Role of Two Transcription Factors (TGA 1a and TGA 2.1) in the Mi-1-Mediated Resistance of Tomato to the Root-Knot Nematode Meloidogyne javanica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Nematode Culture
2.3. VIGS Constructs
2.4. Growth of Agroclones and Agroinfiltration
2.5. Semiquantitative RT-PCR
2.6. Nematode Inoculation and Assessment of Infection Levels
3. Results
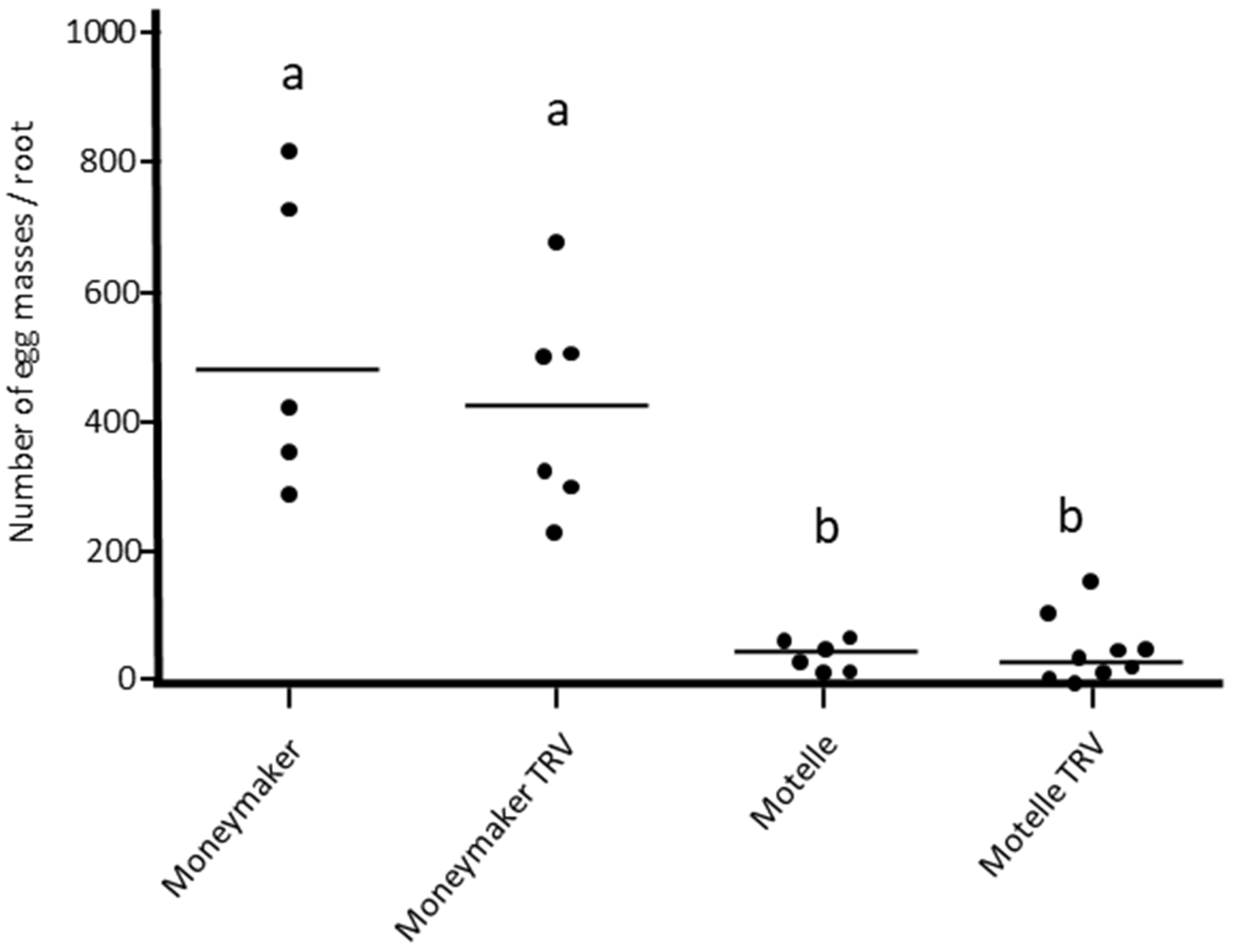
3.1. Assessment of the Effect of Agroinfiltration with the Empty Vector TRV
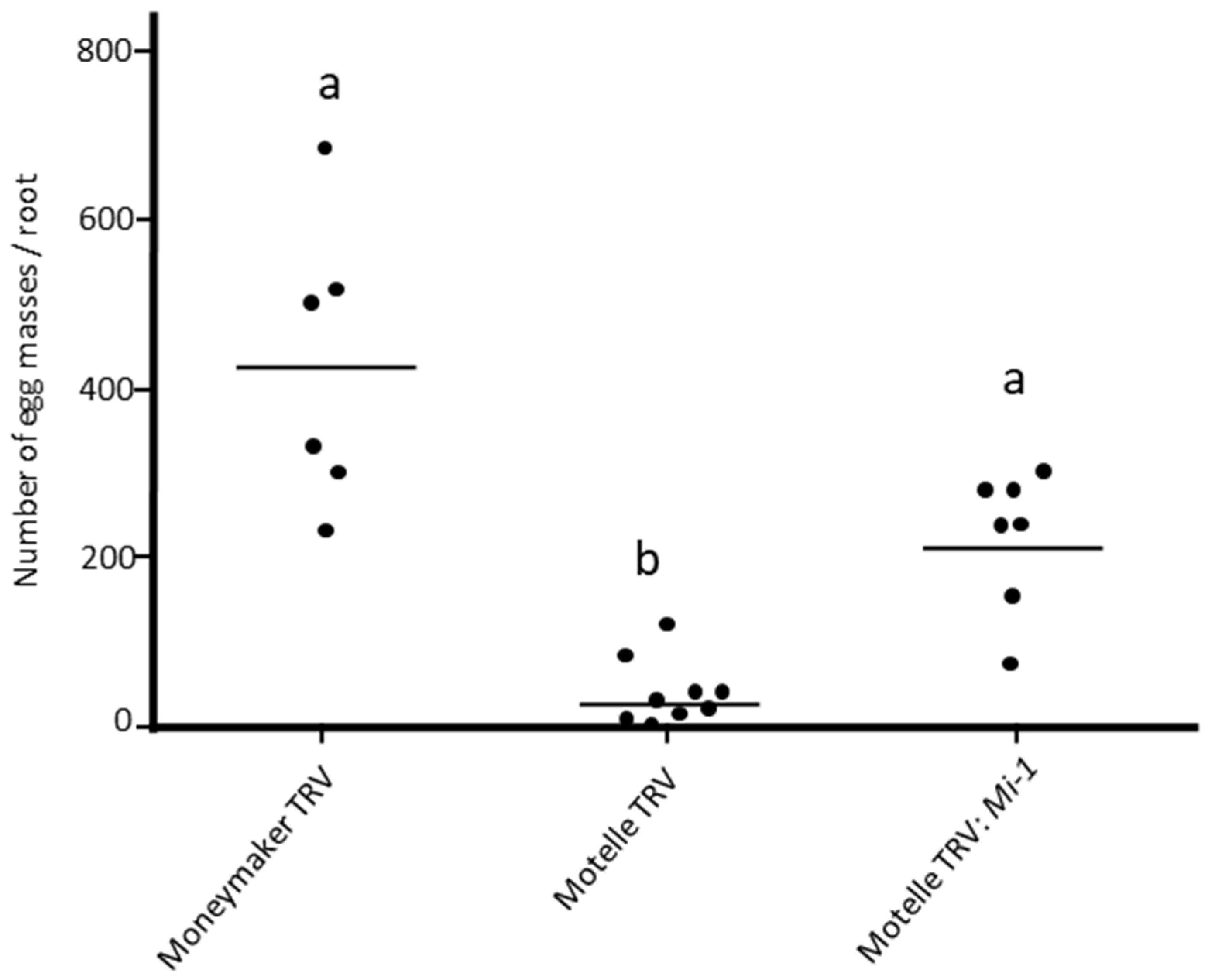
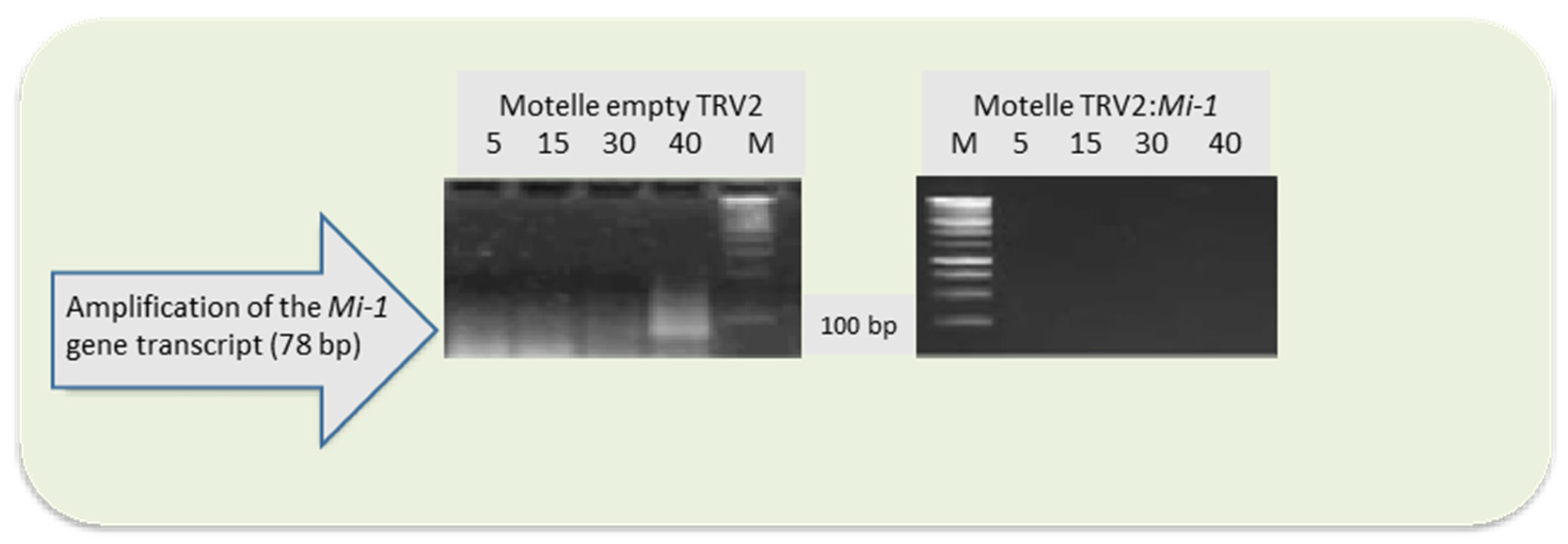
3.2. Silencing of Mi-1 Gene in Tomato Plants
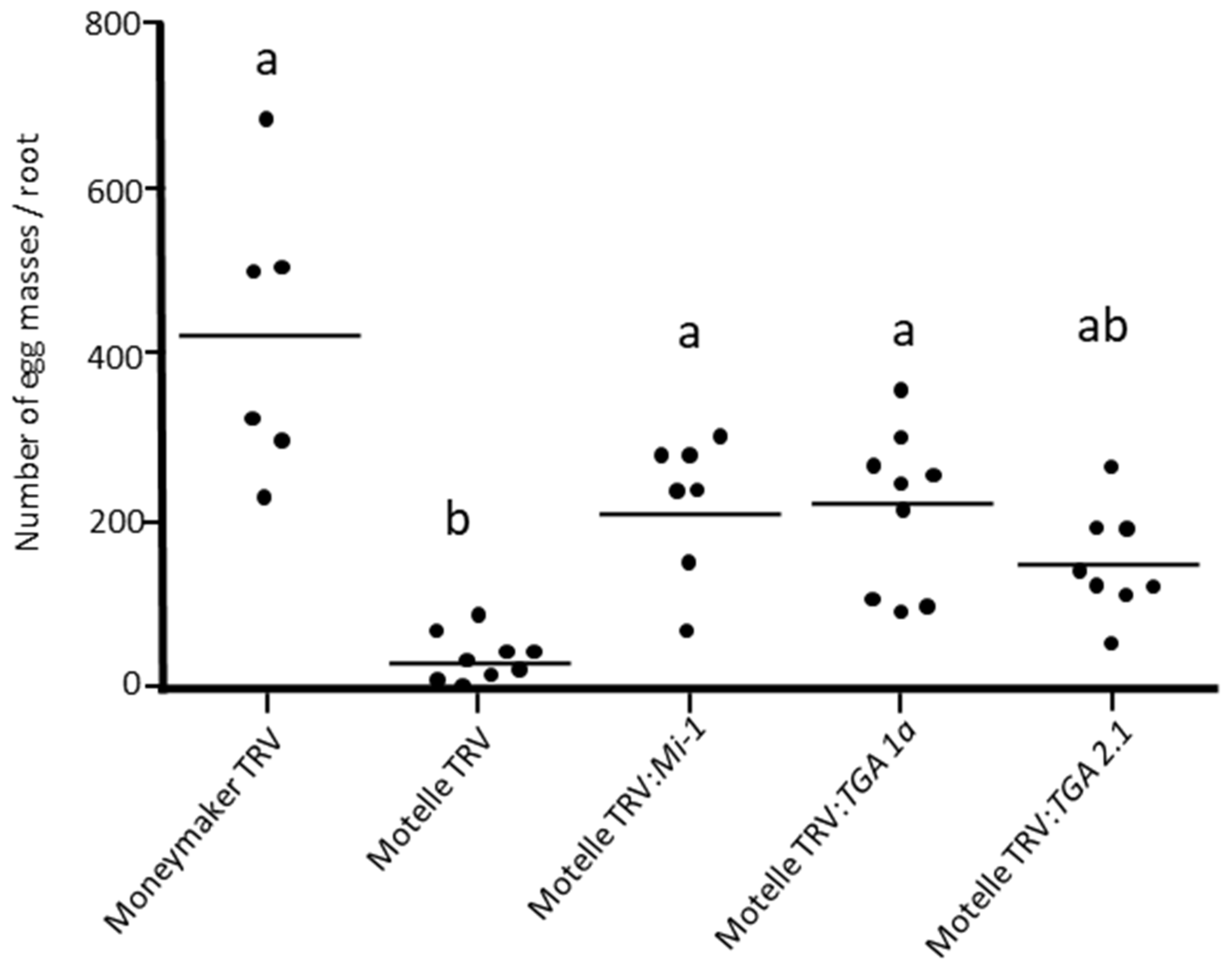
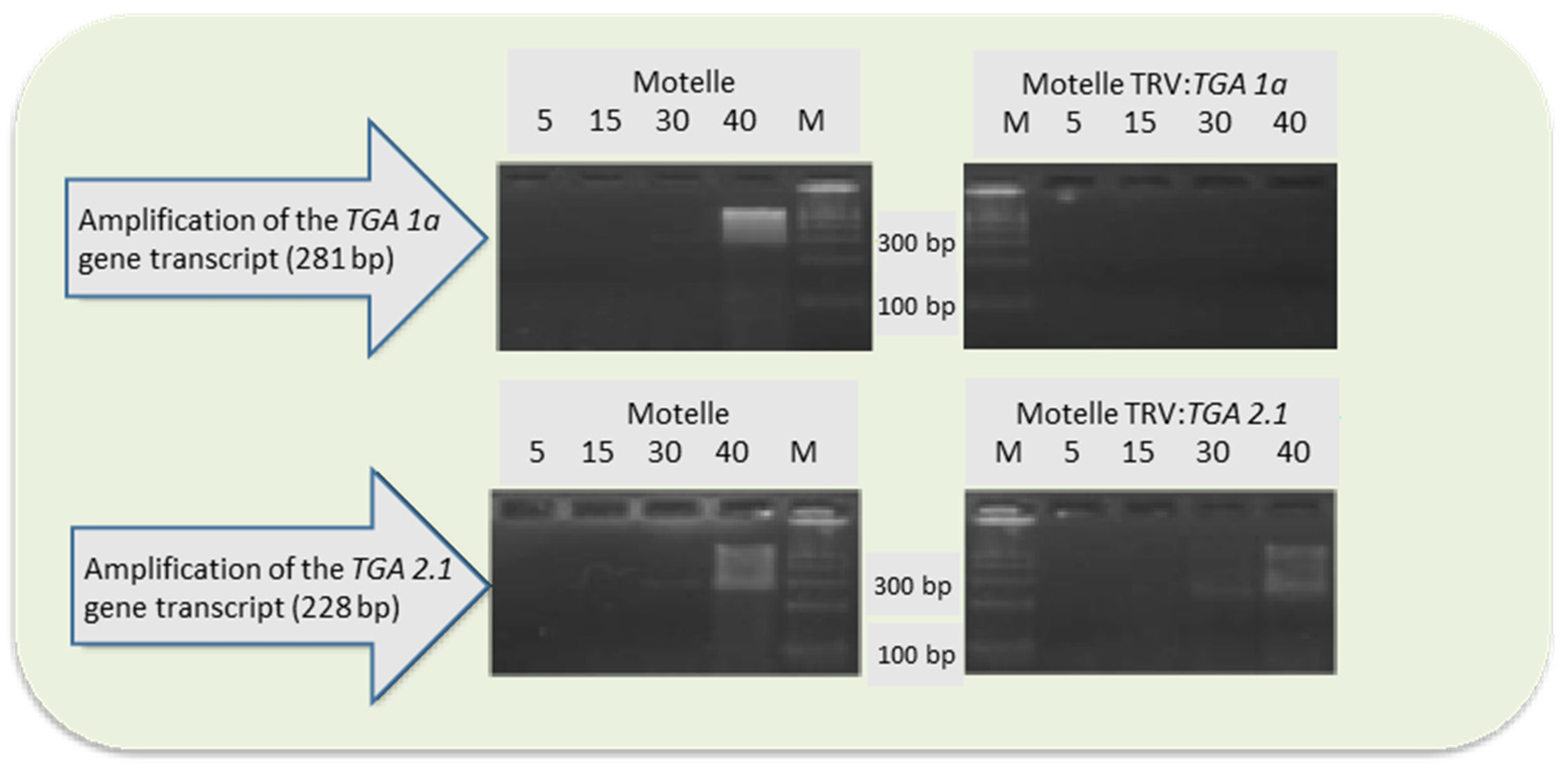
3.3. Silencing of TGA 1a and TGA 2.1 Genes in Tomato Plants
4. Discussion
4.1. Effect of Agroinfiltration with the Empty Vector TRV and TRV-Mi
4.2. The Genes TGA 1a and TGA 2.1 in the Mi-1-Mediated Resistance to M. javanica
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. ISBN 978-94-007-0434-3. [Google Scholar]
- Khan, M.R.; Ahamad, I.; Shah, M.H. Emerging important nematode problems in field crops and their management. In Emerging Trends in Plant Pathology; Singh, K.P., Jahagirdar, S., Sarma, B.K., Eds.; Springer: Singapore, 2021; pp. 33–62. ISBN 978-981-15-6275-4. [Google Scholar]
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bull. 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Kaloshian, I.; Teixeira, M. Advances in plant-nematode interactions with emphasis on the notorious nematode genus Meloidogyne. Phytopathology 2019, 109, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A diverse group of novel and important plant parasites. In Root-Knot Nematodes; CAB International: Wallingford, UK, 2009; pp. 1–17. [Google Scholar]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, A.; Adhikari, T.; Louws, F.; Gorny, A. Meloidogyne enterolobii, a major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef]
- Ebone, L.A.; Kovaleski, M.; Deuner, C.C. Nematicides: History, mode, and mechanism action. Plant Sci. Today 2019, 6, 91–97. [Google Scholar] [CrossRef]
