Seaweed Polysaccharides as Potential Biostimulants in Turnip Greens Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Harvesting and Preparation of Seaweed Biomass for Extraction
2.2. Polysaccharide Extraction
2.2.1. Alginate
2.2.2. Agar
2.2.3. Carrageenan
2.3. Physico-Chemical Characterization of Polysaccharides’ Solutions
2.4. Experimental Conditions
2.5. Growth Parameters of the Obtained Plant Material
2.6. Turnip Greens’ Physiological and Biochemical Characterization
2.6.1. Dry Matter and Ash Content
2.6.2. Total Nitrogen/Protein
2.6.3. Mineral and Trace Element Characterization
2.6.4. FTIR-ATR Analysis
2.6.5. Leaf Pigments Content
Thin-Layer Chromatography (TLC)
Spectrophotometry
2.7. Substrate Characterization
2.7.1. Substrate Density
2.7.2. pH and Electrical Conductivity
2.7.3. Mineral and Trace Element Characterization
2.7.4. Organic Matter Content
2.7.5. Total Nitrogen
2.8. Statistical Analysis
3. Results
3.1. Biostimulant and Biofertilizer Assay in B. napus L.
3.1.1. Biochemical Characterization of the Treatments Applied
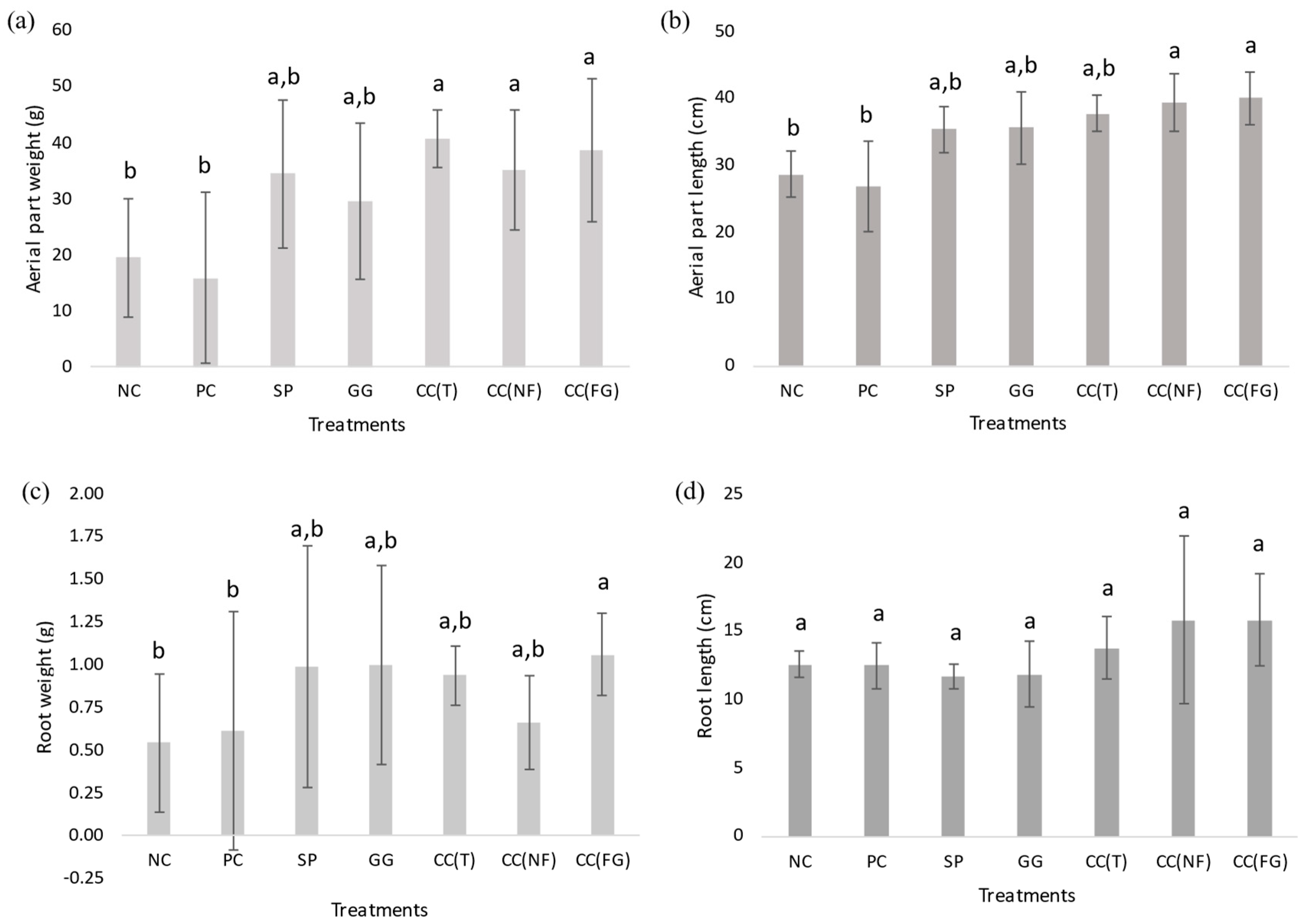
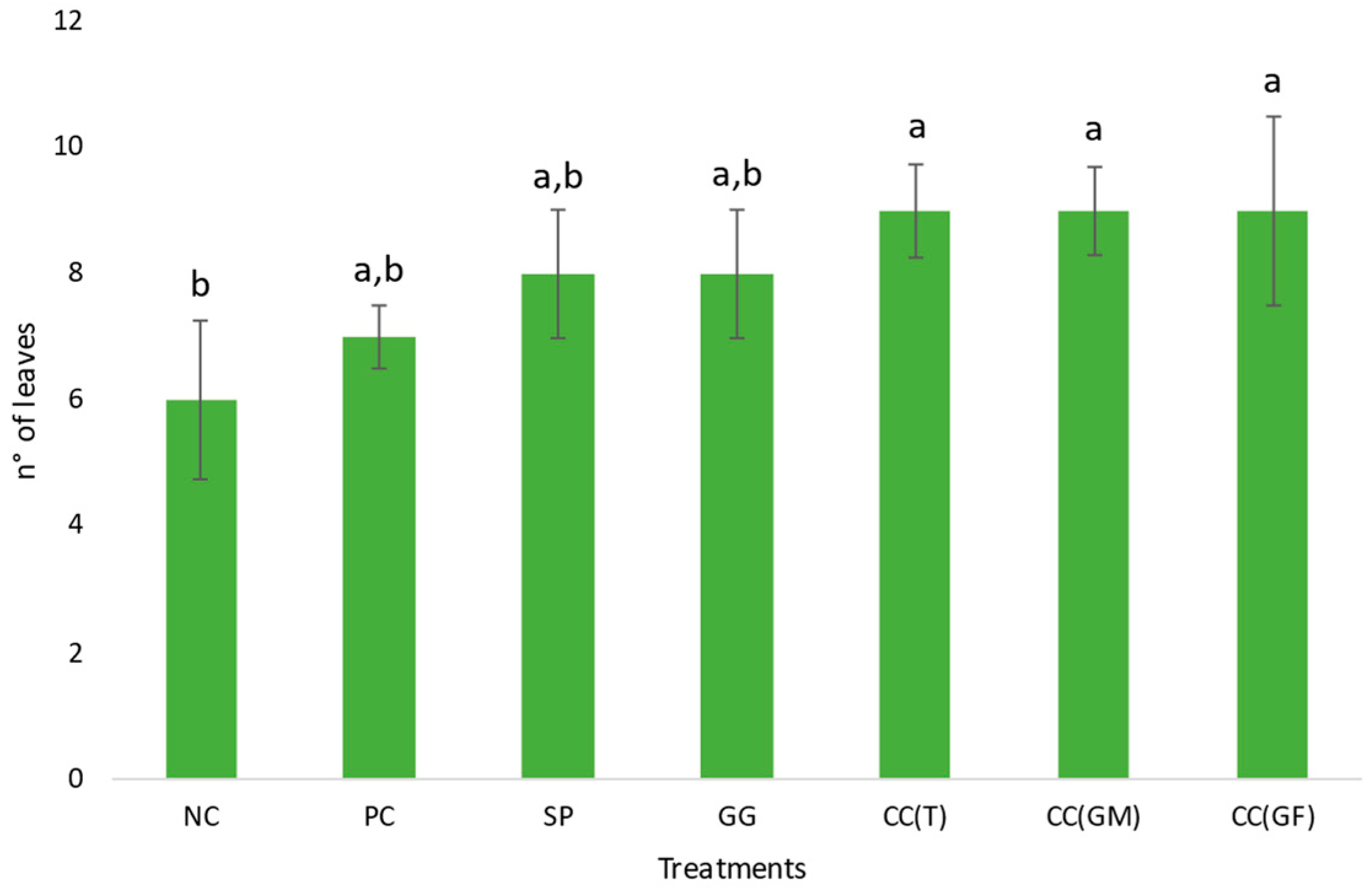
3.1.2. Turnip Greens Parameters
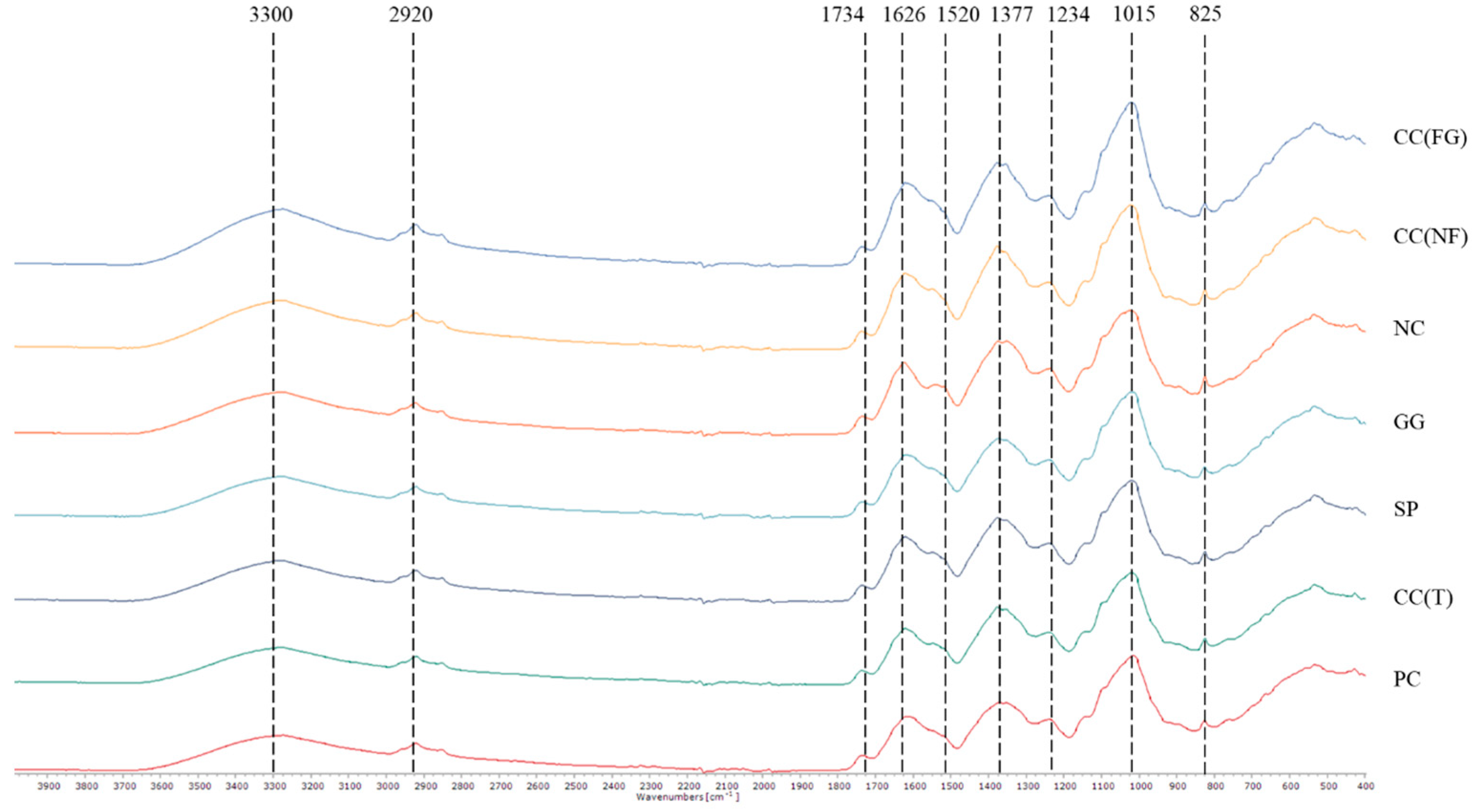
3.1.3. Physiological and Biochemical Characterization of Turnip Greens
Mineral and Trace Element Characterization
Biochemical Characterization of Turnip Greens
Pigment Content
3.2. Substrate Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of Seaweed-Based Biostimulants in Improving Plant and Soil Health: Current Updates and Future Prospective. Int. J. Environ. Sci. Technol. 2022, 19, 12839–12852. [Google Scholar] [CrossRef]
