Abstract
This study investigated the drought protection effects of six fungal and bacterial inoculants and ten consortia thereof on vegetative growth, nutritional status, and tuberization of potato under controlled and field conditions. It was hypothesized that microbial consortia offer improved drought protection as compared with single strains, due to complementary or synergistic effects, with differential impacts also of N fertilization management. Under NO3− fertilization, a 70% reduction in water supply over six weeks reduced shoot and tuber biomass of non-inoculated plants by 30% and 50%, respectively, and induced phosphate (P) limitation compared to the well-watered control. The P nutritional status was significantly increased above the deficiency threshold by three single-strain inoculants and eight consortia. This was associated with the presence of the arbuscular mycorrhizal fungus (AMF) inoculant Rhizophagus irregularis MUCL41833 (five cases) and stimulation of root growth (five cases). Additionally, Bacillus amyloliquefaciens FZB42 and AMF + Pseudomonas brassicacearum 3Re2-7 significantly reduced irreversible drought-induced leaf damage after recovery to well-watered conditions. However, the microbial inoculants did not mitigate drought-induced reductions in tuber biomass, neither in greenhouse nor in field experiments. By contrast, NH4+-dominated fertilization significantly increased tuber biomass under drought stress (534%), which was further increased by additional AMF inoculation (951%). This coincided with (i) improved enzymatic detoxification of drought-induced reactive oxygen species (ROS), (ii) improved osmotic adjustment in the shoot tissue (glycine betaine accumulation), (iii) increased shoot concentrations of ABA, jasmonic acid, and indole acetic acid, involved in drought stress signaling and tuberization, and (iv) reduced irreversible drought-induced leaf damage. Additional application of bacterial inoculants further improved ROS detoxification by increasing the production of antioxidants but stimulated biomass allocation towards shoot growth at the expense of tuber development. The results demonstrated that microbial consortia could increase the probability of drought protection effects influenced by the form of N supply. However, protective effects on vegetative growth do not necessarily translate into yield benefits, which can be achieved by adequate combination of inoculants and fertilizers.
1. Introduction
Potato (Solanum tuberosum L.) is the world’s third most important stable food crop in terms of yield and consumption [1,2]. Root morphology is characterized by a shallow root system which can limit the uptake of water and nutrients from deeper soil layers [3]. This is particularly marked for the acquisition of sparingly soluble nutrients such as phosphorus (P), largely dependent on adaptive responses including root growth promotion, mycorrhizal colonization, and/or root-induced chemical modifications for P mobilization [4]. Since adaptations for solubilization of sparingly soluble soil P forms are not widely expressed in potato plants, the limitations related to root system architecture may explain the high demand of the potato crop for adequate P availability in the rooting zone [5]. This is further exacerbated under drought stress conditions [6], which reduces the solubility and transport of mineral nutrients to the root surface andy inhibits root growth and activity [7]. Drought stress also affects the physiological processes of carbon partitioning, which ultimately inhibits stolon and tuber formation, and finally reduces tuber yield [8]. It has been projected that global potato yield potential will decrease by 10–19% between 2010 and 2039, and by 18–32% from 2040 to 2069 in the absence of any adaptation measure [9,10], suggesting the need for the implementation of suitable approaches to alleviate drought stress in potato.
The application of plant growth-promoting microorganisms (PGPMs) is discussed as a potential perspective to increase drought resistance of crops and stabilize yields in agricultural lands prone to water limitation [11,12]. Various beneficial effects of PGPMs on plant growth and productivity under water deficit have been documented in the literature [13,14]. These microorganisms can colonize roots internally (e.g., arbuscular mycorrhizal (AM) fungi [15] or develop at the root surface (e.g., Bacillus sp.) [16], increasing the accumulation of mineralnutrients, e.g., N and P [17] or stimulating root growth [18], which, in turn, promotes nutrient acquisition. Mechanisms often reported comprise increased production of exopolysaccharides (EPS), phytohormones, and 1-amino-cyclopropane-1-carboxylate (ACC) deaminase that counteract excessive production of ethylene with inhibitory effects on root growth [19,20]. A recent meta-analysis covering 150 published studies revealed superior yield performance of PGPM inoculants in dry climates as compared with temperate climate zones [21]. However, reproducibility of the expected PGPM effects under field conditions remains a major challenge [22].
Recent studies have shown that inoculation with microbial consortia could be more effective than single inoculants in improving plant performance due to possible synergistic interactions between different microbial inoculants [23,24]. Complementary properties of differentially adapted microbial strains may offer an advantage, particularly under variable environmental conditions, as demonstrated by [25].
The performance of microbial inoculants also appears to be influenced by the form of nitrogen (N) supply. In comparison with nitrate as a major N source, ammonium-dominated fertilization has been reported to increase root colonization by some fungal and bacterial PGPMs [26,27]. The microbial inoculants produced phytohormones, stimulated root growth, and promoted rhizosphere acidification to improve the acquisition of sparingly soluble nutrients (i.e., P, Zn, Mn), particularly under neutral or alkaline soil pH, resulting in better plant growth and development [27,28,29]. Particularly for potato cropping systems, fertilizer placement of P and stabilized ammonium have been reported to overcome problems of P limitation [5] with the strongest responsiveness on light- and drought-affected soils [30].
Based on this background information, the present study was conducted with the following objectives:
- (i)
- to characterize the drought-protective potential of microbial strains and strain combinations with PGPM potential under controlled greenhouse conditions.
- (ii)
- to characterize under field conditions the drought-protective potential of the best- performing microbial strains and strain combinations pre-selected in the controlled experiments.
- (iii)
- to characterize the response of plants treated with drought-protective single-strain inoculants and microbial consortia to NH4+ supply, using NO3- fertilization as a control.
- (iv)
- to identify underlying modes of action.
It was hypothesized that (i) microbial consortia will demonstrate superior drought-protective performance compared with single-strain inoculants; (ii) the performance will be further improved by ammonium-dominated fertilization acting via improved nutrient acquisition supporting the expression of physiological drought adaptations.
2. Materials and Methods
The study comprised two greenhouse experiments conducted at the Institute of Crop Science, University of Hohenheim, Stuttgart, Germany (48°42′44.6″ N 9°12′30.4″ E) and one field trial conducted by Agroscope, Nyon, Switzerland (46°23′59″ N 6°14′08″ E) under irrigated and non-irrigated conditions.
2.1. Greenhouse Experiments
The potato cultivar (Solanum tuberosum L. cv Alonso) was used for the greenhouse experiments. The soil used (silty clay–loam, pH.7.1) was collected from a research field at the University of Hohenheim (Heidfeldhof research station) and was sieved with 5 mm mesh size before pot filling. The physicochemical characteristics of the soil are summarized in Supplementary Table S1. Pots were filled with a substrate mixture that comprised 2.5 kg air-dried soil and 2.5 kg washed quartz sand to improve the soil structure. Additional fertilizer supply is reported in the description of the individual experiments.
The method described by [31] was employed to determine the water-holding capacity (WHC) of the substrate. For this purpose, metal cylinders (5 cm Ø and 15 cm length) fitted with a sieve at the bottom were filled with the sand–soil substrate. To prevent the substrate from passing through the sieve, a layer of gravel quartz stones (0.3–0.5 mm in diameter) was added at the bottom.
2.1.1. Potato Culture
Potato tubers (size: 3.5–5.5 cm) were pre-germinated in boxes for 2 weeks at 15 °C in the dark and then 3–4 weeks with illumination until the emergence of 1–2 well-grown sprouts. Suitable sprouts with similar growth were selected and separated from the mother tuber along with a 1–2 cm2 cube of tuber tissue by cutting with a disinfected sharp knife. The cut potato cubes with sprouts were then kept on a clean filter paper for drying for 24 h to induce the formation of a suberin layer, sealing the moist cut surface of tuber tissue to restrict pathogen infections. After drying, potato cubes with sprouts (approx. 10–12 g FW) were selected and planted into culture pots at a depth of 5 cm. After planting, the top surface of the substrate was covered with 300 g coarse quartz sand to minimize evaporation loss and crust formation (Supplementary Figure S3).