- Burns, A.R.; Baker, R.J.; Kitner, M.; Knox, J.; Cooke, B.; Volpatti, J.R.; Vaidya, A.S.; Puumala, E.; Palmeira, B.M.; Redman, E.M.; et al. Selective control of parasitic nematodes using bioactivated nematicides. Nature 2023, 618, 102–109. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Zeferino-Díaz, R.; Alvarado-Rodríguez, J.G.; López-Ruiz, H.; Rojas-Lima, S.; Flores-Castro, K.; del Prado-Vera, I.C.; Alatorre-Rosas, R.; Tut-Pech, F.; Carrillo-Benítez, M.G.; et al. Nematicidal activity of furanoeremophilenes against Meloidogyne incognita and Nacobbus aberrans. Pest Manag. Sci. 2022, 78, 2571–2580. [Google Scholar] [CrossRef]
- Cabrera, J.; Barcala, M.; Fenoll, C.; Escobar, C. The power of omics to identify plant susceptibility factors and to study resistance to root-knot nematodes. Curr. Issues Mol. Biol. 2016, 19, 53–72. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Jiang, Z.; Chen, X.; Li, B.; Xu, L.; Zhang, Z. Cloning and functional analysis of the root-knot nematode resistance gene ntrk1 in tobacco. Physiol. Plant. 2023, 175, e13894. [Google Scholar] [CrossRef]
- Furumizu, C.; Sawa, S. A rapid method for detection of the root-knot nematode resistance gene, Mi-1.2, in tomato cultivars. Plant Biotechnol. 2023, 40, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V. Root-knot nematode resistance genes in tomato and their potential for future use. Annu. Rev. Phytopathol. 1998, 36, 277–293. [Google Scholar] [CrossRef]
- Du, C.; Jiang, J.; Zhang, H.; Zhao, T.; Yang, H.; Zhang, D.; Zhao, Z.; Xu, X.; Li, J. Transcriptomic profiling of Solanum peruvianum LA3858 revealed a Mi-3-mediated hypersensitive response to Meloidogyne incognita. BMC Genom. 2020, 21, 250. [Google Scholar] [CrossRef]
- Jiang, L.; Ling, J.; Zhao, J.; Yang, Y.; Yang, Y.; Li, Y.; Jiao, Y.; Mao, Z.; Wang, Y.; Xie, B. Chromosome-scale genome assembly-assisted identification of Mi-9 gene in Solanum arcanum accession LA2157, conferring heat-stable resistance to Meloidogyne incognita. Plant Biotechnol. J. 2023, 21, 1496–1509. [Google Scholar] [CrossRef]
- Milligan, S.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Ammati, M.; Thomason, I.; Roberts, P. Screening Lycopersicon spp. for new genes imparting resistance to root-knot nematodes (Meloidogyne spp.). Plant Dis. 1985, 69, 112–115. [Google Scholar]
