Smartphone-Based Leaf Colorimetric Analysis of Grapevine (Vitis vinifera L.) Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Color Evaluation and RGB-Based Vegetation Indices
2.3. Chlorophyll Measurement
2.4. Data Analysis
3. Results
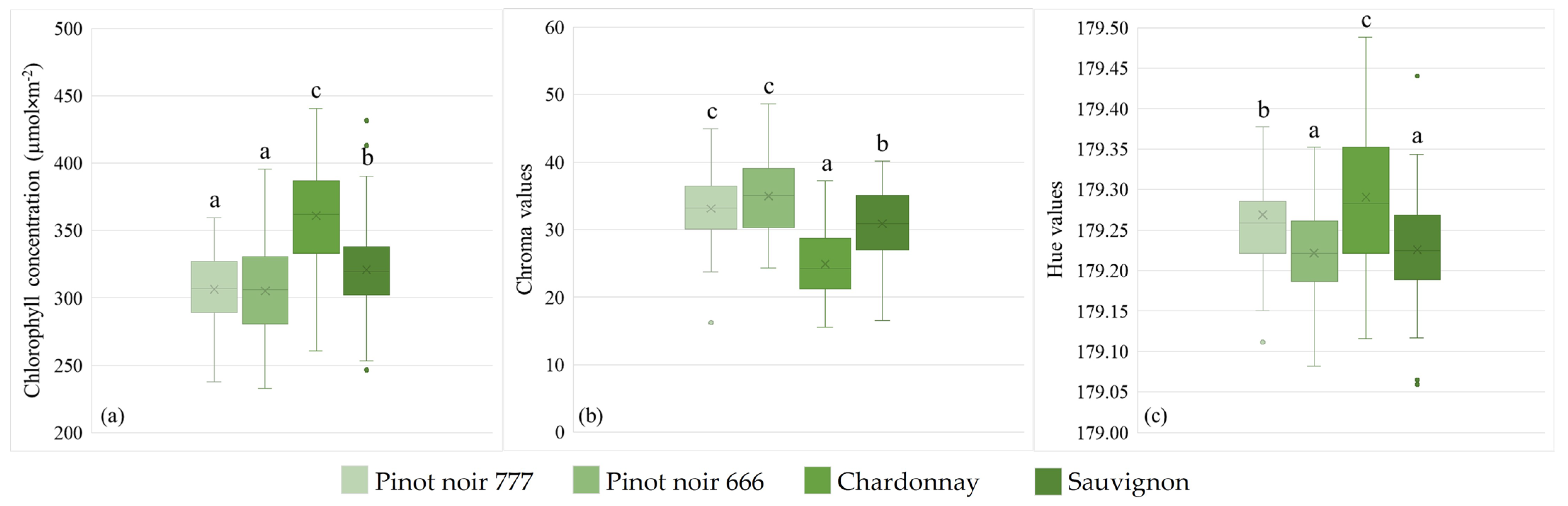
3.1. Chlorophyll Concentration
3.2. Color Values
3.3. Correlation of Vegetation Indices with Chlorophyll Concentration
3.4. Classification of Grapevine Genotypes Based on Their Leaf Color Properties
4. Discussion
4.1. Leaf Color
4.2. Correlation of the Chlorophyll Concentration with Color Indices
4.3. Color-Based Classification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OIV. OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; Office International de la Vigne et du Vin: Paris, France, 2009; p. 177. [Google Scholar]
- Costa, B.R.S.; Oldoni, H.; da Silva, T.M.M.; Farinassi, L.G.; Bognola, I.A.; Bassoi, L.H. How similar is the zoning of different vegetation indices: Defining the optimal framework for monitoring grapevines’ growth within vigorous vineyards. Sci. Hort. 2023, 322, 112404. [Google Scholar] [CrossRef]
- Bertamini, M.; Nedunchezhian, N. Leaf age effects on chlorophyll, Rubisco, photosynthetic electron transport activities and thylakoid membrane protein in field grown grapevine leaves. J. Plant Phys. 2002, 159, 799–803. [Google Scholar] [CrossRef]
- Lashbrooke, J.G.; Young, P.R.; Strever, A.E.; Stander, C.; Vivier, M.A. The development of a method for the extraction of carotenoids and chlorophylls from grapevine leaves and berries for HPLC profiling. Aust. J. Grape Wine Res. 2010, 16, 349–360. [Google Scholar] [CrossRef]
- Taskos, D.G.; Koundouras, S.; Stamatiadis, S.; Zioziou, E.; Nikolaou, N.; Karakioulakis, K.; Theodorou, N. Using active canopy sensors and chlorophyll meters to estimate grapevine nitrogen status and productivity. Prec. Agric. 2015, 16, 77–98. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Franck, N.; Zamorano, D.; Sanhueza, C.; Carvajal, D.E.; Ibacache, A. Rootstock effect on irrigated grapevine yield under arid climate conditions are explained by changes in traits related to light absorption of the scion. Sci. Hortic. 2017, 218, 284–292. [Google Scholar] [CrossRef]
- Ferreira, V.; Pinto-Carnide, O.; Arroyo-García, R.; Castro, I. Berry color variation in grapevine as a source of diversity. Plant Phys. Biochem. 2018, 132, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.N.; Vaillant, N.; Boulay, M.; Clément, C.; Fontaine, F. Alteration of photosynthesis in grapevines affected by esca. Phytopathology 2006, 96, 1060–1066. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Phys. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef]
- Bodor-Pesti, P.; Taranyi, D.; Sárdy, D.N.; Nguyen, L.L.P.; Baranyai, L. Correlation of the Grapevine (Vitis vinifera L.) Leaf Chlorophyll Concentration with RGB Color Indices. Horticulturae 2023, 9, 899. [Google Scholar] [CrossRef]
