Titanium Nanoparticles (TiO2-NPs) as Catalysts for Enhancing Drought Tolerance in Grapevine Saplings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Growing Grafted Grapevine Saplings
2.3. Planting and Cultivation of Saplings
2.4. Characterization of TiO2-NPs and Green Synthesis
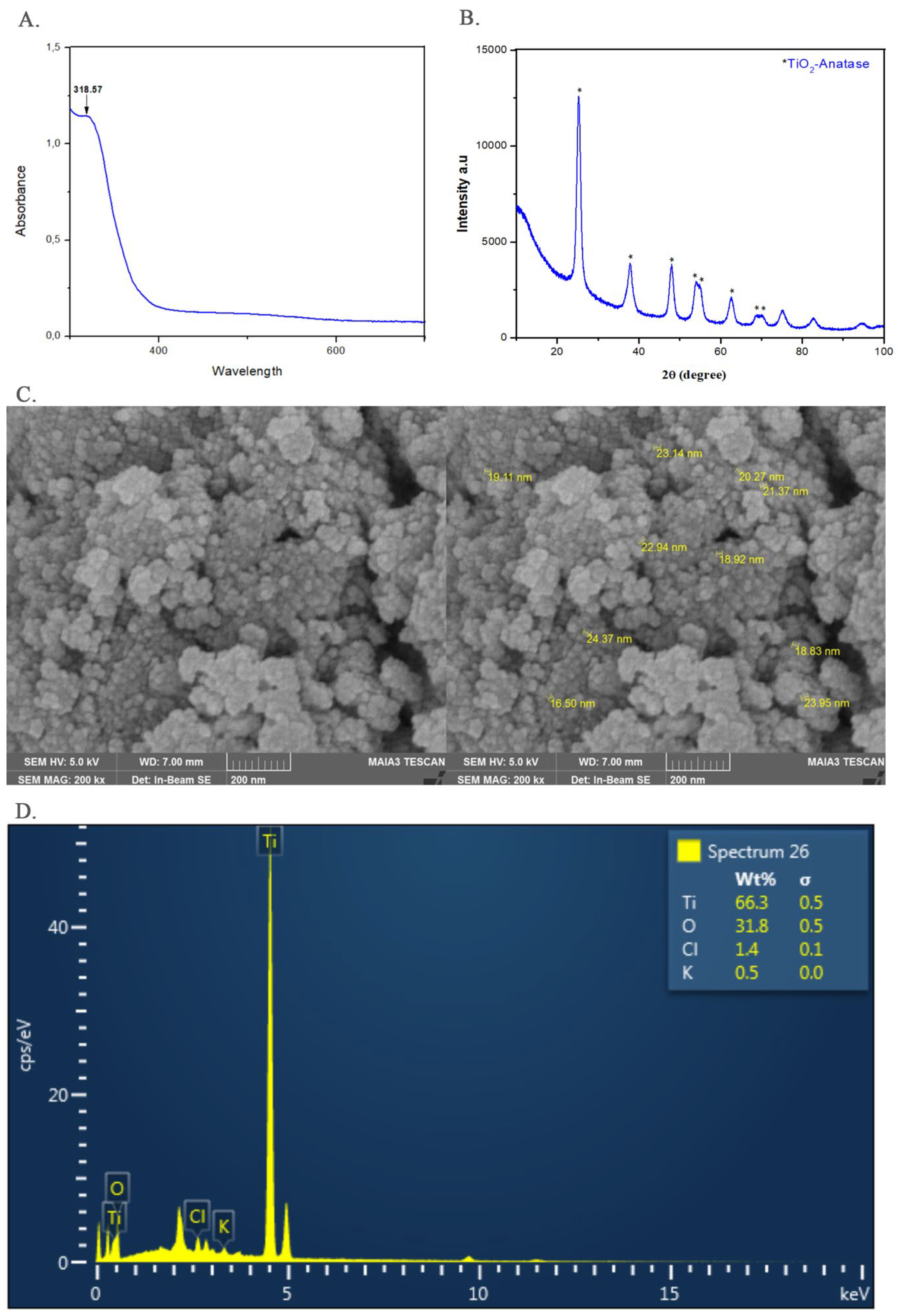
2.4.1. UV–Vis Analysis
2.4.2. XRD Analysis of Powder
2.4.3. SEM Analysis
2.4.4. EDX Analysis
2.5. Preparation and Application of NP Solutions
2.6. Drought Stress Application
2.7. Measurements and Analyses in Saplings
2.7.1. Morphological Analyses
2.7.2. Physiological Analyses
2.7.3. Biochemical Analyses
2.8. Experimental Design and Data Analysis
3. Results
3.1. Characterization Analysis of TiO2-NPs
3.2. Defense Mechanisms in Grapevine Saplings
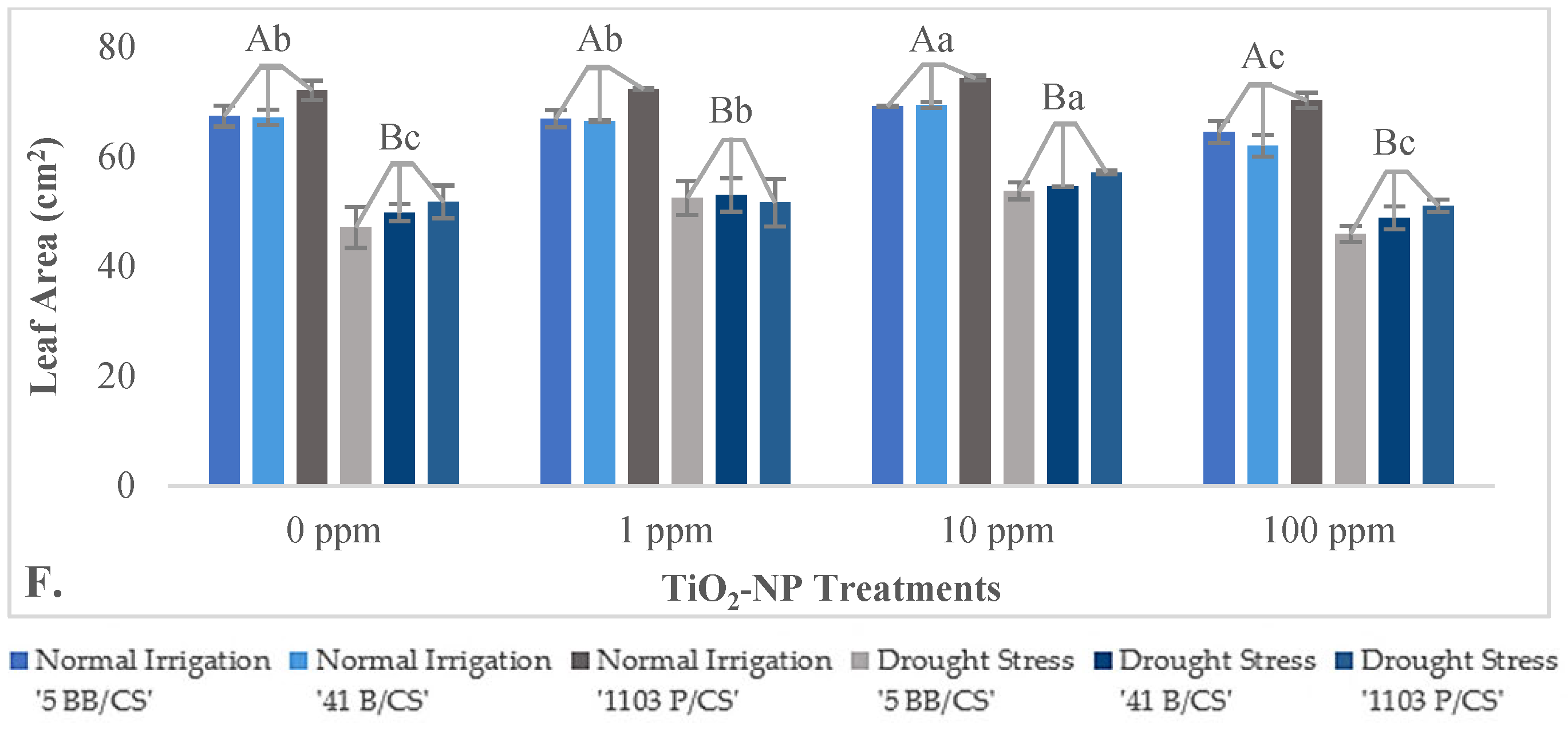
3.2.1. Impact of TiO2-NPs on Growth Traits
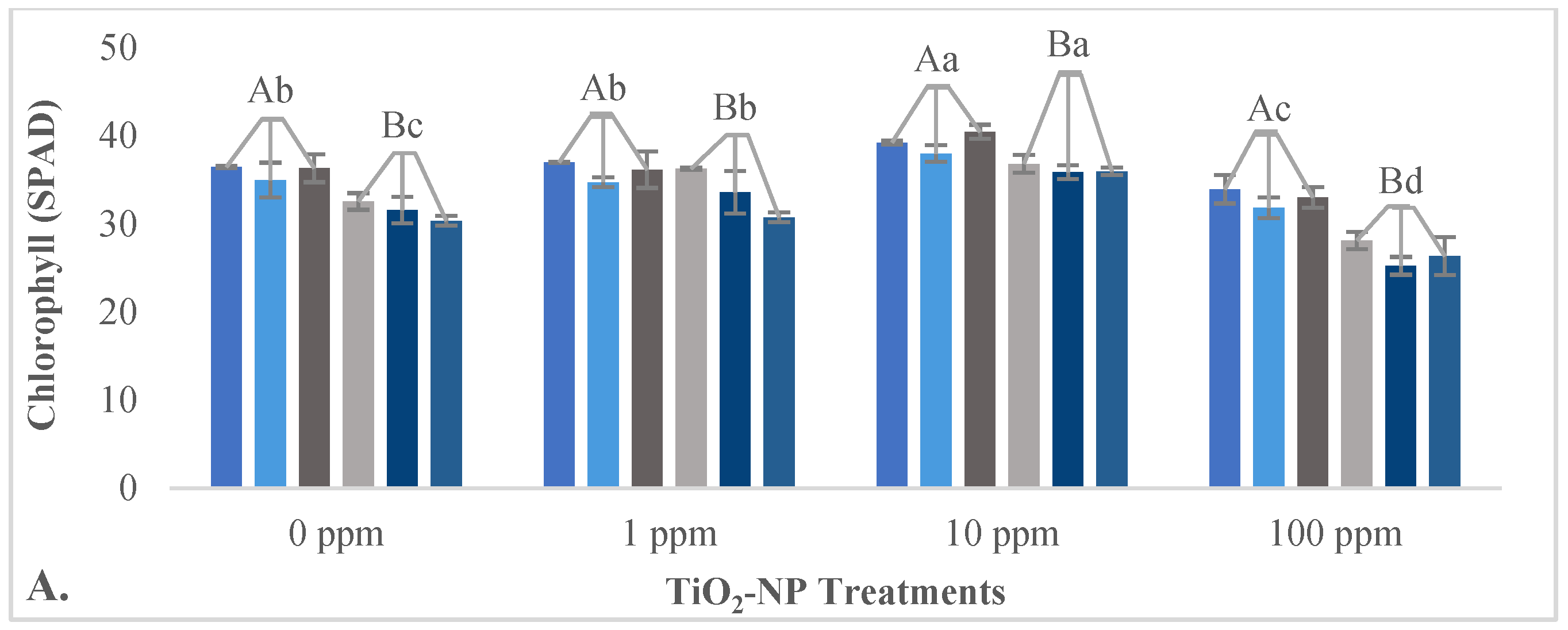
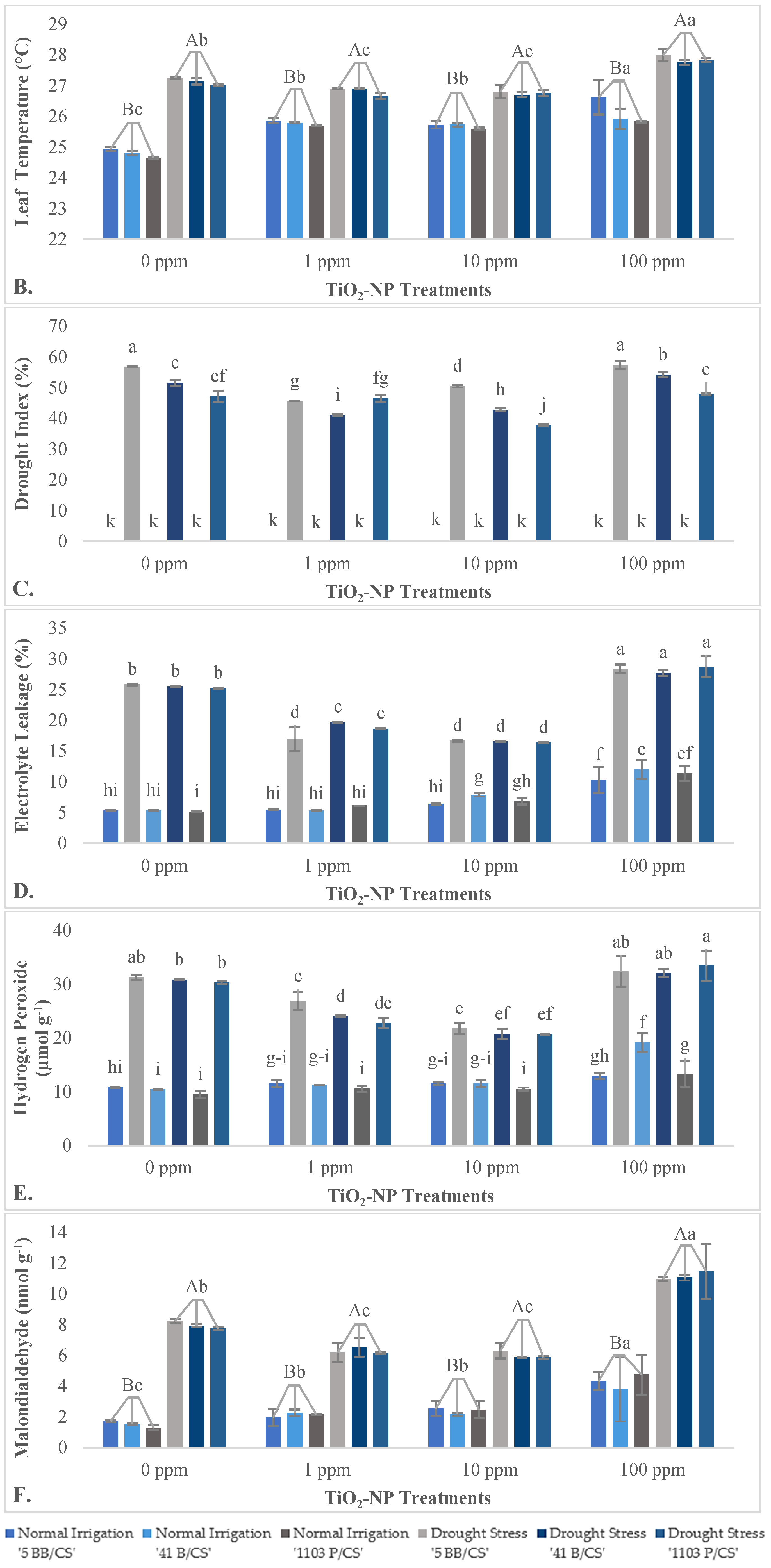
3.2.2. Impact of TiO2-NPs on Physiological Parameters
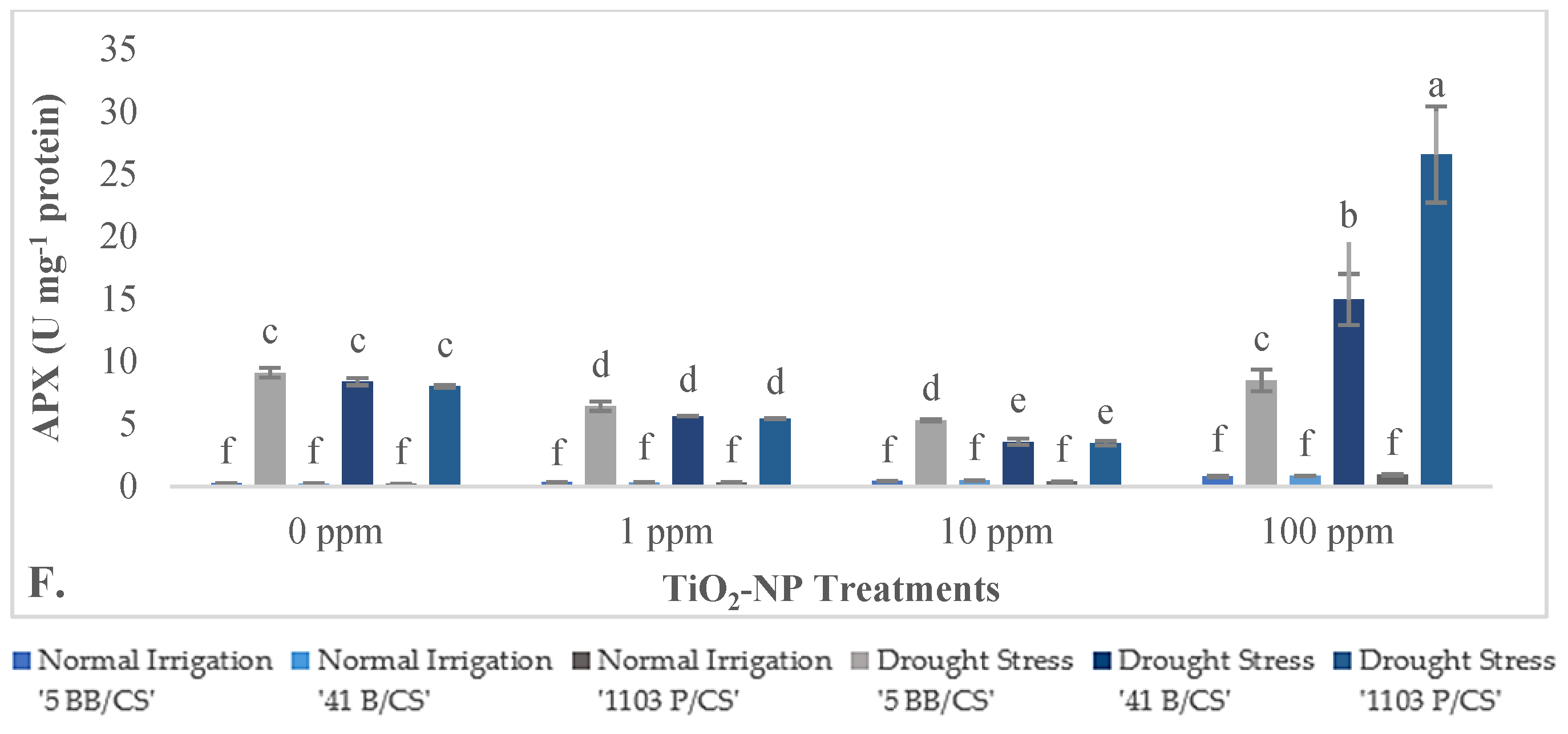
3.2.3. Effects of TiO2-NPs on Biochemical Parameters
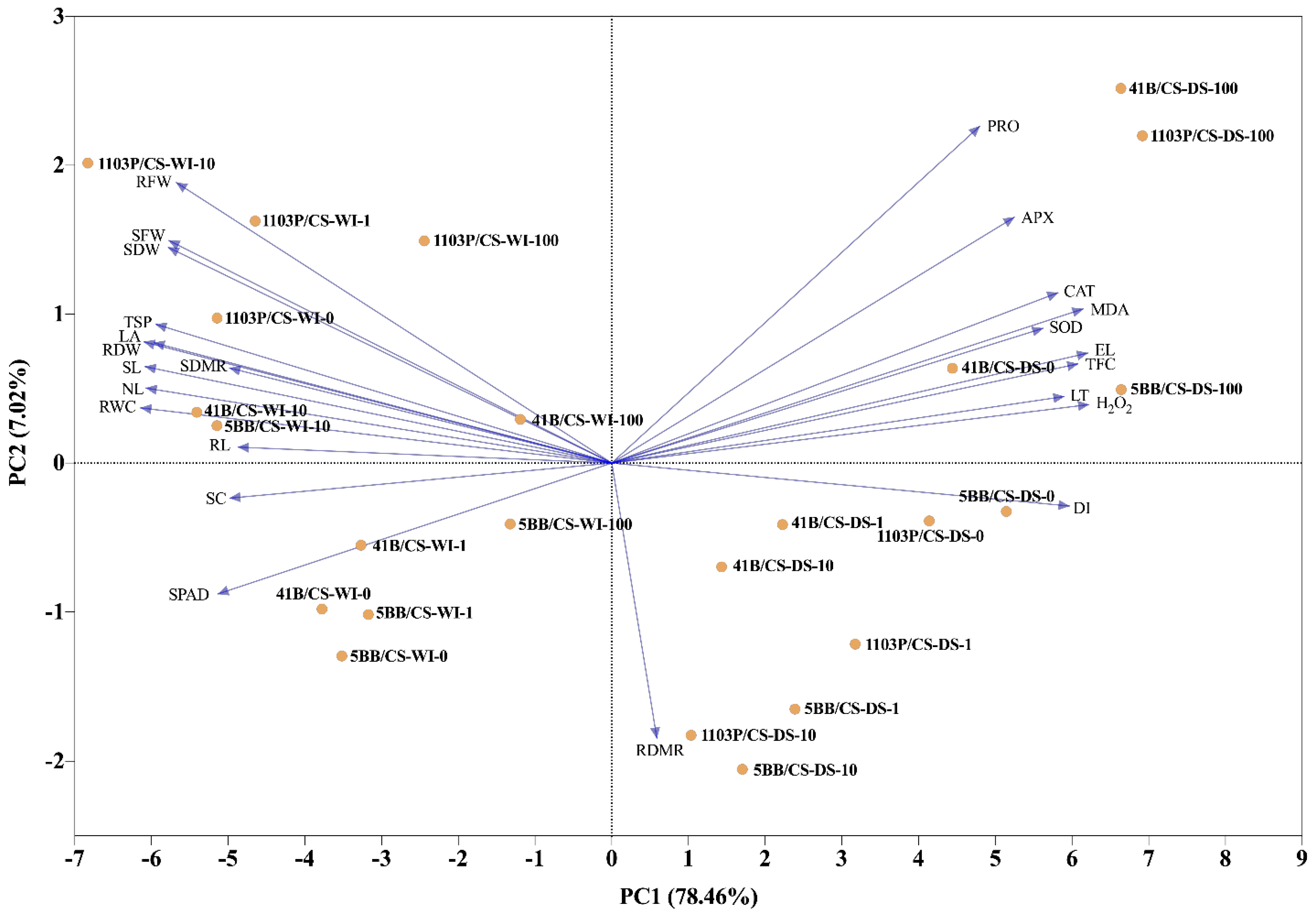
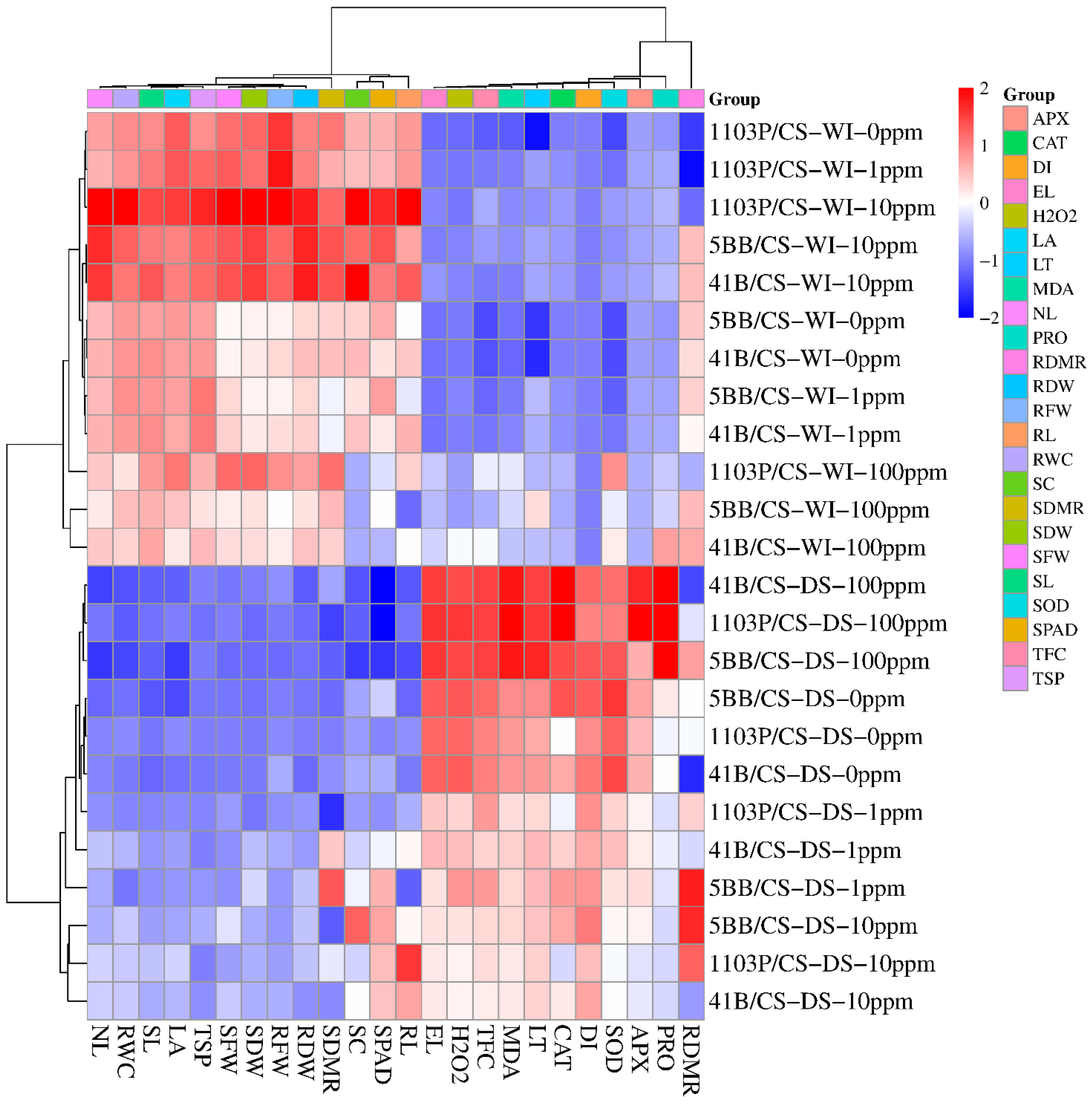
3.3. General Evolution
4. Discussion
4.1. Characterization of TiO2-NPs
4.2. Effects of TiO2-NPs on the Growth Characteristics of Grapevine Saplings
4.3. Effects of TiO2-NPs on Some Physiological and Biochemical Properties
4.4. General Evolution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Haddad, N.; Choukri, H.; Ghanem, M.E.; Smouni, A.; Mentag, R.; Rajendran, K.; Hejjaoui, K.; Maalouf, F.; Kumar, S. High temperature and drought stress effects on growth, yield and nutritional quality with transpiration response to vapor pressure deficit in lentil. Plants 2022, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 23 January 2023).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014—Impacts, Adaptation and Vulnerability: Regional Aspects, 1st ed.; Cambridge University Press: New York, NY, USA, 2014; pp. 1–9. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Clark, R. Physiology of Crop Production, 1st ed.; Food Products Press: Boca Raton, FL, USA, 2006; pp. 1–356. [Google Scholar] [CrossRef]
