Characterization of Changes in Active Ingredients and Mining of Key Metabolites in Bletilla striata under Shading and Drought Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions, Experimental Design, and Sampling
2.2. Determination of Photosynthetic Gas Exchange Parameters
2.3. Determination of Polysaccharides, Total Flavonoids, and Total Phenol Content
2.4. Determination of Militarine Content
2.5. GC-MS-Based Metabolite Analysis
2.5.1. Metabolite Extraction and Derivatization
2.5.2. GC-MS Detection Conditions
3. Results
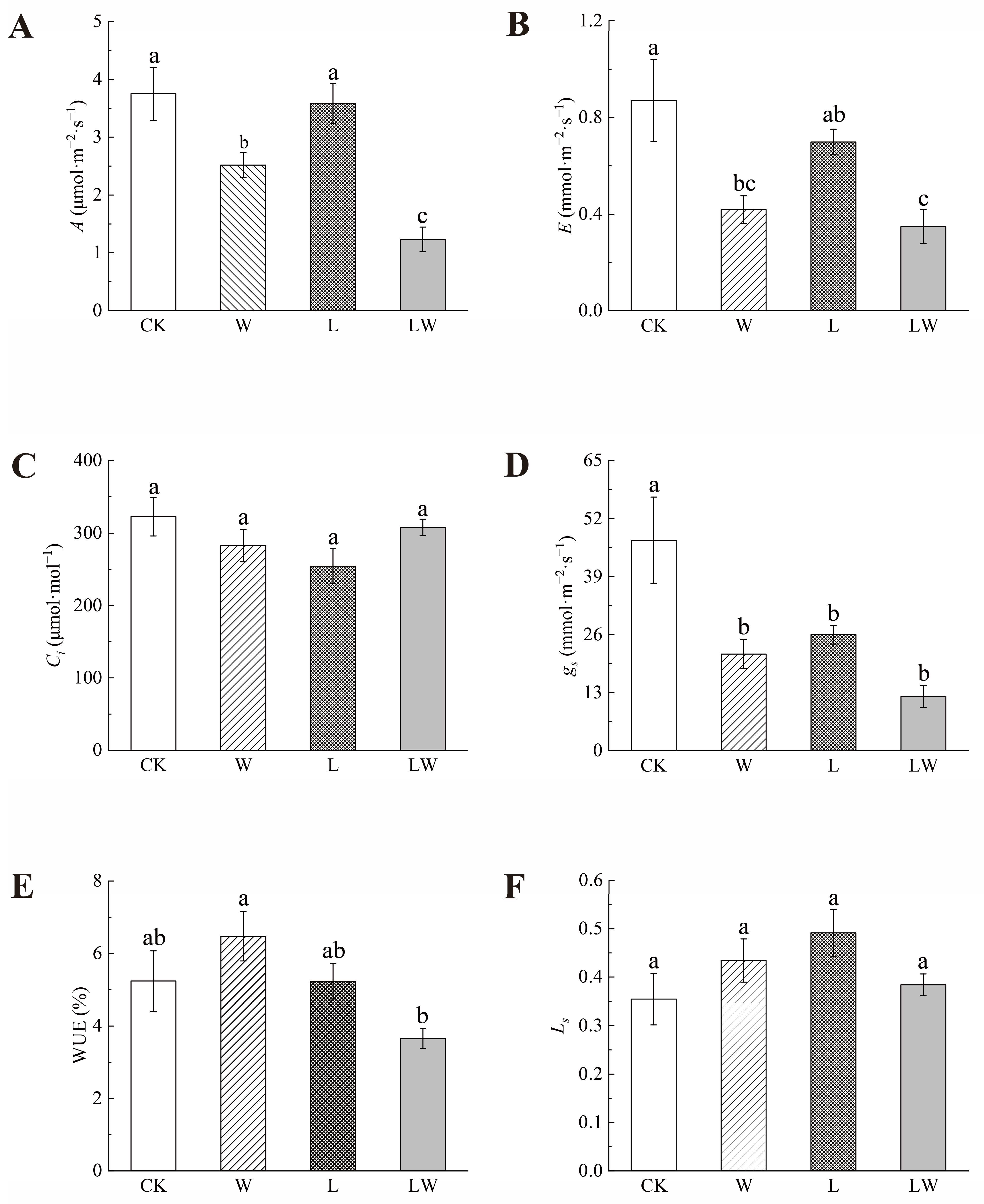
3.1. Effects of Different Stresses on the Photosynthetic Properties of B. striata
3.2. Effect of Different Stresses on the Active Components of BP
3.3. Metabolic Profiling Based on Meteorological Chromatography-Mass Spectrometry
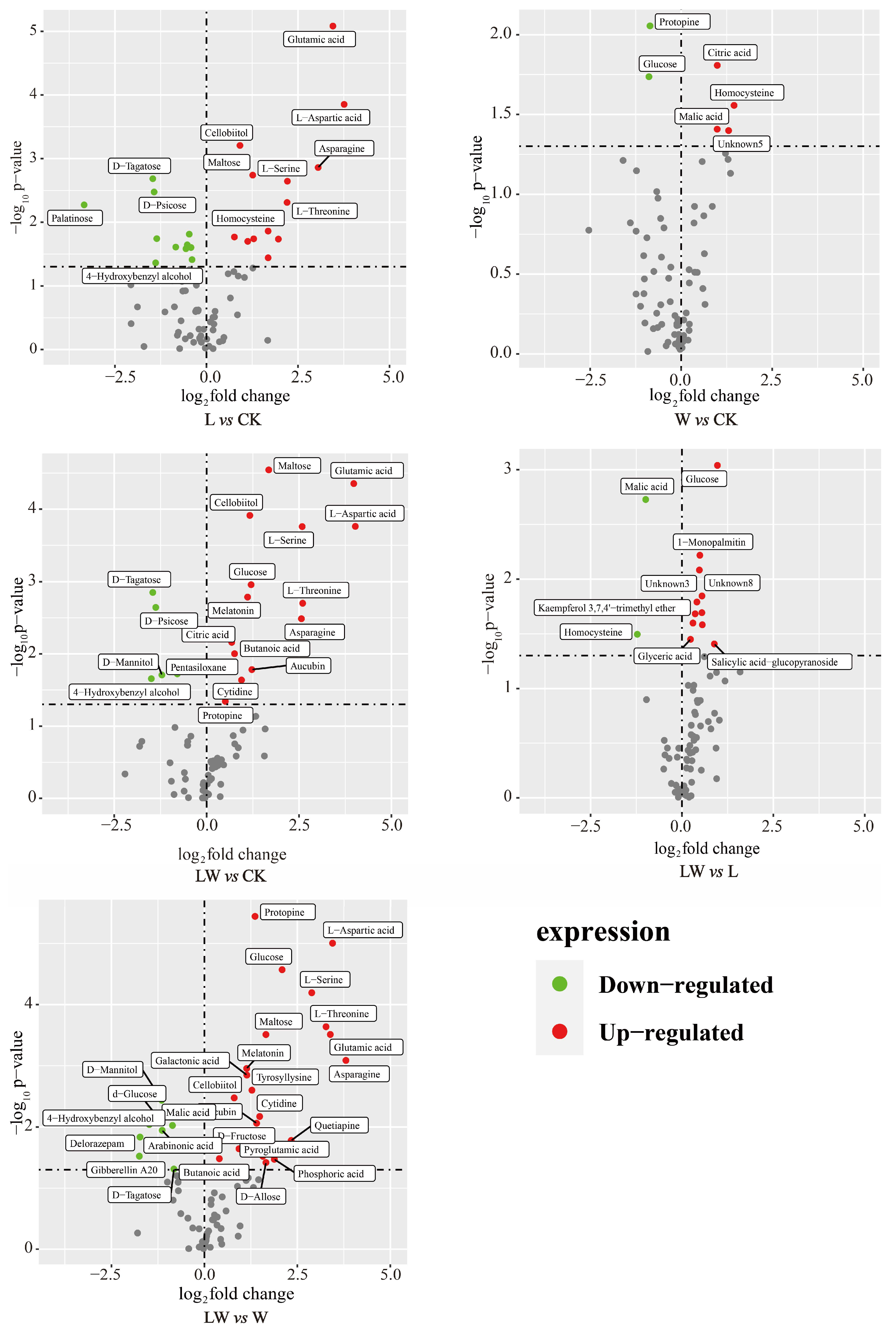
3.4. Changes in Metabolites of BP under Different Stresses
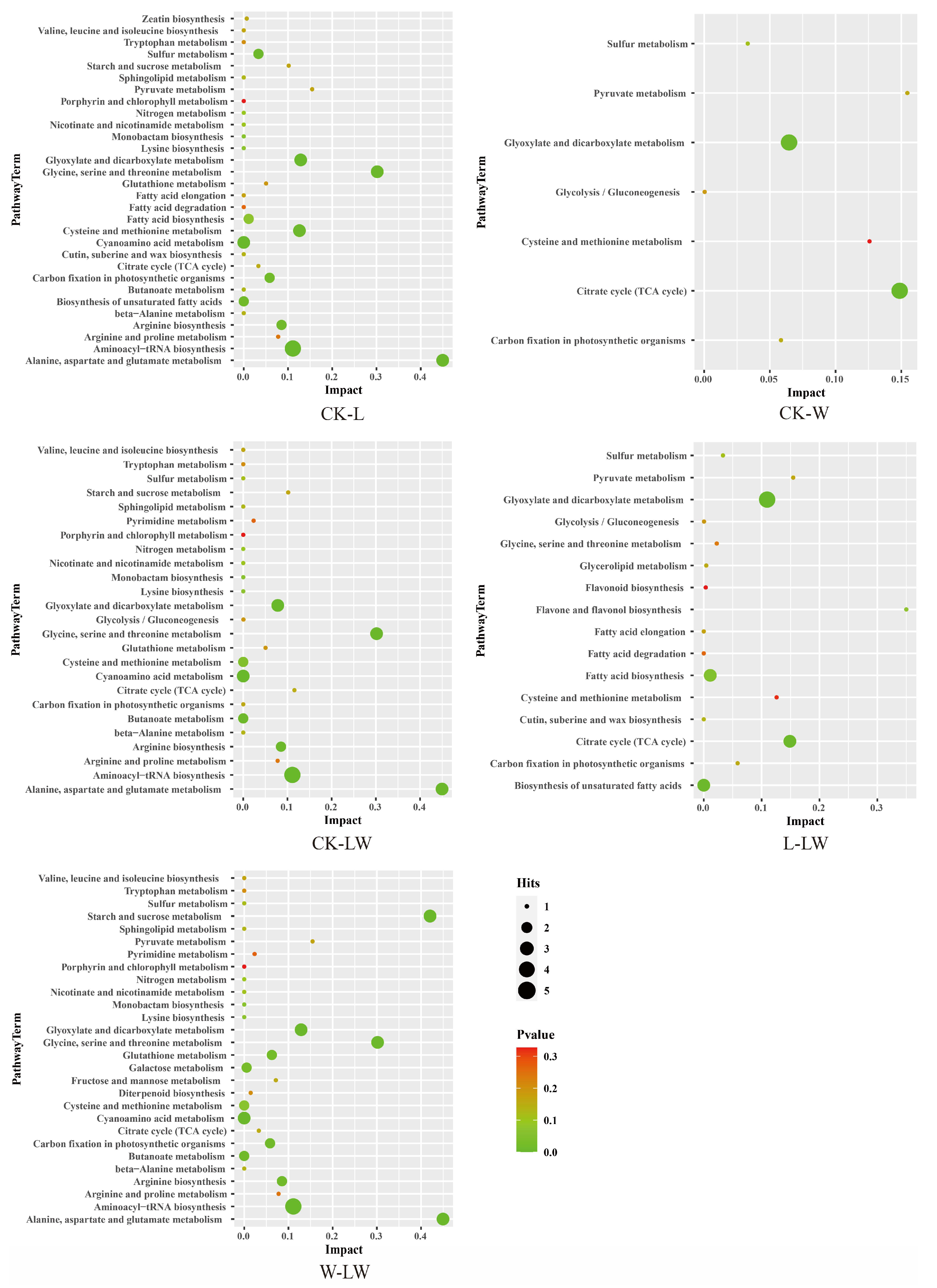
3.5. KEGG-Based Differential Metabolic Pathway Analysis
3.6. Relationship between Metabolic Changes and Active Ingredients
4. Discussion
4.1. Relationship between Changes in Photosynthetic Properties and Active Ingredients in Plants
4.2. Differential Metabolites in Response to Stressful Environments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


| Comparison Group | R2X | R2Y | Q2 |
|---|---|---|---|
| L vs. CK | 0.803 | 0.999 | 0.8 |
| W vs. CK | 0.838 | 0.999 | 0.702 |
| LW vs. CK | 0.942 | 0.999 | 0.902 |
| LW vs. L | 0.874 | 0.999 | 0.757 |
| LW vs. W | 0.902 | 0.999 | 0.954 |
References
- He, X.; Wang, X.; Fang, J.; Zhao, Z.; Huang, L.; Guo, H.; Zheng, X. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017, 195, 20–38. [Google Scholar] [CrossRef]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y. A review of isolation, chemical properties, and bioactivities of polysaccharides from Bletilla striata. BioMed Res. Int. 2020, 2020, 5391379. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Jiang, L.; Xie, Q.; Yuan, H.; Yang, Y.; Zafar, S.; Liu, Y.; Jian, Y.; Li, B.; et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 280, 114263. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China 2020; The Medicine Science and Technology Press of China: Beijing, China, 2020. [Google Scholar]
- Cheng, X. Effects of Light on the Growth and Medicinal Components of Tetrastigma hemsleyanum. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2018. [Google Scholar]
- Poorter, H.; Niinemets, U.; Ntagkas, N.; Siebenkas, A.; Maeenpaeae, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Slavik, B. The Influence of decreasing hydration level on photosynthetic rate in the thalli of the hepatic conocephallum conicum. In Water Stress in Plants; Slavik, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1965; pp. 195–202. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Dinneny, J. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 2019, 35, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Rellan-Alvarez, R.; Lobet, G.; Dinneny, J. Environmental control of root system biology. Annu. Rev. Plant Biol. 2016, 67, 619–642. [Google Scholar] [CrossRef]
- de Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Huston, M. A theory of the spatial and temporal dynamics of plant communities. Prog. Theor. Veg. Sci. 1990, 11, 49–69. [Google Scholar] [CrossRef]
- Quero, J.; Villar, R.; Maranon, T.; Zamora, R. Interactions of drought and shade effects on seedlings of four Quercus species: Physiological and structural leaf responses. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef]
- Holmgren, M.; Scheffer, M.; Huston, M. The interplay of facilitation and competition in plant communities. Ecology 1997, 78, 1966–1975. [Google Scholar] [CrossRef]
- Holmgren, M. Combined effects of shade and drought on tulip poplar seedlings: Trade-off in tolerance or facilitation? Oikos 2000, 90, 67–78. [Google Scholar] [CrossRef]