2.1.2. Maintenance Culture of Inoculants
Microbial strains were provided by participants of the EU project SoLACE GA No 727247 (summarized in Supplementary Table S2) and maintained on suitable agar media: Kings B medium for Pseudomonas strains; pepton/yeast medium (5 g pepton + 1g yeast extract +10 g glucose +1.5 g KH2PO4 + 3.0 g K2HPO4 +15 g agar per liter) for Herbaspirillum and Kosakonia; Potato Dextrose Agar (PDA) for Trichoderma strains and Paraburkholderia. The inoculum of the arbuscular mycorrhizal fungus (AMF) Rhizophagus irregularis MUCL41833 was provided as a spore preparation in a formulation of alginate beads [32]. Bacillus amyloliquefaciens FZB42 (renamed Bacillus velezensis FZB42) was provided as a commercial stabilized endospore formulation (RhizoVital® fl. FZB42, ABITEP, Berlin, Germany).
Microbial strains were provided by participants of the EU project SoLACE GA No 727247 (summarized in Supplementary Table S2) and maintained on suitable agar media: Kings B medium for Pseudomonas strains; pepton/yeast medium (5 g pepton + 1g yeast extract +10 g glucose +1.5 g KH2PO4 + 3.0 g K2HPO4 +15 g agar per liter) for Herbaspirillum and Kosakonia; Potato Dextrose Agar (PDA) for Trichoderma strains and Paraburkholderia. The inoculum of the arbuscular mycorrhizal fungus (AMF) Rhizophagus irregularis MUCL41833 was provided as a spore preparation in a formulation of alginate beads [32]. Bacillus amyloliquefaciens FZB42 (renamed Bacillus velezensis FZB42) was provided as a commercial stabilized endospore formulation (RhizoVital® fl. FZB42, ABITEP, Berlin, Germany).
2.1.3. Preparation and Application of Inoculants
For AMF inoculation, 12.5 g pot−1 of alginate bead formulation with R. irregularis MUCL 41833 was mixed with the substrate during the pot filling (25 ± 15 propagules per fresh alginate bead, or 1000 ± 600 propagules per g of fresh alginate beads) [32]. The remaining inoculants were applied by soil drenching close to the stems of the plants in the 4–5 leaf stage (8 days after planting). For the application of the Trichoderma strain, the mycelium, which had completely covered the agar plate and formed green spores, was scratched from the agar medium and suspended in phosphate-buffered saline solution (PBS). For each experiment, the mycelium from 3 agar plates was suspended to a volume of 400 mL to apply 20 mL suspension per pot. The bacterial strains were applied with suspension aliquots of 20 mL adjusted to 107 cfu mL−1, which resulted in a final application rate of 109 cfu plant−1. The RhizoVital® fl. FZB42 Bacillus formulation was diluted with water before the application according to manufacturer instructions to reach an application concentration of 107 cfu ml−1. For the remaining bacterial strains, agar cubes with bacterial colonies were transplanted from the culture plates to an Erlenmeyer flask filled with 100 mL tryptic soy medium (TSM, Sigma-Aldrich, Darmstadt, Germany) and incubated on a rotary shaker (Multitron, Infors HT, Bottmingen, Switzerland) at 120 rpm for 24 h at 28 °C. Thereafter, the optical density (absorbance 600 nm) of the bacterial suspension was determined spectrophotometrically (NanoDrop 2000C, Thermo Fisher, MA, USA) and adjusted with TSM to reach the absorbance corresponding to 107 cfu mL−1, as previously determined for each strain by plating dilution assays (Supplementary Table S3).
2.1.4. Setup of Pot Experiment I
The first pot experiment was conducted for screening the most effective drought-protective microbial inoculants, improving nutrient uptake, plant performance, and tuberization of potato plants exposed to water limitation. The experiment was conducted from November 2017 to February 2018 with 18 different treatments and four replicates. The plants were grown in a greenhouse under semi-controlled conditions with a total eight hours of additional artificial light, in the morning (05:00–07:00 and 08:00–10:00) as well as in the evening (16:00–18:00 and 19:00–21:00) using 400 W high-pressure sodium lamps (SON-T AGRO 400, Philips Lighting GmbH, Germany). The average light intensity was 275 μM m−1 s−1. The temperature was maintained at 16–28 °C (average temperature 22 °C) during day and night, with an average relative humidity of 41% during the experimental period.
Before pot filling, NPK fertilization was applied by mixing fertilizer solutions with the culture substrate: N (50 mg kg−1 using Ca (NO3)2), P (50 mg kg−1 using Ca(H2PO4)2), and K (150 mg kg−1 using K2SO4). Six microbial inoculants provided by SolACE project partners [33] were tested individually or in combination as specified in Supplementary Table S2. The potato plants were grown for three weeks under well-watered conditions at 70% of the substrate water-holding capacity (WHC), daily adjusted by gravimetric determination (establishment phase), followed by a drought stress period of six weeks (20–25% WHC) and four days recovery at 70% WHC. Non-stressed controls were maintained at 70% WHC throughout the culture period.
2.1.5. Setup of Pot Experiment II
Four promising microbial inoculants (viz. P. brassicacearum 3Re2-7, B. amyloliquefaciens FZB42, Herbaspirillum sp, and R. irregularis MUCL 41833), identified with respect to drought-protective functions during the first experiment, were further investigated in a second pot experiment testing perspectives to further improve the performance of plants in presence of ammonium-dominated N fertilization. The experiment was conducted from May to August 2018 with nine different treatments and five replicates (Supplementary Table S4). Two forms of nitrogen fertilizers were applied as NO3− (50 mg N kg−1 substrate, using Ca (NO3)2) and NH4+ (50 mg N kg−1 substrate, using 3,4-Dimethylpyrazolphosphate (DMPP)-stabilized ammonium sulphate (NovaTec® solub 21, Compo Expert, Münster, Germany). In addition, K (150 mg K kg−1 substrate) was applied as K2SO4, all mixed in the substrate before pot filling. In face of the high soil P status identified during the first experiment and the potential for P solubilization by ammonium-induced rhizosphere acidification on soils with neutral pH [7], no additional P fertilization was applied in this experiment. The plants were watered at 70% WHC for three weeks before the initiation of drought stress for six weeks at 20–25% WHC. Afterwards, all plants were again watered at 70% WHC during a recovery phase for two weeks until harvest. Non-stressed control plants were watered to 70% WHC for the whole duration of the experiment.
2.1.6. Visual Rating of Plant Performance
To quantify the degree of drought-induced damage, a visual scoring scheme was used to estimate plant performance during the drought phase and after recovery. The scoring scheme ranged between 1 = marginal damage and 10 = severe damage, based on plant growth performance and visual symptoms of drought-induced damage (wilting, chlorosis, leaf necrosis). The rating was performed two times for all plants and average scores were used to estimate the final visual plant performance (Supplementary Figure S3).
2.1.7. Harvest of Plant Material
Throughout the experimental period, several non-destructive measurements were carried out such as plant height, total number of leaves per plant−1, and number of damaged (chlorotic, necrotic) leaves recorded during the establishment phase, recovery phase, and before final harvesting of plants. At the end of the recovery phase, the youngest undamaged and fully developed leaves were collected, immediately wraped with aluminum foil, and shock frozen in liquid nitrogen. Subsequently, the leaf samples were stored at −80 °C for analysis of stress metabolites and hormones. For final harvest, plant shoots were cut at their base removing adhering soil particles, and finally weighed for determination of fresh shoot biomass. All shoot samples were oven dried at 60 °C (TKL 10, Ehret GmbH Emmendingen, Germany) until achieving constant weights for recording shoot dry weight. The plants’ roots were carefully washed out of the soil substrate and dried with tissue papers for determination of root fresh weight. For root length measurement, fresh root samples were preserved in 70% (v/v) ethanol. Aliquots of root samples were used for dry weight determination. Tubers were thoroughly washed and dried with tissue papers prior to fresh weight recordings and the number of tubers from each plant was counted.
2.1.8. Root Length Measurement
Aliquots of preserved root samples were cleaned with distilled water to remove ethanol. The root samples were submerged into a water film in a transparent Perspex tray, spread evenly, and scanned using a flatbed scanner (Epson Expression 1000 XL, Tokyo, Japan). Then, the digitalized images were analyzed using the WinRHIZO software package V. 2009c 32-Bit (Regent Instruments, Quebec, QC, Canada) to estimate the total root length.
