Abstract
Allelopathy, a biological phenomenon where plants release chemicals that influence the growth and development of neighboring flora, offers potential natural alternatives for weed management in agriculture. This study investigated the allelopathic effects of Heliotropium indicum leaf and root extracts on the germination, growth, and biochemical parameters of eight cucurbit crops. Results demonstrated that H. indicum extracts generally inhibited seed germination across all cucurbit species, with aqueous extracts showing a stronger effect than methanol extracts in Lagenaria siceraria at 10.66 ± 0.46% (p < 0.001). The treatment also variably affected shoot and root growth, indicating both inhibitory and stimulatory actions, depending on the crop species and extract type. For instance, in methanol extract treatments, Benincasa hispida’s shoot length was significantly reduced, compared to the control, at 2.1 ± 0.14 cm (p < 0.001). Notably, aqueous leaf extracts enhanced chlorophyll content more effectively than root extracts, suggesting a potential application as a biostimulant, to improve photosynthetic efficiency in certain species, for example, Momordica charantia (p < 0.05). The study further explored the antioxidative potential of the extracts by measuring MDA levels, a biomarker for oxidative stress. MDA levels increased significantly across all species in response to both types of extracts, indicating oxidative stress (L. siceraria p < 0.05). These findings suggest the potential of H. indicum extracts as natural herbicides and biostimulants, though their effects are species-specific and dependent on concentration. This research contributes to understanding the complex interactions in plant allelopathy, and highlights the potential of plant-derived extracts in sustainable agriculture.
1. Introduction
Allelopathy describes the biological phenomenon where plants release biochemicals, known as allelochemicals, into their environment to influence the growth of neighboring flora. These compounds, which can originate from plants, microbes, viruses, and fungi, serve a dual role; they can either stimulate or suppress the development of agricultural systems. This influence excludes animal systems, and primarily pertains to plant growth and ecological balance [1]. Sustainable agriculture aims to balance the need for food production with the preservation of environmental health. In this context, allelopathy offers a natural means of controlling weeds, pests, and diseases, reducing the reliance on chemical herbicides and pesticides. For example, the use of cover crops with strong allelopathic properties can suppress weed growth, thereby minimizing the need for chemical weed control [2]. Weeds are a significant challenge in agriculture, particularly in organic farming systems, where they can drastically reduce crop yields [3]. According to Lemerle et al. [4], crop productivity is diminished by weeds by as much as 5% in the most developed nations and up to 25% in the least developed ones. The pervasive issue of weeds has increased reliance on pesticides, including herbicides, to safeguard crops. However, Fishel [5] notes that the overuse of herbicides has given rise to weed resistance, undermining their effectiveness and necessitating the search for alternative weed management strategies. The exploration of allelopathy as a natural herbicide is well-founded, and the allelopathic potential of various crops and trees can be utilized to develop more sustainable weed control methods [6,7]. The issue of weeds extends beyond the loss of crop yields; as stated by Kubiak, Wolna-Maruwka et al. [8], the rapid expansion of agriculture and the associated import and export of plants have inadvertently introduced invasive weeds. These weeds, now recognized as a significant threat to biodiversity, release allelochemicals that can be detrimental to crop and plantation growth. They can inhibit seed germination, disrupt the synthesis of essential proteins and carbohydrates, and impair metabolic pathways, resulting in diminished plant vigor and reduced agricultural output [9,10]. The allelopathic properties of certain weeds have been studied extensively. For instance, Heliotropium species have been reported to exhibit inhibitory effects on the germination and growth of various seeds. Aqueous extract of H. europaeum leaves impede the germination of Cuscuta campestris (dodder) and Raphanus raphanistrum subsp. sativus (radish) seeds [11], while ethanol extract of H. indicum aerial parts could suppress the germination and growth of Lactuca sativa (lettuce) and Vigna mungo (black gram) [12]. However, the specific growth-inhibitory substances within H. indicum have not been isolated, highlighting an area ripe for further study. The concentration and efficacy of allelochemicals are influenced by many factors, including biotic and abiotic stressors, and they vary according to the plant’s age, cultivar, and specific organ. The action mechanisms of crops and allelochemicals are complex and not fully understood; some, such as sorgoleone, have been identified as photosynthesis inhibitors [13]. Notably, contemporary crop cultivars tend to produce fewer allelochemicals than their wild relatives, a consequence of selective breeding for yield rather than defense [6].
The cucurbitaceous vegetables are a significant group in the vegetable kingdom, adapting well to various environments, from arid to humid tropics [14]. These plants are known for their richness in carotenoids, terpenoids, saponins, and phytochemicals. Cucurbit vegetables have been found to have positive effects on human health, with studies showing antioxidant, antidiabetic, anti-inflammatory, and purgative properties [15]. Cucurbit cultivation is thriving in Bangladesh, with 2.57% of the land dedicated to it, yielding 3.73 million tons annually, helping address summer vegetable shortages [14,16].
However, the production of cucurbits faces challenges such as weed infestation, diseases, and insect pests. In some areas, farmers in Bangladesh spend about 25% of their cultivation costs solely on purchasing toxic pesticides [17]. The repeated and prolonged use of these toxic insecticides has drawbacks, including high application costs, environmental pollution, and health hazards [18]. Despite this, there is a lack of knowledge regarding weed allelopathy and its impact on cucurbit crops. It is crucial to fill this knowledge gap in order to better understand how different species of cucurbits respond to weeds. Considering the harmful effects of chemicals on non-target organisms and the environment, it is urgent to develop safer, cheaper, and eco-friendly weed management tools for cucurbit crops in Bangladesh. Hence, the present study aims to investigate the allelopathic effects of H. indicum on various cucurbit crops by focusing on the impact of leaf and root extracts on seed germination and subsequent seedling growth.
2. Materials and Methods
2.1. Sample Collection and Processing
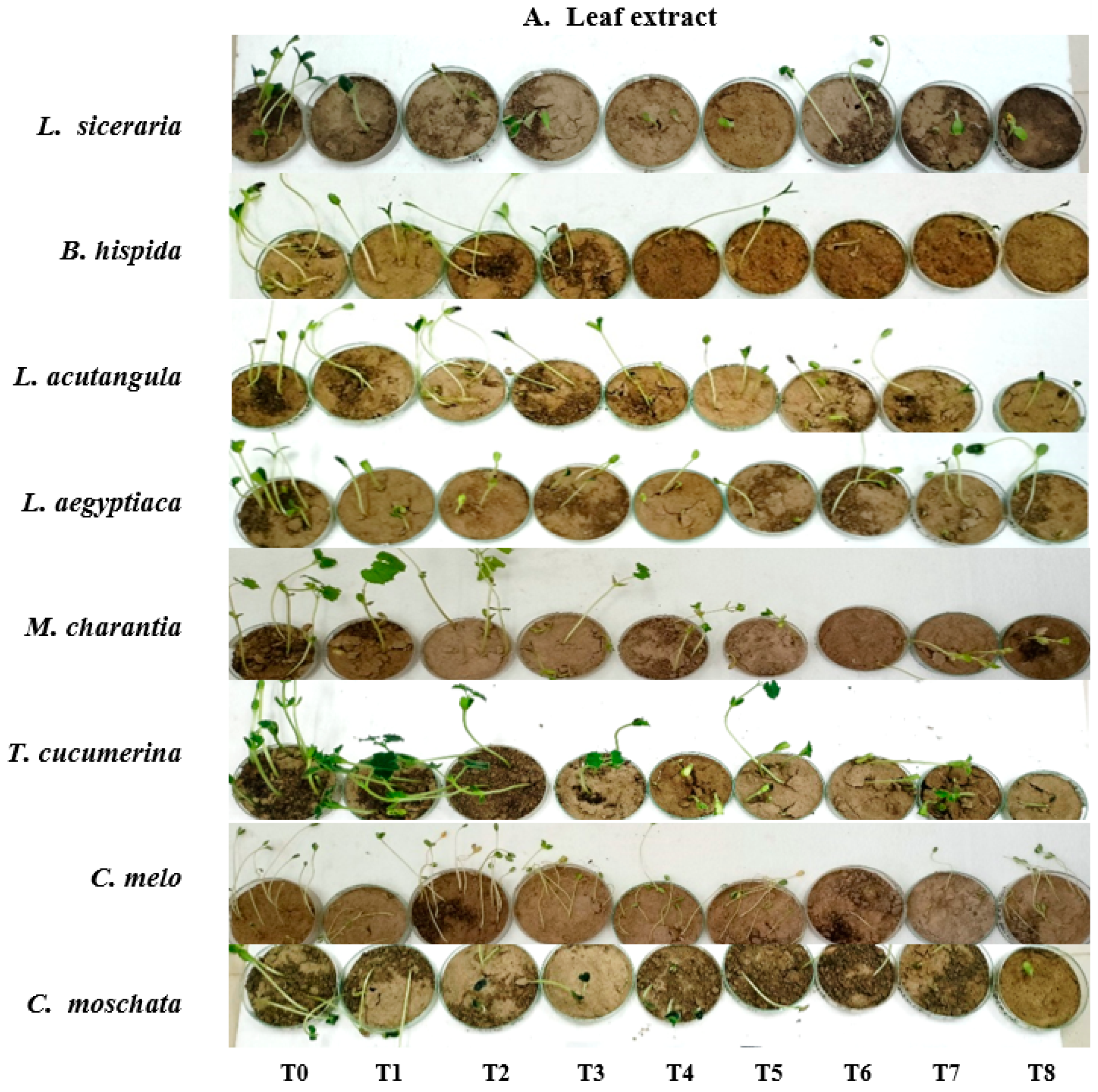
In this study, seed viability was assessed using a float test, where 100 seeds from each of eight cucurbit varieties (Figure 1), purchased from a local hypermarket, were submerged in distilled water in a 200 mL beaker for 5 to 10 min. The viability of seeds was determined by their buoyancy, with floating seeds considered non-viable and sinking seeds viable, and showed a viability percentage ranging from 90–95%. Concurrently, fresh leaves and roots of H. indicum (Figure 2) were collected from the botanical garden at the University of Chittagong. All experimental procedures were carried out at the Department of Botany, University of Chittagong, Chattogram, Bangladesh.