- Domanda, C.; Nuzzo, V.; Montanaro, G.; Failla, O.; Rustioni, L. Trichomes affect grapevine leaf optical properties and thermoregulation. Theor. Experym. Plant Phys. 2023, 35, 299–308. [Google Scholar] [CrossRef]
- Kobayashi, K.D. Are there horticulture-related mobile applications for me? HortTechnology 2013, 23, 399–401. [Google Scholar] [CrossRef]
- Kwon, O.; Park, T. Applications of Smartphone Cameras in Agriculture, Environment, and Food: A review. J. Biosys. Eng. 2017, 42, 330–338. [Google Scholar] [CrossRef]
- Lima, M.J.; Nascimento, C.F.; Rocha, F.R. Feasible photometric measurements in liquid–liquid extraction by exploiting smartphone-based digital images. Anal. Meth. 2017, 9, 2220–2225. [Google Scholar] [CrossRef]
- Crippen, R.E. Calculating the vegetation index faster. Remote Sens. Environ. 1990, 34, 71–73. [Google Scholar] [CrossRef]
- Montero, D.; Aybar, C.; Mahecha, M.D.; Martinuzzi, F.; Söchting, M.; Wieneke, S. A standardized catalogue of spectral indices to advance the use of remote sensing in Earth system research. Sci. Data 2023, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Henrich, V.; Krauss, G.; Götze, C.; Sandow, C. IDB–www.indexdatabase.de, Entwicklung einer Datenbank für Fernerkundungsindizes. AK Fernerkund. Bochum 2012, 4–5, 10. [Google Scholar]
- Sánchez-Sastre, L.F.; Alte da Veiga, N.M.; Ruiz-Potosme, N.M.; Carrión-Prieto, P.; Marcos-Robles, J.L.; Navas-Gracia, L.M.; Martín-Ramos, P. Assessment of RGB vegetation indices to estimate chlorophyll content in sugar beet leaves in the final cultivation stage. AgriEngineering 2020, 2, 128–149. [Google Scholar] [CrossRef]
- Lu, J.; Eitel, J.U.; Engels, M.; Zhu, J.; Ma, Y.; Liao, F.; Tian, Y. Improving Unmanned Aerial Vehicle (UAV) remote sensing of rice plant potassium accumulation by fusing spectral and textural information. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102592. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, X.; Gao, L.; Li, C.; Wang, L.; Zhao, G.; Jiang, Y. Estimation of chlorophyll content in apple leaves based on RGB model using digital camera. Acta Hort. Sin. 2017, 44, 381–390. [Google Scholar] [CrossRef]
- Pazda, A.D.; Thorstenson, C.A. Color intensity increases perceived extraversion and openness for zero-acquaintance judgments. Pers. Indiv. Diff. 2019, 147, 118–127. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhu, Y.; Xu, T. Review of plant identification based on image processing. Arch. Comput. Methods Eng. 2017, 24, 637–654. [Google Scholar] [CrossRef]
- Nasiri, A.; Taheri-Garavand, A.; Fanourakis, D.; Zhang, Y.D.; Nikoloudakis, N. Automated grapevine cultivar identification via leaf imaging and deep convolutional neural networks: A proof-of-concept study employing primary Iranian varieties. Plants 2021, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mi.com/np/redmi-note-9/specs/ (accessed on 17 October 2024).
