Abstract
The Mi-1 gene in tomato confers resistance against insects and nematodes. The mechanisms involved in the functioning of this gene are not completely known, and they differ depending on the damaging organism (insect or nematode). Transcription factors (TF) from different families are essential for plant defence, and the TGAs, members of the Basic Leucine Zipper (bZIP) TF family, are relevant in different pathosystems. In this work, the implication of TGA 1a and TGA 2.1 genes in Mi-1 resistance against the root-knot nematode Meloidogyne javanica was studied, by virus-induced gene silencing (VIGS) based on Tobacco rattle virus (TRV). Results showed that infiltration with the empty TRV vector did not alter Mi-1-mediated resistance, confirming the adequacy of this method. Silencing of the TGA 1a gene resulted in a decrease in resistance to M. javanica, as the numbers of egg masses were significantly higher than those on non-silenced plants. This decrease in resistance was similar to that caused by silencing the Mi-1 gene. However, the silencing of the TGA 2.1 gene caused a limited loss of resistance, with infestation levels intermediate between those of resistant and susceptible varieties. Thus, our results demonstrate the requirement of TGA 1a in Mi-1-mediated resistance to M. javanica, while the incomplete silencing of TGA 2.1 impaired a specific determination of its role.
1. Introduction
Plant-parasitic nematodes (PPNs) are an enormous threat to agriculture worldwide. Crop production losses attributed to nematodes were estimated between 8.8% and 14.6% [1], with a net loss of 2–7% in food-based crops [2]. Over 250 species of PPNs have been identified [3], and root-knot nematodes (RKNs), belonging to the genus Meloidogyne, are one of the most economically important groups of PPNs worldwide [3,4,5]. M. arenaria, M. incognita, M. javanica, and M. hapla are the most important species of RKNs [6], although other threatening Meloidogyne species are emerging [7,8].
Meloidogyne spp. are biotrophic sedentary endoparasites of polyphagous nature, which induce complex feeding structures in the roots of their host plants. Typical symptoms of RKN-induced plant diseases are root deformation due to nodule formation, leading to plant wilting, leaf discoloration, and stunted growth [4]. The wide host range of RKNs combined with their ubiquitous presence, present important challenges for their control [5]. Nematode control still relies on the use of synthetic compounds [9], despite the ban of most traditional nematicides due to their high toxicity to humans and the environment. Furthermore, new chemical compounds are currently still being sought for nematode management [10,11].
Host resistance is a valuable tool for the Integrated Pest Management (IPM) of horticultural crops and increasing knowledge of the plant–RKN interaction creates new possibilities for the control of these parasitic organisms [5,12,13]. The Mi-1.2 gene of tomato (Solanum lycopersicum) confers resistance against nematodes, and it is currently widely used in commercial tomato varieties [14,15]. Together with Mi-1.2, other genes, such as Mi-3 from Solanum peruvianum [16] or Mi-9 from Solanum arcanum [17], can be important resources for breeding tomato resistance against nematodes in the future. The Mi-1.2 gene, like other resistance (R) genes, encodes a coiled-coil domain nucleotide binding site leucine-rich repeat (CC-NLR) protein [18]. Mi-1.2 provides resistance not only against M. incognita, M. arenaria, and M. javanica [19,20,21,22], but also against different phloem-feeding insect pests, such as the potato aphid Macrosiphum euphorbiae, the whitefly Bemisia tabaci, and the tomato psyllid Bactericerca cockerelli [23,24,25]. Additionally, Mi-1.2-like genes can be relevant for resistance in crop plants other than tomato [26,27].
In the case of nematodes, Mi-mediated defence responses are associated with the induction of the hypersensitive response (HR), which does not occur in the case of insects [28,29]. Both HR and Reactive Oxygen Species (ROS) accumulation occur in tomato roots of Mi-1.2-resistant plants after nematode infection but not in the susceptible plants [30]. The genes HsfA1a (Class-A Heat shock factor) and Wfi1 (Whitefly induced 1) are needed for this ROS production and silencing of any of these genes compromises Mi-1.2-mediated resistance [30]. Several other genes are involved in the downstream defence signals in Mi-1.2-mediated resistance to RKNs and insects, such as Sgt1 and Hsp90-1 [31,32] and the WRKY genes SlWRKY72a and b [33]. The gene Rme1 is also required for Mi-1.2-mediated resistance and thought to function also in the early signalling cascade [34]. Common in the Mi-1-mediated resistance to nematodes and insects is the involvement of the SA-signalling pathway [35,36].
Transcription factors (TFs) from different families are essential elements in the activation of the plant defence system and therefore show great potential when it comes to improving the resistance of plants against different types of stresses [37,38,39]. TGA transcription factors are members of the Basic Leucine Zipper (bZIP) transcription factor family and are grouped into five clades according to their structure. TGA transcription factors bind the activation sequence-1 (as-1) motif in the promoter region of pathogenesis-related (PR) genes and interact with the Nonexpressor of PR Genes 1 (NPR) protein, a process affected by redox signals [40,41].
TGA transcription factors are relevant in plant defence in different pathosystems; for example, the recently reported cases of Fusarium wilt in bean (Phaseolus vulgaris) [42]; Pseudomonas syringae pv. actinidiae in kiwifruit (Actinidia chinensis spp.) [43], or Macrophomina phaseolina in soybean (Glycine max) [44]. TGAs are involved in the Mi-3-mediated resistance of S. peruvianum against the root-knot nematode M. incognita [16]. In tomato, TGA factors are important in resistance against different pathogens, such as Ralstonia solanacearum [45], Cladosporium fulvum [46], or Tomato spotted wilt virus (TSWV) [47]. TGA 1a is encoded by the first TGA gene cloned in a plant (tobacco) [48]. Both TGA 1a and TGA 2.2 transcription factors play a role in Pto-mediated resistance to Pseudomonas syringae pv. tomato in tomato [49]. TGAs are important not only in plant defence, but also in other processes such as regulation of fruit growth and ripening [50].