2.1.9. Analysis of Mineral Nutrients
Dried shoot material was homogenized to a fine powder using a grinding mill (Labor Scheibenschwingmühle 100A, Sieb Technik GmbH, Mühlheim-Ruhr, Germany). Samples of dry grinded shoots weighing 250 mg were used for total N determination with a Vario max CN macro-elementar analyser (Elementar Analysensysteme, Hanau, Germany). The shoot P, K, Mg, Ca, Mn, Zn, Cu, Si, and Fe were measured after microwave digestion. Then, 250 mg dry plant material, 2 mL distilled water, 5 mL HNO3, and 4 mL H2O2 were added into digestion tubes, mixed with a vortex mixer and kept for 30 min for reaction. Subsequently, the tubes were put into a microwave digestion unit at 1400 W for 1 h (ETHOS lab Professional Microwave Systems, MLS, Leutkirch, Germany) and then cooled for 30 min. The digested samples were added to conical flasks and the volume was adjusted to 25 mL with dH2O. After shaking and filtration, the samples were ready for analysis. Phosphorous was determined spectrophotometrically (Hitachi U-3300, Tokyo, Japan) according to the method of [34], while K and Ca were measured by flame absorption spectrometry (Eppendorf-ELEX6361, Netheler + Hinz, Hamburg, Germany) and Mg, Mn, Fe, Cu, and Zn were determined by atomic absorption spectrometry (iCE 3000 series, Thermo Fischer, Dreieich, Germany).
2.1.10. Determination of Stress Metabolites and Hormonal Profiling
Plant material collected from undamaged leaves after drought stress recovery was used for the determination of selected stress metabolites after homogenization of shock-frozen plant tissues in liquid nitrogen.
Proline analysis was conducted spectrophotometrically at 520 nm after acetic acid and acid ninhydrin derivatization [35]. Glycine betaine determination was performed according to the spectrophotometric method of [36] with modifications described by [37]. The 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) method was used to evaluate the free radical scavenging activity of antioxidants in plant tissue [29]. Hydrogen peroxide levels were determined spectrophotometrically at 390 nm as described [35]. Ascorbate peroxidase (APX) activity was recorded according to the spectrophotometric method described by [38]. A Spectrophotometer U-3300 (Hitachi, Tokyo, Japan) was used for all spectrophotometric determinations.
UHPLC-MS analysis of phytohormones in shoot tissues was carried out on a Velos LTQ System (Thermo Fisher Scientific, Waltham, MA, United States) fitted with a Synergi Polar column, 4 μ, 150 × 3.0 mm, (Phenomenex, Torrance, CA, USA) according to the method described by [29].
2.2. Field Trial
The field trial was designed as a four-factor experiment with two replications. In addition to potato varieties, three factors were arranged as a randomized complete block design (RCBD). These factors were: (i) inoculation, (ii) irrigation, and (iii) phosphorus supply. The inoculation factor was randomized in the two replication blocks and had four levels: no inoculation, inoculation of consortium 1, consortium 2, and consortium 3. The subfactor irrigation was randomized into the main plots and had two levels: irrigated and rainfed. In addition, the phosphorus fertilization factor displayed two rates of P application, 0 kg ha−1 and 36 kg ha−1 and was randomly applied to the subfactor irrigation. Finally, the variety factor was not randomized and subordinated to the phosphorus factor. It contained cv. Pentland Dell, cv. Sarpo Mira, cv. Maris Piper and cv. Désirée. Each plot was planted with one line of 25 plants for each variety. All the plots were surrounded by buffer rows of cv. Laura. The varieties were planted in the same order inside of each plot: from left to right one line of cv. Sarpo Mira, one line of cv. Désirée, one line of cv. Pentland Dell and online of cv. Maris Piper.
The trial was located at the Agroscope research station (Nyon, Switzerland) on clay–loam soil (40% sand, 37% loam, 23% clay), pH 7.6; Corg 2–3%. Seed tubers were planted at a rate of one seed piece per 0.32 m and with 0.75 m row spacing (Supplementary Figure S2).
2.2.1. Fertilization and Irrigation
Application of phosphorus was carried out at planting at a rate of 36 kg ha−1 (triple superphosphate). Additionally, the following fertilizer rates were applied in all lines: 120 kg ha−1 of nitrogen (urea), 372 kg ha−1 of potassium (potassium chloride), and 20 kg ha−1 of magnesium (34.5% magnesium sulfate + 65.5% magnesium carbonate). Before the soil water content reached the onset of stress for the potato plants (determined empirically), irrigation was performed in irrigated lines at a rate of 20 L per m2. From a water tank filled with the exact amount of water needed for the irrigated plots, individual graduated watering cans were then used to split the water equally between the plots.
2.2.2. Microbial Inoculants, Formulation, Dosage, and Application
Three consortia were inoculated. Consortium 1 contained Rhizophagus irregularis MUCL41833 and Pseudomonas brassicacearum 3Re2-7. Consortium 2 was a combination of Rhizophagus irregularis MUCL41833 and Paraburkholderia phytofirmans PsJN, and consortium 3 was composed of Rhizophagus irregularis MUCL41833, Paraburkholderia phytofirmans PsJN, and Trichoderma asperelloides A. The Trichoderma strain and the two bacterial strains were suspended solutions specified in Supplementary Table S5. Rhizophagus irregularis MUCL41833 was integrated into alginate beads. Preparation of alginate beads was performed according to the protocol of [32]. On the day of planting, the tubers were laid on the soil mechanically with the help of a potato planter. Each tuber was then picked up and dipped in the formulation containing the corresponding inoculant, while 10 alginate beads were placed on the tuber empty spot. The seed potato was then put back in place before the rest of the formulation was poured on top of it. The hills were formed after the application of the inoculum. All the lines were irrigated after hills formation, at a rate of 20 L per m2.
2.3. Experimental Design and Statistical Analysis of Greenhouse Experiments
The first experiment was laid out in a completely randomized block design with four replicates per treatment and 18 different variants. The second experiment was arranged in a completely randomized design using five replicates per treatment with nine treatments. For statistical analyses, the software SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used. Data sets were tested with a one-way ANOVA followed by t-grouping, comparing different microbial treatments (p ≤ 0.05).
2.4. Statistical Analysis of the Field Trial
Soil analyses conducted before planting revealed a high content of phosphorus in the soil. Because of this, we could not generate an impacting difference in soil phosphorus availability. The first analysis of variance revealed no impact of P fertilization on yield and other parameters. To simplify the analysis, the P fertilization treatment was suppressed and considered as two supplementary replications. Yield results were analyzed in R [39] in the environment R studio [40] with the function ssp. plot from the package agricolae [41]. Repetitions were described as blocks, inoculation as the main plot factor, irrigation as the subplot factor, and variety as the subplot factor. Treatments with significant effects were subjected to the least significant difference (LSD) test to reveal significant differences between the corresponding levels, with the LSD test function from the package agricolae.
3. Results
3.1. Pot Experiment I: Plant Growth Parameters
Drought stress significantly reduced shoot dry weight (SDW) as compared to well-watered plants (29%), and no significant effects of microbial inoculants were noticed compared to drought-stressed plants without inoculants (Table 1). However, a general trend for increased shoot elongation rates induced by the microbial inoculants was recorded during the six-week drought stress phase (Figure 1), with significant effects for the single-strain inoculant FZB42 (300%) and the consortia KH (267%) and P&R (420%). Drought stress significantly reduced total tuber weight (50%) without significant effects on the number of tubers per plant. Treatments with microbial inoculants had no effects on total tuber weight or number of tubers per plant (Table 1).

Table 1.
Effect of plant growth-promoting microorganisms on shoot dry weight (SDW), root dry weight (RDW), root length (RL), root/shoot ratio, tuber weight, number of tubers per plant, and individual tuber weight (ITW) of potato under drought stress.
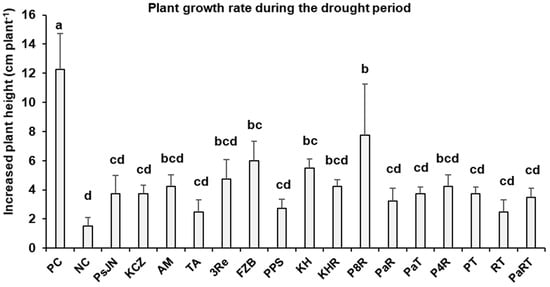
Figure 1.