- Pék, Z.; Helyes, L.; Lugasi, A. Color changes and antioxidant content of vine and postharvest-ripened tomato fruits. HortScience 2010, 45, 466–468. [Google Scholar] [CrossRef]
- Apogee Chlorophyll Meter Support. Available online: https://www.apogeeinstruments.com/chlorophyll-meter-support/#specifications (accessed on 1 February 2023).
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for education and data analysis. Palaeont. Electr. 2001, 4, 1. [Google Scholar]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Pongnumkul, S.; Chaovalit, P.; Surasvadi, N. Applications of smartphone-based sensors in agriculture: A systematic review of research. J. Sens. 2015, 2015, 195308. [Google Scholar] [CrossRef]
- De Bei, R.; Fuentes, S.; Gilliham, M.; Tyerman, S.; Edwards, E.; Bianchini, N.; Collins, C. VitiCanopy: A free computer App to estimate canopy vigor and porosity for grapevine. Sensors 2016, 16, 585. [Google Scholar] [CrossRef]
- Aquino, A.; Millan, B.; Gaston, D.; Diago, M.P.; Tardaguila, J. vitisFlower®: Development and testing of a novel android-smartphone application for assessing the number of grapevine flowers per inflorescence using artificial vision techniques. Sensors 2015, 15, 21204–21218. [Google Scholar] [CrossRef]
- Teixeira, G.; Monteiro, A.; Santos, C.; Lopes, C.M. Leaf morphoanatomy traits in white grapevine cultivars with distinct geographical origin. Ciência Técn. Vitiviníc 2018, 33, 90–101. [Google Scholar] [CrossRef]
- Casanova-Gascón, J.; Martín-Ramos, P.; Martí-Dalmau, C.; Badía-Villas, D. Nutrients assimilation and chlorophyll contents for different grapevine varieties in calcareous soils in the Somontano DO (Spain). Beverages 2018, 4, 90. [Google Scholar] [CrossRef]
- Somogyi, E.; Lázár, J.; Bodor, P.; Kaszab, T. Colour of grapevine (Vitis vinifera L.) accessions influenced by the length of cold storage: Grapevine berry colour measurement. Prog. Agric. Eng. Sci. 2021, 16, 109–116. [Google Scholar] [CrossRef]
- Doğan, A.; Uyak, C. A different approach for grape leaf color. J. Agric. Fac. Gaziosmanpaşa Univ. JAFAG 2020, 37, 44–52. [Google Scholar] [CrossRef]