Virus-induced gene silencing (VIGS) is an easy and affordable technique to study functional genomics in many plants [51]. TRV is a virus with a wide host range and induces mild symptoms in infected plants, making it the virus of choice for VIGS [52,53]. VIGS has been used to study the role of different genes in Mi-1-mediated resistance. This technique was used to demonstrate the requirement of the chaperone Hsp90 in Mi-1-mediated resistance of tomato to nematodes, aphids, and whiteflies [31,32,54]. Li et al. [35] used VIGS to establish the role of MAPK kinases in Mi-1-mediated resistance to potato aphids [35]. In addition, the role of two WRKY TFs in Mi-1-mediated resistance against RKNs and potato aphids was also investigated by VIGS [33].
The potential implication of TGA TFs in Mi-1-mediated resistance to RKNs has not been studied so far. With the aim of deepening the knowledge on the mechanisms involved in this important resistance in tomato, the objective of this work is to unravel the role of TGA1a and TGA 2.2 in Mi-1-mediated resistance to M. javanica, using the TRV-based VIGS methodology.
2. Materials and Methods
2.1. Plant Material
The near-isogenic tomato cultivars Moneymaker (mi-1/mi-1) and Motelle (Mi-1/Mi-1) were used [55,56]. Seeds were germinated in vermiculite at 24 °C, L16:D8 photoperiod, and 70% RH. Ten days after germination, seedlings were transplanted into pots containing a mixture of 45% river sand, 45% pit sand, and 10% organic matter, and incubated for 10 more days at 19 °C, L16:D8 photoperiod, and 70% RH up to the time for agroinfiltration. Seedlings were watered every two days and fertilised with 3 g·L−1 Nutrichem® (Miller Chemical and Fertilizer, Hanover, PA, USA).
2.2. Nematode Culture
Infective-stage juveniles of M. javanica were obtained from roots of infested tomato plants cultivar Marmande, maintained in a growth chamber at 25 °C, L16:D8, and 70% RH. Eight weeks after initial inoculation, the roots with egg masses were washed, cut into pieces, and then macerated in a blender in 10% sodium hypochlorite solution. The resulting liquid was filtered through a strainer to discard the roots. It was then filtered sequentially through 0.070 mm and 0.025 mm pore mesh sieves, retaining the nematode eggs in the last filtration. The eggs were then placed on a circular moist filter paper in a modified Baermann funnel [34] and maintained at room temperature for 48–72 h. The amount and viability of the infective-stage juveniles were assessed by a stereoscopic microscope. The juveniles thus obtained were used to inoculate the plants after gene silencing, as described below.
2.3. VIGS Constructs
The Tobacco Rattle Virus (TRV) was used as a vector for gene silencing. The modified virus vector is composed of pTRV1, containing the replicative part of the virus, and pTRV2, in which a representative sequence of the targeted gene for silencing is introduced [57]. The virus vector constructs pTRV2:TGA 1a and pTRV2:TGA 2.1 were obtained from Dr. Dinesh Kumar [49]. The TGA 1a and TGA 2.1 inserts in the pTRV2 vector were amplified from Nicotiana benthamiana and N. tabacum. Sequencing confirmed that the amplified sequence also corresponded to S. lycopersicum orthologues [58,59]. A pTRV2:PDS construct was used as a visual control of gene silencing, as it silences the gene encoding phytoene desaturase, an enzyme involved in carotenoid biosynthesis resulting in photobleached symptoms when silenced, which was also obtained from Dr. Dinesh Kumar [58].
Each plasmid was introduced into Agrobacterium tumefaciens GV3101 and preserved as agroclones in glycerol at −80 °C. The presence of tomato inserts in the agroclones was confirmed by sequencing, performed at the University of Riverside. The pTRV2:Mi-1 construct used in this work has been described previously [32,35].
2.4. Growth of Agroclones and Agroinfiltration
Agroinfiltration was carried out 20 days after seed germination, when plants had two true leaves. Each agroclone was grown following the methodology previously described for VIGS silencing [32].
The 1:1 mixtures of pTRV1 with each pTRV2 (empty vector or with the gene to be silenced) were infiltrated into the undersides of leaflets and cotyledons using a needleless syringe, taking care to avoid any damage to the plant. After infiltration, plants were kept at 19 °C until nematode inoculation to slow plant growth and thus facilitate the spread of the virus. Plants were watered every 48 h and fertilised every 15 days with Nutrichem® (1 g·L−1). Tomato cultivars Motelle and Moneymaker plants were infiltrated with the empty vector pTRV1 plus pTRV2 (TRV) as a control. Motelle plants were infiltrated with TRV2:PDS, TRV2:Mi-1, TRV2:TGA 1a, and TRV2:TGA 1.2. Three independent experiments were carried out, with 10 plants initially set for each of the silencing constructs. The number of plants finally assessed was lower than 10 because some plants died or became useless.
2.5. Semiquantitative RT-PCR
To evaluate the level of gene expression in silenced plants, RNA was extracted from uninfested roots of infiltrated and non-infiltrated plants. Root samples were kept at −80 °C until use. RNA was extracted following the methodology by Portillo et al. [60]. Briefly, roots were crushed in a mortar with liquid nitrogen and RNA isolated using the TRI Reagent® kit (Sigma-Aldrich, St. Louis, MO, USA), following the manufacturer’s instructions. RNA concentration was measured with a Nanodrop NDI1000 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). The ratio A260/280 was used to estimate the purity of the samples, and samples with values of this ratio between 1.8 and 2 were used to synthesise cDNA. The High-Capacity cDNA Reverse Transcription® kit (Applied Biosystems, Waltham, MA, USA) was used to synthesise cDNA from 2 ng of total RNA. Table 1 lists the primers used for semiquantitative PCR. Their sequences are detailed in another work [49] or they were specifically designed by Dr. Kaloshian for a previous study of our group [32] using the Primer Express® program (Applied Biosystems). Their specificity was checked against the SGN and NCBI databases.

Table 1.