- Cardone, M.F.; Perniola, R.; Catacchio, C.R.; Alagna, F.; Rotunno, S.; Crupi, P.; Antonacci, D.; Velasco, R.; Ventura, M.; Bergamini, C. Grapevine Adaptation to Drought: New Candidate Genes for the Genotype-Dependent Response. In Proceedings of the 42nd World Congress of Vine and Wine, BIO Web of Conferences, Geneva, Switzerland, 15–19 July 2019. [Google Scholar] [CrossRef]
- Calderan, A.; Sivilotti, P.; Braidotti, R.; Mihelčič, A.; Lisjak, K.; Vanzo, A. Managing moderate water deficit increased anthocyanin concentration and proanthocyanidin galloylation in “Refošk” grapes in Northeast Italy. Agric. Water Manag. 2021, 246, 106684. [Google Scholar] [CrossRef]
- Oguz, M.Ç.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. C. R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Semerci, A.; Çiçek, N.; Karahan, F.A.; Özyürek, E.; Arici, Y.K.; Ekmekçi, Y. Some growth and chlorophyll fluorescence parameters of black and hybrid poplar clones under water stress. Turk. J. Agric. For. 2017, 41, 348–356. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, U.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water—use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Martim, S.A.; Santos, M.P.; Pecanha, A.L.; Pommer, C.; Campostrini, E.; Viana, A.P.; Facanha, A.R.; Bressan-Smith, R. Photosynthesis and cell respiration modulated by water deficit in grapevine (Vitis vinifera L.) cv. Cabernet Sauvignon. Braz. J. Plant Physiol. 2009, 21, 95–102. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Morales, J. Climate change (elevated CO2, elevated temperature and moderate drought) triggers the antioxidant enzymes’ response of grapevine cv. Tempranillo, avoiding oxidative damage. Physiol. Plant. 2012, 144, 99–110. [Google Scholar] [CrossRef]
