4.2 | 1.3 | 14.5 | 4.5 | 13.5 | 18.4 | 4.1 | 14.8 | 29.0 |
| Avg | 5.2 | 8.6 | 18.1 | 5.7 | 15.0 | 25.4 | 6.3 | 16.5 | 31.8 |
| STD | 0.8 | 3.3 | 3.3 | 1.3 | 1.0 | 4.8 | 1.0 | 2.4 | 1.8 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 7.3 | 15.6 | 29.1 | 8.6 | 16.8 | 33.4 | 5.6 | 15.4 | 18.2 |
| Min | 5.6 | 12.6 | 22.6 | 4.5 | 13.5 | 18.4 | 4.3 | 8.8 | 10.4 |
| Avg | 6.3 | 14.4 | 25.8 | 5.7 | 15.0 | 25.4 | 5.1 | 12.8 | 14.4 |
| STD | 0.7 | 1.0 | 2.1 | 1.3 | 1.0 | 4.8 | 0.4 | 2.4 | 2.2 |
| Watermelon seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 1.7 | 2.2 | 2.1 | 2.3 | 2.4 | 2.6 | 2.1 | 2.3 | 2.5 |
| Min | 1.4 | 1.2 | 1.5 | 1.2 | 1.4 | 1.7 | 1.3 | 1.6 | 2.5 |
| Avg | 1.5 | 1.7 | 1.8 | 1.6 | 1.9 | 2.1 | 1.8 | 2.0 | 2.4 |
| STD | 0.1 | 0.4 | 0.2 | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 | 2.4 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 1.8 | 2.4 | 2.4 | 2.3 | 2.4 | 2.6 | 2.2 | 2.2 | 2.6 |
| Min | 1.5 | 1.8 | 1.8 | 1.2 | 1.4 | 1.7 | 1.5 | 1.8 | 1.6 |
| Avg | 1.7 | 2.0 | 2.2 | 1.6 | 1.9 | 2.1 | 1.8 | 2.0 | 2.1 |
| STD | 0.1 | 0.2 | 0.2 | 0.4 | 0.3 | 0.3 | 0.2 | 0.1 | 0.3 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 1.9 | 2.1 | 2.1 | 2.3 | 2.4 | 2.6 | 2.2 | 2.4 | 2.7 |
| Min | 1.4 | 1.7 | 1.8 | 1.2 | 1.4 | 1.7 | 1.6 | 1.8 | 2.1 |
| Avg | 1.6 | 1.8 | 2.0 | 1.6 | 1.9 | 2.1 | 1.9 | 2.1 | 2.3 |
| STD | 0.2 | 0.1 | 0.1 | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 1.6 | 2.0 | 2.2 | 2.3 | 2.4 | 2.6 | 2.0 | 2.2 | 2.4 |
| Min | 1.3 | 1.6 | 1.6 | 1.2 | 1.4 | 1.7 | 1.3 | 1.6 | 1.7 |
| Avg | 1.5 | 1.8 | 2.0 | 1.6 | 1.9 | 2.1 | 1.6 | 1.8 | 1.9 |
| STD | 0.1 | 0.1 | 0.2 | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 |
| Lettuce seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 1.4 | 1.9 | 2.8 | 2.3 | 3.2 | 5.9 | 3.1 | 5.1 | 6.8 |
| Min | 1.0 | 1.4 | 1.7 | 1.7 | 1.9 | 4.3 | 2.3 | 3.6 | 5.2 |
| Avg | 1.2 | 1.7 | 2.0 | 2.0 | 2.6 | 5.2 | 2.7 | 4.1 | 6.0 |
| STD | 0.1 | 0.2 | 0.3 | 0.2 | 0.5 | 0.6 | 0.2 | 0.5 | 0.5 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 2.6 | 3.4 | 6.0 | 2.3 | 3.2 | 5.9 | 2.6 | 3.1 | 5.4 |
| Min | 1.5 | 2.3 | 4.2 | 1.7 | 1.9 | 4.3 | 1.8 | 2.1 | 4.6 |