Primers used in semiquantitative RT-PCR to assess the transcription of Mi-1, TGA 1a, and TGA 2.1 genes.
The cDNA amplification by PCR consisted of an initial denaturalization at 94 °C, and three-step cycles: 30 s at 94 °C, 30 s at 62.5 °C, and 30 s at 72 °C. At the end of the amplification cycles, samples were subjected to 3 s extension at 72 °C. For each gene, 4 amplification cycles were used: 5, 15, 30, and 40 cycles. PCR products were analysed by electrophoresis in agarose gels (4% w:v).
2.6. Nematode Inoculation and Assessment of Infection Levels
Plants were inoculated 20 days after infiltration, with approximately 3000 infective-stage juveniles per plant, and maintained at 19 °C for an additional 8 weeks. Plants infiltrated with TRV2:PDS were not inoculated with nematodes.
To assess the level of infection, roots were washed with abundant running water and dried with paper. To count egg masses, roots were stained by immersion in a 0.1 g·L−1 erioglaucine (Sigma-Aldrich, St. Louis, MO, USA) solution [61] for more than 2 h, and then washed with water. Egg masses, stained green, were counted under a stereomicroscope. The number of egg masses was analysed by the non-parametric Kruskal–Wallis test and Dunn’s post test for multiple comparisons, as most data did not meet the requirements to carry out an analysis of variance.
3. Results
3.1. Assessment of the Effect of Agroinfiltration with the Empty Vector TRV
The effect of agroinfiltration on M. javanica infestation was evaluated by comparing the level of infestation in non-infiltrated susceptible Moneymaker and resistant Motelle plants with that of plants of the same varieties infiltrated with the empty pTRV1:pTRV2 vector (TRV). Significant differences in the number of egg masses were detected according to the Kruskal–Wallis test (H = 17.936; df = 3; p = 0.0004534). As the results in Figure 1 show, the number of egg masses on plants infiltrated with the empty TRV and non-infiltrated plants did not differ significantly, regardless of the cultivar. The number of egg masses per root was significantly lower in the Motelle variety compared to Moneymaker, both in infiltrated and non-infiltrated plants. Thus, infiltration with empty TRV did not affect the infestation of tomato by nematodes.
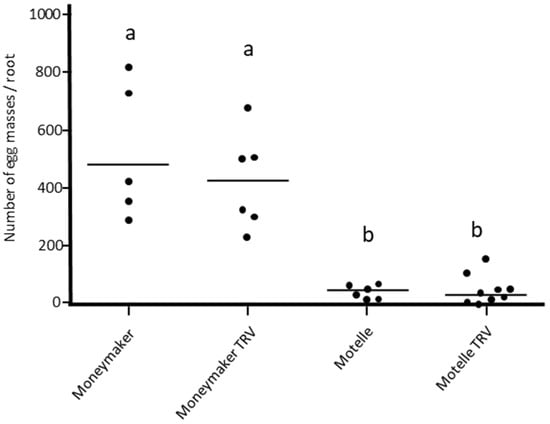
Figure 1.
Number of M. javanica egg masses per root on Moneymaker (mi-1/mi-1) and Motelle (Mi-1/Mi-1) plants infiltrated or not with the empty pTRV1:pTRV2 vector (TRV). Each dot represents the number of egg masses on a root system. The lines indicate the median values. Different letters indicate significant differences (p < 0.05) by the non-parametric Kruskal–Wallis test and Dunn’s post test. This assay was repeated twice with similar results.
For Motelle plants, it was confirmed by semiquantitative PCR that the empty TRV vector did not alter the expression of the Mi-1 gene, as transcript levels were similar in infiltrated and non-infiltrated plants (Figure 2).
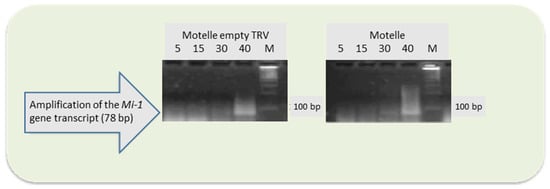
Figure 2.
Agarose gels with transcripts of the Mi-1 gene amplified by semiquantitative PCR from roots of Motelle plants not infiltrated and infiltrated with the TRV empty vector. The number of PCR cycles used is indicated at the top of the figure, as well as the molecular marker (M).
3.2. Silencing of Mi-1 Gene in Tomato Plants
The effect of Mi-1 gene silencing on M. javanica infestation was assessed on 8-week-old plants. Figure 3 shows the number of egg masses per root of Mi-1-silenced plants (Motelle TRV:Mi-1) compared to Moneymaker and Motelle plants infiltrated with the empty vector TRV. Significant differences in the number of egg masses were detected according to the Kruskal–Wallis test (H = 15.706; df = 2; p = 0.0003885).
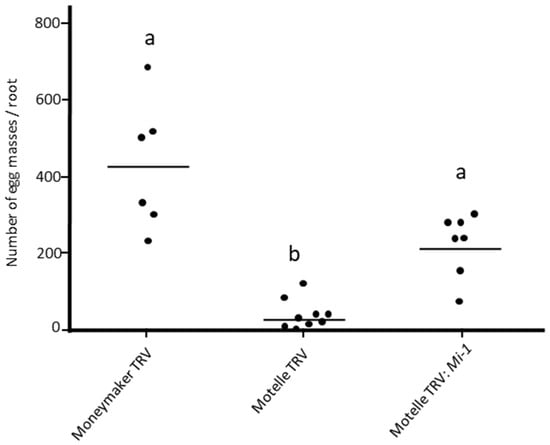
Figure 3.
Number of M. javanica egg masses per root of Moneymaker (mi-1/mi-1) and Motelle (Mi-1/Mi-1) plants infiltrated with the empty pTRV1:pTRV2 vector (TRV) and on Motelle plants agroinfiltrated with the vector pTRV1:pTRV2-Mi (Motelle TRV-Mi). Each dot represents the number of egg masses on a plant. The lines indicate the median values. Different letters indicate significant differences (p < 0.05) by the non-parametric Kruskal–Wallis test and Dunn’s post test. This assay was repeated twice with similar results.