Effect of plant growth-promoting microorganisms on shoot elongation rate of potato measured after 6 weeks of drought stress. The substrates treated with or without (NC = negative control) microbial inoculants viz. P. phytofirmans PsJN (PsJN), Pseudomonas sp. KCZ43 (KCZ), R. irregularis MUCL 41833 (AM), T. asperelloides A (TA), P. brassicacearum 3Re2-7 (3Re), B. amyloliquefaciens FZB42 (FZB), P. protegens 3BS + P. jessenii 17BS (PPS), Kosakonia sp. + Herbaspirillum sp. (KH), Kosakonia sp. + Herbaspirillum sp. + R. irregularis MUCL 41833 (KHR), P. brassicacearum 3Re2-7 + R. irregularis MUCL 41833 (P8R), P. phytofirmans PsJN + R. irregularis MUCL 41833 (PaR), P. phytofirmans PsJN + T. asperelloides A (PaT), Pseudomonas sp. KCZ43 + R. irregularis MUCL 41833 (P4R), P. brassicacearum 3Re2-7 + T. asperelloides A (PT), R. irregularis MUCL 41833 + T. asperelloides A (RT), and P. phytofirmans PsJN + R. irregularis MUCL 41833 + T. asperelloides A (PaRT). PC = positive control under well-watered conditions. Bars represent mean values ± SEM (n = 4). Significant differences (p ≤ 0.05) are indicated by different letters.
Root biomass was significantly affected by drought stress (Table 1) with a significant decline in root dry weight (RDW) as compared to well-watered plants (39%), and a reduction in total root length of 46%. A trend for increased root biomass production, reaching values not significantly different from the well-watered control, was recorded for two single-strain inoculants (3Re27, FZB42) and three consortia (P&R, PaT, P4R) with a similar trend for root length induced by three single-strain inoculants (3Re27, FZB42, TA) and three consortia (P&R, PaT, P4R). The root-to-shoot biomass ratio was significantly increased by 3Re2-7 as compared to controls (Table 1).
3.1.1. Visual Evaluation of Drought Stress Symptoms
To detect irreversible drought-induced leaf damage, evaluations of wilting, chlorosis, and necrosis were performed at the end of the six-week drought stress period and after recovery under well-watered conditions, (Figure 2). Inoculation with microbial consortia PPS, KH, and P8R significantly reduced drought-induced leaf damage by 37%, 41%, and 37%, recorded at the end of the drought stress phase. After re-supplying water to 70% of WHC, the potato plants exhibited rapid recovery particularly from wilting symptoms within four days. However, a general trend for a reduction in irreversible leaf damage after recovery to well-watered conditions was recorded for all inoculants except PT and PaRT, with significant effects induced by the single-strain inoculant FZB42 (40%) and the consortium P&R (50%) reaching final rankings < 2, similar to the well-watered control (PC) (Figure 2).
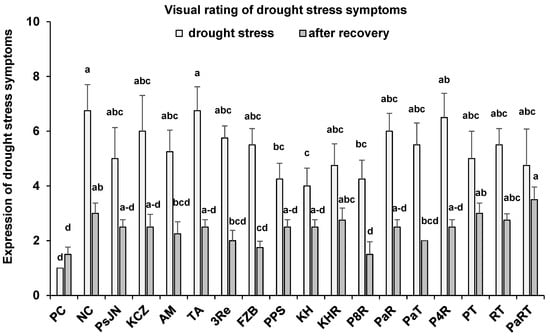
Figure 2.
Visual scoring of drought stress symptoms (wilting, chlorosis, necrosis; 0 = no damage, 10 = severely damaged) at the end of 6-week drought stress and after 1 week of recovery under well-watered conditions. The substrates treated with or without (NC) microbial inoculants viz. P. phytofirmans PsJN (PsJN), Pseudomonas sp. KCZ43 (KCZ), R. irregularis MUCL 41833 (AM), T. asperelloides A (TA), P. brassicacearum 3Re2-7 (3Re), B. amyloliquefaciens FZB42 (FZB), P. protegens 3BS + P. jessenii 17BS (PPS), Kosakonia sp. + Herbaspirillum sp. (KH), Kosakonia sp. + Herbaspirillum sp. + R. irregularis MUCL 41833 (KHR), P. brassicacearum 3Re2-7 + R. irregularis MUCL 41833 (P8R), P. phytofirmans PsJN + R. irregularis MUCL 41833 (PaR), P. phytofirmans PsJN + T. asperelloides A (PaT), Pseudomonas sp. KCZ43 + R. irregularis MUCL 41833 (P4R), P. brassicacearum 3Re2-7 + T. asperelloides A (PT), R. irregularis MUCL 41833 + T. asperelloides A (RT) and P. phytofirmans PsJN + R. irregularis MUCL 41833 + T. asperelloides A (PaRT), PC = positive control under well-watered conditions. Bars represent mean values ± SE (n = 4). Different letters indicate significant differences (p ≤ 0.05).
3.1.2. Mineral Nutritional Status
With the exception of phosphate (P), potassium (K), and magnesium (Mg), the nutritional status of non-inoculated plants was sufficient, as indicated by the shoot mineral nutrient concentrations (Table 2a). However, at the end of the culture period, the N status of well-watered plants with the highest growth activity was critical. Severe phosphate limitation was recorded particularly for the non-inoculated control plants exposed to drought stress (Table 2a), associated with a significant decline (36%) in P shoot accumulation (Table 2b). A general trend for increasing the P nutritional status was recorded for all microbial inoculants (Table 2a), also reflected in increased shoot P accumulation (Table 2b). Significant improvements of the P status above the deficiency threshold were mediated by three out of six single-strain inoculants (AM, 3Re2-7, FZB42) and eight out of ten consortia (PPS, KH, KHR, P&R, PaT, P4R, PT, RT). The AMF Rhizophagus irregularis MUCL 41833 improved the P status in five out of seven inoculants. The single-strain inoculants PsJN and TA were effective only in consortia, but PsJN was ineffective in combination with AMF (Table 2).

Table 2.
Effect of microbial inoculants on shoot concentrations (a) and shoot accumulation of nutrients (b) in potato plants exposed to drought stress treatments with and without microbial inoculants and in well-watered controls.
Although the N status was sufficient in drought-affected plants, shoot nutrient concentrations were further increased by microbial inoculants with significant effects of two single strains (PsJN, AM), and six consortia (PPS, KH, KHR, P&R, PT, RT) for K nutrition and one single-strain (PsJN) and four consortia (PPS, KH, KHR, PT) in case of the N nutritional status. No inoculant effects were detectable for Mg, Mn, and Zn nutrition (Table 2).
3.2. Field Experiment
PGPM inoculation did not impact yield significantly for all tested potato varieties, P fertilization, and irrigation treatments. However, statistical analysis revealed that irrigation (p < 0.001) and variety (p < 0.001) both had an impact on harvested tuber weight (Supplementary Figure S1). Interaction between the different treatments did not provide any significant effect on yield. Post hoc tests revealed that rainfed lines (27.2 t·ha−1) provided less yield in comparison with irrigated ones (36.4 t·ha−1). In addition, cv. Désirée (35.3 t·ha−1) and cv. Maris Piper (34.4 t·ha−1) were not significantly different, but both yielded more than cv. Pentland Dell (30.4 t·ha−1) and cv. Sarpo Mira (27 t·ha−1) which provided similar yields.
3.3. Pot Experiment II
The pot and field experiments with nitrate- or urea-based N fertilization revealed the absence of inoculant effects for alleviation of yield decline in drought-affected potato plants (Table 1; Supplementary Figure S1). Nevertheless, the beneficial impact on nutrient status and vegetative growth observed in the pot experiment suggested further research to optimize the strategy for application of the inoculants. In a follow-up experiment, the potential benefits of stabilized ammonium fertilization reported for potato on drought-affected soils [30] were investigated with a selection of effective single-strain inoculants (AM, FZB42) and strain combinations (AM + 3Re2-7, AM + Herbaspirillum, AM + FZB42). Drought-affected potato plants supplied with stabilized ammonium fertilization with and without microbial inoculants were compared with a well-watered control. Additional controls comprised well-watered and drought-affected plants with nitrate fertilization.