- Beale, D.; Pinu, F.; Kouremenos, K.; Poojary, M.; Narayana, V.; Boughton, B.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Alexander, J.M.; Allard, P.M.; Baines, O.; Baldy, V.; Bardgett, R.D.; Capdevila, P.; Coley, P.D.; David, B.; Defossez, E.; et al. Functional Traits 2.0: The power of the metabolome for ecology. J. Ecol. 2022, 110, 4–20. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in plant stress physiology. Plant Genet. Mol. Biol. 2018, 164, 187–236. [Google Scholar]
- Hsiao, T. Plant responses to water stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Qin, L.; Shen, J.; He, Z.; Shao, Q.; Lin, D. Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb. f. flower. Ind. Crops Prod. 2021, 164, 113388. [Google Scholar] [CrossRef]
- Deng, P.; Yin, R.; Wang, H.; Chen, L.; Cao, X.; Xu, X. Comparative analyses of functional traits based on metabolome and economic traits variation of Bletilla striata: Contribution of intercropping. Front. Plant Sci. 2023, 14, 1147076. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, T.Y.; Wang, X.; Wang, Y.; Gong, Q.; Li, G.; Lin, Q.; Zhu, S.Y. Untargeted metabolomics reveal rhizosphere metabolites mechanisms on continuous ramie cropping. Front. Plant Sci. 2023, 14, 1217956. [Google Scholar] [CrossRef]
- Barron-Gafford, G.; Pavao-Zuckerman, M.; Minor, R.; Sutter, L.; Barnett-Moreno, I.; Blackett, D.; Thompson, M.; Dimond, K.; Gerlak, A.; Nabhan, G.; et al. Agrivoltaics provide mutual benefits across the food–energy–water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Mackova, J.; Vaskova, M.; Macek, P.; Hronkova, M.; Schreiber, L.; Santrucek, J. Plant response to drought stress simulated by ABA application: Changes in chemical composition of cuticular waxes. Environ. Exp. Bot. 2013, 86, 70–75. [Google Scholar] [CrossRef]