Figure 1.
(A) Lagenaria siceraria (bottle gourd); (B) Benincasa hispida (Winter melon); (C) Luffa acutangula (Ridged gourd); (D) Luffa aegyptiaca (Sponge gourd); (E) Momordica charantia (Bitter gourd); (F) Trichosanthes cucumerina (Snake Gourd); (G) Cucumis melo (Muskmelon); and (H) Cucurbita moschata (Pumpkin).

Figure 2.
(A) Whole plant of H. indicum (B), Leaf (left) and root (right).
2.2. Preparation of Aqueous and Methanolic Extracts of Leaf and Root
In the present experiment, 200 g of fresh H. indicum leaf and roots were washed in distilled water to remove dust and other contaminants, and kept in a room temperature of 28–30° to air dry for 24 h (avoiding direct sunlight) until removal of moisture; they were then dried in the oven at 80 °C for 48 h. Then the dried leaves and roots were ground to powder, using an electric grinder, and sieved through an 8.0 mm aperture-size wire-mesh net screen. For sterilization, all the glass jars and beakers were put in an oven at 180° for about 15 min. Ground samples were stored in the airtight glass jars until further use. The powder of the leaves and roots were then soaked in the quantity of 5, 10, 15, and 20 g, separately, in 100 mL of distilled water and 80% methanol for 24 h at room temperature, and stirred continuously. The solutions were filtered through a 2 mm mesh sieve to discard undissolved large particles, and then centrifuged at 3500 rpm for 15 min. Both extracts were stored in conical flasks and refrigerated at 4 °C until further experimentation.
2.3. Preparation of Petri Dishes and Seed Sowing
A total of 64 Petri dishes, each with a diameter of 9 cm, were thoroughly washed, dried, and sterilized in an oven at a temperature of 180 °C for one hour. Inside each Petri dish, a Whatman No.1 filter paper was placed, followed by the addition of garden soil collected from the botanical garden, University of Chittagong. Subsequently, ten seeds were soaked with various aqueous treatments for 24 h and sown in each dish, ensuring equal spacing between the seeds. The experiment was conducted under controlled conditions, with all Petri dishes maintained at a constant room temperature of 23 °C. This setup was preserved for a duration of 9 days, to facilitate the germination process. The experimental design was categorized into eight treatments (T1 to T8).Treatments T1 to T4 consisted of aqueous extracts derived from the leaves and roots, while T5 to T8 comprised methanolic extracts from the same parts of H. indicum. Additionally, a control group (T0) was established, using seeds soaked in distilled water. Throughout the experiment, each dish, including the control, received 5 mL of distilled water to maintain consistent soil moisture. Seed germination was assessed based on the emergence of a radicle exceeding 2 mm in length. After 9 days, the experiment was evaluated for various biochemical markers, including the count of germinated seeds and the growth measurements of roots and shoots. The efficacy of each treatment was quantified using the final germination percentage (FGP), calculated using the formula:
FGP = (Number of seeds germinated/Total number of seeds sown) × 100
This calculation provided a precise measure of germination success for each treatment compared to the control group.
2.4. Measurement of Chlorophyll Content
After 9 days, for each species of crop plant, 0.1 g of fresh leaves was placed in test tubes containing 10 mL of 80% acetone. The tubes were then agitated overnight, using an electric horizontal shaker. The supernatant’s absorbance was measured at wavelengths of 663 nm and 645 nm, using a spectrophotometer (Pharma Spec, UV-Vis 2000, Shimadzu, Japan). The contents of chlorophyll a (Chl a) and chlorophyll b (Chl b) were measured using the equations developed by Arnon [19].
Chl a (mg/g−1) = 12.21 A663 − 2.81 A646
Chl b (mg/g−1) =20.13 A646 − 5.03 A663
A = Absorbance
2.5. Determination of LPO
The lipid peroxidation (LPO) method was adapted from Högberg et al. [20]. In this process, tissue samples were precisely weighed to 1 g and homogenized in 0.15 mol/L cold KCl, using a Yuexin Yuqi FSH-2A homogenizer. The homogenate volume was brought to 2 mL with the addition of 0.3 M Tris-HCL buffer (pH 7.4) and 0.02 mM sodium pyrophosphate. This mixture, containing 0.2 mL of the tissue homogenate, was then incubated at 37 °C in a water bath for 30 min. The reaction was halted by introducing 1 mL of 10% trichloroacetic acid (TCA), and incubated again. After a brief vortex, 1.5 mL of thiobarbituric acid (TBA) was added, and the samples were heated in a boiling water bath for 20 min. This procedure was replicated in three separate trials. Post centrifugation, the formation of color in the reaction mixtures was measured spectrophotometrically at 532 nm. The levels of malondialdehyde (MDA), which interacts with thiobarbituric acid, were quantified and expressed as nmol MDA per mg of protein.
2.6. Statistical Analysis
The experiment was conducted thrice, and the data were analyzed using GraphPad Prism Data Editor for Windows, specifically Version 8.4.3, and by applying Dunnett’s multiple comparison tests, which encompass both one-way and two-way ANOVA. The results were reported as the mean, standard deviation (SD), and p-values. Significance was determined with a threshold of p < 0.05.
3. Results
Table 1 shows that most cucurbit crops had high germination percentages for the control groups (T0), with the exception of L. siceraria (70.00 ± 0.81) and B. hispida (60.00 ± 0.71). Other cucurbit crops that showed high percentages were L. acutangula (80.00 ± 0.76), L. aegyptiaca (90.00 ± 0.80), M. charantia (90.00 ± 0.85), T. cucumerina (100.00 ± 0.00), C. melo (100.00 ± 0.00), and C. moschata (60.00 ± 0.78). Treatment groups T1 to T4, which were treated with aqueous extract (AQE), generally showed a reduction in germination percentage across all crops, compared to the control. For example, L. siceraria dropped to 10.66 ± 0.46% in T1 and remained around this value through T4, indicating a potential inhibitory effect of the aqueous extract on germination (Figure 3).

Table 1.
Germination percent of eight cucurbit crops exposed to distilled water (T0) and different concentrations of aqueous and methanol leaf extracts of H. indicum at 9 days.

Figure 3.
The inhibitory effect of H. indicum aqueous & and methanol leaf (A) and root (B) extracts on germination of cucurbits.
The methanol extract (MTE) treatment groups T5 to T9 showed similar variable percentages of germination (Figure 3). In T5, T. cucumerina exhibited 50.00 ± 0.81% of germination, but in T4 it increased slightly to 50.66 ± 0.71%, indicating some variation in the response to the methanol extract that is statistically significant (p < 0.001), compared to the control group. The most drastic reductions in germination were observed in C. moschata, with T8 showing a germination percentage of 10.60 ± 0.47, which is notably lower than the control (Table 1 and Figure 3).
Table 2 showcases the shoot elongation of eight different receptor cucurbit crops when exposed to the control (T0) and varying concentrations of aqueous and methanol leaf extracts of H. indicum at 9 days (Figure 4). For the control treatment (T0), which is the baseline with just distilled water, the shoot lengths were as follows: L. siceraria (10.2 ± 0.16 cm), B. hispida (13.9 ± 0.08 cm), L. acutangula (13.1 ± 0.08 cm), L. aegyptiac (11.4 ± 0.08 cm), M. charantia (22.4 ± 0.08 cm), T. cucumerina (16.9 ± 0.08 cm), C. melo (12.4 ± 0.08 cm), and C. moschata (11.03 ± 0.12 cm).

Table 2.
Shoot Elongation of receptor cucurbit crops in distilled water (T0) and different concentrations of aqueous and methanol leaf extracts of H. indicum, at 9 days.

Figure 4.
The inhibitory effect of H. indicum aqueous and methanol leaf extracts on shoot growth and root growth on eight cucurbits after 9 days.
Upon treatment with aqueous extract (T1–T4), there was a general decline in shoot elongation across all crops, compared to the control. For instance, L. siceraria’s shoot length reduced from 10.2 cm in the control to 8.53 cm (p < 0.05) in T1, and had further decreased to 4.53 cm (p < 0.01) by T4.
Methanol extract treatments (T5–T9) also affected shoot elongation variably. For example, T. cucumerina had a shoot length of 11.1 cm in T5, which was lower than the control, and then further decreased to 6.1 cm in T6. Notably, L. acutangula showed an increase in shoot length in treatment T1 to 19.0 ± 0.16 cm, which is higher than the control, but this effect was not maintained in the subsequent treatments. The greatest reduction in shoot length was seen in B. hispida in treatment T6, with a length of 2.1 ± 0.14 cm (p < 0.001), significantly lower than the control length (Table 2 and Figure 4).