The number of egg masses per root was significantly higher on Mi-1-silenced Motelle plants (Motelle TRV:Mi-1) than on Motelle plants infiltrated with the empty vector (Motelle TRV). In contrast, the level of nematode infestation was not significantly different when comparing Mi-1 silenced Motelle plants with Moneymaker plants infiltrated with the empty vector (Moneymaker TRV). This indicates the loss of resistance in Motelle plants by Mi-1 gene silencing. While the number of egg masses was higher on Moneymaker plants infiltrated with the empty vector than on Mi-1-silenced Motelle plants, this difference was not statistically significant.
To confirm the silencing of the Mi-1 gene, its expression was analysed in Motelle plants infiltrated with the empty vector and in Mi-1-silenced Motelle plants, by semiquantitative RT-PCR. Figure 4 shows the results. Transcripts of the Mi-1 gene were detected in Motelle plants infiltrated with the empty TRV vector, while in roots of Mi-1-silenced Motelle plants, no Mi-1 transcripts were observed, indicating reduced expression of Mi-1 in the latter roots.
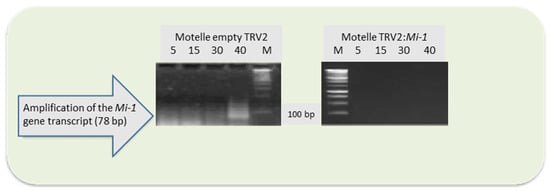
Figure 4.
Agarose gels with transcripts of the Mi-1 gene amplified by semiquantitative PCR from roots of Motelle plants infiltrated with the TRV empty vector and Mi-1-silenced Motelle plants (Motelle TRV:Mi-1). The number of PCR cycles used is indicated at the top of the figure, as well as the molecular marker (M).
3.3. Silencing of TGA 1a and TGA 2.1 Genes in Tomato Plants
Figure 5 shows the number of M. javanica egg masses on 8-week-old Motelle plants, with the genes TGA 1a or TGA 2.1 silenced. These egg mass numbers were compared with those counted on roots of Moneymaker:TRV, MotelleTRV, and MotelleTRV:Mi-1. Significant differences in the number of egg masses were detected according to the Kruskal–Wallis test (H = 24.088; df = 4; p = 0.0000767).
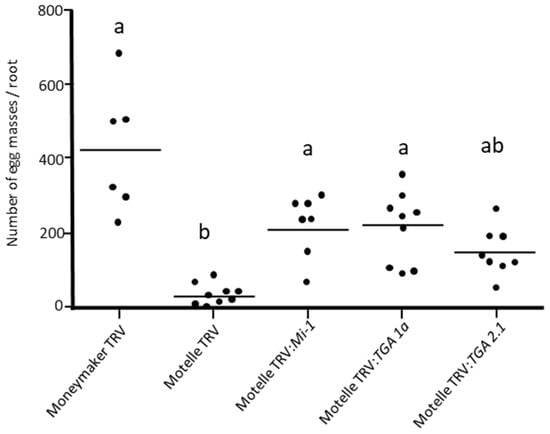
Figure 5.
Number of M. javanica egg masses per root of Moneymaker (mi-1/mi-1) and Motelle (Mi-1/Mi-1) plants infiltrated with the empty pTRV1:pTRV2 vector (TRV) and on Motelle plants agroinfiltrated with pTRV1:pTRV2-Mi-1 (Motelle TRV:Mi-1), pTRV1:pTRV2-TGA 1a (Motelle TRV:TGA 1a), and pTRV1:pTRV2:TGA 2.1 (Motelle TRV:TGA 2.1). Each dot represents the number of egg masses on a plant. The lines indicate the median values. Different letters indicate significant differences (p < 0.05) by the non-parametric Kruskal–Wallis test and Dunn’s post test. This assay was repeated twice with similar results.
Silencing of the TGA 1a gene in Motelle plants (Motelle TRV2:TGA 1a) resulted in a significant increase in the nematode infestation compared to the resistant non-silenced Motelle (Motelle TRV). Infestation levels on Motelle TRV2:TGA 1a were close (although slightly lower) to those of the susceptible Moneymaker plants infiltrated with the empty vector (Moneymaker TRV), as differences were statistically not significant. Silencing of the TGA 1a gene in the resistant Motelle resulted in an increase in the number of egg masses, which was almost equal to that registered on Motelle plants with the Mi-1 gene silenced (Motelle TRV:Mi-1).
In contrast, when the TGA 2.1 gene was silenced, it was observed that the difference with the non-silenced Motelle plants (Motelle TRV) was not significantly different. Interestingly, the difference in nematode infestation levels between TGA 2.1-silenced plants was also not significantly different from the susceptible Moneymaker (Moneymaker TRV). The levels of infestation in TGA 2.1-silenced plants showed an intermediate level of resistance loss between that of the resistant and susceptible varieties.
Semiquantitative RT-PCR was used to check gene silencing by evaluating the gene transcript levels in the silenced plants compared to non-silenced control plants. Figure 6 shows the results. The amplified transcripts of the TGA 1a gene in non-silenced Motelle plants were detected at 30 cycles onwards. No transcripts appeared in the roots of Motelle TRV2:TGA 1a, even at 40 cycles. This result was consistent with the number of nematode egg masses, which was significantly higher in Motelle TGA 1a-silenced plants compared to non-silenced control plants.
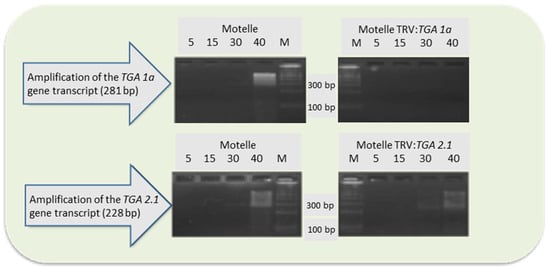
Figure 6.