| Avg | 2.0 | 2.7 | 5.1 | 2.0 | 2.6 | 5.2 | 2.1 | 2.5 | 4.9 |
| STD | 0.3 | 0.4 | 0.5 | 0.2 | 0.5 | 0.6 | 0.3 | 0.3 | 0.3 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 2.4 | 3.6 | 6.0 | 2.3 | 3.2 | 5.9 | 4.2 | 6.0 | 8.1 |
| Min | 1.5 | 1.4 | 4.2 | 1.7 | 1.9 | 4.3 | 2.6 | 4.7 | 6.1 |
| Avg | 1.9 | 2.5 | 4.9 | 2.0 | 2.6 | 5.2 | 3.3 | 5.3 | 7.1 |
| STD | 0.3 | 0.6 | 0.7 | 0.2 | 0.5 | 0.6 | 0.5 | 0.5 | 0.7 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 2.6 | 2.8 | 6.0 | 2.3 | 3.2 | 5.9 | 2.4 | 2.6 | 5.1 |
| Min | 1.5 | 1.8 | 4.1 | 1.7 | 1.9 | 4.3 | 1.4 | 1.8 | 4.2 |
| Avg | 1.9 | 2.5 | 5.1 | 2.0 | 2.6 | 5.2 | 1.9 | 2.3 | 4.6 |
| STD | 0.3 | 0.3 | 0.5 | 0.2 | 0.5 | 0.6 | 0.3 | 0.3 | 0.3 |
| Pak choi seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 1.6 | 1.8 | 2.1 | 2.4 | 4.5 | 9.1 | 2.0 | 3.9 | 10.7 |
| Min | 1.3 | 1.5 | 1.4 | 1.1 | 1.5 | 7.0 | 1.5 | 2.6 | 7.1 |
| Avg | 1.5 | 1.6 | 1.8 | 1.5 | 3.0 | 7.9 | 1.7 | 3.2 | 8.6 |
| STD | 0.1 | 0.1 | 0.2 | 0.4 | 1.0 | 0.7 | 0.2 | 0.5 | 1.3 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 2.0 | 4.2 | 9.2 | 2.4 | 4.5 | 9.1 | 2.2 | 3.7 | 8.2 |
| Min | 1.5 | 2.5 | 7.3 | 1.1 | 1.5 | 7.0 | 1.4 | 2.5 | 6.5 |
| Avg | 1.8 | 3.1 | 7.9 | 1.5 | 3.0 | 7.9 | 1.8 | 3.1 | 7.6 |
| STD | 0.2 | 0.6 | 0.6 | 0.4 | 1.0 | 0.7 | 0.3 | 0.5 | 0.5 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 1.7 | 3.4 | 8.7 | 2.4 | 4.5 | 9.1 | 3.2 | 4.6 | 10.5 |
| Min | 1.2 | 2.4 | 6.7 | 1.1 | 1.5 | 7.0 | 2.3 | 2.6 | 8.8 |
| Avg | 1.5 | 2.9 | 7.8 | 1.5 | 3.0 | 7.9 | 2.6 | 4.0 | 9.7 |
| STD | 0.2 | 0.3 | 0.7 | 0.4 | 1.0 | 0.7 | 0.3 | 0.6 | 0.6 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 1.6 | 3.4 | 8.3 | 2.4 | 4.5 | 9.1 | 1.7 | 2.7 | 7.3 |
| Min | 1.4 | 2.3 | 7.1 | 1.1 | 1.5 | 7.0 | 1.3 | 2.1 | 6.3 |
| Avg | 1.5 | 2.9 | 7.6 | 1.5 | 3.0 | 7.9 | 1.4 | 2.4 | 6.8 |
| STD | 0.1 | 0.4 | 0.4 | 0.4 | 1.0 | 0.7 | 0.1 | 0.2 | 0.3 |
Number of sample, n = 15; Unit = cm.

Table A3.
Comparison of leaf canopy temperature using thermal sensor of six seedling varieties with different stress conditions.
Table A3.
Comparison of leaf canopy temperature using thermal sensor of six seedling varieties with different stress conditions.
