Exploring the Role of Calcium in the Physiology of Tulipa: A Comparative Study across Different Cultivars
Abstract
:1. Introduction
2. Materials and Methods
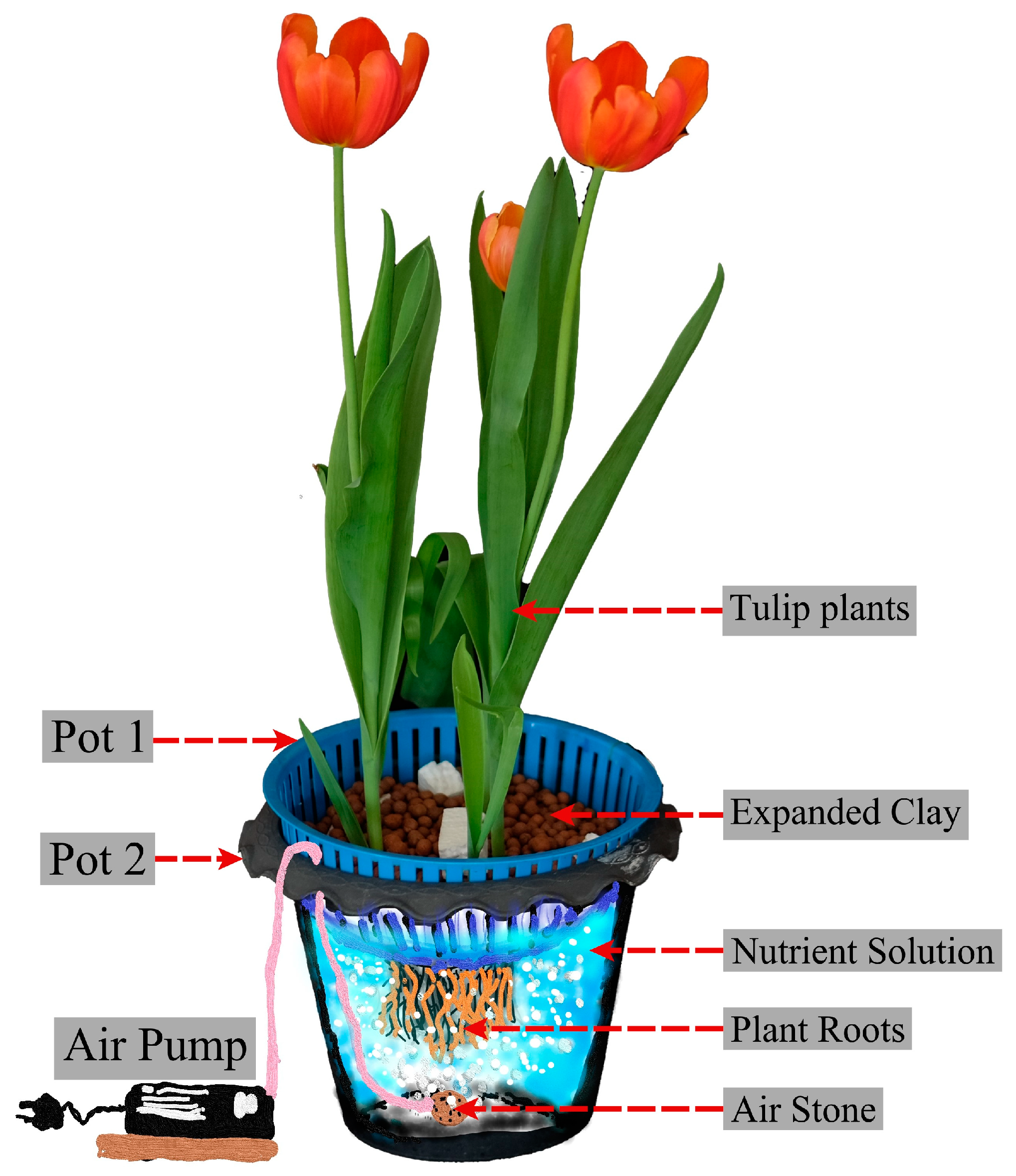
2.1. Plant Materials, Growth Conditions, and Treatment
2.2. Measurement of Plant Morphology and Growth Characteristics
2.3. Chemical Analysis
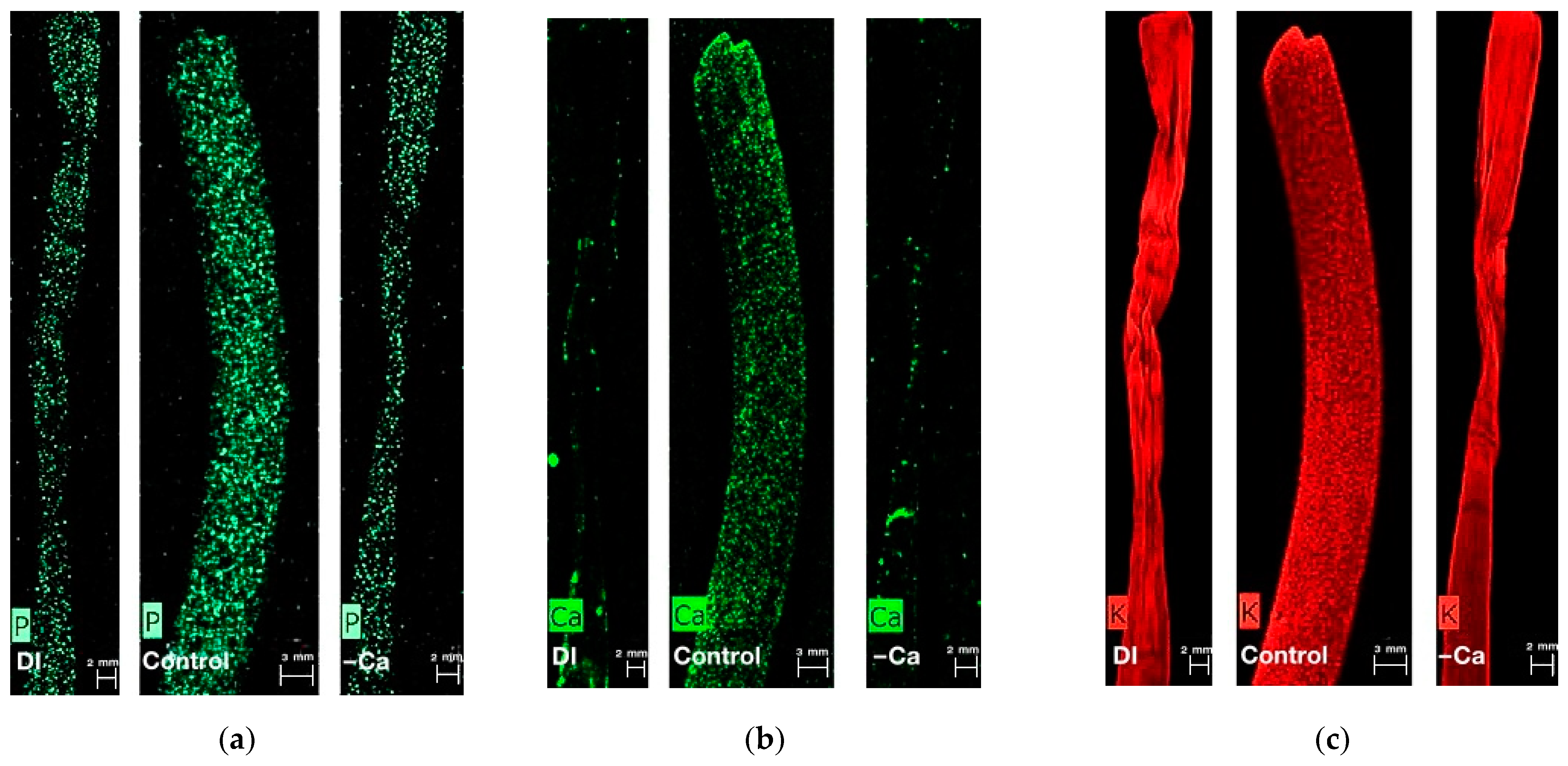