Table 3 outlines the effects of various treatments with aqueous and methanol H. indicum leaf extracts on the root length of eight cucurbit crops, at 9 days (Figure 4). On the other hand, the control treatment (T0), untreated shows the initial root lengths: L. siceraria (8.1 ± 0.21 cm), B. hispida (9.13 ± 0.12 cm), L. acutangula (7.06 ± 0.04 cm), L. aegyptiaca (8.1 ± 0.08 cm), M. charantia (6.06 ± 0.04 cm), T. cucumerina (5.43 ± 0.09 cm), C. melo (6 ± 0.08 cm), and C. moschata (10.23 ± 0.20 cm). For the aqueous-extract treatments (T1–T4), the root lengths varied, with some reductions and some increases, compared to the control. In the case of L. siceraria, the root length decreased in T1 to 6.46 ± 0.12 cm (p < 0.001) but had increased to 8.36 ± 0.12 cm by T4, which is close to the control length.

Table 3.
Root Length of eight cucurbit crops in distilled water (T0) and different concentrations of aqueous and methanol H. indicum leaf extracts, at 9 days.
Methanol extract treatments (T5–T9) also varied in their impact. M. charantia’s root length decreased to 3.3 ± 0.21 cm (p < 0.001) in T5, which is significantly less than the control, but by T8 the root length had increased to 9.03 ± 0.12 cm, which is comparable to the control. Notably, some treatments, like T3 for L. acutangula and T8 for B. hispida, showed an increase in root length compared to the control, with 8.0 ± 0.08 cm and 9.43 ± 0.16 cm, respectively. However, in some cases, such as B. hispida in T6, a significant reduction in root length to 4.01 ± 0.08 cm (p < 0.001) was observed, indicating a strong effect of the methanol extract at this concentration (Table 3 and Figure 4).
Table 4 reports the germination percentage of eight cucurbit crops treated with distilled water (T0) and different concentrations of aqueous and methanolic root extracts of H. indicum at 9 days (Figure 3). The control group (T0) had the following germination percentages: L. siceraria (70.00 ± 0.81), B. hispida (60.66 ± 1.69), L. acutangula (80.00 ± 0.81), L. aegyptiaca (90.00 ± 0.81), M. charantia (90.00± 0.85), T. cucumerina (90.66 ± 0.47), C. melo (90.033 ± 0.47), and C. moschata (60.00 ± 0.81). Treatments with aqueous extract (T1–T4) displayed a general decline in germination percentages for all species, compared to the control. For example, L. siceraria showed a decrease to 40.00 ± 0.77% (p < 0.05) in T1 and further, to 20.00 ± 0.78% (p < 0.05), by T4.

Table 4.
Germination percentage of eight cucurbit crops in distilled water (T0) and different concentrations of aqueous and methanolic root extracts of H. indicum, at 9 days.
Methanol extract treatments (T5–T8) continued this trend of reduced germination percentages. T. cucumerina dropped from a control value of 90.66 ± 0.85 to 10.66 ± 0.47% (p < 0.001) in T7. Certain treatments, like T8 for C. melo and L. acutangula, resulted in a substantial germination percentage decrease, compared to the control (20.33 ± 0.47% and 20.00 ± 0.81%, (p < 0.05), respectively), suggesting some specific concentrations of the extracts might not significantly affect germination (Table 4 and Figure 3).
Table 5 presents the shoot length of eight cucurbit crops when treated with distilled water (T0) and various concentrations of aqueous and methanolic root extracts of H. indicum, at 9 days (Figure 5). The control group (T0) shows standard shoot lengths: L. siceraria (10.2 ± 0.16 cm), B. hispida (14.2 ± 0.16 cm), L. acutangula (13 ± 0.08 cm), L. aegyptiaca (11.4 ± 0.08 cm), M. charantia (22.4 ± 0.08 cm), T. cucumerina (17.1 ± 0.08 cm), C. melo (12.3 ± 0.16 cm), and C. moschata (11.1 ± 0.08 cm). Treatment T1, which involved aqueous extract, increased the shoot length in L. siceraria to 14.66 ± 0.12 cm, but decreased B. hispida to 10.33 ± 0.04 cm (p < 0.01). Subsequent treatments (T2–T8) showed fluctuations in shoot lengths across the species. For instance, L. acutangula experienced a notable increase to 15.30 ± 0.08 cm at T4.

Table 5.
Shoot Length of eight cucurbit crops in distilled water (T0) and different concentrations of aqueous and methanolic root extracts of H. indicum, at 9 days.

Figure 5.
The inhibitory effect of H. indicum aqueous and methanol root extracts on shoot growth and root growth on eight cucurbits after 9 days.
Methanol extract treatments (T5–T9) also showed variability in their effects, with some treatments resulting in shoot lengths that were shorter than the control. For example, C. moschata decreased to 3.46 ± 0.12 cm (p < 0.001) at T7.
Many treatments resulted in significant changes in shoot length compared to the control, such as the decrease in M. charantia to 4.06 ± 0.04 cm (p < 0.001) at T4. Some treatments, such as T8, had less of an impact, as seen in the shoot length of L. siceraria at 3.6 ± 0.08 cm (p < 0.001), which was closer to the control measurement (Table 5 and Figure 5). Table 6 details the root length of eight cucurbit crops subjected to distilled water (control) and various concentrations of aqueous and methanolic root extracts of H. indicum, at 9 days. The control (T0) readings show the baseline root lengths for the crops: L. siceraria (8.13 ± 0.12 cm), B. hispida (9.1 ± 0.08 cm), L. acutangula (7.1 ± 0.08 cm), L. aegyptiaca (8.1 ± 0.08 cm), M. charantia (6.1 ± 0.08 cm), T. cucumerina (5.36 ± 0.09 cm), C. melo (6.13 ± 0.12 cm), and C. moschata (10.4 ± 0.08 cm). Aqueous extract treatments (T1–T4) influenced the root length in varying ways. For instance, B. hispida’s root length increased from 9.1 cm in the control to 9.16 ± 0.12 cm in T7, which was non-significant and almost the same result. In L. acutangula, the root length decreased to 4.4 ± 0.08 cm (p < 0.001) in T2, but then increased to 10.2 ± 0.08 cm in T4.

Table 6.
Root length of eight cucurbit crops in distilled water (T0) and different concentrations of aqueous and methanolic root extracts of H. indicum, at 9 days.
Methanol extract treatments (T5–T9) also showed a differential impact on root length. M. charantia’s root length decreased to 3.1 ± 0.08 cm (p < 0.001) in T7, but was higher in T4, at 10.13 ± 0.12 cm.
Notable significant changes in root length include the increase in L. siceraria to 9.13 ± 0.12 cm in T6 and the decrease in C. melo to 1.6 ± 0.08 cm (p < 0.001) in T7. A treatment like T5 did not significantly affect the root length for certain species, such as C. moschata, suggesting that the effect of the extract may vary, depending on the concentration and the specific crop (Table 6 and Figure 5).