- Mishra, A.; Sahni, S.; Kumar, S.; Prasad, B.D. Seaweed—An Eco-Friendly Alternative of Agrochemicals in Sustainable Agriculture. Curr. J. Appl. Sci. Technol. 2020, 39, 71–78. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor Activity of Sargassum vulgare and Acanthophora spicifera Extracts and Their Prospects for Use in Vegetable Crops for Sustainable Crop Production. J. Appl. Phycol. 2021, 33, 639–651. [Google Scholar] [CrossRef]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid Bioassays to Evaluate the Plant Growth Promoting Activity of Ascophyllum nodosum (L.) Le Jol. Using a Model Plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed Extract Stimuli in Plant Science and Agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Crouch, I.J.; Beckett, R.P.; Van Staden, J. Effect of Seaweed Concentrate on the Growth and Mineral Nutrition of Nutrient-Stressed Lettuce. J. Appl. Phycol. 1990, 2, 269–272. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed Polysaccharides and Derived Oligosaccharides Stimulate Defense Responses and Protection against Pathogens in Plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; Swan, R.; McCormack, R.; Mellon, R. Biostimulant Activity of Brown Seaweed Species from Strangford Lough: Compositional Analyses of Polysaccharides and Bioassay of Extracts Using Mung Bean (Vigno mungo L.) and Pak Choi (Brassica rapa Chinensis L.). J. Appl. Phycol. 2012, 24, 1081–1091. [Google Scholar] [CrossRef]
- Rolland, F.; Moore, B.; Sheen, J. Sugar Sensing and Signaling in Plants. Plant Cell 2002, 14, S185–S205. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of Carrageenan (E 407) and Processed Eucheuma Seaweed (E 407a) as Food Additives. EFSA J. 2018, 16, e05238. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of Alginic Acid and Its Sodium, Potassium, Ammonium and Calcium Salts (E 400–E 404) as Food Additives. EFSA J. 2017, 15, e05049. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.; et al. Re-evaluation of Agar (E 406) as a Food Additive. EFSA J. 2016, 14, e04645. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Smidsrød, O. Uronic Acid Sequence in Alginate from Different Sources. Carbohydr. Res. 1974, 32, 217–225. [Google Scholar] [CrossRef]