- Gabriel, M.; Kulczynski, S.; Muniz, M.; Boiteux, L.; Carneiro, R. Reaction of a heterozygous tomato hybrid bearing the Mi-1.2 gene to 15 Meloidogyne species. Plant Pathol. 2020, 69, 944–952. [Google Scholar] [CrossRef]
- Roberts, P.; Thomason, I. Variability in reproduction of isolates of Meloidogyne incognita and Meloidogyne javanica on resistant tomato genotypes. Plant Dis. 1986, 70, 547–551. [Google Scholar] [CrossRef]
- Santos, D.; da Silva, P.; Abrantes, I.; Maleita, C. Tomato Mi-1.2 gene confers resistance to Meloidogyne luci and M. ethiopica. Eur. J. Plant Pathol. 2020, 156, 571–580. [Google Scholar] [CrossRef]
- Casteel, C.L.; Walling, L.L.; Paine, T.D. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 2006, 121, 67–72. [Google Scholar] [CrossRef]
- Nombela, G.; Williamson, V.M.; Muñiz, M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant Microbe Interact. 2003, 16, 645–649. [Google Scholar] [CrossRef]
- Rossi, M.; Goggin, F.; Milligan, S.; Kaloshian, I.; Ullman, D.; Williamson, V. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA 1998, 95, 9750–9754. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.Q.; Hussain, A.; Akram, A.; Hussain, S.; Naqvi, R.Z.; Amin, I.; Saeed, M.; Mansoor, S. Cotton Mi-1.2-like gene: A potential source of whitefly resistance. Gene 2023, 851, 146983. [Google Scholar] [CrossRef] [PubMed]
- Goggin, F.; Jia, L.; Shah, G.; Hebert, S.; Williamson, V.; Ullman, D. Heterologous expression of the Mi-1.2 gene from tomato confers resistance against nematodes but not aphids in eggplant. Mol. Plant Microbe Interact. 2006, 19, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dropkin, V.H. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne. Reversal by temperature. Phytopathology 1969, 59, 1632–1637. [Google Scholar]
- de Ilarduya, O.M.; Xie, Q.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, X.-C.; Cao, J.-J.; Yin, L.-L.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Heat shock factor HsfA1a is essential for R gene-mediated nematode resistance and triggers H2O2 production. Plant Physiol. 2018, 176, 2456–2471. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.K.; Li, Q.; Liu, Y.; Dinesh-Kumar, S.P.; Kaloshian, I. The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 2007, 144, 312–323. [Google Scholar] [CrossRef]