- Fahim, S.; Ghanbari, A.; Naji, A.M.; Shokohian, A.A.; Lajayer, H.M.; Gohari, G.; Hano, C. Multivariate discrimination of some grapevine cultivars under drought stress in Iran. Horticulturae 2022, 8, 871. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Vitic. 2002, 53, 268–274. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Souza-da Costa, B.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Nanotechnology: Recent advances in viticulture and enology. J. Sci. Food Agric. 2021, 101, 6156–6166. [Google Scholar] [CrossRef]
- Rikabad, M.M.; Pourakbar, L.; Moghaddam, S.S.; Popovic-Djordjevic, J. Agrobiological, chemical and antioxidant properties of saffron (Crocus sativus L.) exposed to TiO2 nanoparticles and ultraviolet-B stress. Ind. Crops Prod. 2019, 137, 137–143. [Google Scholar] [CrossRef]
- Karamian, R.; Ghasemlou, F.; Amiri, H. Physiological evaluation of drought stress tolerance and recovery in Verbascum sinuatum plants treated with methyl jasmonate, salicylic acid and titanium dioxide nanoparticles. Off. J. Soc. Bot. Ital. 2020, 154, 277–287. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications-An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Bhavyasree, P.G.; Xavier, T.S. Green synthesis of copper oxide/carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon 2020, 6, e03323. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigena, M.K.; Hagen, T.M. Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Anekonda, T.S. Resveratrol-a boon for treating Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Castangia, I.; Marongiu, F.; Manca, M.L.; Pompei, R.; Angius, F.; Ardu, A.; Fadda, A.M.; Manconi, M.; Ennas, G. Combination of grape extract-silver nanoparticles and liposomes: A totally green approach. Eur. J. Pharm. Sci. 2017, 97, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Arrieta, J.; Florido, A.; Pérez-Ràfols, C.; Serrano, N.; Fiol, N.; Poch, J.; Villaescusa, I. Green synthesis of Ag nanoparticles using grape stalk waste extract for the modification of screen-printed electrodes. Nanomaterials 2018, 8, 946. [Google Scholar] [CrossRef] [PubMed]
- Ollat, N.; Geny, L.; Soyer, J. Les boutures fructifères de vigne: Validation d, un modèle d, etude du development de la physiologie de la vigne, I Caractèristiques de l’appareil vegetative. J. Int. Sci. Vigne Vin 1998, 32, 1–9. [Google Scholar]