- Farquhar, G.; Sharkey, T. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Chaves, M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Dong, B.; Yang, H.; Liu, H.; Qiao, Y.; Zhang, M.; Wang, Y.; Xie, Z.; Liu, M. Effects of shading stress on grain number, yield, and photosynthesis during early reproductive growth in wheat. Crop Sci. 2019, 59, 363–378. [Google Scholar] [CrossRef]
- Kleinwachter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2014, 35, 121–131. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.; Yang, L.; Sun, Z.; Zhang, T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crops Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Hosseini, M.; Samsampour, D.; Ebrahimi, M.; Abadia, J.; Khanahmadi, M. Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 2018, 156, 124–134. [Google Scholar] [CrossRef]
- Shahrajabian, M.; Kuang, Y.; Cui, H.; Fu, L.; Sun, W. Metabolic changes of active components of important medicinal plants on the basis of traditional Chinese medicine under different environmental stresses. Curr. Org. Chem. 2023, 27, 782–806. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczuk-Nowicka, E.; Arasimowicz-Jelonek, M.; Tanwar, U.; Floryszak-Wieczorek, J. Plant homocysteine, a methionine precursor and plant’s hallmark of metabolic disorders. Front. Plant Sci. 2022, 13, 1044944. [Google Scholar] [CrossRef]
- Lopez-Bucio, J.; Nieto-Jacobo, M.; Ramirez-Rodriguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xie, S. Influence of water stress on the citric acid metabolism related gene expression in the ponkan fruits. Agric. Sci. 2014, 5, 1513. [Google Scholar] [CrossRef]
- Timpa, J.; Burke, J.; Quisenberry, J.; Wendt, C. Effects of water stress on the organic acid and carbohydrate compositions of cotton plants. Plant Physiol. 1986, 82, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Aziz, A.; The, K.; Lam, S.; Cha, T. Metabolites re-programming and physiological changes induced in Scenedesmus regularis under nitrate treatment. Sci. Rep. 2018, 8, 9746. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, K.; Rodziewicz, P.; Swarcewicz, B.; Sawikowska, A.; Krajewski, P.; Marczak, L.; Ciesiolka, D.; Kuczynska, A.; Mikolajczak, K.; Ogrodowicz, P.; et al. Analysis of drought-induced proteomic and metabolomic changes in barley (Hordeum vulgare L.) leaves and roots unravels some aspects of biochemical mechanisms involved in drought tolerance. Front. Plant Sci. 2016, 7, 1108. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Jin, P.; Chen, X.; Zhang, T.; Zhong, K.; Liu, P.; Chen, J.; Yang, J. Comparative proteomic analysis of Nicotiana benthamiana plants under Chinese wheat mosaic virus infection. BMC Plant Biol. 2021, 21, 51. [Google Scholar] [CrossRef]
- Wang, M.; Zang, L.; Jiao, F.; Perez-Garcia, M.; Oge, L.; Hamama, L.; Le, G.; Sakr, S.; Chen, J. Sugar signaling and post-transcriptional regulation in plants: An overlooked or an emerging topic? Front. Plant Sci. 2020, 11, 578096. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, R.; Chen, L.; Deng, P.; Cao, X.; Xu, X. Characterization of Changes in Active Ingredients and Mining of Key Metabolites in Bletilla striata under Shading and Drought Stresses. Horticulturae 2024, 10, 163. https://doi.org/10.3390/horticulturae10020163
Yin R, Chen L, Deng P, Cao X, Xu X. Characterization of Changes in Active Ingredients and Mining of Key Metabolites in Bletilla striata under Shading and Drought Stresses. Horticulturae. 2024; 10(2):163. https://doi.org/10.3390/horticulturae10020163
Chicago/Turabian StyleYin, Ruoyong, Leiru Chen, Pengfei Deng, Xiaoqing Cao, and Xiaoniu Xu. 2024. "Characterization of Changes in Active Ingredients and Mining of Key Metabolites in Bletilla striata under Shading and Drought Stresses" Horticulturae 10, no. 2: 163. https://doi.org/10.3390/horticulturae10020163
APA StyleYin, R., Chen, L., Deng, P., Cao, X., & Xu, X. (2024). Characterization of Changes in Active Ingredients and Mining of Key Metabolites in Bletilla striata under Shading and Drought Stresses. Horticulturae, 10(2), 163. https://doi.org/10.3390/horticulturae10020163