- Pascual, S.; Rodríguez-Álvarez, C.I.; Kaloshian, I.; Nombela, G. Hsp90 gene is required for Mi-1-mediated resistance of tomato to the whitefly Bemisia tabaci. Plants 2023, 12, 641. [Google Scholar] [CrossRef]
- Bhattarai, K.; Atamian, H.; Kaloshian, I.; Eulgem, T. WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 2010, 63, 229–240. [Google Scholar] [CrossRef]
- de Ilarduya, O.M.; Moore, A.; Kaloshian, I. The tomato Rme1 locus is required for Mi-1-mediated resistance to root-knot nematodes and the potato aphid. Plant J. 2001, 27, 417–425. [Google Scholar] [CrossRef]
- Li, Q.; Xie, Q.-G.; Smith-Becker, J.; Navarre, D.A.; Kaloshian, I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant Microbe Interact. 2006, 19, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alvarez, C.I.; Lopez-Climent, M.F.; Gomez-Cadenas, A.; Kaloshian, I.; Nombela, G. Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull. Entomol. Res. 2015, 105, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Lanna, R. An overview of plant resistance to plant-pathogenic bacteria. Trop. Plant Pathol. 2023, 48, 243–259. [Google Scholar] [CrossRef]
- Viswanath, K.; Kuo, S.; Tu, C.; Hsu, Y.; Huang, Y.; Hu, C. The role of plant transcription factors in the fight against plant viruses. Int. J. Mol. Sci. 2023, 24, 8433. [Google Scholar] [CrossRef] [PubMed]
- Budimir, J.; Treffon, K.; Nair, A.; Thurow, C.; Gatz, C. Redox-active cysteines in TGACG-BINDING FACTOR 1 (TGA1) do not play a role in salicylic acid or pathogen-induced expression of TGA1-regulated target genes in Arabidopsis thaliana. New Phytol. 2021, 230, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Gatz, C. From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant Microbe Interact. 2013, 26, 151–159. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Li, Z.; Feng, M.; Ge, W.; Zhong, C.; Xue, R. Genome-wide identification of the TGA genes in common bean (Phaseolus vulgaris) and revealing their functions in response to Fusarium oxysporum f. sp. phaseoli infection. Front. Genet. 2023, 14, 1137634. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, C.; Liu, L.; Huang, D.; Ma, C.; Li, R.; Huang, L. Genome-wide identification of the TGA gene family in kiwifruit (Actinidia chinensis spp.) and revealing its roles in response to Pseudomonas syringae pv. actinidiae (Psa) infection. Int. J. Biol. Macromol. 2022, 222, 101–113. [Google Scholar] [CrossRef]