3.1. Effect of Leaf Extract of H. indicum on Chlorophyll Content of Tested Crops
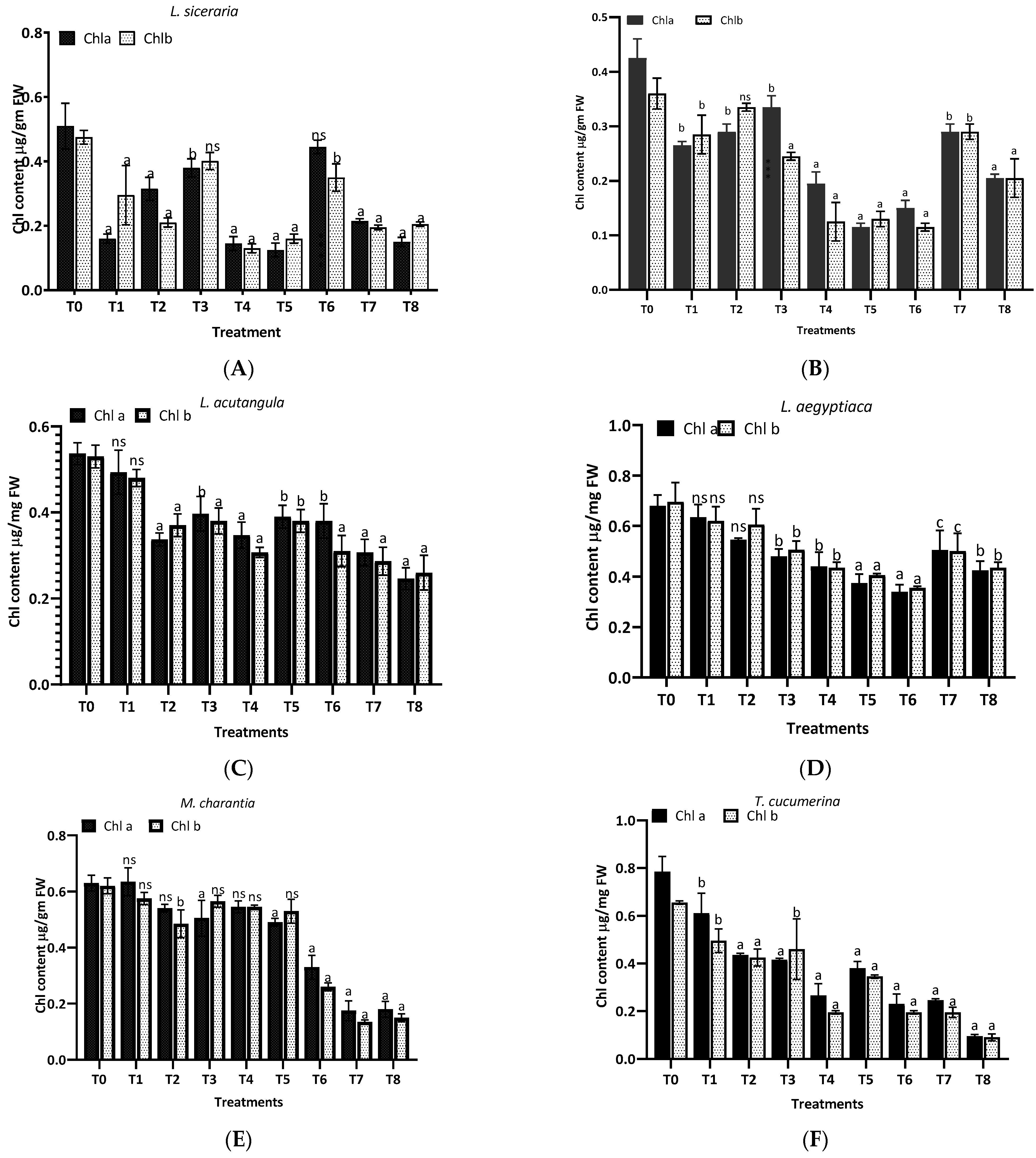
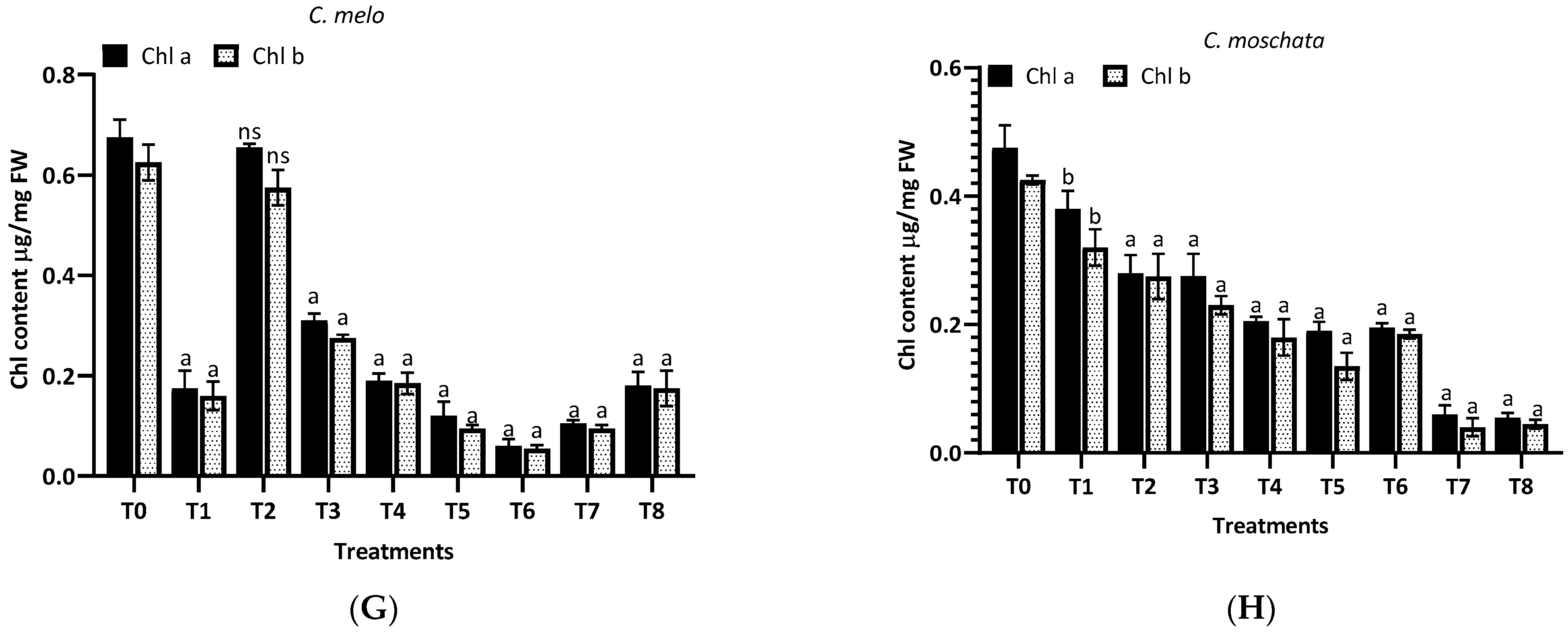
The results from Figure 6 reveal the differential impact of aqueous (T1–T4) and methanol (T5-T8) extracts of H. indicum leaves on the chlorophyll content in eight plant species. For L. siceraria (A), the treatments showed variability in the chlorophyll content, with several treatments significantly differing from the control (T0). Treatment T3 resulted in the highest chlorophyll a content, with p < 0.001, indicating a highly significant difference. Chlorophyll b also showed substantial variations, especially in T3 and T6. In the case of B. hispida (B), treatments T1, T2, T3 and T7 notably increased chlorophyll a content significantly, with significance (p < 0.001). Chlorophyll b levels followed a similar trend in the aqueous extract, but in methanolic extracts highly significant differences were found. Again, for L. acutangula (C), no significant differences (ns) were observed for any treatments in chlorophyll a and b content, suggesting that this species might be less responsive to the extracts. L. aegyptiaca (D) displayed a complex response pattern. While T5 had a highly significant effect on chlorophyll a content (p < 0.0001), T6 presented a very significant effect (p < 0.001) on chlorophyll b content. Other treatments varied, with T8 showing a significant impact (p < 0.05) on chlorophyll b.
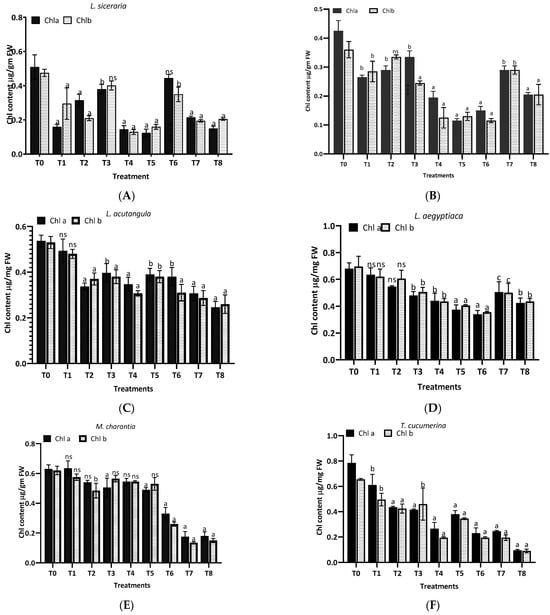
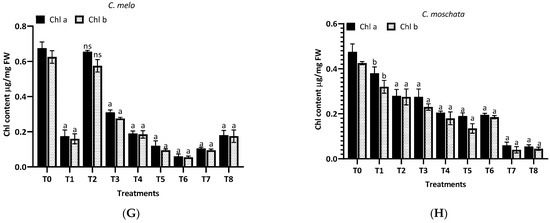
Figure 6.
Treatment of aqueous (T1–T4) and methanol (T5–T8) extracts of the leaf of H. indicum on the chlorophyl content; a = p < 0.0001, b = p < 0.001, and c = p < 0.05; ns indicates not significant. (A) (Lagenaria siceraria), (B) (Benincasa hispida), (C) (Luffa acutangula), (D) (Luffa aegyptiaca), (E) (Momordica charantia) (F) (Trichosanthes cucumerina), (G) (Cucumis melo), and (H) (Cucurbita moschata).
Furthermore, in M. charantia (E), the majority of treatments did not significantly alter chlorophyll a levels, whereas chlorophyll b levels were significantly increased by treatment T5 (b, p < 0.001). Other treatments were not significant. For T. cucumerina (F), treatments T2, T3, T5, T6, and T7 resulted in a substantial increase in chlorophyll a content (a, p < 0.0001), with T7 also showing a very significant increase in chlorophyll b content (b, p < 0.001). Treatment T8 significantly affected chlorophyll a content (c, p < 0.05). In the case of C. melo (G) exhibited significant increases in chlorophyll a content with treatments T2, T3, T5, and T6 (a, p < 0.0001), while chlorophyll b content did not significantly change with any treatment. Finally, C. moschata (H) showed a very significant increase in chlorophyll a content with treatments T3 and T7 (b, p < 0.001), and a significant increase with T5 and T6 (c, p < 0.05). Chlorophyll b content was significantly increased by treatment T7 (c, p < 0.05), with other treatments not showing significant changes.