Agarose gels with transcripts of the TGA 1a and TGA 2.1 genes amplified by semiquantitative PCR from roots of Motelle plants infiltrated with the TRV2 empty vector (Motelle TRV2), TGA 1a-silenced Motelle plants (Motelle TRV2:TGA 1a), and TGA 2.1-silenced Motelle plants (Motelle TRV2:TGA 2.1). The number of PCR cycles used is indicated at the top of the figure, as well as the molecular marker (M).
In non-silenced Motelle plants, transcripts of the TGA 2.1 gene slightly appeared from cycle 30, as for the TGA 1a gene. However, transcripts were also observed in TGA 2.1-silenced Motelle plants at 30 cycles, and more clearly after 40 cycles. This result indicated a lower level of TGA 2.1 silencing, which contributed to the lower number of egg masses on TGA 2.1-silenced plants.
4. Discussion
4.1. Effect of Agroinfiltration with the Empty Vector TRV and TRV-Mi
Agroinfiltration with the empty TRV2 vector did not alter basal defence or Mi-1-mediated resistance to M. javanica, as the number of egg masses did not change on the roots of Moneymaker (mi-1/-mi-1) or Motelle (Mi-1/Mi-1) plants infiltrated with TRV. Furthermore, the differences between the susceptible Moneymaker and resistant Motelle plants were maintained, as expected. This supports the suitability of the TRV vector for VIGS studies with nematodes. These results agree with those obtained previously with aphids [35], whiteflies [32], and with the same nematode species used in this study [31], confirming that the TRV vector does not alter the resistance conferred by the Mi-1 gene to nematodes and insects. Similarly, in other plant species, infiltration with the TRV empty vector did not affect resistance to pathogens mediated by different R genes, such as the N gene conferring resistance to tobacco mosaic virus, TMV [58], or the SacMi gene, involved in resistance against M. incognita in Solanum aculeatissimum [62].
In contrast, silencing the Mi-1 gene in Motelle plants caused a considerable reduction of resistance to M. javanica, as the number of egg masses increased compared to non-silenced Motelle, although the infestation level was slightly lower than that observed in the susceptible Moneymaker.
In parallel to the partial loss of resistance to M. javanica, semiquantitative PCR showed a significant reduction of Mi-1 expression levels in plants agroinfiltrated with the TRV:Mi-1 construct. This loss of resistance is only partial because silencing by the TRV vector in tomato is not uniform, as shown in previous works [31,32,35]. This phenotype is species dependent as a more uniform silencing has been reported in N. benthamiana [57,58,63]. This patchy silencing has been demonstrated by silencing the PDS gene [32,58].
4.2. The Genes TGA 1a and TGA 2.1 in the Mi-1-Mediated Resistance to M. javanica
Silencing of the TGA 1a gene in tomato Motelle cultivar resulted in the loss of resistance to M. javanica since nematode infestation levels recorded were almost identical to those registered after silencing of the Mi-1 gene, and only slightly lower than those on the susceptible Moneymaker. Thus, we demonstrated that TF TGA 1a is involved in Mi-1-mediated resistance to M. javanica.
The TGA TFs function by activating promoters of several plant defence genes [40,41,64,65,66,67]. It is known that the TGA gene family is quite extensive, and its members have extensive roles in plants [68]. Ekengren et al. [49] proposed a model for the function of TGA TFs in the resistance mediated by the Pto gene to the phytopathogenic bacteria P. syringae pv. tomato. In this model, the TGA 1a and TGA 2.2 genes in particular were regulated by transcriptional activation and through the NPR1 protein. The two TGA TFs function as heterodimers interacting with NPR1 [49]. This same function has been shown in other pathosystems, mostly in the model plant Arabidopsis [69,70,71,72,73,74]. However, the results obtained in the present work suggest that the TGA 1a and TGA 2.1 genes would not act in tomato resistance to M. javanica following the Ekengren’s model; they would not function as heterodimers, since silencing TGA 1a alone resulted in the loss of nematode resistance. This work supports a diverse function for these TFs in plant resistance. For instance, TGA TFs are regulated, independently of NPR1, in Arabidopsis defence against P. syringae and Hyaloperonospora arabidopsidis [68].
Apparently, NPR1 always needs to interact with TFs, including positive regulators, such as TGA TFs [68,74]. It is also known that SA activates NPR1 [74,75,76]. In addition, the function of NPR1 seems to be highly conserved in the plant kingdom, since homologs of NPR1 have been found in various cultivated plants, such as rice, tobacco, tomato, apple, and citrus [58,75,77]. However, in the pathosystem studied in this work, it is known that the Mi-1-mediated resistance to RKNs does not require SA [78,79], and therefore this seems to suggest that TGAs would not function through the NPR1 protein in Mi-1-mediated resistance to M. javanica.
The partial loss of resistance to M. javanica observed in Motelle plants in which TGA 1a was silenced was correlated with a decrease in the TGA 1a expression levels. This reduction in gene expression has also been reported in different works that study the involvement of different genes in resistance processes through semiquantitative and quantitative analyses. For example, when studying the function of SlSERK3A and SlSERK3B in bacterial and nematode innate immunity [80], the roles of the HsfAs gene in the Mi-1-mediated resistance to nematodes [30], or the role of Hsp90 in the Mi-1-mediated resistance to nematodes, aphids, and whiteflies [31,32].
In contrast to what we observed for the TGA1a gene, TGA 2.1-silenced Motelle plants showed infestation levels intermediate between those of the resistant non-silenced Motelle and the susceptible Moneymaker, although differences were not statistically significant. The inefficient silencing of TGA 2.1, as demonstrated by semiquantitative PCR, makes it difficult to draw meaningful conclusions regarding the role of TGA 2.1 in Mi-1-mediated resistance to M. javanica.