- Lawaju, B.; Lawrence, K.; Lawrence, G.; Klink, V. Harpin-inducible defense signaling components impair infection by the ascomycete Macrophomina phaseolina. Plant Physiol. Biochem. 2018, 129, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Huang, M.; Hu, X.; Zhang, Y.; Wang, Y.; Li, P.; Chen, S.; Zhang, D.; Cao, S.; Zhu, W.; et al. A Ralstonia solanacearum effector targets TGA transcription factors to subvert salicylic acid signaling. Plant Cell 2022, 34, 1666–1683. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.-Q.; Chen, X.-L.; Zhang, H.; Chai, X.-F.; Jiang, J.-B.; Xu, X.-Y.; Li, J.-F. Transcriptome analysis of the Cf-12-mediated resistance response to Cladosporium fulvum in Tomato. Front. Plant Sci. 2017, 7, 2012. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Shen, Y.; Wang, X.; Zhang, S.; Li, Y.; Islam, M.; Wang, J.; Zhao, P.; Zhan, X.; Zhang, F.; et al. A new NLR gene for resistance to tomato spotted wilt virus in tomato (Solanum lycopersicum). Theor. Appl. Genet. 2022, 135, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Lam, E.; Chua, N.-H. Two tobacco dna-binding proteins with homology to the nuclear factor CREB. Nature 1989, 340, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Liu, Y.; Schiff, M.; Dinesh-Kumar, S.; Martin, G. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003, 36, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Lemaire-Chamley, M.; Koutouan, C.; Jorly, J.; Assali, J.; Yoshida, T.; Nogueira, M.; Tohge, T.; Ferrand, C.; Peres, L.; Asamizu, E.; et al. A chimeric TGA repressor slows down fruit maturation and ripening in tomato. Plant Cell Physiol. 2022, 63, 120–134. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Mysore, K.S. Tobacco rattle virus-based virus-induced gene silencing in Nicotiana benthamiana. Nat. Protoc. 2014, 9, 1549–1562. [Google Scholar] [CrossRef]
- Shi, G.; Hao, M.; Tian, B.; Cao, G.; Wei, F.; Xie, Z. A methodological advance of tobacco rattle virus-induced gene silencing for functional genomics in plants. Front. Plant Sci. 2021, 12, 671091. [Google Scholar] [CrossRef]