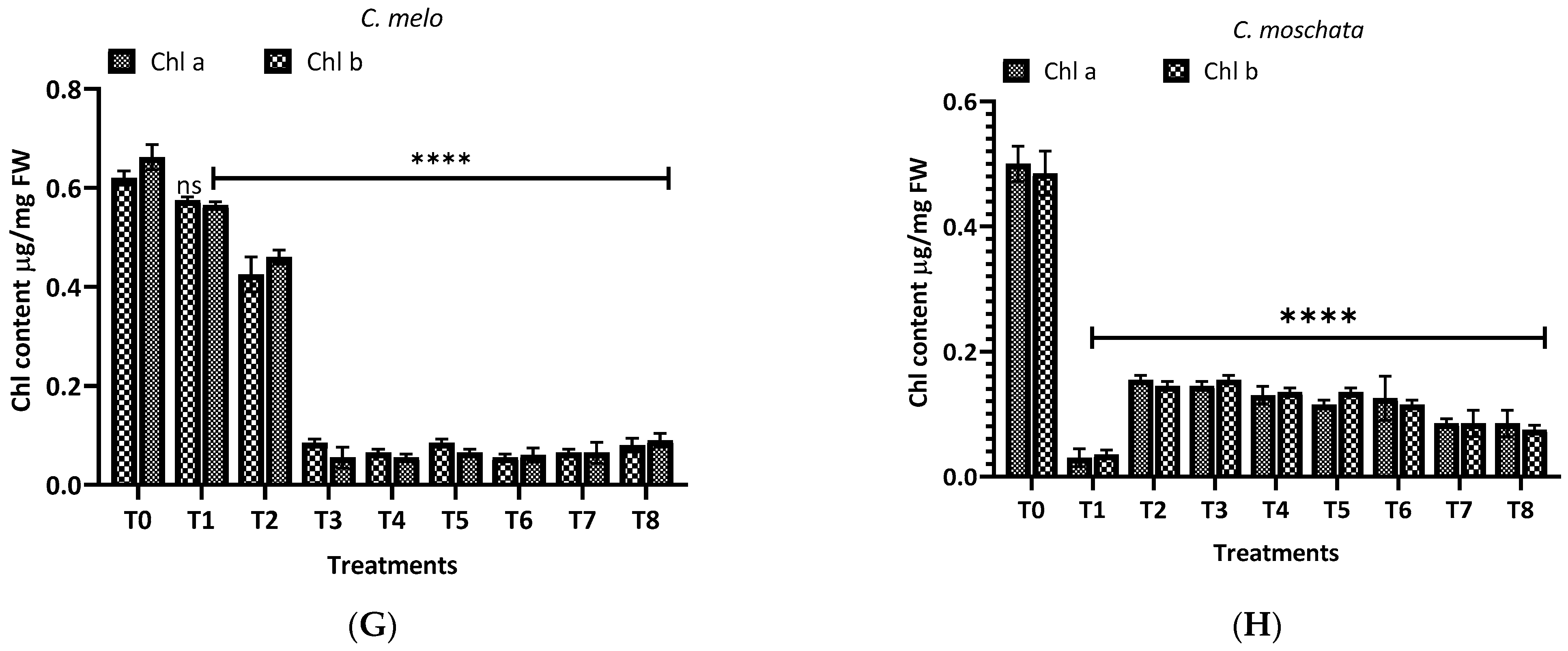
3.2. Effect of Root Extract of H. indicum on Chlorophyll Content of Tested Crops
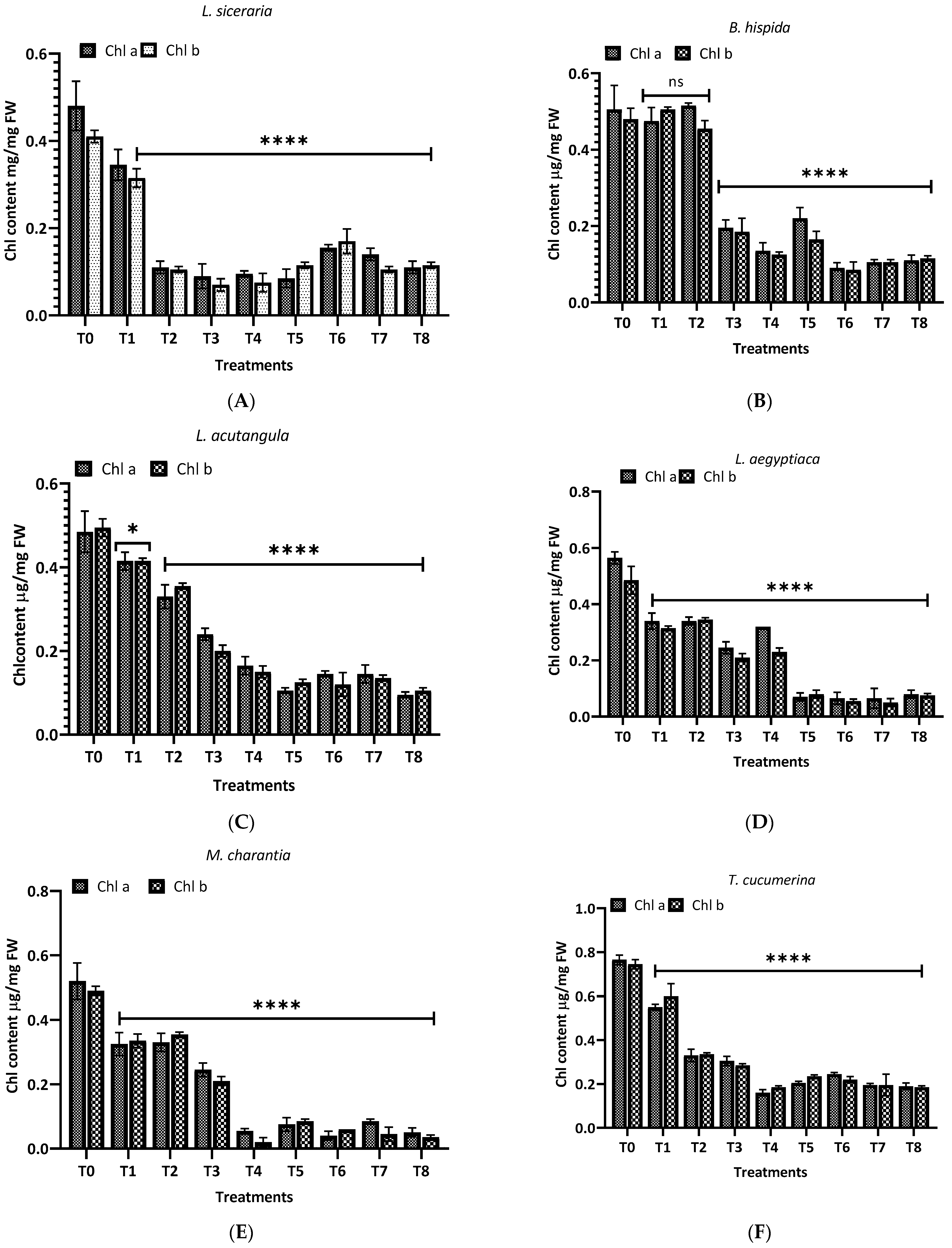
The impact of aqueous and methanol extracts of H. indicum root on the chlorophyll content of eight cucurbit plants was investigated, with the results shown in Figure 7. For L. siceraria (A), the control (T0) exhibited the highest chlorophyll a and b content. Upon treatment, a marked decline in both chlorophyll a and b was observed, with the aqueous extracts (T1–T4) showing a highly significant reduction (p < 0.0001); in the case of B. hispida (B), the chlorophyll content in the control group remained high. Interestingly, the treatments did not significantly affect the chlorophyll a and b levels at T1 and T2. Still, for chlorophyll a and b, a significant decrease was observed in the T5–T8 methanol extract treatments (p < 0.0001), suggesting a differential sensitivity between the chlorophyll types to the methanol extracts. In L. acutangular (C), the control group showed high levels of both chlorophylls. Following treatment, the chlorophyll a level showed a significant decline at T3 and T4 (p < 0.05), and a more pronounced effect at T5–T8 (p < 0.0001). Chlorophyll b levels followed a similar pattern, but with less variance among treatments. For L. aegyptiaca (D), chlorophyll levels in the control group were relatively higher, compared to treated groups. The treatments caused a significant reduction in chlorophyll content, particularly with the methanol extracts (T5–T8), where the decrease in chlorophyll a and b was highly significant (p < 0.0001).
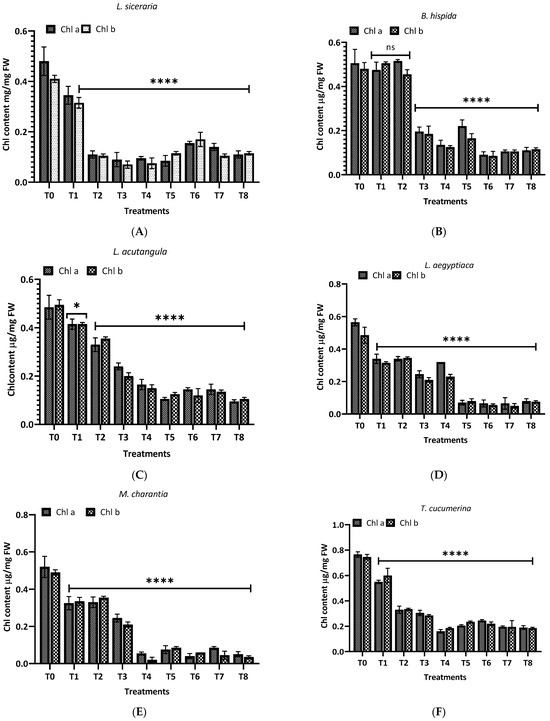
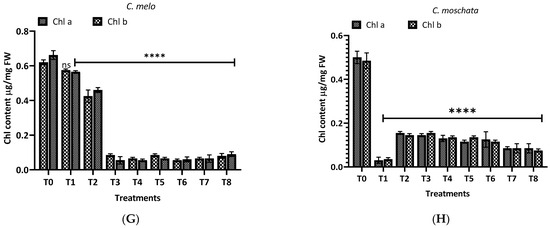
Figure 7.