| Pepper seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 23.0 | 23.9 | 25.0 | 25.0 | 29.0 | 33.0 | 27.7 | 31.9 | 33.9 |
| Min | 22.3 | 23.2 | 24.8 | 24.4 | 28.0 | 31.9 | 26.9 | 30.6 | 32.8 |
| Avg | 22.7 | 23.6 | 24.9 | 24.6 | 28.4 | 32.5 | 27.3 | 31.2 | 33.4 |
| STD | 0.2 | 0.3 | 0.1 | 0.2 | 0.3 | 0.4 | 0.3 | 0.5 | 0.4 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 23.7 | 27.6 | 31.8 | 25.0 | 29.0 | 33.0 | 27.7 | 27.7 | 31.0 |
| Min | 22.9 | 26.8 | 30.7 | 24.4 | 28.0 | 31.9 | 26.9 | 26.8 | 29.9 |
| Avg | 23.2 | 27.2 | 31.3 | 24.6 | 28.4 | 32.5 | 27.3 | 27.2 | 30.5 |
| STD | 0.3 | 0.3 | 0.4 | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 23.8 | 30.0 | 32.0 | 25.0 | 29.0 | 33.0 | 30.0 | 32.0 | 34.0 |
| Min | 22.2 | 29.3 | 31.3 | 24.4 | 28.0 | 31.9 | 28.9 | 31.1 | 33.7 |
| Avg | 23.3 | 29.7 | 31.7 | 24.6 | 28.4 | 32.5 | 29.4 | 31.4 | 33.8 |
| STD | 0.5 | 0.3 | 0.2 | 0.2 | 0.3 | 0.4 | 0.4 | 0.3 | 0.1 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 23.8 | 29.1 | 24.9 | 25.0 | 29.0 | 33.0 | 29.8 | 31.8 | 34.1 |
| Min | 23.0 | 28.0 | 24.8 | 24.4 | 28.0 | 31.9 | 29.0 | 31.2 | 33.6 |
| Avg | 23.3 | 28.5 | 24.8 | 24.6 | 28.4 | 32.5 | 29.3 | 31.4 | 33.8 |
| STD | 0.3 | 0.4 | 0.1 | 0.2 | 0.3 | 0.4 | 0.3 | 0.2 | 0.2 |
| Cucumber seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 23.8 | 24.5 | 25.5 | 30.5 | 33.2 | 34.4 | 29.8 | 34.0 | 36.5 |
| Min | 23.3 | 23.7 | 25.3 | 28.7 | 31.0 | 31.9 | 28.7 | 30.9 | 34.2 |
| Avg | 23.5 | 24.2 | 25.4 | 29.5 | 32.0 | 33.3 | 29.4 | 32.2 | 34.9 |
| STD | 0.2 | 0.3 | 0.1 | 0.6 | 0.8 | 0.9 | 0.3 | 1.0 | 0.7 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 30.6 | 30.9 | 31.9 | 30.5 | 33.2 | 34.4 | 31.2 | 32.5 | 33.2 |
| Min | 28.2 | 28.6 | 30.6 | 28.7 | 31.0 | 31.9 | 28.8 | 30.5 | 30.4 |
| Avg | 29.3 | 29.6 | 31.2 | 29.5 | 32.0 | 33.3 | 30.1 | 31.2 | 32.3 |
| STD | 0.9 | 0.8 | 0.5 | 0.6 | 0.8 | 0.9 | 0.8 | 0.7 | 0.9 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 28.3 | 30.6 | 31.9 | 30.5 | 33.2 | 34.4 | 31.2 | 33.0 | 35.2 |
| Min | 26.8 | 28.6 | 29.5 | 28.7 | 31.0 | 31.9 | 29.4 | 31.5 | 34.0 |
| Avg | 27.8 | 29.8 | 30.7 | 29.5 | 32.0 | 33.3 | 30.3 | 32.4 | 34.8 |
| STD | 0.5 | 0.7 | 0.7 | 0.6 | 0.8 | 0.9 | 0.6 | 0.6 | 0.4 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 30.5 | 30.8 | 32.2 | 30.5 | 33.2 | 34.4 | 31.2 | 33.1 | 35.5 |
| Min | 27.2 | 28.1 | 30.0 | 28.7 | 31.0 | 31.9 | 30.4 | 32.5 | 34.9 |
| Avg | 28.9 | 29.2 | 31.1 | 29.5 | 32.0 | 33.3 | 30.7 | 32.8 | 35.1 |
| STD | 1.2 | 1.0 | 0.8 | 0.6 | 0.8 | 0.9 | 0.3 | 0.2 | 0.2 |
| Tomato seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 22.8 | 23.7 | 25.2 | 24.5 | 30.1 | 31.5 | 27.5 | 31.8 | 34.0 |