- Fuentes, S.; Hernández-Montes, E.; Escalona, J.M.; Bota, J.; Viejo, C.G.; Poblete-Echeverría, C.; Medrano, H. Automated grapevine cultivar classification based on machine learning using leaf morpho-colorimetry, fractal dimension and near-infrared spectroscopy parameters. Comput. Electron. Agric. 2018, 151, 311–318. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Torres-Huerta, N.; Araya-Alman, M.; Verdugo-Vásquez, N.; Moreno-Simunovic, Y.; Valdés-Gómez, H.; Acevedo-Opazo, C. Leaf morpho-colorimetric characterization of different grapevine varieties through changes on plant water status. Horticulturae 2021, 7, 315. [Google Scholar] [CrossRef]
- Kawashima, S.; Nakatani, M. An algorithm for estimating chlorophyll content in leaves using a video camera. Ann. Bot. 1998, 81, 49–54. [Google Scholar] [CrossRef]
- Guendouz, A.; Bendada, H.; Benniou, R. A comparative study of chlorophyll content estimation in barley (Hordeum vulgare L.) genotypes based on RGB (Red, Green, Blue) image analysis. Agric. Sci. Digest A Res. J. 2022, 42, 68–71. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Klouvatou, A.; Vekkos, K.A.; Manetas, Y.; Karabourniotis, G. The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann. Bot. 2006, 98, 257–265. [Google Scholar] [CrossRef]
- Preiner, D.; Safner, T.; Karoglan Kontić, J.; Marković, Z.; Šimon, S.; Maletić, E. Analysis of phyllometric parameters efficiency in discrimination of Croatian native V. vinifera cultivars. Vitis 2014, 53, 215–217. [Google Scholar] [CrossRef]
- Reiczigel, Z.; Szekszárdi, A.; Bisztray, G.D.; Ladányi, M.; Bálo, B.; Bodor, P. Szőlőfajták Ampelometriai Mutatóinak Statisztikai Elemzése. XXIII. Növénynemesítési Tudományos Nap; MTA Agrártudományok Osztálya Növénynemesítési Tudományos Bizottság, Magyar Növénynemesítők Egyesülete: Budapest, Hungary, 2017; p. 137. [Google Scholar]
- Németh, M. Ampelográfiai Album. Termesztett Borszőlőfajták 1; Mezőgazdasági Kiadó: Budapest, Hungary, 1967; p. 235. [Google Scholar]
- Németh, M. Ampelográfiai Album. Termesztett Borszőlőfajták 2; Mezőgazdasági Kiadó: Budapest, Hungary, 1970; p. 272. [Google Scholar]
- Németh, M. Ampelográfiai Album 3. Alany-, Direkt Termő és Csemegeszőlő-Fajták; Mezőgazdasági Kiadó: Budapest, Hungary, 1975; p. 358. [Google Scholar]
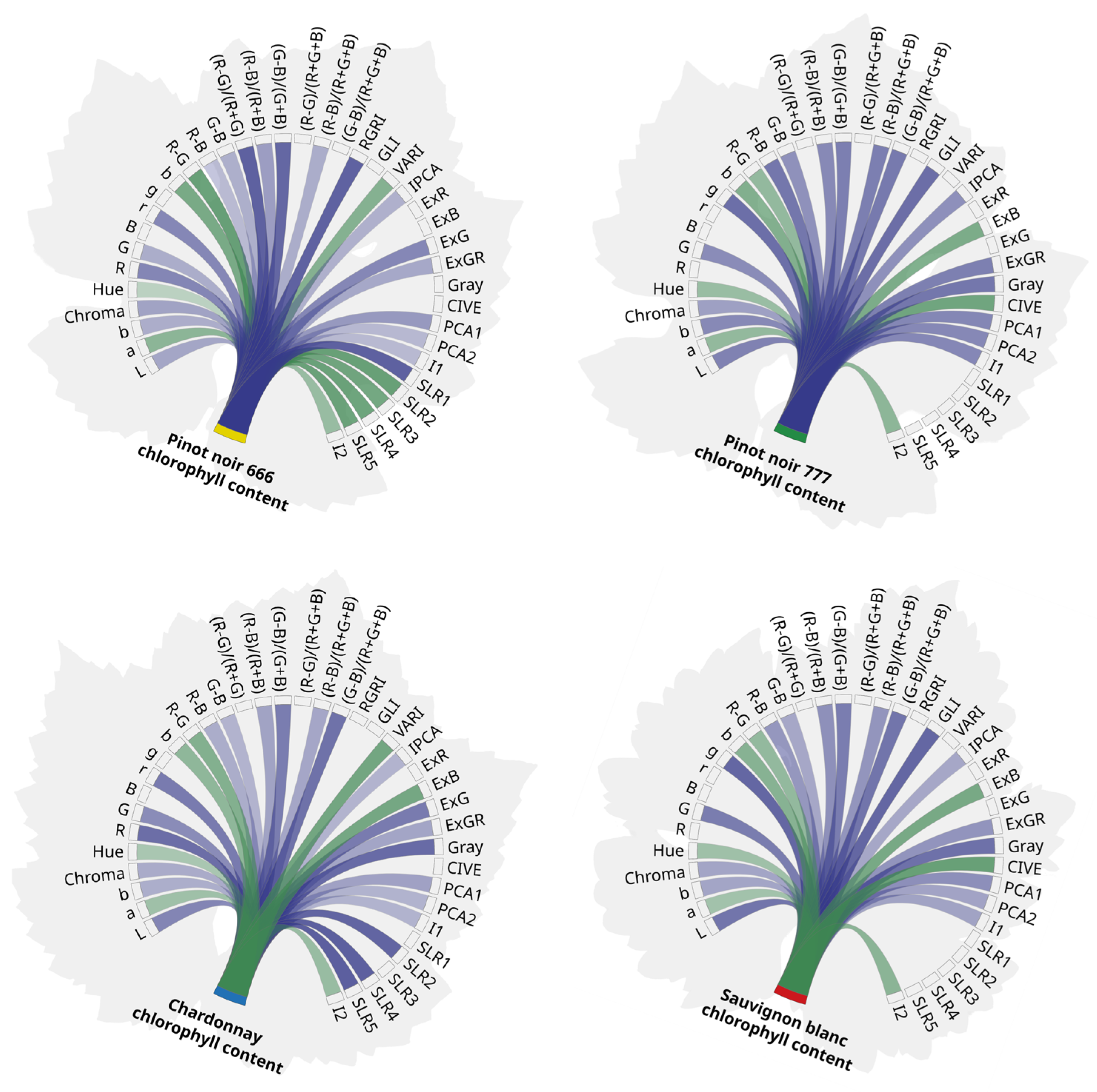
| Nr. | Color Attribute | Vitis vinifera L. | ‘Chardonnay’ | ‘Sauvignon Blanc’ | ‘Pinot Noir’ 666 | ‘Pinot Noir’ 777 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L* | −0.4943 | ** | −0.4126 | ** | −0.3275 | ** | −0.5912 | ** | −0.3963 | ** |