2.4. Determination of Element Mapping
2.5. Statistical Analysis
3. Results
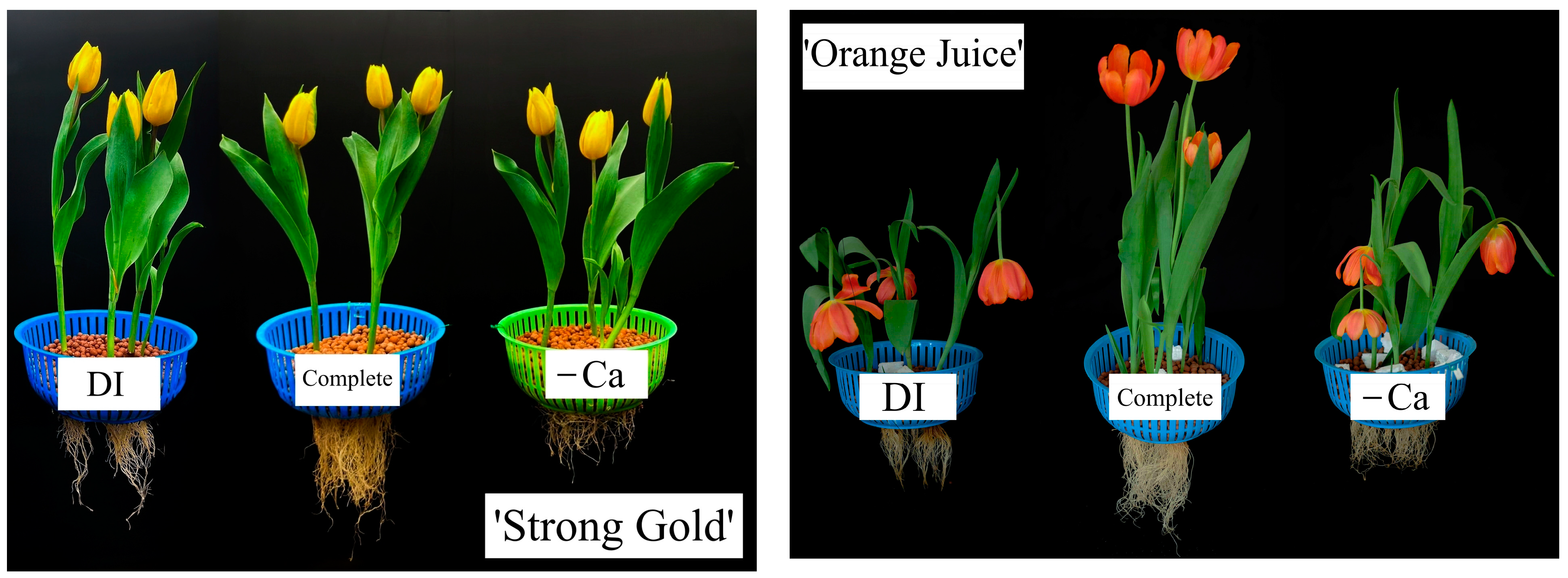
3.1. Vegetative Growth
3.2. Flowering
3.3. New Bulb Qualities
3.4. Photosynthetic Rate, Stomatal Conductance, and Transpiration Rate
3.5. Nutritional Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coskuncelebi, K.; Terzioglu, S.; Turkmen, Z.; Makbul, S.; Usta, A. A comparative study on two closely relative Tulipa L. taxa from NE Anatolia. Plant Syst. Evol. 2008, 276, 191–198. [Google Scholar] [CrossRef]
- Statista. Area Used for Production of Tulip Bulbs in the Netherlands from 2008 to 2019 (in 100 Hectares). 2019. Available online: https://www.statista.com/statistics/641905/total-area-used-for-production-of-tulip-bulbs-in-the-netherlands/ (accessed on 15 October 2023).