| Min | 22.0 | 23.1 | 24.2 | 22.8 | 28.5 | 29.9 | 26.6 | 30.3 | 32.3 |
| Avg | 22.5 | 23.4 | 24.6 | 23.7 | 29.2 | 30.8 | 27.1 | 31.1 | 33.2 |
| STD | 0.3 | 0.2 | 0.3 | 0.5 | 0.6 | 0.5 | 0.3 | 0.6 | 0.5 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 23.7 | 27.5 | 31.7 | 24.5 | 30.1 | 31.5 | 27.6 | 27.6 | 30.9 |
| Min | 22.7 | 26.6 | 30.5 | 22.8 | 28.5 | 29.9 | 26.9 | 26.7 | 29.7 |
| Avg | 23.2 | 27.1 | 31.1 | 23.7 | 29.2 | 30.8 | 27.2 | 27.1 | 30.4 |
| STD | 0.3 | 0.3 | 0.4 | 0.5 | 0.6 | 0.5 | 0.3 | 0.3 | 0.4 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 23.7 | 30.1 | 32.1 | 24.5 | 30.1 | 31.5 | 29.9 | 32.1 | 33.9 |
| Min | 22.2 | 29.4 | 31.4 | 22.8 | 28.5 | 29.9 | 29.0 | 31.2 | 33.6 |
| Avg | 23.2 | 29.8 | 31.8 | 23.7 | 29.2 | 30.8 | 29.4 | 31.6 | 33.7 |
| STD | 0.5 | 0.3 | 0.2 | 0.5 | 0.6 | 0.5 | 0.4 | 0.3 | 0.1 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 23.7 | 29.0 | 24.8 | 24.5 | 30.1 | 31.5 | 29.7 | 31.9 | 34.0 |
| Min | 22.9 | 27.9 | 24.7 | 22.8 | 28.5 | 29.9 | 28.9 | 31.3 | 33.5 |
| Avg | 23.3 | 28.4 | 24.7 | 23.7 | 29.2 | 30.8 | 29.3 | 31.6 | 33.7 |
| STD | 0.3 | 0.4 | 0.1 | 0.5 | 0.6 | 0.5 | 0.3 | 0.2 | 0.2 |
| Watermelon seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 26.0 | 27.3 | 29.3 | 26.3 | 29.4 | 32.6 | 27.3 | 30.9 | 33.7 |
| Min | 25.2 | 24.6 | 27.5 | 24.8 | 27.3 | 30.5 | 25.9 | 28.9 | 30.6 |
| Avg | 25.6 | 26.1 | 28.4 | 25.7 | 28.2 | 31.6 | 26.7 | 30.2 | 32.3 |
| STD | 0.3 | 0.9 | 0.6 | 0.6 | 0.8 | 0.7 | 0.5 | 0.7 | 0.9 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 26.4 | 28.1 | 32.0 | 26.3 | 29.4 | 32.6 | 28.0 | 28.0 | 31.3 |
| Min | 24.6 | 27.3 | 30.8 | 24.8 | 27.3 | 30.5 | 27.2 | 27.1 | 30.2 |
| Avg | 25.3 | 27.8 | 31.4 | 25.7 | 28.2 | 31.6 | 27.6 | 27.5 | 30.8 |
| STD | 0.5 | 0.3 | 0.4 | 0.6 | 0.8 | 0.7 | 0.3 | 0.3 | 0.3 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 25.0 | 28.6 | 31.7 | 26.3 | 29.4 | 32.6 | 30.5 | 32.5 | 34.6 |
| Min | 23.9 | 27.4 | 30.0 | 24.8 | 27.3 | 30.5 | 29.4 | 31.7 | 34.2 |
| Avg | 24.5 | 28.0 | 30.7 | 25.7 | 28.2 | 31.6 | 29.9 | 32.0 | 34.4 |
| STD | 0.4 | 0.5 | 0.5 | 0.6 | 0.8 | 0.7 | 0.4 | 0.3 | 0.1 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 26.9 | 28.6 | 30.6 | 26.3 | 29.4 | 32.6 | 28.9 | 30.0 | 32.3 |
| Min | 25.1 | 28.5 | 28.5 | 24.8 | 27.3 | 30.5 | 27.5 | 29.4 | 31.8 |
| Avg | 26.1 | 28.6 | 29.7 | 25.7 | 28.2 | 31.6 | 28.1 | 29.6 | 32.0 |
| STD | 0.7 | 0.1 | 0.7 | 0.6 | 0.8 | 0.7 | 0.4 | 0.2 | 0.2 |
| Lettuce seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 25.3 | 26.2 | 27.3 | 26.8 | 31.5 | 35.6 | 27.8 | 31.8 | 33.7 |