- Singh, A.K.; Ghosh, D.; Chakraborty, S. Optimization of Tobacco Rattle Virus (TRV)-based virus-induced gene silencing (VIGS) in tomato. Methods Mol. Biol. 2022, 2408, 133–145. [Google Scholar] [CrossRef]
- de Ilarduya, O.M.; Nombela, G.; Hwang, C.; Williamson, V.; Muniz, M.; Kaloshian, I. Rme1 is necessary for Mi-1-mediated resistance and acts early in the resistance pathway. Mol. Plant Microbe Interact. 2004, 17, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Laterrot, H. Near isogenic tomato lines in Moneymaker type with different genes for disease resistances. Rep. Tomato Gen. Coop. 1987, 37, 91. [Google Scholar]
- Ho, J.; Weide, R.; Ma, H.; Vanwoedragen, M.; Lambert, K.; Koornneef, M.; Zabel, P.; Williamson, V. The Root-knot nematode resistance gene (Mi) in tomato-construction of a molecular linkage map and identification of dominant cDNA markers in resistant genotypes. Plant J. 1992, 2, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burch-Smith, T.; Schiff, M.; Feng, S.; Dinesh-Kumar, S. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 2004, 279, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.; Fenoll, C.; Escobar, C. Evaluation of different RNA extraction methods for small quantities of plant tissue: Combined effects of reagent type and homogenization procedure on RNA quality-integrity and yield. Physiol. Plant. 2006, 128, 1–7. [Google Scholar] [CrossRef]
- Omwega, C.; Thomason, I.; Roberts, P. A nondestructive technique for screening bean germplasm for resistance to the nematode Meloidogyne incognita utilizing a nontoxic egg mass stain. J. Nematol. 1988, 20, 654. [Google Scholar]
- Zhou, X.; Liu, J.; Bao, S.; Yang, Y.; Zhuang, Y. Molecular cloning and characterization of a wild eggplant Solanum aculeatissimum NBS-LRR gene, involved in plant resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2018, 19, 583. [Google Scholar] [CrossRef]
- Lu, R.; Malcuit, I.; Moffett, P.; Ruiz, M.; Peart, J.; Wu, A.; Rathjen, J.; Bendahmane, A.; Day, L.; Baulcombe, D. High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 2003, 22, 5690–5699. [Google Scholar] [CrossRef]