- Niedziela, C.E.; Nelson, P.V.; Dickey, D.A. Growth, Development, and Mineral Nutrient Accumulation and Distribution in Tulip from Planting through Postanthesis Shoot Senescence. Int. J. Agron. 2015, 2015, 341287. [Google Scholar] [CrossRef]
- Bakker, M. Teelt Information; CNB Information: Lisse, The Netherlands, 1991; Volume 9. [Google Scholar]
- De Hertogh, A.A. Holland Bulb Forcer’s Guide, 5th ed.; The International Flower-Bulb Center: Hillegom, The Netherlands, 1996. [Google Scholar]
- Klougart, A. Calcium uptake of tulips during forcing. Acta Hort. 1980, 109, 89–95. [Google Scholar] [CrossRef]
- Nelson, P.V.; Niedziela, C.E., Jr. Effects of calcium source and temperature regime on calcium deficiency during hydroponic forcing of tulip. Sci. Hortic. 1998, 73, 137–150. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 1986; pp. 243–254. [Google Scholar]
- Bergmann, W. Nutritional Disorders of Plants; Gustav Fischer: New York, NY, USA, 1992; pp. 132–151. [Google Scholar]
- Ohyama, T.; Ito, M.; Kobayashi, K.; Araki, S.; Yasuyoshi, S.; Sasaki, O.; Yamazaki, T.; Sayoma, K.; Tamemura, R.; Izuno, Y.; et al. Analytical procedures of N, P, K content in plant and manure materials using H2SO4-H2O2 Kjeldahl digestion Method. Bull. Fac. Agri. Niigata Univ. 1991, 43, 111–120. [Google Scholar]
- Gibson, J.L.; Dharmalingam, S.P.; Williams-Rhodes, A.L.; Whipker, B.E.; Nelson, P.V.; Dole, J.M. Nutrient Deficiencies in Bedding Plants; Ball Publishing: Chicago, IL, USA, 2007; 384p. [Google Scholar]
- Duan, S.; Zhang, C.; Song, S.; Ma, C.; Zhang, C.; Xu, W.; Bondada, B.; Wang, L.; Wang, S. Understanding calcium functionality by examining growth characteristics and structural aspects in calcium-deficient grapevine. Sci. Rep. 2022, 12, 3233. [Google Scholar] [CrossRef] [PubMed]
- Ruamrungsri, S. Physiological Studies of Plant Nutrition and Metabolism in Narcissus cv. ‘Garden Giant’; Niigata University: Niigata, Japan, 1997. [Google Scholar]
- Zhang, X.P.; Ma, C.X.; Sun, L.R.; Hao, F.S. Roles and mechanisms of Ca2+ in regulating primary root growth of plants. Plant Signal Behav. 2020, 15, 1748283. [Google Scholar] [CrossRef]
- Stael, S.; Rocha, A.G.; Robinson, A.J.; Kmiecik, P.; Vothknecht, U.C.; Teige, M. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Lett. 2011, 585, 3935. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wan, S.; Li, X. The Significance of Calcium in Photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. [Google Scholar] [CrossRef]
- Charles, S.A.; Halliwell, B. Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other enzymes of the calvin cycle. Biochem. J. 1980, 188, 775–779. [Google Scholar] [CrossRef]
- Kreimer, G.; Melkonian, M.; Holtum, J.A.M.; Latzko, E. Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol. 1988, 86, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Popelkova, H.; Boswell, N.; Yocum, C. Probing the topography of the photosystem II oxygen evolving complex: PsbO is required for efficient calcium protection of the manganese cluster against dark-inhibition by an artificial reductant. Photosynth. Res. 2011, 110, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Tyryshkin, A.M.; Watt, R.K.; Baranov, S.V.; Dasgupta, J.; Hendrich, M.P.; Dismukes, G.C. Spectroscopic evidence for Ca2+ involvement in the assembly of the Mn4Ca cluster in the photosynthetic water-oxidizing complex. Biochemistry 2006, 45, 12876–12889. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Haumann, M. Eight steps preceding O-O bond formation in oxygenic photosynthesis–a basic reaction cycle of the photosystem ii manganese complex. Biochim. Biophys. Acta. 2007, 1767, 472. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, C.; Rocca, N.L.; Andreucci, F.; Deák, Z.; Vass, I.; Rascio, N.; Barbato, R. The extreme halophyte Salicornia veneta is depleted of the extrinsic PsbQ and PsbP proteins of the oxygen-evolving complex without loss of functional activity. Ann. Bot. 2009, 103, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.B.; Powikrowska, M.; Krogholm, K.S.; Naumann-Busch, B.; Schjoerring, J.K.; Husted, S.; Jensen, P.E.; Pedas, P.R. Photosystem II Functionality in Barley Responds Dynamically to Changes in Leaf Manganese Status. Front. Plant Sci. 2016, 7, 1772. [Google Scholar] [CrossRef]
- Nagao, R.; Suzuki, T.; Okumura, A.; Niikura, A.; Iwai, M.; Dohmae, N.; Tomo, T.; Shen, J.R.; Ikeuchi, M.; Enami, I. Topological analysis of the extrinsic PsbO, PsbP and PsbQ proteins in a green algal PSII complex by cross-linking with a water-soluble carbodiimide. Plant Cell Physiol. 2010, 51, 718–727. [Google Scholar] [CrossRef]
- Ifuku, K.; Nakatsu, T.; Kato, H.; Sato, F. Crystal structure of the PsbP protein of photosystem II from Nicotiana tabacum. EMBO Rep. 2004, 5, 362. [Google Scholar] [CrossRef]
- Heredia, P.; Rivas, J.D.L. Calcium-dependent conformational change and thermal stability of the isolated PsbO protein detected by FTIR spectroscopy. Biochemistry 2003, 42, 11831–11838. [Google Scholar] [CrossRef]
- Kentaro, I.; Takumi, N. Structural Coupling of Extrinsic Proteins with the Oxygen-Evolving Center in Photosystem II. Front. Plant Sci. 2016, 7, 84. [Google Scholar] [CrossRef]
- Miqyass, M.; Marosvölgyi, M.A.; Nagel, Z.; Yocum, C.F.; Gorkom, H.J.V. S-state dependence of the calcium requirement and binding characteristics in the oxygen-evolving complex of photosystem II. Biochemistry 2008, 47, 7915. [Google Scholar] [CrossRef] [PubMed]
- Millikan, C.R.; Hanger, B.C.; Bjarnason, E.N. Interaction between calcium level and nitrogen sources on growth and 45Ca distribution in subterranean clover. Aust. J. Biol. Sci. 1969, 22, 535–544. [Google Scholar] [CrossRef]
- Jakobsen, S.T. Interaction between phosphate and calcium in nutrient uptake by plant roots. Commun. Soil Sci. Plant Anal. 2008, 10, 141–152. [Google Scholar] [CrossRef]
- Robson, A.D.; Edwards, D.G.; Loneragan, J.F. Calcium stimulation of phosphate absorption by annual legumes. Aust. J. Agric. Res. 1970, 21, 601–612. [Google Scholar] [CrossRef]
| Nutrient Solution | Concentration (mg L−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Mo | Cu | Zn | B | Mn | |
| DI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Complete | 224 | 62 | 235 | 160 | 24 | 32 | 0.05 | 0.03 | 0.13 | 0.27 | 0.11 |
| Ca deficiency | 224 | 62 | 235 | 0 | 24 | 32 | 0.05 | 0.03 | 0.13 | 0.27 | 0.11 |