| Min | 24.6 | 25.5 | 27.1 | 24.9 | 28.0 | 32.9 | 26.6 | 30.1 | 32.3 |
| Avg | 25.0 | 25.9 | 27.2 | 26.0 | 30.2 | 34.0 | 27.3 | 31.1 | 33.0 |
| STD | 0.2 | 0.3 | 0.1 | 0.6 | 1.3 | 0.9 | 0.4 | 0.6 | 0.5 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 28.6 | 29.8 | 31.8 | 26.8 | 31.5 | 35.6 | 28.9 | 30.8 | 32.8 |
| Min | 22.9 | 27.2 | 30.6 | 24.9 | 28.0 | 32.9 | 27.0 | 26.9 | 30.4 |
| Avg | 26.5 | 28.7 | 31.0 | 26.0 | 30.2 | 34.0 | 27.8 | 29.1 | 31.9 |
| STD | 1.8 | 1.0 | 0.4 | 0.6 | 1.3 | 0.9 | 0.6 | 1.4 | 0.8 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 25.2 | 31.4 | 33.4 | 26.8 | 31.5 | 35.6 | 31.6 | 33.8 | 36.0 |
| Min | 23.6 | 29.0 | 32.6 | 24.9 | 28.0 | 32.9 | 28.6 | 31.9 | 34.5 |
| Avg | 24.7 | 30.1 | 33.1 | 26.0 | 30.2 | 34.0 | 29.8 | 33.0 | 35.3 |
| STD | 0.5 | 0.8 | 0.2 | 0.6 | 1.3 | 0.9 | 1.0 | 0.6 | 0.5 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 25.5 | 30.8 | 32.5 | 26.8 | 31.5 | 35.6 | 28.2 | 30.1 | 32.5 |
| Min | 24.7 | 29.7 | 29.6 | 24.9 | 28.0 | 32.9 | 27.4 | 29.5 | 31.9 |
| Avg | 25.1 | 30.2 | 30.9 | 26.0 | 30.2 | 34.0 | 27.7 | 29.8 | 32.1 |
| STD | 0.3 | 0.4 | 0.9 | 0.6 | 1.3 | 0.9 | 0.3 | 0.2 | 0.2 |
| Pak choi seedling | |||||||||
| Days | 4th | 9th | 15th | 4th | 9th | 15th | 4th | 9th | 15th |
| Light | 50 | 50 | 50 | 250 | 250 | 250 | 450 | 450 | 450 |
| Max | 25.1 | 26.0 | 30.2 | 26.7 | 30.4 | 32.0 | 29.3 | 32.0 | 32.1 |
| Min | 24.4 | 25.3 | 27.1 | 26.1 | 29.7 | 29.9 | 27.6 | 30.0 | 31.0 |
| Avg | 24.8 | 25.7 | 28.8 | 26.4 | 30.0 | 31.1 | 28.5 | 31.0 | 31.6 |
| STD | 0.2 | 0.3 | 1.1 | 0.2 | 0.2 | 0.7 | 0.5 | 0.6 | 0.4 |
| Nutrient | 3 | 3 | 3 | 1 | 1 | 1 | 6 | 6 | 6 |
| Max | 25.8 | 29.7 | 31.4 | 26.7 | 30.4 | 32.0 | 27.8 | 30.7 | 32.6 |
| Min | 24.9 | 28.9 | 29.8 | 26.1 | 29.7 | 29.9 | 26.4 | 28.4 | 30.8 |
| Avg | 25.3 | 29.3 | 30.6 | 26.4 | 30.0 | 31.1 | 27.1 | 29.3 | 31.7 |
| STD | 0.3 | 0.3 | 0.5 | 0.2 | 0.2 | 0.7 | 0.5 | 0.8 | 0.6 |
| Temp. | 20 | 20 | 20 | 25 | 25 | 25 | 30 | 30 | 30 |
| Max | 26.6 | 29.9 | 31.9 | 26.7 | 30.4 | 32.0 | 30.0 | 32.0 | 33.4 |
| Min | 24.8 | 28.6 | 29.6 | 26.1 | 29.7 | 29.9 | 28.3 | 30.1 | 30.9 |
| Avg | 25.9 | 29.2 | 30.6 | 26.4 | 30.0 | 31.1 | 29.4 | 31.3 | 32.6 |
| STD | 0.5 | 0.4 | 0.7 | 0.2 | 0.2 | 0.7 | 0.6 | 0.6 | 0.9 |
| Water | high | high | high | normal | normal | normal | low | low | low |
| Max | 26.9 | 29.6 | 30.8 | 26.7 | 30.4 | 32.0 | 29.2 | 31.3 | 33.4 |
| Min | 24.8 | 28.3 | 28.6 | 26.1 | 29.7 | 29.9 | 27.4 | 29.6 | 30.2 |
| Avg | 25.9 | 29.0 | 29.7 | 26.4 | 30.0 | 31.1 | 28.2 | 30.5 | 31.7 |
| STD | 0.8 | 0.4 | 0.7 | 0.2 | 0.2 | 0.7 | 0.6 | 0.6 | 1.1 |
Number of sample, n = 15; Unit = Degree Celsius (°C).