- Droge-Laser, W.; Snoek, B.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Kogel, K.; Langen, G. Induced disease resistance and gene expression in cereals. Cell. Microbiol. 2005, 7, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.; Tada, Y.; Loake, G. Post-translational protein modification as a tool for transcription reprogramming. New Phytol. 2010, 186, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Shearer, H.L.; Cheng, Y.T.; Wang, L.; Liu, J.; Boyle, P.; Després, C.; Zhang, Y.; Li, X.; Fobert, P.R. Arabidopsis clade I TGA transcription factors regulate plant defenses in an NPR1-independent fashion. Mol. Plant Microbe Interact. 2012, 25, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Despres, C.; DeLong, C.; Glaze, S.; Liu, E.; Fobert, P. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 2000, 12, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Delaney, T. Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. Plant J. 2002, 32, 151–163. [Google Scholar] [CrossRef]
- Shearer, H.; Wang, L.; DeLong, C.; Despres, C.; Fobert, P. NPR1 enhances the DNA binding activity of the Arabidopsis bZIP transcription factor TGA7. Botany 2009, 87, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Kinkema, M.; Li, X.; Dong, X. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6523–6528. [Google Scholar] [CrossRef]
- Zhou, J.; Trifa, Y.; Silva, H.; Pontier, D.; Lam, E.; Shah, J.; Klessig, D. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol. Plant Microbe Interact. 2000, 13, 191–202. [Google Scholar] [CrossRef]
- Zhou, P.; Zavaliev, R.; Xiang, Y.; Dong, X. Seeing is believing: Understanding functions of NPR1 and its paralogs in plant immunity through cellular and structural analyses. Curr. Opin. Plant Biol. 2023, 73, 102352. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Kumar, S.; Zavaliev, R.; Wu, Q.; Zhou, Y.; Cheng, J.; Dillard, L.; Powers, J.; Withers, J.; Zhao, J.; Guan, Z.; et al. Structural basis of NPR1 in activating plant immunity. Nature 2022, 605, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.; Fitzgerald, H.; Yadav, R.; Canlas, P.; Dong, X.; Ronald, P. Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J. 2001, 27, 101–113. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Xie, Q.-G.; Mantelin, S.; Bishnoi, U.; Girke, T.; Navarre, D.A.; Kaloshian, I. Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant Microbe Interact. 