| 2 | a* | 0.6731 | ** | 0.5448 | ** | 0.5599 | ** | 0.4395 | ** | 0.4746 | ** |
| 3 | b* | −0.6853 | ** | −0.6386 | ** | −0.5868 | ** | −0.6349 | ** | −0.4322 | ** |
| 4 | Chroma | −0.7187 | ** | −0.6211 | ** | −0.5889 | ** | −0.5776 | ** | −0.5562 | ** |
| 5 | Hue | 0.6308 | ** | 0.5978 | ** | 0.5430 | ** | 0.7038 | ** | 0.5133 | ** |
| 6 | R | −0.2175 | ** | −0.2872 | * | −0.1274 | n.s. | −0.4158 | ** | −0.1281 | n.s. |
| 7 | G | −0.5463 | ** | −0.4529 | ** | −0.3690 | ** | −0.6264 | ** | −0.4509 | ** |
| 8 | B | 0.1702 | ** | 0.0760 | n.s. | 0.1783 | n.s. | 0.0401 | n.s. | 0.1896 | n.s. |
| 9 | r | −0.1918 | ** | −0.3587 | ** | −0.1759 | n.s. | −0.4223 | ** | −0.1544 | n.s. |
| 10 | g | −0.2929 | ** | −0.1698 | n.s. | −0.2487 | * | −0.0250 | n.s. | −0.2899 | ** |
| 11 | b | 0.4679 | ** | 0.4559 | ** | 0.4072 | ** | 0.3324 | ** | 0.3838 | ** |
| 12 | R-G | 0.5969 | ** | 0.3877 | ** | 0.4789 | ** | 0.2380 | * | 0.4789 | ** |
| 13 | R-B | −0.5712 | ** | −0.6262 | ** | −0.5121 | ** | −0.7073 | ** | −0.3352 | ** |
| 14 | G-B | −0.7112 | ** | −0.6664 | ** | −0.5997 | ** | −0.6445 | ** | −0.4795 | ** |
| 15 | (R − G)/(R + G) | 0.0817 | n.s. | −0.1066 | n.s. | 0.0548 | n.s. | −0.2154 | * | 0.0883 | n.s. |
| 16 | (R − B)/(R + B) | −0.5261 | ** | −0.5777 | ** | −0.4713 | ** | −0.5431 | ** | −0.4320 | ** |
| 17 | (G − B)/(G + B) | −0.4280 | ** | −0.3660 | ** | −0.3657 | ** | −0.2497 | * | −0.3818 | ** |
| 18 | (R − G)/(R + G + B) | 0.1571 | ** | −0.0196 | n.s. | 0.1313 | n.s. | −0.1316 | n.s. | 0.1652 | n.s. |
| 19 | (R − B)/(R + G + B) | −0.5525 | ** | −0.5944 | ** | −0.4999 | ** | −0.6279 | ** | −0.3805 | ** |
| 20 | (G − B)/(R + G + B) | −0.3807 | ** | −0.3103 | * | −0.3264 | ** | −0.1571 | n.s. | −0.3484 | ** |
| 21 | RGRI | 0.0941 | n.s. | −0.1056 | n.s. | 0.0549 | n.s. | −0.2221 | * | 0.1030 | n.s. |
| 22 | GLI | −0.3034 | ** | −0.1722 | n.s. | −0.2516 | * | −0.0310 | n.s. | −0.2884 | ** |
| 23 | VARI | 0.1080 | * | 0.3007 | * | 0.1470 | n.s. | 0.4012 | ** | 0.0260 | n.s. |
| 24 | IPCA | −0.7098 | ** | −0.6669 | ** | −0.5989 | ** | −0.6489 | ** | −0.4759 | ** |
| 25 | ExR | 0.1571 | ** | −0.0196 | n.s. | 0.1313 | n.s. | −0.1316 | n.s. | 0.1652 | n.s. |
| 26 | ExB | 0.3807 | ** | 0.3103 | ** | 0.3264 | ** | 0.1571 | n.s. | 0.3484 | ** |