5. Conclusions
The present work provided new insights into the mechanisms involved in the Mi-1-mediated resistance to plant-parasitic nematodes, demonstrating that the transcription factor TGA 1a gene is required for the correct functioning of such a resistance against M. javanica. Despite having confirmed the adequacy of gene silencing by the TRV virus in these types of studies, the partial silencing of the TGA 2.1 gene impaired the determination of its role in the Mi-1-mediated resistance to M. javanica. More research is needed to unravel the metabolic pathways involved in the complex process of the tomato resistance mediated by Mi-1, a gene whose function is unique and exceptional as it confers resistance to harmful organisms of a very diverse nature and, as such, provides the basis for a multiple resistance that is undoubtedly useful for IPM in agriculture.
Author Contributions
Conceptualization, G.N.; methodology, G.N.; validation, G.N., M.E. and S.P.; investigation, M.E.; resources, G.N.; writing—original draft preparation, S.P.; writing—review and editing, S.P. and G.N.; visualization, S.P.; supervision, G.N.; project administration, G.N.; funding acquisition, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Project (AGL2007-65854/AGR) from the Plan Nacional I+D+I, Spanish Ministry of Education and Science. Mariana Emiliozzi was financially supported by a pre-doctoral fellowship (JAEPre_08_00957) from the JAE Program of CSIC (Spain).
Data Availability Statement
Data are contained within the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to S. Dinesh Kumar (Yale University, New Haven, CT, USA) for providing the original TRV constructs used to silence the TGA 1a, TGA2.1, and PDS genes. We also thank Isgouhi Kaloshian (University of California, Riverside, USA) for providing the primers for Mi-1 gene silencing, and for her supervision along different phases of this work, including a critical review of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. ISBN 978-94-007-0434-3. [Google Scholar]
- Khan, M.R.; Ahamad, I.; Shah, M.H. Emerging important nematode problems in field crops and their management. In Emerging Trends in Plant Pathology; Singh, K.P., Jahagirdar, S., Sarma, B.K., Eds.; Springer: Singapore, 2021; pp. 33–62. ISBN 978-981-15-6275-4. [Google Scholar]
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bull. 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Kaloshian, I.; Teixeira, M. Advances in plant-nematode interactions with emphasis on the notorious nematode genus Meloidogyne. Phytopathology 2019, 109, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A diverse group of novel and important plant parasites. In Root-Knot Nematodes; CAB International: Wallingford, UK, 2009; pp. 1–17. [Google Scholar]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, A.; Adhikari, T.; Louws, F.; Gorny, A. Meloidogyne enterolobii, a major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef]
- Ebone, L.A.; Kovaleski, M.; Deuner, C.C. Nematicides: History, mode, and mechanism action. Plant Sci. Today 2019, 6, 91–97. [Google Scholar] [CrossRef]
- Burns, A.R.; Baker, R.J.; Kitner, M.; Knox, J.; Cooke, B.; Volpatti, J.R.; Vaidya, A.S.; Puumala, E.; Palmeira, B.M.; Redman, E.M.; et al. Selective control of parasitic nematodes using bioactivated nematicides. Nature 2023, 618, 102–109. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Zeferino-Díaz, R.; Alvarado-Rodríguez, J.G.; López-Ruiz, H.; Rojas-Lima, S.; Flores-Castro, K.; del Prado-Vera, I.C.; Alatorre-Rosas, R.; Tut-Pech, F.; Carrillo-Benítez, M.G.; et al. Nematicidal activity of furanoeremophilenes against Meloidogyne incognita and Nacobbus aberrans. Pest Manag. Sci. 2022, 78, 2571–2580. [Google Scholar] [CrossRef]
- Cabrera, J.; Barcala, M.; Fenoll, C.; Escobar, C. The power of omics to identify plant susceptibility factors and to study resistance to root-knot nematodes. Curr. Issues Mol. Biol. 2016, 19, 53–72. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Jiang, Z.; Chen, X.; Li, B.; Xu, L.; Zhang, Z. Cloning and functional analysis of the root-knot nematode resistance gene ntrk1 in tobacco. Physiol. Plant. 2023, 175, e13894. [Google Scholar] [CrossRef]
- Furumizu, C.; Sawa, S. A rapid method for detection of the root-knot nematode resistance gene, Mi-1.2, in tomato cultivars. Plant Biotechnol. 2023, 40, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V. Root-knot nematode resistance genes in tomato and their potential for future use. Annu. Rev. Phytopathol. 1998, 36, 277–293. [Google Scholar] [CrossRef]
- Du, C.; Jiang, J.; Zhang, H.; Zhao, T.; Yang, H.; Zhang, D.; Zhao, Z.; Xu, X.; Li, J. Transcriptomic profiling of Solanum peruvianum LA3858 revealed a Mi-3-mediated hypersensitive response to Meloidogyne incognita. BMC Genom. 2020, 21, 250. [Google Scholar] [CrossRef]
- Jiang, L.; Ling, J.; Zhao, J.; Yang, Y.; Yang, Y.; Li, Y.; Jiao, Y.; Mao, Z.; Wang, Y.; Xie, B. Chromosome-scale genome assembly-assisted identification of Mi-9 gene in Solanum arcanum accession LA2157, conferring heat-stable resistance to Meloidogyne incognita. Plant Biotechnol. J. 2023, 21, 1496–1509. [Google Scholar] [CrossRef]
- Milligan, S.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Ammati, M.; Thomason, I.; Roberts, P. Screening Lycopersicon spp. for new genes imparting resistance to root-knot nematodes (Meloidogyne spp.). Plant Dis. 1985, 69, 112–115. [Google Scholar]
- Gabriel, M.; Kulczynski, S.; Muniz, M.; Boiteux, L.; Carneiro, R. Reaction of a heterozygous tomato hybrid bearing the Mi-1.2 gene to 15 Meloidogyne species. Plant Pathol. 2020, 69, 944–952. [Google Scholar] [CrossRef]
- Roberts, P.; Thomason, I. Variability in reproduction of isolates of Meloidogyne incognita and Meloidogyne javanica on resistant tomato genotypes. Plant Dis. 1986, 70, 547–551. [Google Scholar] [CrossRef]