3.3.1. Plant Growth Parameters
Under well-watered conditions, the form of N supply had no significant impact on SDW, RDW, and tuber yield (Table 3), but RL was significantly reduced by NH4+ supply (22%) (Table 3). Drought stress significantly reduced SDW preferentially in NH4+-treated plants (33.4%), while RDW remained unaffected with a general trend for increased RL, particularly under NH4+ supply (34.2%). However, by far the largest drought-induced biomass reduction was recorded for tuber yield in plants with NO3− fertilization (97.7%) and 84.9% under NH4+ supply, without significant effects on the number of tubers per plant. As compared with NO3− supply, NH4+ fertilization significantly increased tuber weight under drought stress (534%), which was further increased by AM inoculation (951%) but reached only 24–25% of the well-watered controls. The remaining inoculants had no significant effects on tuber weight under drought stress. Only FZB tended to increase SDW (Table 3).

Table 3.
Effect of plant growth-promoting microorganisms and form of N fertilizer on shoot dry weight (SDW), root dry weight (RDW), root length (RL), tuber weight, number of tuber, and individual tuber weight (ITW) under drought stress conditions.
In well-watered plants, the relative biomass distribution between roots, shoots, and tubers showed a clear dominance of tuber biomass (60%) and was not affected by the form of N supply. Under drought stress, relative root and shoot biomass allocation increased at the expense of tuber biomass. The total biomass of the drought-affected plants was very similar in all treatments, but compared with NO3− supply, biomass allocation for tuber formation increased under NH4+ fertilization, particularly in combination with single-strain AM inoculation. By contrast, for the remaining inoculants, shoot biomass allocation increased at the expense of tuber biomass in the NH4+ variants, while root biomass remained largely unaffected (Table 4).

Table 4.
Total fresh plant biomass and relative biomass distribution of potato plants at harvest inoculation in plants exposed to drought.
3.3.2. Rating of Drought Stress Symptoms
As a more objective criterion for the evaluation of visual drought stress symptoms, the number and percentage of leaves expressing senescence symptoms (wilting, intense chlorosis, and necrosis) was determined at the end of the drought stress period. Drought stress significantly increased the number of senescent leaves without differences between the forms of N supply. However, the application of microbial inoculants in the ammonium variants decreased the degree of leaf damage with significant reductions in the treatments with AM, AM+Her, and AM+FZB42 as compared with the non-inoculated control (Figure 3).
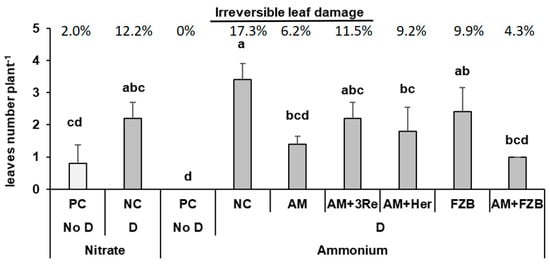
Figure 3.
Visual evaluation of irreversibly damaged leaf number (chlorosis, necrosis) and percentage relative to the total number of leaves at the end of the 6-week drought period. The substrates treated with or without microbial inoculants viz. R. irregularis MUCL 41833 (AM), R. irregularis MUCL 41833 + P. brassicacearum 3Re2-7 (AM+3Re), R. irregularis MUCL 41833 + Herbaspirillum sp. (AM+Her), and R. irregularis MUCL 41833 + B. amyloliquefaciens FZB42 (AM+FZB) under well-watered (PC No D) and drought (NC D) conditions. Bars represent mean values ± SEM (n = 5), different letters indicate significant differences (p ≤ 0.05).
3.3.3. Plant Nutritional Status
Under well-watered conditions, potato plants exhibited N, P, K, and Mg shoot concentrations below the reported deficiency thresholds, while the other nutrients reached the threshold (Ca, Mn, Cu) or were markedly above (Fe, Zn). The form of N supply had no significant effects on nutrient concentrations and content in the shoot tissue in well-watered plants (Table 5). After recovery from the drought stress treatment, the concentration of N, P, and K was significantly increased compared to the well-watered controls but reached the deficiency threshold level only for N. However, NH4+ supply in drought-affected plants was associated with a significantly lower shoot content of all nutrients as compared with NO3− fertilization. In most cases, microbial inoculants provided no significant increase in shoot nutrient concentration or content. Only the AM-FZB combination increased the content of macronutrients in the shoot tissue as compared with the non-inoculated control with significant effects for N, Mg, and Ca and the highest increase (36%) for P (Table 5).

Table 5.
Effect of plant growth-promoting microorganisms and form of N fertilizer on shoot nutrient concentrations (A) and content (B) in potato plants under drought stress.
3.3.4. Stress Metabolites in Leaves
Drought stress significantly increased the accumulation of reactive oxygen species (ROS: H2O2) in leaf tissues of potato plants as compared to well-watered conditions (610–740%), still detectable even after a two-week recovery period, independent of the form of N supply (Figure 4a). Application of microbial consortia gradually decreased H2O2 concentrations with significant effects for the treatments with FZB42 and the combinations of AM and bacterial inoculants (Figure 4a). A trend for increased shoot accumulation of the osmo-protectant glycine betaine was detectable in drought-affected plants even after 2 weeks recovery under well-watered conditions, independent of the N supply form (Figure 4c). However, only in the presence of microbial inoculants, glycine betaine concentrations were significantly increased by 200–330%. In contrast to glycine betaine, the accumulation of the osmo-protective amino acid proline was not affected by drought stress treatments but was significantly increased by NH4+ fertilization and reached the highest levels in AM-inoculated plants (Figure 4b). The other inoculants had no significant effects on proline accumulation, which was three orders of magnitude lower as compared with glycine betaine (Figure 4b).
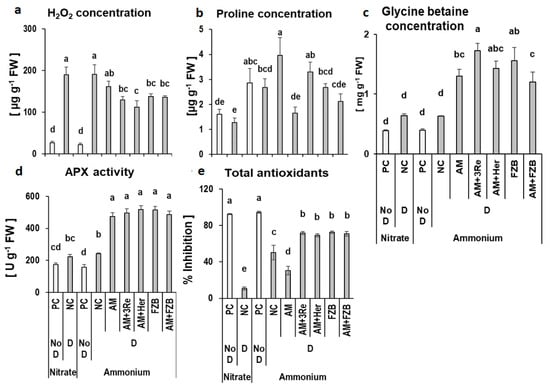
Figure 4.
Effect of plant growth-promoting microorganisms and form of N fertilizer on leaf H2O2 concentration (a), proline concentration (b), glycine betaine concentration (c), ascorbate peroxidase (APX) activity (d), and total antioxidants (e) in potato plants under drought stress conditions. The substrates treated with or without microbial inoculants viz. R. irregularis MUCL 41833 (AM), R. irregularis MUCL 41833 + P. brassicacearum 3Re2-7 (AM+3Re), R. irregularis MUCL 41833 + Herbaspirillum sp (AM+Her), and R. irregularis MUCL 41833 + B. amyloliquefaciens FZB42 (AM+FZB) under well-watered (NoD) and drought (D) conditions. PC = well-watered controls without inoculants, NC = drought-stressed controls without inoculants. Bars represent mean values ± SEM (n = 5), different letters indicate significant differences (p ≤ 0.05).
Following declining concentrations of H2O2, all microbial inoculants significantly increased the activity of ascorbate peroxidase (APX) in the leaf tissue (95–114%), responsible for enzymatic detoxification of H2O2 to H2O, which was only marginally increased in drought-affected plants without microbial inoculation (28–54%) (Figure 4d). However, drought stress significantly reduced the non-enzymatic, anti-oxidative potential after drought recovery, particularly in plants with NO3− supply followed by the combination of AM inoculation with NH4+ fertilization and NH4+-fed plants. By contrast, the other inoculants significantly increased the accumulation of antioxidants compared with the non-inoculated control (Figure 4e).
3.3.5. Hormonal Status of Drought-Affected Plants
The leaf hormonal status was recorded for a selection of inoculant treatments showing the strongest effects on vegetative growth or tuber formation. Among the tested hormones, gibberellic acid, zeatin, and salicylic acid remained below the detection limits in drought-affected plants after two weeks of recovery under well-watered conditions (Figure 5). The concentrations of indole acetic acid (IAA), abscisic acid (ABA), and jasmonic acid (JA) were not affected by the form of N supply. However, AM inoculation significantly increased the shoot concentrations of the detected phytohormones by approximately 50% without further effects by combined application with the bacterial inoculant FZB42 (Figure 5).