References
- Hussain, M.; Bhat, N. World population statistics: Some key findings. Res. J. Soc. Sci. 2018, 9, 16–25. [Google Scholar]
- Wang, X.J.; Kang, M.Z.; Lewlomphaisarl, U.; Hua, J.; Wang, H.Y. Optimal control of plant growth in a plant factory using a plant model. In Proceedings of the 2022 Australian & New Zealand Control Conference (ANZCC), Gold Coast, Australia, 24–25 November 2022; pp. 166–170. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Yang, R.Y.; Hughes, J.D.A.; Easdown, W.J.; Holmer, R. The importance of vegetables in ensuring both food and nutritional security in attainment of the Millennium Development Goals. Food Secur. 2011, 3, 491–501. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C. Tapping the economic and nutritional power of vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- Dhandevi, P.E.M.; Jeewon, R. Fruit and vegetable intake: Benefits and progress of nutrition education interventions-narrative review article. Iran. J. Public Health 2015, 44, 1309. [Google Scholar]
- Gregorio, N.O.; Herbohn, J.L.; Harrison, S.R. Guide to Quality Seedling Production in Smallholder Nurseries; Visayas State University: Leyte, Philippines, 2010; pp. 1–43. [Google Scholar]
- Huang, K.L.; Yang, C.L.; Kuo, C.M. Plant factory crop scheduling considering volume, yield changes and multi-period harvests using Lagrangian relaxation. Biosyst. Eng. 2020, 200, 328–337. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: New York, NY, USA, 2015; pp. 1–405. [Google Scholar]
- Park, Y.; Gómez, C.; Runkle, E.S. Indoor production of ornamental seedlings, vegetable transplants, and microgreens. In Plant Factory Basics, Applications and Advances; Academic Press: New York, NY, USA, 2022; pp. 351–375. [Google Scholar]
- Singh, H.; Sethi, S.; Kaushik, P.; Fulford, A. Grafting vegetables for mitigating environmental stresses under climate change: A review. J. Water Clim. Chang. 2020, 11, 1784–1797. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and future of plant stress detection: An overview from remote sensing to positron emission tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Bhattacharya, A. Effect of low-temperature stress on germination, growth, and phenology of plants: A review. In Physiological Processes in Plants under Low Temperature Stress; Springer: Singapore, 2022; pp. 1–106. [Google Scholar]
- Blum, A.; Blum, A. Plant water relations, plant stress and plant production. In Plant Breeding for Water-Limited Environments; Springer: New York, NY, USA, 2011; pp. 11–52. [Google Scholar]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, J.; Luoranen, J. Seedling Production and the Field Performance of Seedlings. Forests 2018, 9, 740. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean Seed Vigor: Uniformity and Growth as Key Factors to Improve Yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef]
- Houetohossou, S.C.A.; Houndji, V.R.; Hounmenou, C.G.; Sikirou, R.; Kakaï, R.L.G. Deep learning methods for biotic and abiotic stresses detection and classification in fruits and vegetables: State of the art and perspectives. Artif. Intell. Agric. 2023, 9, 46–60. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Langan, P.; Bernád, V.; Walsh, J.; Mangina, E.; Negrão, S. Capturing crop adaptation to abiotic stress using image-based technologies. Open Biol. 2022, 12, 210353. [Google Scholar] [CrossRef]
- Rossi, R.; Costafreda-Aumedes, S.; Leolini, L.; Leolini, C.; Bindi, M.; Moriondo, M. Implementation of an algorithm for automated phenotyping through plant 3D-modeling: A practical application on the early detection of water stress. Comput. Electron. Agric. 2022, 197, 106937. [Google Scholar] [CrossRef]
- Mahlein, A.K. Plant disease detection by imaging sensors–parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef]
- Barbedo, J.G.A.; Garcia, J. Digital image processing techniques for detecting, quantifying and classifying plant diseases. SpringerPlus 2013, 2, 660. [Google Scholar] [CrossRef]
- Gebejes, A.; Huertas, R. Texture characterization based on grey-level co-occurrence matrix. Databases 2013, 9, 375–378. [Google Scholar]
- Sun, Y.; Tong, C.; He, S.; Wang, K.; Chen, L. Identification of nitrogen, phosphorus, and potassium deficiencies based on temporal dynamics of leaf morphology and color. Sustainability 2018, 10, 762. [Google Scholar] [CrossRef]
- Latte, M.V.; Shidnal, S.; Anami, B.S. Rule based approach to determine nutrient deficiency in paddy leaf images. Int. J. Agric. Technol. 2017, 13, 227–245. [Google Scholar]