Treatment of aqueous (T1–T4) and methanol (T5–T8) extracts of the root of H. indicum on the chlorophyl content of 8 cucurbit plants (A–H). * = p < 0.05; **** = p < 0.0001; T0 = control. (A) (Lagenaria siceraria), (B) (Benincasa hispida), (C) (Luffa acutangula), (D) (Luffa aegyptiaca), (E) (Momordica charantia) (F) (Trichosanthes cucumerina), (G) (Cucumis melo), and (H) (Cucurbita moschata).
Further, in M. charantia (E), the control exhibited the highest chlorophyll a and b content. A dramatic reduction in chlorophyll content was observed in response to the treatments, with the methanol extracts (T5–T8) showing a highly significant reduction (p < 0.0001) for both chlorophyll types. Again, for T. cucumerina (F), chlorophyll a and b levels in the control remained high, similar to M. charantia. Post treatment, there was a pronounced decrease in chlorophyll content, with methanol extracts (T5–T8) exhibiting a highly significant reduction (p < 0.0001) in both chlorophyll a and b. For C. melo (G), the control group showed the highest chlorophyll a and b levels. Upon treatment, chlorophyll levels did not differ significantly (ns), but chlorophyll b levels were affected considerably by methanol extracts (T5–T8), with a notable reduction (p < 0.0001). In the case of C. moschat (H), the control group showed the highest chlorophyll a and b content. Treatments led to a significant decline in chlorophyll a and b, particularly with the methanol extract treatments (T5–T8), where the decrease was highly significant (p < 0.0001).
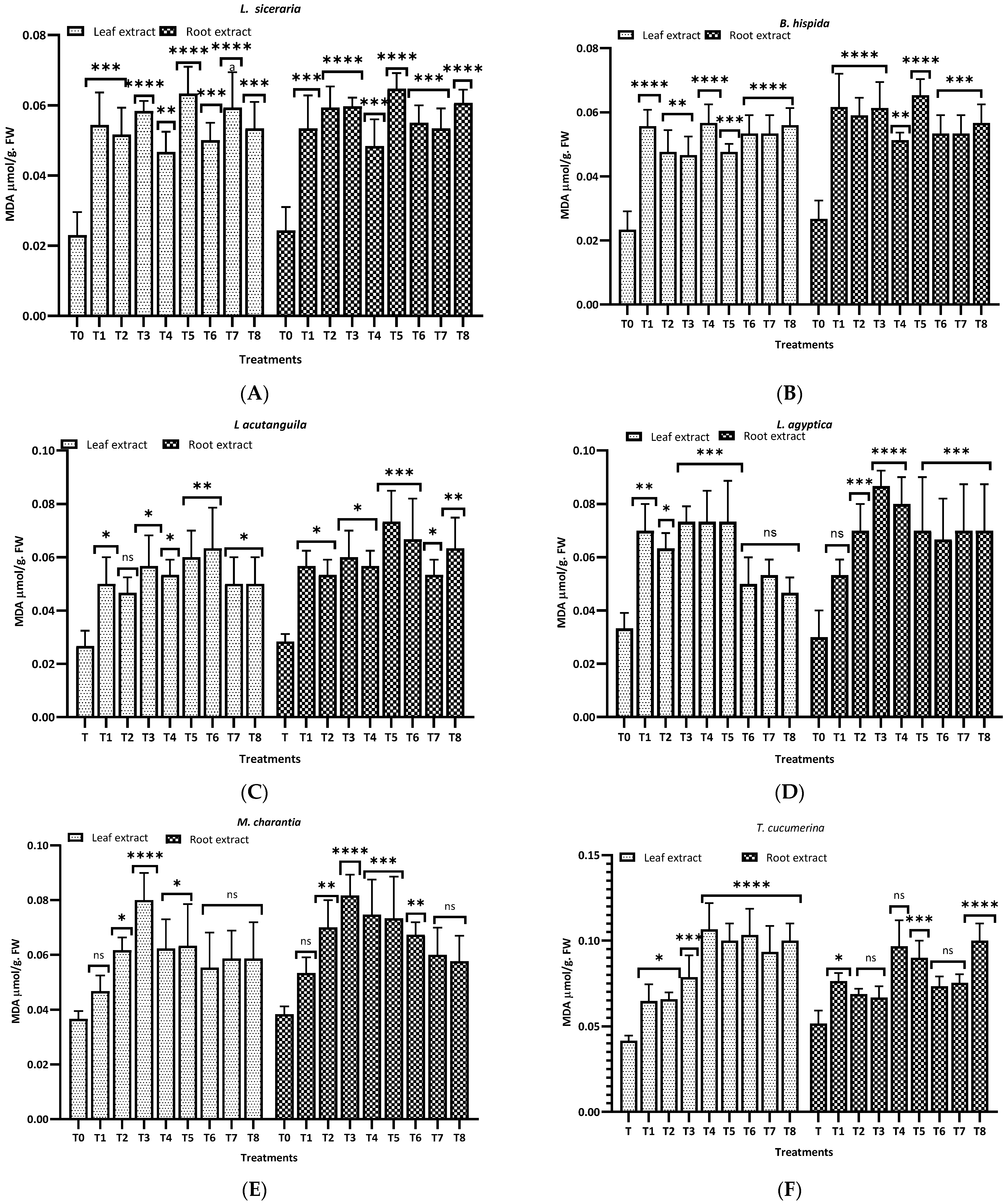
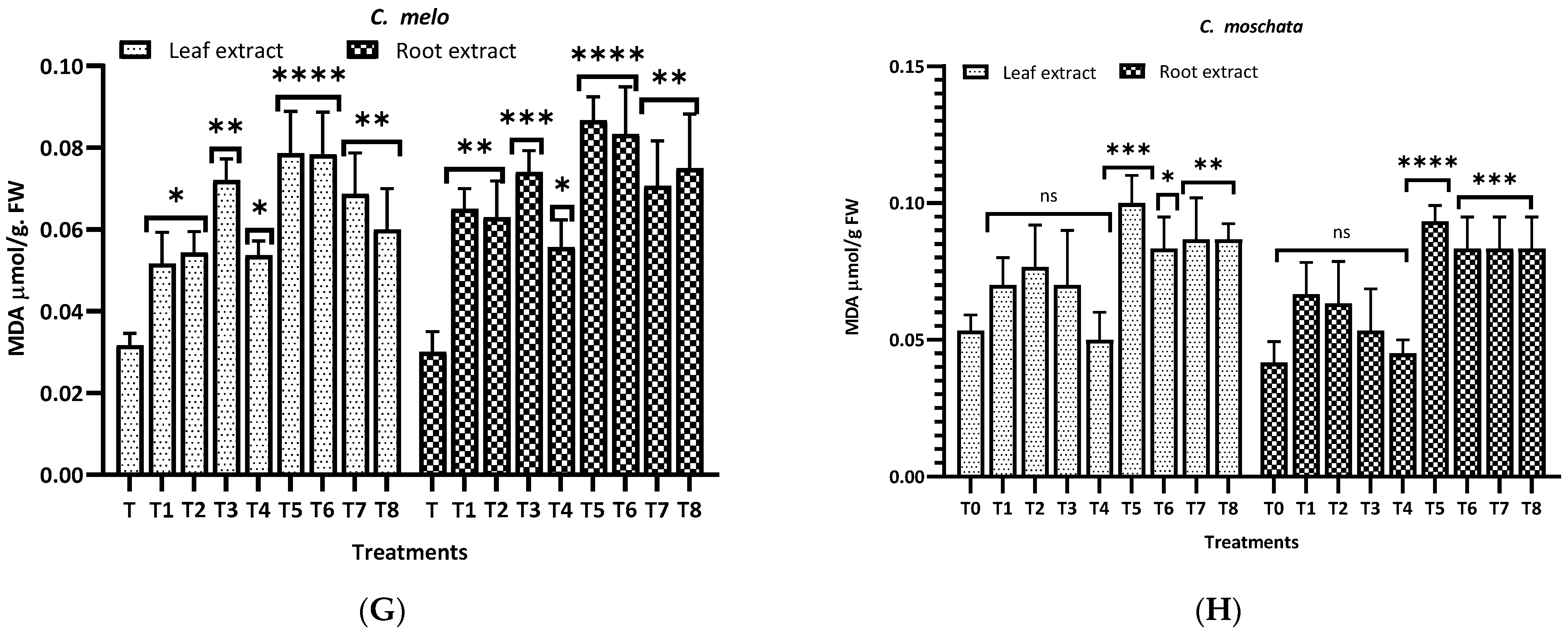
3.3. MDA Levels
Figure 8 illustrates the malondialdehyde (MDA) levels observed in this study for different plants and treatments. For L. siceraria (A), both leaf and root extracts showed a significant increase in MDA content across all treatments, compared to the control, with the highest significance observed in T3, T5, and T7 for both, and in T2 and T7 for root extracts (p < 0.0001). In B. hispida (B), the MDA levels also increased significantly in response to the treatments, with T1, T7, T4 and T8 (leaf) and T1, T2, T3 and T5 (root) showing the most significant effects (p < 0.0001). For L. acutangula (C), the results were more varied; some treatments did not significantly affect T2 (leaf) MDA levels (ns), while others, like T5, and T6 (root) showed high significance (p < 0.001). Interestingly, T1 demonstrated a significant decrease in MDA content (p < 0.05). L. aegyptiaca (D) displayed a pattern where the leaf extract treatments (T1–T4) generally resulted in higher MDA levels (p < 0.05 to p < 0.001), whereas T3 and T4 of root extracts showed extremely significant increases (p < 0.0001) of MDA levels, compared to control.