| 27 | ExG | −0.1918 | ** | −0.3587 | ** | −0.1759 | n.s. | −0.4223 | ** | −0.1544 | n.s. |
| 28 | ExGR | −0.5505 | ** | −0.5891 | ** | −0.4956 | ** | −0.6043 | ** | −0.3848 | ** |
| 29 | Gray | −0.3574 | ** | −0.2721 | ** | −0.3056 | ** | −0.1188 | n.s. | −0.3350 | ** |
| 30 | CIVE | 0.2852 | ** | 0.1581 | n.s. | 0.2420 | * | 0.0148 | n.s. | 0.2838 | ** |
| 31 | PCA1 | −0.5283 | ** | −0.5662 | ** | −0.4683 | ** | −0.5222 | ** | −0.4190 | ** |
| 32 | PCA2 | −0.6808 | ** | −0.6639 | ** | −0.5809 | ** | −0.6902 | ** | −0.4281 | ** |
| 33 | I1 | −0.6762 | ** | −0.6627 | ** | −0.5781 | ** | −0.6933 | ** | −0.4227 | ** |
| 34 | SLR1 | 0.0813 | n.s. | −0.1085 | n.s. | 0.0552 | n.s. | −0.2149 | * | 0.0879 | n.s. |
| 35 | SLR2 | −0.0898 | n.s. | −0.2421 | * | 0.0637 | n.s. | 0.2312 | * | −0.0663 | n.s. |
| 36 | SLR3 | −0.0272 | * | −0.1631 | n.s. | 0.1408 | n.s. | 0.2803 | ** | −0.0301 | n.s. |
| 37 | SLR4 | −0.0687 | n.s. | −0.2089 | * | 0.0933 | n.s. | 0.2520 | * | −0.0548 | n.s. |
| 38 | SLR5 | −0.0717 | n.s. | −0.2051 | * | 0.0881 | n.s. | 0.2513 | * | −0.0562 | n.s. |
| 39 | I2 | 0.4466 | ** | 0.5067 | ** | 0.4351 | ** | 0.5106 | ** | 0.4472 | ** |
| Parameters | Discriminant Analysis | Kernel Function | |||
|---|---|---|---|---|---|
| Linear | Polynomial | Radial | Sigmoid | ||
| Training | |||||
| CIE-L*a*b* | 68.66 | 65.09 | 70.56 | 43.09 | |
| RGB | 70.19 | 65.22 | 71.97 | 40.00 | |
| Derived RGB | 66.84 | 55.91 | 64.31 | 23.19 | |
| PCA | 39.53 | 39.03 | 43.69 | 25.63 | |
| Miscellaneous | 56.75 | 49.97 | 60.16 | 33.09 | |
| All | 73.25 | 63.56 | 72.91 | 40.50 | |
| Validation | |||||
| CIE-L*a*b* | 68.5 | 67.75 | 60.50 | 64.63 | 41.75 |
| RGB | 64.5 | 67.88 | 59.88 | 66.63 | 40.00 |
| Derived RGB | 62.5 | 64.75 | 52.13 | 60.00 | 22.88 |
| PCA | 54.5 | 38.38 | 38.13 | 39.88 | 24.25 |
| Miscellaneous | 61.25 | 54.88 | 47.13 | 55.38 | 31.25 |
| All | 62.75 | 71.63 | 58.38 | 66.38 | 39.88 |
| Literature | ‘Pinot Noir’ 777 | ‘Pinot Noir’ 666 | ‘Chardonnay’ | ‘Sauvignon Blanc’ | |
|---|---|---|---|---|---|
| R | present study | 45.42 | 38.84 | 41 | 35.99 |
| Fuentes et al. [36] | - | - | 118.7 | 94.9 | |
| Gutiérrez-Gamboa et al. [37]—stressed | 64.37 * | 68.96 | 71.83 | ||
| Gutiérrez-Gamboa et al. [37]—non-stressed | 75.92 * | 81.24 | 75.94 | ||
| G | present study | 84.87 | 78.43 | 70.45 | 69.94 |