- Gioele, C.; Marilena, S.; Valbona, A.; Nunziacarla, S.; Andrea, S.; Antonio, M. Gracilaria gracilis, Source of Agar: A Short Review. Curr. Org. Chem. 2017, 21, 380–386. [Google Scholar] [CrossRef]
- McHugh, D.J. Production and Utilization of Products from Commercial Seaweeds; Food and Agriculture Organization: Rome, Italy, 1987. [Google Scholar]
- Batool, M.; El-Badri, A.M.; Hassan, M.U.; Haiyun, Y.; Chunyun, W.; Zhenkun, Y.; Jie, K.; Wang, B.; Zhou, G. Drought Stress in Brassica napus: Effects, Tolerance Mechanisms, and Management Strategies. J. Plant Growth Regul. 2023, 42, 21–45. [Google Scholar] [CrossRef]
- Rakow, G. Species Origin and Economic Importance of Brassica. In Brassica; Springer: Berlin/Heidelberg, Germany, 2004; pp. 3–11. [Google Scholar]
- Sun, R. Economic/Academic Importance of Brassica rapa. In The Brassica Rapa Genome; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–15. [Google Scholar]
- Pereira, L. Portuguese Seaweeds Website (MACOI). Available online: http://www.flordeutopia.pt/macoi/default.php (accessed on 7 June 2020).
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ Carbohydrate Polymers as Plant Growth Promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Fernández, C. The Retreat of Large Brown Seaweeds on the North Coast of Spain: The Case of Saccorhiza polyschides. Eur. J. Phycol. 2011, 46, 352–360. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development, 1229th ed.; Food and Agriculture Organization: Rome, Italy, 2021; ISBN 978-92-5-134710-2. [Google Scholar]