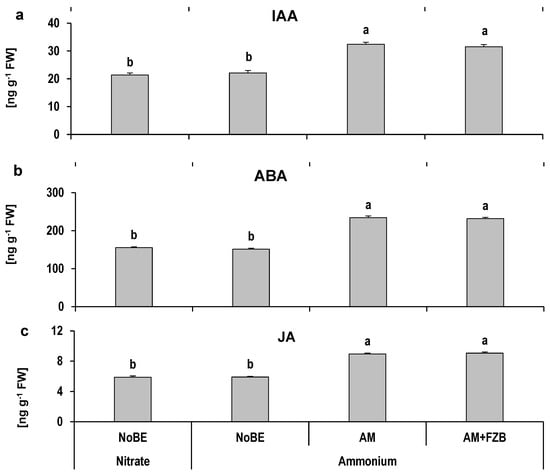
Figure 5.
Effects of plant growth-promoting microorganisms on accumulation of phytohormones indole acetic acid—IAA (a), Abscisic acid—ABA (b), and jasmonic acid—JA (c) in fresh leaf tissue of potato plants under drought stress. The substrates treated without (NoBE) or with microbial inoculants viz. R. irregularis MUCL 41833 (AM) and R. irregularis MUCL 41833 + Bacillus. amyloliquefaciens FZB42 (AM+FZB). Bars represent mean values ± SEM (n = 5), different letters indicate significant differences (p ≤ 0.05).
4. Discussion
In facing increasing challenges for agricultural production related to climate change, the use of selected microbial inoculants to increase abiotic stress tolerance of crops is a strategy extensively discussed in the recent past [12,42]. Although a wide range of drought-protective properties triggered by plant-microbial interactions is documented in the literature, the limited reproducibility of the expected effects under field conditions remains a major challenge [43,44]. Factors determining the successful establishment of plant–inoculant interactions [45] in the rhizosphere are still poorly understood. This applies similarly for the exploitation of complementary or synergistic effects of a combination of inoculants with different properties, combinations with non-microbial biostimulants or different fertilizers [25,27,28,42,46]. In this context, the present study aimed to investigate the drought protection potential of selected bacterial and fungal inoculants with documented plant-beneficial properties, applied as single-strains or as consortia, under nitrate and ammonium forms of nitrogen (N) supply.
4.1. Screening for the Effects of Drought Protection Inoculants under NO3− Fertilization
In a standardized greenhouse screening system, significant reductions, both in shoot and root growth of drought-affected potato plants (Table 1) indicated the impact of severe drought stress. By contrast, moderate water limitation usually leads to adaptive reductions in shoot growth and can even stimulate root development [47]. Inhibition of root growth is one of the most important factors limiting the acquisition of sparingly soluble nutrients such as P and K [48] under conditions of severe drought. Accordingly, P deficiency was characteristic for drought-affected plants (Table 2), and restoration of the P status was the most widespread beneficial effect of the various microbial inoculants used in the present study. This may be attributed in part to the improved spatial acquisition of P mediated by the AMF inoculant (Rhizophagus irregularis MUCL4833) and to bacterial strains having mycorrhizal helper functions and/or P solubilizing properties [49,50,51], as well as the stimulation of root growth as recorded, e.g., for Bacillus amyloliquefaciens FZB42 or Pseudomonas brassicacearum 3Re2-7 inoculants (Table 1). As a confirmation of earlier studies, underlining the superior performance of microbial consortia over single-strain inoculants [25], improved P acquisition was more frequently recorded for microbial consortia (eight out of ten inoculant combinations) as compared with three out of six investigated single strains. However, the recorded increase in P shoot concentrations was not significantly different between single-strain inoculants vs. consortia (Table 2). The improved P nutritional status may explain a general trend for increased vegetative shoot growth of inoculated plants recorded during the drought period (Figure 1). By contrast, other drought protection functions, such as reducing irreversible drought-induced leaf damage, were more restricted to certain inoculants or consortia, such as FZB42, KH, or P&R (Figure 2). Unfortunately, none of the recorded benefits on vegetative growth induced by the inoculants could be translated into improvements of tuber yield, which was reduced by 50% under drought stress as compared to non-stressed plants (Table 1). This observation was also confirmed in the field experiment conducted in Switzerland (Supplementary Figure S1).
4.2. Effects of Drought Protection Inoculants under NH4+ Fertilization
Although various beneficial traits of the selected inoculants were identified, supporting the vegetative development of drought-affected potato plants, the obvious lack of responses in tuber yield in the pot and field experiments suggests the need for further optimization of the inoculation strategy. Therefore, in a second experiment, stabilized NH4+ fertilization was applied instead of NO3− supply. The N-form modification was selected because certain benefits of NH4+ nutrition had been reported for potato, especially on light- and drought-affected soils [30]. Root-induced rhizosphere acidification, triggered by NH4+-dominated N fertilization, may also contribute to Ca-P solubilization at a recorded soil pH of 7.1 [7], thereby counteracting P deficiency detected in the drought-affected potato plants (Table 2). Moreover, beneficial effects of NH4+-fertilization on root colonization and auxin production of various inoculants (e.g., strains of Trichoderma, Bacillus, and Pseudomonas) have been repeatedly reported in the literature [27,46]. Consequently, a commercial ammonium sulfate fertilizer, stabilized with the synthetic nitrification inhibitor DMPP (3,4-dimethylpyrazolphosphate), was applied in combination with a selection of single-strain inoculants (AM, FZB42) and consortia (AM+3Re27, AM+Herbaspirillum sp., AM+FZB42), showing beneficial effects in the previous screening experiment. In terms of the high potentially plant-available P content of the substrate (Supplementary Table 1) in the optimum range [52] and the expected additional P solubilization induced by ammonium fertilization, no additional P fertilization was applied in this case.
4.3. Effects on Plant Growth and Nutritional Status
Under well-watered conditions, no effect of N fertilization form (NO3− or NH4+) was noticed on plant biomass production, nutritional status, or tuber yield at the end of the 11-week culture period. This corroborates the results of [53] and [54] on tuber yield. The N, P, K, and Mg nutritional status detected by leaf analysis was in the deficiency range [55] and may reflect the intense translocation of shoot nutrients to the developing tubers, which comprised 60% of the total plant biomass (Table 4). The analysis of the P nutritional status did not reveal any indication of P solubilization induced by NH4+ fertilization (Table 3). A high pH buffering capacity of the respective soil, counteracting NH4+-induced rhizosphere acidification, may offer an explanation in this case [7]. Intensification of proton extrusion via local root proliferation induced by placement of NH4+ fertilizers has been discussed as a strategy to overcome these limitations [56].
Drought stress reduced the total plant biomass by approximately 60%, independent of the form of N supply or microbial inoculant. However, a massive N-form effect was recorded for biomass partitioning between shoot, roots, and tubers of drought-affected plants (Table 4). Tuber biomass reached 3% of the total plant fresh weight under NO3− fertilization, but it reached 23% in the presence of NH4+ and even 32% in NH4+-fertilized plants with AM inoculation, leading to the highest tuber biomass production in drought-affected plants. In contrast to the first pot experiment, this was not associated with an increased P status of AM-inoculated plants (Table 5), suggesting that benefits in tuber yield could not be related to improved AM-mediated P acquisition in this case. Obviously, the significant contribution of the AMF inoculant to P acquisition was only possible via improved spatial acquisition of the more soluble fertilizer P sources, supplied only in the first pot experiment [7] but not for native soil P as an exclusive P source in the second pot experiment. No comparable effects were detectable for the other microbial inoculants.