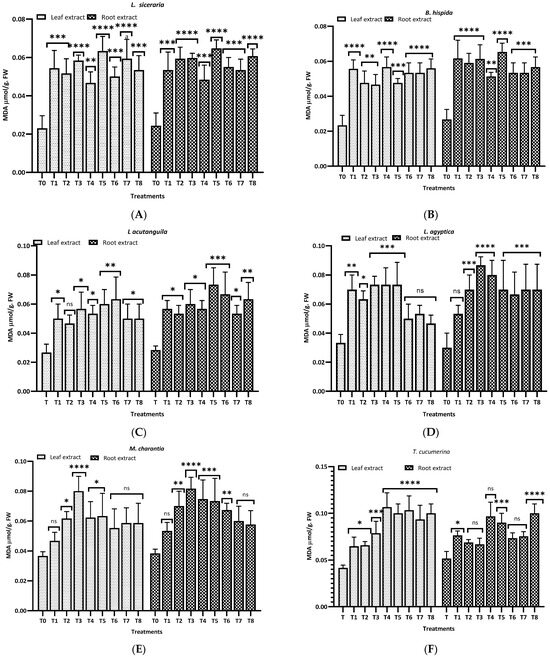
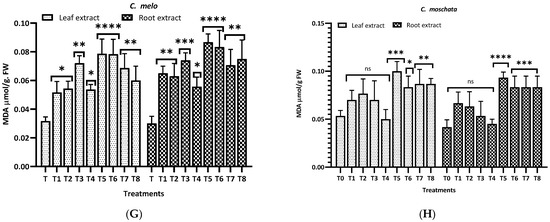
Figure 8.
Treatment of aqueous (T1–T4) and methanol (T5–T8) extracts of the leaf of H. indicum on the proline and MDA content of 8 cucurbit plants (A–H). * = p < 0.05, ** = p < 0.01, *** = p < 0.001, and **** = p < 0.0001 indicate significance levels between the treatments and control. ns = not significant. (A) (Lagenaria siceraria), (B) (Benincasa hispida), (C) (Luffa acutangula), (D) (Luffa aegyptiaca), (E) (Momordica charantia) (F) (Trichosanthes cucumerina), (G) (Cucumis melo), and (H) (Cucurbita moschata).
In M. charantia (E), significant increases in MDA content were observed in treatments T2, T4, T5, and T7 (p < 0.05 to p < 0.001), with the highest significance in T4 (p < 0.001), for both extracts. Conversely, several treatments did not significantly affect MDA levels (ns). For T. cucumerina (F), the MDA response was profound, particularly in treatments T4, T6, T7, and T8, with all showing extremely significant increases (p < 0.0001). However, some treatments did not significantly alter MDA content (ns). C. melo (G) showed significant variation in MDA levels, with the most pronounced increases in T5 and T6 (p < 0.0001) in both cases. Other treatments also significantly elevated MDA content (p < 0.01), while T1, T2 (leaf), and T4 (both) were less impactful, yet still significant (p < 0.05). Lastly, C. moschata (H) exhibited significant increases in MDA content with treatments T5 to T8 (p < 0.05 to p < 0.0001), indicating a strong response to both aqueous and methanol leaf extracts. Notably, T1 and T4 did not significantly affect the MDA levels (ns).
4. Discussion
In an integrated analysis of Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, the collective data present a multidimensional perspective on the allelopathic influence of H. indicum root extracts on cucurbit crops. The tables detail the germination percentage, shoot elongation, and root length of eight cucurbit crops, offering insight into the phytotoxic and potential phytopromotive effects of both aqueous and methanolic extracts.
4.1. Germination Percentage
Table 1 and Table 4 reveals a general trend of inhibited germination across most cucurbit species when treated with H. indicum extracts. This suppression of seed germination is consistent with the allelopathic effects reported in the literature, where plant extracts containing allelochemicals can hinder the germination of neighboring plants, a strategy that could be utilized for weed control in agricultural settings [21].
4.2. Shoot and Root Growth
Table 2, Table 3, Table 4, Table 5 and Table 6 (except for Table 4) describe varying effects on shoot and root growth. While some treatments notably reduced shoot length, suggesting an inhibitory action of the extracts, others showed no significant impact. or even increased shoot length, indicating a possible stimulatory or hormetic effect [22]. The data from Table 6 are particularly revealing, as they show a biphasic response in root length to different extract concentrations, a phenomenon well-documented in hormesis studies [23].
4.3. Species-Specific Responses
The species-specific responses highlighted in the tables underscore the selective nature of allelopathy. For instance, while L. siceraria exhibited increased root length under certain treatments, other species such as C. melo showed a decrease. This specificity suggests that the extracts contain a complex mixture of compounds, each with a distinct mode of action, and that the species vary in their sensitivity to these compounds [24,25].
4.4. Implications for Sustainable Agriculture
These findings have significant implications for sustainable agriculture. The inhibitory effects on germination and growth could be harnessed for developing natural herbicides, offering an eco-friendly alternative to synthetic options [26]. Conversely, the growth-promoting effects observed at certain concentrations and in specific species point to the potential use of H. indicum extracts as bio-stimulants, enhancing crop growth under stress conditions [27].
4.5. Biochemical Interactions and Synergistic Effects
The differential biochemical interactions between the extracts and the plant species suggest that synergistic effects may play a role in the observed results. The presence of various secondary metabolites in the extracts could interact in a synergistic or antagonistic manner, affecting the overall outcome on the plant growth [28].
The present study has demonstrated the potential of H. indicum root and leaf extracts to modulate the chlorophyll content in a range of cucurbit species. These findings contribute to the growing body of evidence supporting the phytostimulatory effect of plant extracts on photosynthetic pigments. The chlorophyll-enhancing effects of H. indicum root extracts observed in L. siceraria, B. hispida, and other cucurbit species align with those reported by [29], who found similar bio-stimulatory effects in other medicinal plants. The increased chlorophyll content could be attributed to the presence of phytochemicals in the extracts, which are known to stimulate chlorophyll synthesis or reduce chlorophyll degradation [29].
Comparing the efficacy of root and leaf extracts, it is evident that both exert a positive influence on chlorophyll content, though the aqueous leaf extracts consistently showed more pronounced effects across the species. This observation is in harmony with the findings which reported that leaf extracts, rich in growth-promoting substances like cytokinins and gibberellins, could enhance chlorophyll content more effectively than root extracts [30].
The differential response of cucurbit plants to aqueous and methanol extracts suggests that the solvent used for extraction plays a critical role in the bioavailability of active compounds. This is supported by researchers who indicated that polar solvents tend to extract a higher concentration of hydrophilic phytochemicals, which might be more effective in influencing chlorophyll content [31].
Interestingly, C. moschata and C. melo exhibited significant increases in chlorophyll content when treated with H. indicum leaf extracts. These results parallel those of research in which similar cucurbit species responded favorably to herbal extracts, suggesting a species-specific affinity for certain phytochemicals that promote photosynthetic activity [32].
However, the responses of L. aegyptiaca and T. cucumerina to the methanol extracts were comparatively less pronounced, which may indicate a variation in the extract’s phytochemical profile, as noted by other researchers. The lower chlorophyll enhancement could also result from the presence of compounds in methanol extracts that may inhibit chlorophyll biosynthesis or accelerate chlorophyll breakdown [33].
The application of plant extracts, such as those from H. indicum, as biostimulants in agriculture. could be a sustainable alternative to chemical treatments, reducing environmental impact and enhancing the nutritional quality of crops [34].
The present study elucidates the impact of H. indicum root and leaf extracts on the malondialdehyde (MDA) content in eight cucurbit species. MDA is a well-established biomarker for oxidative stress, often correlated with lipid peroxidation, which can adversely affect cellular functions and plant health. Allelochemicals can cause membrane lipid peroxidation by throwing off the balance between the process of making free radicals and the process of discarding them in plant tissue. MDA, also known as malondialdehyde, is a result of this mechanism. The amount of malondialdehyde (MDA) in the cucurbit crop seedlings that were treated with both extracts was much higher than in the control group. In addition, the concentration of MDA exposed to treated crops showed a significant dependence on the dosage [35]. Following our research, we found that both extracts can increase the activity of antioxidant enzymes in cucurbit seedlings, speed up the build-up of reactive oxygen species inside cells, and ultimately damage the integrity and functionality of membranes, making them more permeable. The significant increase in MDA content across all cucurbit species treated with both types of extracts suggests that H. indicum has adverse effects on crops [36].