- Carrington, E.; Grace, S.P.; Chopin, T. Life History Phases and the Biomechanical Properties of the Red Alga Chondrus crispus (Rhodophyta). J. Phycol. 2001, 37, 699–704. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T.; Amado, A.M.; Ribeiro-Claro, P.J.A. A Comparative Analysis of Phycocolloids Produced by Underutilized versus Industrially Utilized Carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009, 21, 599–605. [Google Scholar] [CrossRef]
- Brown, M.T.; Neish, A.; Harwood, D. Comparison of Three Techniques for Identifying Isomorphic Phases of Chondrus crispus (Gigartinaceae). J. Appl. Phycol. 2004, 16, 447–450. [Google Scholar] [CrossRef]
- Sivagnanavelmurugan, M.; Radhakrishnan, S.; Palavesam, A.; Arul, V.; Immanuel, G. Characterization of Alginic Acid Extracted from Sargassum wightii and Determination of Its Anti-Viral Activity of Shrimp Penaeus monodon Post Larvae against White Spot Syndrome Virus. Int. J. Curr. Res. Life Sci. 2018, 7, 1863–1872. [Google Scholar]
- Li, H.; Yu, X.; Jin, Y.; Zhang, W.; Liu, Y. Development of an Eco-Friendly Agar Extraction Technique from the Red Seaweed Gracilaria lemaneiformis. Bioresour. Technol. 2008, 99, 3301–3305. [Google Scholar] [CrossRef]
- Pereira, L.; Van De Velde, F. Portuguese Carrageenophytes: Carrageenan Composition and Geographic Distribution of Eight Species (Gigartinales, Rhodophyta). Carbohydr. Polym. 2011, 84, 614–623. [Google Scholar] [CrossRef]
- Mamede, M.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Seaweed Polysaccharides on Seed Germination of Brassica napus L. Algal. Res. 2023, 76, 103288. [Google Scholar] [CrossRef]
- Cunniff, P. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis Chemical and Microbiological Properies. Part 2; Black, A., Evans, D.D., White, J.L., Ensmingert, L.E., Clark, F.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1979; pp. 1149–1176. [Google Scholar]
- PortFIR—INSA. Available online: http://Portfir.Insa.Pt/ (accessed on 10 January 2024).