- Korkmaz, N.; Ceylan, Y.; Taslimi, P.; Karadağ, A.; Bülbül, A.S.; Şen, F. Biogenic nano silver: Synthesis, characterization, antibacterial, antibiofilms, and enzymatic activity. Adv. Powder Technol. 2020, 31, 2942–2950. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Met. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Vara Prasad, P.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef]
- Mohammadi, H.; Esmailpour, M.; Gheranpaye, A. Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov. 2016, 107, 385–396. [Google Scholar] [CrossRef]
- Cochetel, N.; Ghan, R.; Toups, H.S.; Degu, A.; Tillett, R.L.; Schlauch, K.A.; Cramer, G.R. Drought tolerance of the grapevine, Vitis champinii cv. Ramsey, is associated with higher photosynthesis and greater transcriptomic responsiveness of abscisic acid biosynthesis and signaling. BMC Plant Biol. 2020, 20, 55. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Javed, B.; Mashwani, Z.R.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; et al. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Earl, H.J. A precise gravimetric method for simulating drought stress in pot experiments. Crop Sci. 2003, 43, 1868–1873. [Google Scholar] [CrossRef]
- International Plant Genetic Resources Institute (IPGRI). Descriptors for Grapevine (Vitis ssp.), 1st ed.; International Plant Genetic Resources Institute: Rome, Italy, 1997; p. 62. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b in leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.C. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stres studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S.; Veselova, M.V.; Bulgakov, V.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. The rol-B gene-induced over production of resveratrol in Vitis amurensis transformed cells. J. Biotechnol. 2007, 128, 681–692. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.R. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-Induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Özden, M.; Demirel, U.; Kahraman, U. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifoli. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Gong, Y.; Toivonen, P.M.A.; Lau, O.L.; Wiersma, P.A. Antioxidant system level in ‘Braeburn’ apple in related to its browing disorder. Bot. Bull. Acad. Sin. 2001, 42, 259–264. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Evgenidis, G.; Traka-Mavrona, E.; Koutsika-Sotiriou, M. Principal component and cluster analysis as a tool in the assessment of tomato hybrids and cultivars. Int. J. Agron. 2011, 9, 1–7. [Google Scholar] [CrossRef]
- Saka, A.; Shifera, Y.; Jule, L.T.; Badassa, B.; Nagaprasad, N.; Shanmugam, R.; Dwarampudi, L.P.; Seenivasan, V.; Ramaswamy, K. Biosynthesis of TiO2 nanoparticles by Caricaceae (Papaya) shell extracts for antifungal application. Sci. Rep. 2022, 12, 15960. [Google Scholar] [CrossRef]
- Rajkumar, S.; Venkatraman, M.R.; Suguna, K.; Karuppasamy, P.; Pandian, M.S.; Ramasamy, P. Synthesis of Ag-incorporated TiO2 nanoparticles by simple green approach as working electrode for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2022, 33, 4965–4973. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Alasiri, A.S.; Ahmad, J.; Alqahtani, A.A.; Abdullah, M.M.; Abdel-Wahab, B.A.; Pathak, K.; Saikia, S.; Das, A.; Sarma, H.; et al. Green Synthesis of Titanium Dioxide Nanoparticles Using Ocimum sanctum Leaf Extract: In Vitro Characterization and Its Healing Efficacy in Diabetic Wounds. Molecules 2022, 27, 7712. [Google Scholar] [CrossRef]
- Anbumani, D.; Dhandapani, K.V.; Manoharan, J.; Babujanarthanam, R.; Bashir, A.K.H.; Muthusamy, K.; Alfarhan, A.; Kanimozhi, K. Green synthesis and antimicrobial efficacy of titanium dioxide nanoparticles using Luffa acutangula leaf extract. J. King Saud Univ. Sci. 2022, 34, 101896. [Google Scholar] [CrossRef]
- Ghomrasni, N.B.; Chivas-Joly, C.; Devoille, L.; Hochepied, J.F.; Feltin, N. Challenges in sample preparation for measuring nanoparticles size by scanning electron microscopy from suspensions, powder form and complex media. Powder Technol. 2020, 359, 226–237. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Zheng, L.; Mingyu, S.; Chao, L.; Liang, C.; Huang, H.; Xiao, W.; Xiaoqing, L.; Yang, F.; Gao, F.; Hong, F. Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol. Trace Elem. Res. 2007, 119, 68–76. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Sauret-Gueto, S.; Calder, G.; Harberd, N.P. Transient gibberellin application promotes Arabidopsis thaliana hypocotyl cell elongation without maintaining transverse orientation of microtubules on the outer tangential wall of epidermal cells. Plant J. 2012, 69, 628–639. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Fellet, G.; Zavalloni, C.; Carlino, E.; Musetti, R. Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 332. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Nabati, J.; Mirmiran, S.M.; Hojjat, H. Titanium dioxide nanoparticles mitigates the adverse effects of salinity stress on grass pea (Lathyrus sativus L.) germination and seedling development. Azarian J. Agric. 2020, 7, 17–25. [Google Scholar] [CrossRef]
- Yaqoob, S.; Ullah, F.; Mehmood, S.; Mahmood, T.; Ullah, M.; Khattak, A.; Zeb, M.A. Effect of waste water treated with TiO2 nanoparticles on early seedling growth of Zea mays L. J. Water Reuse Desalination 2017, 8, 424–431. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Aghili, R.; Nabavi, M. Physiological effects of TiO2 nanoparticles on wheat (Triticum aestivum). Tech. J. Eng. Appl. Sci. 2013, 3, 1365–1370. [Google Scholar]
- AlKahtani, M.D.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.; Abdelaal, K.A. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Bhat, J.A.; Guan, Y.; Jan, B.L.; Tyagi, A.; Ahmad, P. Nitric oxide and spermine revealed positive defense interplay for the regulation of the chromium toxicity in soybean (Glycine max L.). Environ. Pollut. 2022, 308, 119602. [Google Scholar] [CrossRef]
- Mohammadi, R.; Maali-Amiri, R.; Abbasi, A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 2013, 152, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, Z.; Xie, Z.; Tian, X.; Eneji, A.; Duan, L. Coronatine alleviates polyethylene glycol-induced water stress in two rice (Oryza sativa L.) cultivars. J. Agron. Crop Sci. 2008, 194, 360–368. [Google Scholar] [CrossRef]