4.4. Effects of AMF on Oxidative Stress Protection and Osmotic Adjustment
In the drought-stressed control plants, a massive N-form-independent increase in ROS (H2O2) accumulation (610–740%), associated with a reduction in total antioxidants in young, fully developed leaves, was still detectable two weeks after drought stress recovery (Figure 4). This suggests an incomplete re-establishment of ROS detoxification in the drought-affected plants during the recovery phase. By contrast, the analysis of physiological stress indicators in inoculated plants revealed a significant contribution of the AMF inoculant to the systemic induction of adaptations towards drought-induced ROS detoxification and osmotic adjustment (Figure 4), as similarly reported in other studies [57,58]. This finding indicates a persisting stress-priming effect of the AMF inoculant, detectable even two weeks after recovery to well-watered conditions. AMF inoculation increased the activity of ascorbate peroxidase as a central component for enzymatic detoxification of H2O2 via conversion to H2O [59]. Additionally, the shoot accumulation of glycine betaine was significantly increased (Figure 4), which combines functions in osmo-protection and oxidative stress defense [60]. Accordingly, this was associated with a significant increase in ABA and JA concentrations (Figure 5) known to be involved in ROS defense signaling and synthesis of osmolytes [60,61,62]. A concomitant decline in drought-induced leaf damage (Figure 3) underlined the drought protection potential of the observed physiological modifications induced by the AMF inoculant. Similarly, upregulation of ABA and JA accumulation by AM inoculation has been reported in various plant species and is additionally discussed as a factor in promoting AMF colonization [57,58] by the downregulation of plant defense against biotrophic fungi [63].
4.5. Effects of AMF on Tuberization
Shoot accumulation of proline, known as osmo-protectant, under drought and salinity stress increased in response to NH4+ fertilization, particularly in drought-affected plants with AMF inoculation (Figure 4). However, the recorded concentration of proline was in the nano-molar range and three orders of magnitude lower as compared with the osmo-protectant glycine betaine, suggesting another role of proline accumulation in this case. Apart from functions in osmotic adjustment and ROS protection, proline accumulation has also been reported in the context of N re-translation during the induction of leaf senescence [64], which is also stimulated by ABA and JA [65]. Since the carbon partitioning from source leaves to developing tubers as sink organs can be promoted by increasing the ABA-GA ratio [66], the increased JA and ABA concentration in AM-inoculated plants (Figure 5) may be responsible for the induction of preferential assimilation and nutrient translocation from source leaves to tubers. (Table 4). The related initiation of leaf senescence may explain the increased tuber biomass and leaf accumulation of proline as a consequence of the intensification of this process in AM-inoculated plants. In general, the AM inoculant promoted a hormonal signature in the shoot tissue, characteristic of both the induction of drought stress adaptations and stimulation of tuberization. Increased IAA concentrations (Figure 5) have been reported to stimulate the growth of stolons, while a high ABA/GA ratio (with GA concentrations below the detection limit in our study) was shown to favor tuber growth [67,68].
4.6. Effects of Bacterial Inoculants and Microbial Consortia
A completely different scenario was recorded for the bacterial inoculants or inoculant consortia. Compared with the single-strain AMF inoculant, the potential for ROS detoxification was further increased by bacterial inoculants and consortia. This was indicated by additional leaf accumulation of antioxidants together with increased APX activities, resulting in the lowest levels of ROS (H2O2) accumulation (Figure 4). In contrast to glycine betaine, proline concentrations were not significantly increased and associated with the lowest values for tuber biomass. This metabolic pattern suggests a particularly efficient ROS detoxification as previously reported, e.g., for other Bacillus-based PGPR inoculants [69,70], promoting vegetative growth and counteracting stress-induced leaf senescence [71]. The delayed leaf senescence and stimulation of vegetative growth may also explain the shift in resource allocation favoring shoot biomass production at the expense of tuber growth in these treatments (Table 4).
A similar situation may apply to the potato plants in experiment 1 supplied with nitrate and P fertilization known to support cytokinin synthesis, which stimulates shoot growth, delays leaf senescence, reduces ABA [72], and consequently suppresses tuberization at higher concentrations [54]. It remains to be established whether the observed promotion of vegetative growth at the expense of tuber formation is characteristic only for the early stages of tuberization investigated in the present study and may be compensated in later stages of plant development. However, the absence of inoculant effects recorded under field conditions (Supplementary Figure S1) suggests suboptimal effects also in the long run.
4.7. Concluding Remarks
The results of the present study demonstrate that the investigated inoculants were able to mitigate drought stress in potato. The application of microbial consortia obviously increased the probability of the expression of beneficial and complementary effects, but the absolute effect size was not necessarily superior to the use of efficient single-strain inoculants. The observed benefits comprised (i) an improved P nutritional status, (ii) root growth stimulation by selected bacterial inoculants, (iii) improved osmotic adjustment (AMF and bacteria), (iv) improved enzymatic ROS detoxification (AMF and bacteria), (v) non-enzymatic ROS detoxification via production of antioxidants (mainly bacteria), and (vi) hormonal signatures (with increased levels of ABA, jasmonic, and indole acetic acid), promoting the induction of drought stress adaptations and tuberization by AMF inoculation. The benefits of microbial inoculants concerning final tuber yield were particularly expressed under ammonium fertilization and restricted to single-strain AMF inoculation, while microbial consortia rather counteracted stress-induced leaf senescence and promoted vegetative growth at the expense of tuberization. The results suggest significant and selective interactions of microbial inoculants with factors and plant signalling pathways not only involved in the induction of drought adaptations but also in tuberization. More detailed knowledge of the respective interactions seems to be necessary for the successful selection and application of suitable microbial inoculants as drought protectants in potato production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10010102/s1, Figure S1: Tuber yield, in tons per hectare, for the field trial conducted in Switzerland. Potato varieties are presented separately along the horizontal axis, while irrigated and rainfed treatments were split between top and bottom panels, respectively. Colours represent control (red) or inoculations of consortia (R.irreg = Rhizophagus irregularis MUCL 41833; P. brass = Pseudomonas brassicacearum 3Re2-7; P. phyt = Paraburkholderia phytofirmans PsJN; T. asper = Trichoderma asperelloides A, Figure S2: The potato field experiment with drip irrigation vs rainfed conditions during early growth (a) and 45 days after planting (b), Figure S3: Pot experiments: Regeneration of potato plants (cv Alonso) from tuber cuttings (a), rooting in planting substrate (b) and young potato plant in the 4–5 leaf stage used for soil drenching with inoculants (c). Potato plant at 9 weeks after sowing grown under well-watered conditions (d) and after 6 weeks of drought stress with 20–25 % substrate water-holding capacity (e); Table S1: Physical and chemical properties of the soil used for the greenhouse experiments, Table S2: Treatments, microbial strains and strain combinations used for experiment I, Table S3: Optical density corresponding to 107 cfu mL−1 for each bacterial inoculant strain (pure liquid TSM as blank to adjust the NanoDrop™ spectrophotometer), Table S4: Treatments, microbial strains and strain combinations used for experiment II, Table S5: Formulation and dosage of PGPM strains in the field trial.
Author Contributions
A.A.M., M.W. and G.N. conceived and designed the experiments. A.A.M. and A.A. conducted the experiments, performed the analyses, and collected the data. N.M. supported analyses of stress metabolites and hormones. B.D. and G.D. conducted the field experiment, K.M., S.D. and W.V. provided microbial inoculants and developed formulations. A.A.M. prepared a first draft of the manuscript and G.N., M.W., U.L., S.D., G.D., W.V., K.M. and F.N. read and edited the manuscript. All authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Union, Horizon2020 Project “SoLACE” (Solutions for improving Agroecosystem and Crop Efficiency for water and nutrient use), Grant Agreement No. 727247 and a Faculty Scholarship of the Faculty of Agricultural Sciences, University of Hohenheim, Stuttgart, Germany.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors K.M. and W.V. were employed by the companies Sourcon Padena GmbH, Sindelfinger Str. 3, 72070 Tübingen, Germany and Agrobiota, Vor dem Kreuzberg 17, 72070 Tübingen, Germany. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- FAO. FAO Statistical Databases FAOSTAT. 2014. Available online: http://faostat3.fao.org/ (accessed on 20 October 2023).