- Lucas, M.D.; Sequeira, E.M. Determinação Do Cu, Zn, Mn, Fe, Ca, Mg, K, e Na Totais Das Plantas Por Espectrofotometria de Absorção Atómica e Fotometria de Chama. Pedologia 1976, 11, 163–169. [Google Scholar]
- Ribas, M.C.; Veiga, M.E.; Curto, A.; Oliveira, E.; Barbeitos, M.M.; Ferreira, M.; Pacheco, C.; Peralta, M.F.; Duarte, M. Métodos de Análise de Material Vegetal e Terras. Pedologia 1988, 11, 163–169. [Google Scholar]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Cotas, J.; Figueirinha, A.; Pereira, L.; Batista, T. The Effect of Salinity on Fucus ceranoides (Ochrophyta, Phaeophyceae) in the Mondego River (Portugal). J. Oceanol. Limnol. 2019, 37, 881–891. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Patanè, C. Effect of Application of Biostimulants on the Biomass, Nitrate, Pigments, and Antioxidants Content in Radish and Turnip Microgreens. Agronomy 2023, 13, 145. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- EN 13652:2001; Soil Improvers and Growing Media—Extraction of Water Soluble Nutrients and Elements. European Committee for Standardization: Brussels, Belgium, 2001; pp. 1–18.
- Póvoas, I.; Barral, M.F. Métodos de Análise de Solos; Instituto de Investigação Científica Tropical: Lisbon, Portugal, 1992. [Google Scholar]
- Laboratório Químico Agrícola Rebelo da Silva. Sector de Fertilidade Do Solo; DGSA—Ministério da Agricultura: Lisbon, Portugal, 1977. [Google Scholar]
- Cornforth, I.S.; Stephen, R.C.; Barry, T.N.; Baird, G.A. Mineral Content of Swedes, Turnips, and Kale. New Zealand J. Exp. Agric. 1978, 6, 151–156. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T. Thin-Layer Chromatography of Natural Pigments: New Advances. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 1521–1541. [Google Scholar] [CrossRef]
- Quach, H.T.; Steeper, R.L.; Griffin, G.W. An Improved Method for the Extraction and Thin-Layer Chromatography of Chlorophyll a and b from Spinach. J. Chem. Educ. 2004, 81, 385. [Google Scholar] [CrossRef]
- Tarragó-Celada, J.; Novell, J.M.F. Colour, chlorophyll, and chromatography. Sci Educ. 2019, 47, 41–45. [Google Scholar]
- Khan, N.; Ray, R.L.; Sargani, G.R.; Ihtisham, M.; Khayyam, M.; Ismail, S. Current Progress and Future Prospects of Agriculture Technology: Gateway to Sustainable Agriculture. Sustainability 2021, 13, 4883. [Google Scholar] [CrossRef]
- Lorenzo, J.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef]
- Kaidi, S.; Bentiss, F.; Jama, C.; Khaya, K.; Belattmania, Z.; Reani, A.; Sabour, B. Isolation and Structural Characterization of Alginates from the Kelp Species Laminaria ochroleuca and Saccorhiza polyschides from the Atlantic Coast of Morocco. Colloids Interfaces 2022, 6, 51. [Google Scholar] [CrossRef]