Moreover, the study’s findings contribute to the growing interest in using plant-based biostimulants to enhance stress tolerance in crops. Such biostimulants can activate the plant’s own defense systems, potentially leading to less reliance on synthetic agrochemicals, and contributing to sustainable agriculture practices [29].
5. Conclusions
Based on the integrated analysis of H. indicum root and leaf extracts on cucurbit crops, the study demonstrates a multifaceted impact on plant growth and development. H. indicum extracts generally inhibit seed germination, suggesting their potential application as natural herbicides. However, the effects on shoot and root growth vary among species, indicating both inhibitory and potentially stimulatory actions, reflecting the complex nature of allelopathy. Notably, the extracts enhance chlorophyll content in certain cucurbit species, particularly with aqueous leaf extracts, underscoring their potential as biostimulants to improve photosynthetic efficiency. Moreover, these same extracts may lead to an elevation in MDA levels, which increased significantly across all species in response to both types of extracts, indicating oxidative stress. These findings suggest the potential of H. indicum extracts as natural herbicides and biostimulants, though their effects are species-specific and dependent on concentration. This research contributes to understanding the complex interactions in plant allelopathy and highlights the potential of plant-derived extracts in sustainable agriculture. Future research should focus on identifying specific bioactive compounds responsible for these effects, and on validating their practical applications in agriculture through field trials.
Author Contributions
Conceptualization: P.A.; data curation: P.A., T.S., R.B. and A.M.A.A.; formal analysis: P.A. and T.S.; investigation: P.A. and T.S.; supervision: P.A.; writing—original draft: P.A., T.S. and R.B.; writing—review and editing: P.A. and A.M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The article contains all of the data.
Acknowledgments
The authors would like to extend sincere thanks to the chairman of the department of Botany, University of Chittagong, Chittagong-4331, Bangladesh, for providing all laboratory facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aci, M.M.; Sidari, R.; Araniti, F.; Lupini, A. Emerging trends in allelopathy: A genetic perspective for sustainable agriculture. Agronomy 2022, 12, 2043. [Google Scholar] [CrossRef]
- Ain, Q.; Mushtaq, W.; Shadab, M.; Siddiqui, M. Allelopathy: An alternative tool for sustainable agriculture. Physiol. Mol. Biol. Plants 2023, 29, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Jabran, K.; Jabran, K. Allelopathy: Introduction and concepts. In Manipulation of Allelopathic Crops for Weed Control; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–12. [Google Scholar]
- Lemerle, D.; Verbeek, B.; Orchard, B. Ranking the ability of wheat varieties to compete with Lolium rigidum. Weed Res. 2001, 41, 197–209. [Google Scholar] [CrossRef]
- Fishel, F.M. Pesticide use trends in the US: Global comparison. EDIS 2007, 2007, 3. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Bio/Technol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Dhillon, N.P.; Srimat, S.; Laenoi, S.; Bhunchoth, A.; Phuangrat, B.; Warin, N.; Deeto, R.; Chatchawankanphanich, O.; Jom, K.N.; Sae-tan, S.; et al. Resistance to three distinct Begomovirus species in the agronomical superior tropical pumpkin line AVPU1426 developed at the world vegetable center. Agronomy 2021, 11, 1256. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The problem of weed infestation of agricultural plantations vs. the assumptions of the European biodiversity strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Abbas, T.; Nadeem, M.; Tanveer, A.; Syed, S.; Zohaib, A.; Farooq, N.; Shehzad, M. Allelopathic influence of aquatic weeds on agro-ecosystems: A review. Planta Daninha 2017, 35. [Google Scholar] [CrossRef]
- Khan, S.; Ali, K.W.; Shinwari, M.I.; Khan, R.A.; Rana, T. Environmental, ecological and evolutionary effects of weeds allelopathy. Int. J. Botany Stud. 2019, 4, 77–84. [Google Scholar]
- Abdulghader, K.; Nojavan, M.; Naghshbandi, N. Chemical stress induced by heliotrope (Heliotropium europaeum L.) allelochemicals and increased activity of antioxidant enzymes. Pak. J. Biol. Sci. PJBS 2008, 11, 915–919. [Google Scholar] [CrossRef][Green Version]
- Das, S.; Coku, A. Allelopathic and antimicrobial evaluation of two Indian weeds–Heliotropium indicum L. and Synedrella nodiflora L. Gaertn with phytochemical studies. Am. J. PharmTech Res. 2014, 4, 367–377. [Google Scholar]
- Choudhary, C.S.; Behera, B.; Raza, M.B.; Mrunalini, K.; Bhoi, T.K.; Lal, M.K.; Nongmaithem, D.; Pradhan, S.; Song, B.; Das, T.K. Mechanisms of allelopathic interactions for sustainable weed management. Rhizosphere 2023, 25, 100667. [Google Scholar] [CrossRef]
- Mondal, T.; Bachchu, M.A.A.; Ara, R.; Uddin, M.N.; Hossain Bhuyain, M.M.; Sultana, R. Monitoring and eco-friendly management of cucurbit fruit fly, Bactrocera cucurbitae (Coquillett) on bitter gourd. J. Asiat. Soc. Bangladesh Sci. 2023, 48, 67–82. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef]
- Haque, M.M.; Hoque, M.Z. Vegetable Production and Marketing Channels in Bangladesh: Present Scenario, Problems, and Prospects; Seminar Paper; Bangabandhu Sheikh Mujibur Rahman Agricultural University: Gazipur, Bangladesh, 2021. [Google Scholar]
- Nasiruddin, M.; Alam, A.K.M.; Khorsheduzzaman, A.K.M.; Rahaman, Z.; Karim, A.N.M.R.; Jasmine, H.S.; Rajott, E.G. Integrated Management of Cucurbit Fruit Fly, B. Cucurbitae Coquillett in Bangladesh. IPM CRSP Bangladesh Site Technical Bulletin No. 1. 2004, p. 16. Available online: https://www.researchgate.net/publication/265542679 (accessed on 10 January 2024).
- Saxena, S.; Chamoli, A.; Kunjwal, S.S. Pesticide Pollution and It’s Effects on Environment and Human Health: A Review. Uttar Pradesh J. Zool. 2023, 44, 33–39. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Högberg, J.; Larson, R.E.; Kristoferson, A.; Orrenius, S. NADPH-dependent reductase solubilized from microsomes by peroxidation and its activity. Biochem. Biophys. Res. Commun. 1974, 56, 836–842. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef]
- Ogunsanya, H.Y.; Motti, P.; Li, J.; Trinh, H.K.; Xu, L.; Bernaert, N.; Van Droogenbroeck, B.; Murvanidze, N.; Werbrouck, S.P.; Mangelinckx, S. Belgian endive-derived biostimulants promote shoot and root growth in vitro. Sci. Rep. 2022, 12, 8792. [Google Scholar] [CrossRef]
- Nweke, C.O.; Ogbonna, C.J. Statistical models for biphasic dose-response relationships (hormesis) in toxicological studies. Ecotoxicol. Environ. Contam. 2017, 12, 39–55. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Shan, Z.; Zhou, S.; Shah, A.; Arafat, Y.; Arif Hussain Rizvi, S.; Shao, H. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy 2023, 13, 2358. [Google Scholar] [CrossRef]
- Akter, P.; Ahmed, A.A.; Promie, F.K.; Haque, M.E. Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L. Agronomy 2023, 13, 381. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Richards, L.A.; Glassmire, A.E.; Ochsenrider, K.M.; Smilanich, A.M.; Dodson, C.D.; Jeffrey, C.S.; Dyer, L.A. Phytochemical diversity and synergistic effects on herbivores. Phytochem. Rev. 2016, 15, 1153–1166. [Google Scholar] [CrossRef]
- Sarkar, C.; Mondal, M.; Khanom, B.; Hossain, M.M.; Hossain, M.S.; Sureda, A.; Islam, M.T.; Martorell, M.; Kumar, M.; Sharifi-Rad, J. Heliotropium indicum L.: From farm to a source of bioactive compounds with therapeutic activity. Evid. Based Complement. Altern. Med. 2021, 2021, 9965481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Le Thi, H.; Teruya, T.; Suenaga, K. Two potent allelopathic substances in cucumber plants. Sci. Hortic. 2011, 129, 894–897. [Google Scholar] [CrossRef]
- Iman Radha, J.; Rawnaq Ahmed, I.; Hala Muzher, Y. Physiological responses of Helianthus anuus L. plants under allelopathic effect of Cucurbita moschata. Revis Bionatura 2023, 8, 108. [Google Scholar]
- Christ, B.; Hörtensteiner, S. Mechanism and significance of chlorophyll breakdown. J. Plant Growth Regul. 2014, 33, 4–20. [Google Scholar] [CrossRef]
- keya Tudu, C.; Dey, A.; Pandey, D.K.; Panwar, J.S.; Nandy, S. Role of plant derived extracts as biostimulants in sustainable agriculture: A detailed study on research advances, bottlenecks and future prospects. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 159–179. [Google Scholar]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. 2020, 10, 11332. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).