2008, 21, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Bhattarai, K.; Jhaveri, T.; Kaloshian, I. Mi-1-mediated resistance to Meloidogyne incognita in tomato may not rely on ethylene but hormone perception through ETR3 participates in limiting nematode infection in a susceptible host. PLoS ONE 2013, 8, e63281. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-C.; Kaloshian, I. The tomato leucine-rich repeat receptor-like kinases SlSERK3A and SlSERK3B have overlapping functions in bacterial and nematode innate immunity. PLoS ONE 2014, 9, e93302. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence | Fragment Length a | SGN b | Source |
|---|---|---|---|---|---|
| Mi-1 | Mi-RT-F | 5′-AGAGGAGGGAACGATCTTCAGA-3′ | 78 | U564276 | [32] |
| Mi-RT-R | 5′-AAGCAAAGTTCAACCAAAATGCT-3′ | ||||
| TGA 1a | TGA 1a-RT-F | 5′-GGGCTTACTCGCCTTGGGGG-3′ | 281 | U570984 | [49] |
| TGA 1a-RT-R | 5′-CCAACGCGTTCAAAAGTTGCTCGG-3′ | ||||
| TGA 2.1 | TGA 2.1-RT-F | 5′-CCAGACAATCAGCCCGTGCTCT-′ | 228 | U571820 | [49] |
| TGA 2.1-RT-R | 5′-TTCTGCACTTCCACAAGCACATACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, S.; Emiliozzi, M.; Nombela, G. Role of Two Transcription Factors (TGA 1a and TGA 2.1) in the Mi-1-Mediated Resistance of Tomato to the Root-Knot Nematode Meloidogyne javanica. Horticulturae 2024, 10, 134. https://doi.org/10.3390/horticulturae10020134
Pascual S, Emiliozzi M, Nombela G. Role of Two Transcription Factors (TGA 1a and TGA 2.1) in the Mi-1-Mediated Resistance of Tomato to the Root-Knot Nematode Meloidogyne javanica. Horticulturae. 2024; 10(2):134. https://doi.org/10.3390/horticulturae10020134
Chicago/Turabian StylePascual, Susana, Mariana Emiliozzi, and Gloria Nombela. 2024. "Role of Two Transcription Factors (TGA 1a and TGA 2.1) in the Mi-1-Mediated Resistance of Tomato to the Root-Knot Nematode Meloidogyne javanica" Horticulturae 10, no. 2: 134. https://doi.org/10.3390/horticulturae10020134
APA StylePascual, S., Emiliozzi, M., & Nombela, G. (2024). Role of Two Transcription Factors (TGA 1a and TGA 2.1) in the Mi-1-Mediated Resistance of Tomato to the Root-Knot Nematode Meloidogyne javanica. Horticulturae, 10(2), 134. https://doi.org/10.3390/horticulturae10020134