- Devina, N.; Eriwati, Y.K.; Santosa, A.S. The Purity and Viscosity of Sodium Alginate Extracted from Sargassum Brown Seaweed Species as a Basic Ingredient in Dental Alginate Impression Material. J. Phys. Conf. Ser. 2018, 1073, 052012. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Mamede, M.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Seaweed Polysaccharides in Agriculture: A Next Step towards Sustainability. Appl. Sci. 2023, 13, 6594. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-Related Proteins and Peptides as Promising Tools for Engineering Plants with Multiple Stress Tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R. Essential Nutrients for Plant Growth: Nutrient Functions and Deficiency Symptoms. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; College of Tropical Agriculture and Human Resources, University of Hawaii: Manoa, HI, USA, 2000; pp. 31–55. [Google Scholar]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Thye, K.-L.; Wan Abdullah, W.M.A.N.; Balia Yusof, Z.N.; Wee, C.-Y.; Ong-Abdullah, J.; Loh, J.-Y.; Cheng, W.-H.; Lamasudin, D.U.; Lai, K.-S. λ-Carrageenan Promotes Plant Growth in Banana via Enhancement of Cellular Metabolism, Nutrient Uptake, and Cellular Homeostasis. Sci. Rep. 2022, 12, 19639. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil Bulk Density as Related to Soil Texture, Organic Matter Content and Available Total Nutrients of Coimbatore Soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere Control of Soil Nitrogen Cycling: A Key Component of Plant Economic Strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.; Fournier, J. The Algal Polysaccharide Carrageenans Can Act as an Elicitor of Plant Defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]



| Treatment | Concentration |
|---|---|
| Negative control (Tap water) | - |
| Positive control (“Profertil”) | 1.5% (v/v) |
| Alginate solution | 0.50 mg/mL |
| Agar solution | 0.50 mg/mL |
| Carrageenan (tetrasporophyte) solution | 0.25 mg/mL |
| Carrageenan (non-fructified thalli) solution | 0.50 mg/mL |
| Carrageenan (female gametophyte) solution | 0.50 mg/mL |
| Treatment | Concentration (mg/mL) | pH | EC (µS/cm) | Viscosity (mPa.s) |
|---|---|---|---|---|
| Negative control | - | 5.86 | 302 | 1.00 |
| Positive control | 1.5% (v/v) | 7.30 | 1685 | 5.10 |
| Alginate solution | 0.50 | 3.70 | 109 | 3.60 |
| Agar solution | 0.50 | 5.83 | 73 | 8.40 |
| Carrageenan (tetrasporophyte) solution | 0.25 | 9.34 | 100 | 10.80 |
| Carrageenan (non-fructified thalli) solution | 0.50 | 9.56 | 184 | 9.00 |
| Carrageenan (female gametophyte) solution | 0.50 | 9.86 | 191 | 9.00 |
| Sample | AP Length/Weight | R Length/Weight | AP Weight/R Weight | AP Length/R Length |
|---|---|---|---|---|
| NC | 1.46 | 23.27 | 36.20 | 2.28 |
| PC | 1.68 | 20.45 | 26.09 | 2.15 |
| SP | 1.03 | 11.91 | 35.04 | 3.02 |
| GG | 1.20 | 11.94 | 29.77 | 3.00 |
| CC(T) | 0.92 | 14.73 | 43.69 | 2.74 |
| CC(NF) | 1.12 | 24.01 | 53.54 | 2.49 |
| CC(FG) | 1.03 | 14.94 | 36.62 | 2.53 |
| Treatments | NC | PC | SP | GG | CC(T) | CC(NF) | CC(FG) | Literature Values | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Dry matter (%) | 5.30 ± 0.00 ab | 5.34 ± 0.01 ab | 3.42 ± 2.31 b | 7.19 ± 1.68 a | 5.80 ± 0.04 ab | 6.66 ± 0.05 a | 6.77 ± 0.43 a | 6.00 | [33] |