| Fuentes et al. [36] | - | - | 129 | 119.2 | |
| Gutiérrez-Gamboa et al. [37]—stressed | 67.72 * | 74.35 | 75.94 | ||
| Gutiérrez-Gamboa et al. [37]—non-stressed | 78.92 * | 85.36 | 77.77 | ||
| B | present study | 38.21 | 27.82 | 36.89 | 26.23 |
| Fuentes et al. [36] | - | - | 13.1 | 27.9 | |
| Gutiérrez-Gamboa et al. [37]—stressed | 47.53 * | 46.86 | 49.06 | ||
| Gutiérrez-Gamboa et al. [37]—non-stressed | 48.04 * | 50.34 | 49.49 | ||
| L* | present study | 32.23 | 29.46 | 26.82 | 26.21 |
| Fuentes et al. [36] | - | - | 57.6 | 56.1 | |
| Gutiérrez-Gamboa et al. [37]—stressed | - | - | - | ||
| Gutiérrez-Gamboa et al. [37]—non-stressed | - | - | - | ||
| a* | present study | −24.09 | −24.69 | −18.61 | −21.82 |
| Fuentes et al. [36] | - | - | −8.6 | −19.3 | |
| Gutiérrez-Gamboa et al. [37]—stressed | - | - | - | ||
| Gutiérrez-Gamboa et al. [37]—non-stressed | - | - | - | ||
| b* | present study | 22.37 | 24.66 | 16.43 | 21.73 |
| Fuentes et al. [36] | - | - | 13.1 | 27.9 | |
| Gutiérrez-Gamboa et al. [37]—stressed | - | - | - | ||
| Gutiérrez-Gamboa et al. [37]—non-stressed | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodor-Pesti, P.; Taranyi, D.; Vértes, G.; Fazekas, I.; Nyitrainé Sárdy, D.Á.; Deák, T.; Varga, Z.; Baranyai, L. Smartphone-Based Leaf Colorimetric Analysis of Grapevine (Vitis vinifera L.) Genotypes. Horticulturae 2024, 10, 1179. https://doi.org/10.3390/horticulturae10111179
Bodor-Pesti P, Taranyi D, Vértes G, Fazekas I, Nyitrainé Sárdy DÁ, Deák T, Varga Z, Baranyai L. Smartphone-Based Leaf Colorimetric Analysis of Grapevine (Vitis vinifera L.) Genotypes. Horticulturae. 2024; 10(11):1179. https://doi.org/10.3390/horticulturae10111179
Chicago/Turabian StyleBodor-Pesti, Péter, Dóra Taranyi, Gábor Vértes, István Fazekas, Diána Ágnes Nyitrainé Sárdy, Tamás Deák, Zsuzsanna Varga, and László Baranyai. 2024. "Smartphone-Based Leaf Colorimetric Analysis of Grapevine (Vitis vinifera L.) Genotypes" Horticulturae 10, no. 11: 1179. https://doi.org/10.3390/horticulturae10111179
APA StyleBodor-Pesti, P., Taranyi, D., Vértes, G., Fazekas, I., Nyitrainé Sárdy, D. Á., Deák, T., Varga, Z., & Baranyai, L. (2024). Smartphone-Based Leaf Colorimetric Analysis of Grapevine (Vitis vinifera L.) Genotypes. Horticulturae, 10(11), 1179. https://doi.org/10.3390/horticulturae10111179