- Santos, D.; da Silva, P.; Abrantes, I.; Maleita, C. Tomato Mi-1.2 gene confers resistance to Meloidogyne luci and M. ethiopica. Eur. J. Plant Pathol. 2020, 156, 571–580. [Google Scholar] [CrossRef]
- Casteel, C.L.; Walling, L.L.; Paine, T.D. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 2006, 121, 67–72. [Google Scholar] [CrossRef]
- Nombela, G.; Williamson, V.M.; Muñiz, M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant Microbe Interact. 2003, 16, 645–649. [Google Scholar] [CrossRef]
- Rossi, M.; Goggin, F.; Milligan, S.; Kaloshian, I.; Ullman, D.; Williamson, V. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA 1998, 95, 9750–9754. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.Q.; Hussain, A.; Akram, A.; Hussain, S.; Naqvi, R.Z.; Amin, I.; Saeed, M.; Mansoor, S. Cotton Mi-1.2-like gene: A potential source of whitefly resistance. Gene 2023, 851, 146983. [Google Scholar] [CrossRef] [PubMed]
- Goggin, F.; Jia, L.; Shah, G.; Hebert, S.; Williamson, V.; Ullman, D. Heterologous expression of the Mi-1.2 gene from tomato confers resistance against nematodes but not aphids in eggplant. Mol. Plant Microbe Interact. 2006, 19, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dropkin, V.H. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne. Reversal by temperature. Phytopathology 1969, 59, 1632–1637. [Google Scholar]
- de Ilarduya, O.M.; Xie, Q.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, X.-C.; Cao, J.-J.; Yin, L.-L.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Heat shock factor HsfA1a is essential for R gene-mediated nematode resistance and triggers H2O2 production. Plant Physiol. 2018, 176, 2456–2471. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.K.; Li, Q.; Liu, Y.; Dinesh-Kumar, S.P.; Kaloshian, I. The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 2007, 144, 312–323. [Google Scholar] [CrossRef]
- Pascual, S.; Rodríguez-Álvarez, C.I.; Kaloshian, I.; Nombela, G. Hsp90 gene is required for Mi-1-mediated resistance of tomato to the whitefly Bemisia tabaci. Plants 2023, 12, 641. [Google Scholar] [CrossRef]
- Bhattarai, K.; Atamian, H.; Kaloshian, I.; Eulgem, T. WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 2010, 63, 229–240. [Google Scholar] [CrossRef]
- de Ilarduya, O.M.; Moore, A.; Kaloshian, I. The tomato Rme1 locus is required for Mi-1-mediated resistance to root-knot nematodes and the potato aphid. Plant J. 2001, 27, 417–425. [Google Scholar] [CrossRef]
- Li, Q.; Xie, Q.-G.; Smith-Becker, J.; Navarre, D.A.; Kaloshian, I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant Microbe Interact. 2006, 19, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alvarez, C.I.; Lopez-Climent, M.F.; Gomez-Cadenas, A.; Kaloshian, I.; Nombela, G. Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull. Entomol. Res. 2015, 105, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Lanna, R. An overview of plant resistance to plant-pathogenic bacteria. Trop. Plant Pathol. 2023, 48, 243–259. [Google Scholar] [CrossRef]
- Viswanath, K.; Kuo, S.; Tu, C.; Hsu, Y.; Huang, Y.; Hu, C. The role of plant transcription factors in the fight against plant viruses. Int. J. Mol. Sci. 2023, 24, 8433. [Google Scholar] [CrossRef] [PubMed]
- Budimir, J.; Treffon, K.; Nair, A.; Thurow, C.; Gatz, C. Redox-active cysteines in TGACG-BINDING FACTOR 1 (TGA1) do not play a role in salicylic acid or pathogen-induced expression of TGA1-regulated target genes in Arabidopsis thaliana. New Phytol. 2021, 230, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Gatz, C. From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant Microbe Interact. 2013, 26, 151–159. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Li, Z.; Feng, M.; Ge, W.; Zhong, C.; Xue, R. Genome-wide identification of the TGA genes in common bean (Phaseolus vulgaris) and revealing their functions in response to Fusarium oxysporum f. sp. phaseoli infection. Front. Genet. 2023, 14, 1137634. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, C.; Liu, L.; Huang, D.; Ma, C.; Li, R.; Huang, L. Genome-wide identification of the TGA gene family in kiwifruit (Actinidia chinensis spp.) and revealing its roles in response to Pseudomonas syringae pv. actinidiae (Psa) infection. Int. J. Biol. Macromol. 2022, 222, 101–113. [Google Scholar] [CrossRef]
- Lawaju, B.; Lawrence, K.; Lawrence, G.; Klink, V. Harpin-inducible defense signaling components impair infection by the ascomycete Macrophomina phaseolina. Plant Physiol. Biochem. 2018, 129, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Huang, M.; Hu, X.; Zhang, Y.; Wang, Y.; Li, P.; Chen, S.; Zhang, D.; Cao, S.; Zhu, W.; et al. A Ralstonia solanacearum effector targets TGA transcription factors to subvert salicylic acid signaling. Plant Cell 2022, 34, 1666–1683. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.-Q.; Chen, X.-L.; Zhang, H.; Chai, X.-F.; Jiang, J.-B.; Xu, X.-Y.; Li, J.-F. Transcriptome analysis of the Cf-12-mediated resistance response to Cladosporium fulvum in Tomato. Front. Plant Sci. 2017, 7, 2012. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Shen, Y.; Wang, X.; Zhang, S.; Li, Y.; Islam, M.; Wang, J.; Zhao, P.; Zhan, X.; Zhang, F.; et al. A new NLR gene for resistance to tomato spotted wilt virus in tomato (Solanum lycopersicum). Theor. Appl. Genet. 2022, 135, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Lam, E.; Chua, N.-H. Two tobacco dna-binding proteins with homology to the nuclear factor CREB. Nature 1989, 340, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Liu, Y.; Schiff, M.; Dinesh-Kumar, S.; Martin, G. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003, 36, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Lemaire-Chamley, M.; Koutouan, C.; Jorly, J.; Assali, J.; Yoshida, T.; Nogueira, M.; Tohge, T.; Ferrand, C.; Peres, L.; Asamizu, E.; et al. A chimeric TGA repressor slows down fruit maturation and ripening in tomato. Plant Cell Physiol. 2022, 63, 120–134. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Mysore, K.S. Tobacco rattle virus-based virus-induced gene silencing in Nicotiana benthamiana. Nat. Protoc. 2014, 9, 1549–1562. [Google Scholar] [CrossRef]