| Ashes (%) | 22.74 ± 0.02 a | 20.45 ± 0.07 a | 19.52 ± 0.02 a | 19.36 ± 0.28 a | 20.23 ± 0.05 a | 19.47 ± 0.07 a | 18.48 ± 0.10 a | 13.50 | [33] |
| N (%) | 5.68 ± 0.05 a | 5.27 ± 0.12 ab | 5.23 ± 0.06 ab | 4.86 ± 0.01 b | 5.37 ± 0.03 ab | 5.16 ± 0.03 b | 4.88 ± 0.05 b | 3.23 | [43] |
| Protein (%) | 35.50 ± 0.31 a | 32.91 ± 0.72 ab | 32.66 ± 0.34 ab | 30.34 ± 0.03 b | 33.56 ± 0.19 ab | 32.25 ± 0.19 ab | 30.47 ± 0.34 b | 33.33 | [33] |
| P (%) | 0.84 ± 0.00 ab | 0.87 ± 0.01 a | 0.81 ± 0.01 ab | 0.73 ± 0.02 b | 0.80 ± 0.00 ab | 0.75 ± 0.01 b | 0.76 ± 0.00 b | 0.75 | [33] |
| Ca (%) | 1.46 ± 0.01 a | 1.33 ± 0.01 ab | 1.28 ± 0.20 ab | 1.23 ± 0.05 ab | 1.29 ± 0.00 ab | 1.23 ± 0.03 ab | 1.20 ± 0.02 b | 1.67 | [33] |
| Mg (%) | 0.30 ± 0.01 a | 0.27 ± 0.00 ab | 0.23 ± 0.01 b | 0.24 ± 0.00 b | 0.26 ± 0.01 ab | 0.28 ± 0.01 ab | 0.27 ± 0.01 ab | 0.17 | [33] |
| K (%) | 8.58 ± 0.16 a | 7.31 ± 0.12 ab | 8.23 ± 0.17 ab | 7.43 ± 0.29 ab | 8.17 ± 0.07 ab | 6.88 ± 0.02 b | 7.24 ± 0.17 b | 5.00 | [33] |
| Na (%) | 0.39 ± 0.03 a | 0.41 ± 0.00 a | 0.44 ± 0.00 a | 0.34 ± 0.03 a | 0.39 ± 0.10 a | 0.90 ± 0.52 a | 0.36 ± 0.02 a | 0.67 | [33] |
| Cu (mg/kg) | 35.25 ± 0.15 ab | 36.80 ± 0.50 ab | 33.25 ± 0.45 b | 34.70 ± 0.20 ab | 35.50 ± 0.80 ab | 35.40 ± 1.00 ab | 38.00 ± 0.30 a | NI | NI |
| Zn (mg/kg) | 118.15 ± 1.25 a | 81.00 ± 1.60 ab | 77.05 ± 0.15 b | 77.75 ± 1.15 b | 81.10 ± 1.50 ab | 77.25 ± 0.15 b | 81.85 ± 0.65 ab | 87.40 | [43] |
| Fe (mg/kg) | 149.40 ± 1.30 a | 99.35 ± 0.25 ab | 93.70 ± 6.80 ab | 96.75 ± 0.75 ab | 91.50 ± 3.20 b | 94.95 ± 2.45 ab | 91.25 ± 1.35 b | NI | NI |
| Mn (mg/kg) | 119.75 ± 2.15 a | 58.80 ± 2.60 b | 89.50 ± 0.20 ab | 78.15 ± 1.25 b | 92.90 ± 2.30 ab | 93.25 ± 1.45 ab | 71.90± 1.40 b | 98.70 | [43] |
| Reference Wave Number (cm−1) | Bond | Wave Number Observed (cm−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| CC (FG) | CC (NF) | NC | GG | SP | CC (T) | PC | ||
| 3334 | Cellulose | sh | sh | sh | sh | 3286 | 3278 | 3274 |
| 2917 | Cellulose | 2921 | 2921 | 2921 | 2921 | 2921 | 2920 | 2921 |
| 1734 | Pectins with ester | 1736 | 1736 | 1735 | 1736 | 1736 | 1736 | 1736 |
| 1626 | Free carboxyl groups | 1620 | 1621 | 1624 | 1619 | 1622 | 1622 | 1617 |
| 1520 | Lignin and phenolic backbone | sh | sh | 1540 | sh | sh | sh | sh |
| 1371–1314 | Cellulose and xyloglucan | 1377 | 1377 | 1351 | 1375 | 1376 | 1376 | 1352 |
| 1234 | Proteins | 1240 | 1240 | 1238 | 1239 | 1239 | 1239 | 1238 |
| 1015 | Polysaccharides, sugars, and pectins | 1021 | 1021 | 1023 | 1019 | 1020 | 1019 | 1016 |
| 825 | NA | 825.3 | 825 | 824.8 | 825.5 | 825.2 | 825.1 | 825.6 |
| 770 | Phenyl groups | nd | nd | nd | nd | nd | nd | nd |
| Rf Observed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nº * | Visible Color | NC | PC | SP | GG | CC (T) | CC (NF) | CC (FG) | Rf Literature | Pigment | Reference |
| 1 | light green | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.04 | NI | NI | NI |
| 2 | light grey | 0.07 | 0.08 | 0.09 | 0.10 | 0.10 | 0.12 | 0.14 | NI | NI | NI |
| 3 | light yellow | nd | nd | 0.16 | 0.17 | 0.20 | 0.20 | 0.22 | 0.18 | Neoxanthin | [44] |