- Zahed, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Dolatkhah, D.A.; Hosseini, M.S.M.; Pazoki, A. Exogenous chitosan nanoparticles drought stress through changing yield, biochemical attributes, and fatty acid profile of common bean (Phaseolus vulgaris L.) cultivars. Gesunde Pflanz. 2023, 75, 2463–2476. [Google Scholar] [CrossRef]
- Ahmed, K.B.M.; Khan, M.M.A.; Shabbir, A.; Ahmad, B.; Uddin, M.; Azam, A. Comparative effect of foliar application of silicon, titanium and zinc nanoparticles on the performance of vetiver-a medicinal and aromatic plant. Silicon 2023, 15, 153–166. [Google Scholar] [CrossRef]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticle at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Hojjat, H. Effect of nano silver on seed germination and seedling growth in fenugreek seed. ETP Int. J. Food Eng. 2015, 1, 106–110. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russ. Agric. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 2007, 12, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, D.; Nayyar, H. Comparative response of maize and rice genotypes to heat stress: Status of oxidative stress and antioxidants. Acta Physiol. Plant. 2012, 34, 75–86. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; El-Naggar, M.H. Diet and reproductive biology of the Starred Agama, Laudakia stellio (Linnaeus, 1758) (Squamata: Agamidae), in the northern Sinai, Egypt. Zool. Middle East 2013, 59, 136–143. [Google Scholar] [CrossRef]
- Ching, H.; Dandekar, A. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Akte, J.; Yasmin, S.; Bhuiyan, M.J.H.; Khatun, F.; Roy, J.; Goswami, K. In vitro screening of rice genotypes using polyethylene glycol under drought stress. Progress. Agric. 2016, 27, 128–135. [Google Scholar] [CrossRef]
- Nekrasova, G.; Ushakova, O.; Ermakov, A.; Uimin, M.; Byzov, I. Effects of copper (II) ions and copper oxide nanoparticles on Elodea densa Planch. Russ. J. Ecol. 2011, 42, 458–463. [Google Scholar] [CrossRef]
- Shobha, G.; Moses, V.; Ananda, S. Biological synthesis of copper nanoparticles and its impact-a Review. Int. J. Pharm. Sci. Invent. 2014, 3, 28–38. [Google Scholar]