- Shi, G.; Hao, M.; Tian, B.; Cao, G.; Wei, F.; Xie, Z. A methodological advance of tobacco rattle virus-induced gene silencing for functional genomics in plants. Front. Plant Sci. 2021, 12, 671091. [Google Scholar] [CrossRef]
- Singh, A.K.; Ghosh, D.; Chakraborty, S. Optimization of Tobacco Rattle Virus (TRV)-based virus-induced gene silencing (VIGS) in tomato. Methods Mol. Biol. 2022, 2408, 133–145. [Google Scholar] [CrossRef]
- de Ilarduya, O.M.; Nombela, G.; Hwang, C.; Williamson, V.; Muniz, M.; Kaloshian, I. Rme1 is necessary for Mi-1-mediated resistance and acts early in the resistance pathway. Mol. Plant Microbe Interact. 2004, 17, 55–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laterrot, H. Near isogenic tomato lines in Moneymaker type with different genes for disease resistances. Rep. Tomato Gen. Coop. 1987, 37, 91. [Google Scholar]
- Ho, J.; Weide, R.; Ma, H.; Vanwoedragen, M.; Lambert, K.; Koornneef, M.; Zabel, P.; Williamson, V. The Root-knot nematode resistance gene (Mi) in tomato-construction of a molecular linkage map and identification of dominant cDNA markers in resistant genotypes. Plant J. 1992, 2, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burch-Smith, T.; Schiff, M.; Feng, S.; Dinesh-Kumar, S. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 2004, 279, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.; Fenoll, C.; Escobar, C. Evaluation of different RNA extraction methods for small quantities of plant tissue: Combined effects of reagent type and homogenization procedure on RNA quality-integrity and yield. Physiol. Plant. 2006, 128, 1–7. [Google Scholar] [CrossRef]
- Omwega, C.; Thomason, I.; Roberts, P. A nondestructive technique for screening bean germplasm for resistance to the nematode Meloidogyne incognita utilizing a nontoxic egg mass stain. J. Nematol. 1988, 20, 654. [Google Scholar]
- Zhou, X.; Liu, J.; Bao, S.; Yang, Y.; Zhuang, Y. Molecular cloning and characterization of a wild eggplant Solanum aculeatissimum NBS-LRR gene, involved in plant resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2018, 19, 583. [Google Scholar] [CrossRef]
- Lu, R.; Malcuit, I.; Moffett, P.; Ruiz, M.; Peart, J.; Wu, A.; Rathjen, J.; Bendahmane, A.; Day, L.; Baulcombe, D. High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 2003, 22, 5690–5699. [Google Scholar] [CrossRef]
- Droge-Laser, W.; Snoek, B.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Kogel, K.; Langen, G. Induced disease resistance and gene expression in cereals. Cell. Microbiol. 2005, 7, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.; Tada, Y.; Loake, G. Post-translational protein modification as a tool for transcription reprogramming. New Phytol. 2010, 186, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Shearer, H.L.; Cheng, Y.T.; Wang, L.; Liu, J.; Boyle, P.; Després, C.; Zhang, Y.; Li, X.; Fobert, P.R. Arabidopsis clade I TGA transcription factors regulate plant defenses in an NPR1-independent fashion. Mol. Plant Microbe Interact. 2012, 25, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Despres, C.; DeLong, C.; Glaze, S.; Liu, E.; Fobert, P. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 2000, 12, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Delaney, T. Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. Plant J. 2002, 32, 151–163. [Google Scholar] [CrossRef]
- Shearer, H.; Wang, L.; DeLong, C.; Despres, C.; Fobert, P. NPR1 enhances the DNA binding activity of the Arabidopsis bZIP transcription factor TGA7. Botany 2009, 87, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Kinkema, M.; Li, X.; Dong, X. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6523–6528. [Google Scholar] [CrossRef]
- Zhou, J.; Trifa, Y.; Silva, H.; Pontier, D.; Lam, E.; Shah, J.; Klessig, D. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol. Plant Microbe Interact. 2000, 13, 191–202. [Google Scholar] [CrossRef]
- Zhou, P.; Zavaliev, R.; Xiang, Y.; Dong, X. Seeing is believing: Understanding functions of NPR1 and its paralogs in plant immunity through cellular and structural analyses. Curr. Opin. Plant Biol. 2023, 73, 102352. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Kumar, S.; Zavaliev, R.; Wu, Q.; Zhou, Y.; Cheng, J.; Dillard, L.; Powers, J.; Withers, J.; Zhao, J.; Guan, Z.; et al. Structural basis of NPR1 in activating plant immunity. Nature 2022, 605, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.; Fitzgerald, H.; Yadav, R.; Canlas, P.; Dong, X.; Ronald, P. Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J. 2001, 27, 101–113. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Xie, Q.-G.; Mantelin, S.; Bishnoi, U.; Girke, T.; Navarre, D.A.; Kaloshian, I. Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant Microbe Interact. 2008, 21, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Bhattarai, K.; Jhaveri, T.; Kaloshian, I. Mi-1-mediated resistance to Meloidogyne incognita in tomato may not rely on ethylene but hormone perception through ETR3 participates in limiting nematode infection in a susceptible host. PLoS ONE 2013, 8, e63281. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-C.; Kaloshian, I. The tomato leucine-rich repeat receptor-like kinases SlSERK3A and SlSERK3B have overlapping functions in bacterial and nematode innate immunity. PLoS ONE 2014, 9, e93302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).