| 4 | bright yellow | 0.57 | 0.57 | 0.57 | 0.57 | 0.68 | 0.68 | 0.69 | 0.15–0.35 | Xanthophyll | [45,46] |
| 5 | light green | 0.62 | 0.62 | 0.62 | 0.62 | 0.73 | 0.73 | 0.75 | 0.32–0.42 | Chlorophyll b | [45,46] |
| 6 | faded green | 0.66 | nd | 0.66 | 0.66 | 0.77 | 0.77 | 0.79 | NI | NI | NI |
| 7 | dark green | 0.74 | 0.74 | 0.74 | 0.74 | 0.84 | 0.83 | 0.84 | 0.44–0.59 | Chlorophyll a | [45,46] |
| 8 | light grey | 0.83 | nd | 0.83 | nd | 0.91 | 0.91 | nd | 0.49 | Pheophytin b | [45] |
| 9 | dark grey | 0.91 | 0.91 | 0.91 | 0.91 | 0.95 | 0.95 | 0.95 | 0.60 | Pheophytin a | [45] |
| 10 | light grey | 0.95 | 0.95 | 0.95 | 0.95 | nd | nd | nd | NI | NI | NI |
| 11 | golden | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.98 | 0.98 | 0.95–0.98 | β-carotene | [44,45,46] |
| Pigments (mg/100 g) | NC | PC | SP | GG | CC(T) | CC(NF) | CC(GF) |
|---|---|---|---|---|---|---|---|
| Chlorophyll a | 4.346 ± 0.01 b | 4.458 ± 0.01 ab | 5.233 ± 0.01 ab | 4.303 ± 0.01 b | 6.916 ± 0.01 a | 5.516 ± 0.01 ab | 5.914 ± 0.01 ab |
| Chlorophyll b | 1.503 ± 0.01 ab | 1.399 ± 0.01 b | 1.729 ± 0.01 ab | 1.361 ± 0.01 b | 2.301 ± 0.01 a | 1.841 ± 0.01 ab | 1.851 ± 0.01 ab |
| Anthocyanins | 0.011 ± 0.01 a | 0.010 ± 0.01 a | 0.011 ± 0.01 a | 0.009 ± 0.01 a | 0.016 ± 0.01 a | 0.012 ± 0.01 a | 0.014 ± 0.01 a |
| Carotenoids | 0.936 ± 0.01 b | 1.013 ± 0.01 b | 1.231 ± 0.01 ab | 1.056 ± 0.01 ab | 1.448 ± 0.01 a | 1.230 ± 0.01 ab | 1.426 ± 0.01 ab |
| Soil Sample | NC | PC | SP | GG | CC(T) | CC(NF) | CC(FG) |
|---|---|---|---|---|---|---|---|
| Ds (g/L) | 945.19 ± 29.58 a | 804.97 ± 14.01 ab | 767.59 ± 10.83 b | 771.10 ± 4.21 b | 836.16 ± 68.54 ab | 802.81 ± 4.18 ab | 837.85 ± 16.07 ab |
| ms at 60 mL (g) | 56.71 ± 1.77 a | 48.30 ± 0.84 a | 46.06 ± 0.65 a | 46.27 ± 0.25 a | 50.17 ± 4.11 a | 48.17 ± 0.25 a | 50.27 ± 0.96 a |
| OM (%) | 34.97 ± 1.33 a | 24.17 ± 0.73 b | 27.22 ± 1.89 ab | 23.52 ± 0.50 b | 19.54 ± 1.16 b | 27.67 ± 7.40 b | 23.53 ± 0.84 b |
| N (%) | 0.42 ± 0.01 a | 0.40 ± 0.02 a | 0.44 ± 0.02 a | 0.41 ± 0.02 a | 0.35 ± 0.00 a | 0.33 ± 0.01 a | 0.37 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamede, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Polysaccharides as Potential Biostimulants in Turnip Greens Production. Horticulturae 2024, 10, 130. https://doi.org/10.3390/horticulturae10020130
Mamede M, Cotas J, Pereira L, Bahcevandziev K. Seaweed Polysaccharides as Potential Biostimulants in Turnip Greens Production. Horticulturae. 2024; 10(2):130. https://doi.org/10.3390/horticulturae10020130
Chicago/Turabian StyleMamede, Mariana, João Cotas, Leonel Pereira, and Kiril Bahcevandziev. 2024. "Seaweed Polysaccharides as Potential Biostimulants in Turnip Greens Production" Horticulturae 10, no. 2: 130. https://doi.org/10.3390/horticulturae10020130
APA StyleMamede, M., Cotas, J., Pereira, L., & Bahcevandziev, K. (2024). Seaweed Polysaccharides as Potential Biostimulants in Turnip Greens Production. Horticulturae, 10(2), 130. https://doi.org/10.3390/horticulturae10020130