- Franchetti, B.; Ntouskos, V.; Giuliani, P.; Herman, T.; Barnes, L.; Pirri, F. Vision Based Modeling of Plants Phenotyping in Vertical Farming under Artificial Lighting. Sensors 2019, 19, 4378. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Chatterjee, S.S.; Pai, P.; Varshney, A.; Juikar, S.; Prasad, V.; Bhadra, B.; Dasgupta, S. Viable smart sensors and their application in data driven agriculture. Comput. Electron. Agric. 2022, 198, 107096. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, X.; He, B.; Liang, D.; Zhang, D.; Huang, L. A System for Diagnosis of Wheat Leaf Diseases Based on Android Smartphone. In Proceedings of the International Symposium on Optical Measurement Technology and Instrumentation, Beijing, China, 9–11 May 2016. [Google Scholar]
- Naik, H.S.; Zhang, J.; Lofquist, A.; Assefa, T.; Sarkar, S.; Ackerman, D.; Singh, A.; Singh, A.K.; Ganapathysubramanian, B. A real-time phenotyping framework using machine learning for plant stress severity rating in soybean. Plant Methods 2017, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Watchareeruetai, U.; Noinongyao, P.; Wattanapaiboonsuk, C.; Khantiviriya, P.; Duangsrisai, S. Identification of Plant Nutrient Deficiencies Using Convolutional Neural Networks; IEEE: Krabi, Thailand, 2018; pp. 1–4. [Google Scholar]
- Islam, M.; Anh, D.; Wahid, K.; Bhowmik, P. Detection of potato diseases using image segmentation and multiclass support vector machine. In Proceedings of the 2017 IEEE 30th Canadian Conference on Electrical and Computer Engineering (CCECE), Windsor, ON, Canada, 30 April–3 May 2017; pp. 1–4. [Google Scholar]
- Wernick, M.N.; Yang, Y.; Brankov, J.G.; Yourganov, G.; Strother, S.C. Machine learning in medical imaging. IEEE Signal Process. Mag. 2010, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Chemura, A.; Mutanga, O.; Sibanda, M.; Chidoko, P. Machine learning prediction of coffee rust severity on leaves using spectroradiometer data. Trop. Plant Pathol. 2018, 43, 117–127. [Google Scholar] [CrossRef]
- Wicaksono, Y.; Wahono, R.; Suhartono, V. Color and texture feature extraction using gabor filter-local binary patterns for image segmentation with fuzzy C-means. J. Intell. Syst. 2015, 1, 15–21. [Google Scholar]
- Sharma, J.; Upadhyay, A.K.; Adsule, P.G.; Sawant, S.D.; Sharma, A.K.; Satisha, J.; Yadav, D.S.; Ramteke, S.D. Effect of climate change on grape and its value-added products. In Climate-Resilient Horticulture: Adaptation and Mitigation Strategies; Springer: New Delhi, India, 2013; pp. 67–80. [Google Scholar]
- Karadağ, K.; Tenekeci, M.E.; Taşaltın, R.; Bilgili, A. Detection of pepper fusarium disease using machine learning algorithms based on spectral reflectance. Sustain. Comput. Inform. Syst. 2020, 28, 100299. [Google Scholar] [CrossRef]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus Huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, P.; Hazra, R.; Kumar, A. Plant Leaf Disease Detection Using Gabor Wavelet Transform. In Proceedings of the 3rd International Conference on Swarm, Evolutionary, and Memetic Computing, Bhubaneswar, India, 20–22 December 2012; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Son, K.H.; Kim, E.Y.; Oh, M.M. Growth and development of cherry tomato seedlings grown under various combined ratios of red to blue LED lights and fruit yield and quality after transplanting. J. Bio-Environ. Control 2018, 27, 54–63. [Google Scholar] [CrossRef]
- Liu, N.; Ji, F.; Xu, L.; He, D. Effects of LED light quality on the growth of pepper seedling in plant factory. Int. J. Agric. Biol. Eng. 2019, 12, 44–50. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched led lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- An, S.; Hwang, H.; Chun, C.; Jang, Y.; Lee, H.J.; Wi, S.H.; Yeo, K.H.; Yu, I.H.; Kwack, Y. Evaluation of air temperature, photoperiod and light intensity conditions to produce cucumber scions and rootstocks in a plant factory with artificial lighting. Horticulturae 2021, 7, 102. [Google Scholar] [CrossRef]
- Mickens, M.A.; Torralba, M.; Robinson, S.A.; Spencer, L.E.; Romeyn, M.W.; Massa, G.D.; Wheeler, R.M. Growth of red pak choi under red and blue, supplemented white, and artificial sunlight provided by LEDs. Sci. Hortic. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Islam, S.; Reza, M.N.; Chowdhury, M.; Islam, M.N.; Ali, M.; Kiraga, S.; Chung, S.O. Image processing algorithm to estimate ice-plant leaf area from RGB images under different light conditions. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 924, p. 012013. [Google Scholar]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A., Jr. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chilli peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Fang, H.; Baret, F.; Plummer, S.; Schaepman-Strub, G. An overview of global leaf area index (LAI): Methods, products, validation, and applications. Rev. Geophys. 2019, 57, 739–799. [Google Scholar] [CrossRef]