- Kamalizadeh, M.; Bihamta, M.; Peyghambari, S.; Hadian, J. The effect of different levels of titanium dioxide nanoparticle on production of two majore phenolic compound in Dragonhead herb (Dracocephalum moldavica L.). Iran. J. Med. Aromat. Plants Res. 2015, 31, 428–435. [Google Scholar]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Jiang, H.S.; Yin, L.Y.; Ren, N.N.; Zhao, S.T.; Li, Z.; Zhi, Y.; Shao, H.; Li, W.; Gontero, B. Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 2017, 11, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Faraji, J.; Sepehri, A. Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Hamouda, I.; Badri, M.; Mejri, M.; Cruz, C.; Siddique, K.H.M.; Hessini, K. Salt tolerance of Beta macrocarpa is associated with efficient osmotic adjustment and increased apoplastic water content. Plant Biol. 2016, 18, 369–375. [Google Scholar] [CrossRef]
- Ansari, F.A.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daler, S.; Kaya, O.; Korkmaz, N.; Kılıç, T.; Karadağ, A.; Hatterman-Valenti, H. Titanium Nanoparticles (TiO2-NPs) as Catalysts for Enhancing Drought Tolerance in Grapevine Saplings. Horticulturae 2024, 10, 1103. https://doi.org/10.3390/horticulturae10101103
Daler S, Kaya O, Korkmaz N, Kılıç T, Karadağ A, Hatterman-Valenti H. Titanium Nanoparticles (TiO2-NPs) as Catalysts for Enhancing Drought Tolerance in Grapevine Saplings. Horticulturae. 2024; 10(10):1103. https://doi.org/10.3390/horticulturae10101103
Chicago/Turabian StyleDaler, Selda, Ozkan Kaya, Nesrin Korkmaz, Tuğba Kılıç, Ahmet Karadağ, and Harlene Hatterman-Valenti. 2024. "Titanium Nanoparticles (TiO2-NPs) as Catalysts for Enhancing Drought Tolerance in Grapevine Saplings" Horticulturae 10, no. 10: 1103. https://doi.org/10.3390/horticulturae10101103
APA StyleDaler, S., Kaya, O., Korkmaz, N., Kılıç, T., Karadağ, A., & Hatterman-Valenti, H. (2024). Titanium Nanoparticles (TiO2-NPs) as Catalysts for Enhancing Drought Tolerance in Grapevine Saplings. Horticulturae, 10(10), 1103. https://doi.org/10.3390/horticulturae10101103