| Factor | Plant Height (cm) | Root Length (cm) | Leaf Area (cm2) | |
|---|---|---|---|---|
| Cultivar | Strong Gold | 43.9 a | 17.1 a | 209.6 b |
| Orange Juice | 39.8 b | 15.5 b | 266.9 a | |
| LSD 0.05 | * | * | * | |
| Nutrient solution | DI | 40.5 b | 15.0 b | 233.9 ab |
| Complete | 43.3 a | 20.9 a | 260.5 a | |
| Ca deficiency | 41.7 ab | 13.9 b | 220.3 b | |
| LSD 0.05 | * | * | * | |
| Cultivar × Nutrient solution | * | * | * | |
| Factor | Flower Stalk Length (cm) | Flower Abortion (%) | Stem Toppling (%) | |
|---|---|---|---|---|
| Cultivar | Strong Gold | 33.6 b | 0 | 0 |
| Orange Juice | 37.5 a | 8 | 33 | |
| LSD 0.05 | * | |||
| Nutrient solution | DI | 35.0 a | 9 | 29 |
| Complete | 35.6 a | 0 | 0 | |
| Ca deficiency | 36.1 a | 4 | 21 | |
| LSD 0.05 | * | - | - | |
| Cultivar × Nutrient solution | * | - | - | |
| Factor | Bulb Fresh Weight (g) | Bulb Circumference (cm) | |
|---|---|---|---|
| Cultivar | Strong Gold | 6.9 b | 7.15 a |
| Orange Juice | 8.0 a | 7.18 a | |
| LSD 0.05 | * | NS | |
| Nutrient solution | DI | 7.21 b | 6.58 b |
| Complete | 8.63 a | 8.43 a | |
| Ca deficiency | 6.44 b | 6.48 b | |
| LSD 0.05 | * | * | |
| Cultivar × Nutrient solution | * | * | |
| Factor | Photosynthetic Rate (µmol m−2 s−1) | Stomatal Conductance (mol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | |
|---|---|---|---|---|
| Cultivar | Strong Gold | 3.36 b | 0.97 b | 0.11 b |
| Orange Juice | 5.04 a | 1.61 a | 0.15 a | |
| LSD 0.05 | * | * | * | |
| Nutrient solution | DI | 3.62 c | 1.16 b | 0.12 b |
| Complete | 4.73 a | 1.32 ab | 0.12 b | |
| Ca deficiency | 4.23 b | 1.39 a | 0.15 a | |
| LSD 0.05 | * | * | * | |
| Cultivar × Nutrient solution | * | * | * | |
| Treatment | Total N (%) | Total P (%) | Total K (%) | Total Ca (%) | Total Mg (%) |
|---|---|---|---|---|---|
| DI | 0.77 b | 2.28 b | 28.58 b | 5.60 b | 1.50 b |
| Complete | 1.97 a | 3.97 a | 38.87 a | 9.86 a | 2.47 b |
| Ca deficiency | 0.83 b | 1.63 b | 39.58 a | 6.25 b | 5.65 a |
| CV (%) | 18.03 | 15.77 | 9.89 | 15.79 | 38.95 |
| LSD 0.05 | * | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inkham, C.; Wichapeng, W.; Panjama, K.; Ruamrungsri, S. Exploring the Role of Calcium in the Physiology of Tulipa: A Comparative Study across Different Cultivars. Horticulturae 2024, 10, 13. https://doi.org/10.3390/horticulturae10010013
Inkham C, Wichapeng W, Panjama K, Ruamrungsri S. Exploring the Role of Calcium in the Physiology of Tulipa: A Comparative Study across Different Cultivars. Horticulturae. 2024; 10(1):13. https://doi.org/10.3390/horticulturae10010013
Chicago/Turabian StyleInkham, Chaiartid, Weerasak Wichapeng, Kanokwan Panjama, and Soraya Ruamrungsri. 2024. "Exploring the Role of Calcium in the Physiology of Tulipa: A Comparative Study across Different Cultivars" Horticulturae 10, no. 1: 13. https://doi.org/10.3390/horticulturae10010013
APA StyleInkham, C., Wichapeng, W., Panjama, K., & Ruamrungsri, S. (2024). Exploring the Role of Calcium in the Physiology of Tulipa: A Comparative Study across Different Cultivars. Horticulturae, 10(1), 13. https://doi.org/10.3390/horticulturae10010013