- Gu, S.; Zuo, W.; Guo, S.; Chen, Y.; Chen, C.; Zhang, L. Learning dynamic guidance for depth image enhancement. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 3769–3778. [Google Scholar]
- Li, J.; Tang, L. Developing a low-cost 3D plant morphological traits characterization system. Comput. Electron. Agric. 2017, 143, 1–13. [Google Scholar] [CrossRef]
- Piazzoli, G. Thermal Imaging to Monitor Soil and Canopy Temperature under Mulching and Natural Soil Cover Conditions. Doctoral Dissertation, Instituto Superior de Agronomia, Universidade de Lisboa, Lisbon, Portugal, 2022. [Google Scholar]
- Singh, V.; Sharma, N.; Singh, S. A review of imaging techniques for plant disease detection. Artif. Intell. Agric. 2020, 4, 229–242. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, photosynthetic physiology and biochemistry of nine herbaceous plants under water stress. Front. Plant Sci. 2023, 14, 1147208. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. Potential of biochar application to mitigate salinity stress in eggplant. HortScience 2020, 55, 1946–1955. [Google Scholar] [CrossRef]
- Zheng, G.; Moskal, L.M. Retrieving leaf area index (LAI) using remote sensing: Theories, methods and sensors. Sensors 2009, 9, 2719–2745. [Google Scholar] [CrossRef] [PubMed]
- Parkash, V.; Singh, S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Kausar, A.; Ashraf, M.Y.; Ali, I.; Niaz, M.; Abbass, Q. Evaluation of sorghum varieties/lines for salt tolerance using physiological indices as screening tool. Pak. J. Bot. 2012, 44, 47–52. [Google Scholar]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Dikšaitytė, A.; Viršilė, A.; Žaltauskaitė, J.; Januškaitienė, I.; Juozapaitienė, G. Growth and photosynthetic responses in Brassica napus differ during stress and recovery periods when exposed to combined heat, drought and elevated CO2. Plant Physiol. Biochem. 2019, 142, 59–72. [Google Scholar] [CrossRef]
- Wang, C.; He, J.; Zhao, T.H.; Cao, Y.; Wang, G.; Sun, B.; Yan, X.; Guo, W.; Li, M.H. The smaller the leaf is, the faster the leaf water loses in a temperate forest. Front. Plant Sci. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Gratani, L.; Crescente, M.F.; Puglielli, G.; Varone, L. Daily Temperature Effect on Seedling Growth Dynamic of Three Invasive Alien Species. Front. Plant Sci. 2022, 13, 837449. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Rahman, M.; Fakhar, A.; Rafique, M.; et al. Plant growth-promoting rhizobacteria eliminate the effect of drought stress in plants: A review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Mesa, T.; Polo, J.; Arabia, A.; Caselles, V.; Munné-Bosch, S. Differential physiological response to heat and cold stress of tomato plants and its implication on fruit quality. J. Plant Physiol. 2022, 268, 153581. [Google Scholar] [CrossRef]
- Virk, G.; Snider, J.L.; Chee, P.; Jespersen, D.; Pilon, C.; Rains, G.; Roberts, P.; Kaur, N.; Ermanis, A.; Tishchenko, V. Extreme temperatures affect seedling growth and photosynthetic performance of advanced cotton genotypes. Ind. Crops Prod. 2021, 172, 114025. [Google Scholar] [CrossRef]
- Haj Sghaier, A.; Tarnawa, Á.; Khaeim, H.; Kovács, G.P.; Gyuricza, C.; Kende, Z. The Effects of Temperature and Water on the Seed Germination and Seedling Development of Rapeseed (Brassica napus L.). Plants 2022, 11, 2819. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, J.; Dayananda, B.; Li, J. Effect of light intensities on the photosynthesis, growth and physiological performances of two maple species. Front. Plant Sci. 2022, 13, 999026. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Guo, S.; Li, M.; Tian, Z.; Han, B.; Tang, X.; Liu, B. Effects of Light Intensity on Seedling Emergence and Early Growth of Liquidambar formosana Hance. Forests 2023, 14, 867. [Google Scholar] [CrossRef]
- Franchetti, B.; Pirri, F. Detection and localization of tip-burn on large lettuce canopies. Front. Plant Sci. 2022, 13, 874035. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant Nutrition: An Effective Way to Alleviate Abiotic Stress in Agricultural Crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.D.; Idso, S.; Reginato, R.; Pinter, P. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).