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for sustainable global food security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Zarzyńska, K.; Boguszewska-Mańkowska, D.; Nosalewicz, A. Differences in size and architecture of the potato cultivars root system and their tolerance to drought stress. Plant Soil Environ. 2017, 63, 159–164. [Google Scholar] [CrossRef]
- Hopkins, B.G.; Horneck, D.A.; MacGuidwin, A.E. Improving phosphorus use efficiency through potato rhizosphere modification and extension. Am. J. Potato Res. 2014, 91, 161–174. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2020, 63, 97–119. [Google Scholar] [CrossRef]
- van der Linden, C.G.; Anithakumari, A.M.; van Culemborg, M.; Visser, R.G.F. Dissecting the genetics of abiotic stress tolerance in potato. In Proceedings of the Plant & Animal Genomes XIXth Conference, San Diego, CA, USA, 15–19 January 2011. [Google Scholar]
- Neumann, G.; Römheld, V. Root-induced changes in the availability of nutrients in the rhizosphere. In Plant Roots: The Hidden Half; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 617–649. [Google Scholar]
- Aliche, E.B.; Theeuwen, T.P.; Oortwijn, M.; Visser, R.G.; van der Linden, C.G. Carbon partitioning mechanisms in potato under drought stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Wolf, J. Climate change impact on global potato production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, H.B. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Armada, E.; Portela, G.; Roldán, A.; Azcón, R. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 2014, 232, 640–648. [Google Scholar] [CrossRef]
- Bukovská, P.; Bonkowski, M.; Konvalinková, T.; Beskid, O.; Hujslová, M.; Püschel, D.; Jansa, J. Utilization of organic nitrogen by arbuscular mycorrhizal fungi is there a specific role for protists and ammonia oxidizers. Mycorrhiza 2018, 28, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Brookes, P.C.; Xu, J. Arbuscular mycorrhizal fungal hyphae alter soil bacterial community and enhance polychlorinated biphenyls dissipation. Front. Microbiol. 2016, 7, 939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fimognari, L.; de Almeida, J.; Jensen, C.N.G.; Compant, S.; Oliveira, T.; Liu, F. Effect of Bacillus paralicheniformis on soybean (Glycine max) roots colonization, nutrient uptake and water use efficiency under drought stress. J. Agron. Crop Sci. 2023, 209, 547–565. [Google Scholar] [CrossRef]
- Barea, J.M.; Toro, M.; Orozco, M.O.; Campos, E.; Azcón, R. The application of isotopic (32 P and 15 N) dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutr. Cycl. Agroecosyst. 2002, 63, 35–42. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, J. Prospecting the roles of metabolites and additives in future bioformulations for sustainable agriculture. Appl. Soil Ecol. 2016, 107, 405–407. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer: Berlin/Heidelberg, Germany, 2007; pp. 329–339. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef]
- Weinmann, M.; Neumann, G. Bio-effectors to optimize the mineral nutrition of crop plants. In Achieving Sustainable Crop Nutrion; Rengel, Z., Ed.; Burleigh Dodds Science Publishing Limited: Camebridge, UK, 2020; pp. 589–690. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Nuti, M. Ecosystem functions of microbial consortia in sustainable agriculture. Agronomy 2020, 10, 1902. [Google Scholar] [CrossRef]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Weinmann, M. Microbial consortia versus single-strain inoculants: An advantage in PGPM-assisted tomato production. Agronomy 2019, 9, 105. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Müller, T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: From lab to field. Chem. Biol. Technol. Agric. 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Gomez-Genao, N.; Moradtalab, N.; Wanke, D.; Chrobaczek, V.; Ahmed, A.; Ludewig, U. The role of N form supply for PGPM-host plant interactions in maize. J. Plant Nutr. Soil Sci. 2019, 182, 908–920. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Nkebiwe, P.M.; Kuhlmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Moradtalab, N.; Ahmed, A.; Geistlinger, J.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Synergisms of microbial consortia, N forms, and micronutrients alleviate oxidative damage and stimulate hormonal cold stress adaptations in maize. Front. Plant Sci. 2020, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Baumgartner, N. Unterfussdüngung bei Kartoffeln. BLW 2017, 12, 52–53. [Google Scholar]
- Öhlinger, R. Maximum Water-Holding Capacity. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Heidelberg, Germany, 1996; pp. 385–386. [Google Scholar]
- De Jaeger, N.; De la Providencia, I.E.; Rouhier, H.; Declerck, S. Co-entrapment of Trichoderma harzianum and Glomus sp. within alginate beads: Impact on the arbuscular mycorrhizal fungi life cycle. J. Appl. Microbiol. 2011, 111, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Microbial Inoculants Provided by Solace Project Partners. Available online: https://www.solace-eu.net (accessed on 17 October 2018).
- Gericke, S.; Kurmies, B. Die kolorimetrische phosphorsäurebestimmung mit ammonium-vanadat-molybdat und ihre Anwendung in der Pflanzenanalyse. Z. Pflanzenernährung Düngung Bodenkd. 1952, 59, 235–247. [Google Scholar]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Valadez-Bustos, M.G.; Aguado-Santacruz, G.A.; Tiessen-Favier, A.; Robledo-Paz, A.; Muñoz-Orozco, A.; Rascón-Cruz, Q.; Santacruz-Varela, A. A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal. Biochem. 2016, 498, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Boominathan, R.; Doran, P.M. Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol. 2002, 156, 205–215. [Google Scholar] [CrossRef] [PubMed]
- CR Team. Team RDC. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Allaire, J. RStudio: Integrated development environment for R. Boston MA 2012, 770, 165–171. [Google Scholar]
- de Mendiburu, F. Package ‘agricolae’. In R Package, Version; R foundation for Statistical Computing: Vienna, Austria, 2019; Volume 1. [Google Scholar]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Velivelli, S.L.; Sessitsch, A.; Prestwich, B.D. The role of microbial inoculants in integrated crop management systems. Potato Res. 2014, 57, 291–309. [Google Scholar] [CrossRef]
- Overbeek, W.; Jeanne, T.; Hogue, R.; Smith, D.L. Effects of Microbial Consortia, Applied as Fertilizer Coating, on Soil and Rhizosphere Microbial Communities and Potato Yield. Front. Agron. 2021, 3, 714700. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef]
- Bradáčová, K.; Kandeler, E.; Berger, N.; Ludewig, U.; Neumann, G. Microbial consortia inoculants stimulate early growth of maize depending on nitrogen and phosphorus supply. Plant Soil Environ. 2020, 66, 105–112. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef]
- Mackay, A.D.; Barber, S.A. Soil moisture effects on root growth and phosphorus uptake by com. Agron. J. 1985, 77, 519–523. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ramesh, A.; Johri, B.N. Isolation and characterization of plant growth-promoting Bacillus amyloliquefaciens strain sks_bnj_1 and its influence on rhizosphere soil properties and nutrition of soybean (Glycine max L. Merrill). J. Virol. Microbiol. 2013, 2013, 446006. [Google Scholar] [CrossRef]
- Thonar, C.; Lekfeldt, J.D.S.; Cozzolino, V.; Kundel, D.; Kulhánek, M.; Mosimann, C.; Mäder, P. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Yusran, Y.; Roemheld, V.; Mueller, T. Effects of Pseudomonas sp.” Proradix” and Bacillus Amyloliquefaciens FZB42 on the Establishment of AMF Infection, Nutrient Acquisition and Growth of Tomato Affected by Fusarium Oxysporum Schlecht f. sp. Radicis-lycopersici Jarvis and Shoemaker. 2009. Available online: https://escholarship.org/uc/item/8g70p0zt (accessed on 20 September 2020).
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten e.V. Speyer, Germany). Standpunkt. Phosphordüngung nach Bodenuntersuchung und Pflanzenbedarf; VDLUFA Verlag: Darmsatdt, Germany, 2018. [Google Scholar]
- Gao, Y.; Jia, L.; Hu, B.; Alva, A.; Fan, M. Potato stolon and tuber growth influenced by nitrogen form. Plant Prod. Sci. 2014, 17, 138–143. [Google Scholar] [CrossRef]
- Krauss, A. Interaction of nitrogen nutrition, phytohormones, and tuberization. Potato Physiol. 1985, S, 209–230. [Google Scholar]
- Wolf, B. Diagnostic Techniques for Improving Crop Production, 1st ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Fernández-Lizarazo, J.C.; Moreno-Fonseca, L.P. Mechanisms for tolerance to water-deficit stress in plants inoculated with arbuscular mycorrhizal fungi. A review. Agron. Colomb. 2016, 34, 179–189. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Rajasheker, G.; Jawahar, G.; Jalaja, N.; Kumar, S.A.; Kumari, P.H.; Punita, D.L.; Kishor, P.B.K. Role and regulation of osmolytes and ABA interaction in salt and drought stress tolerance. In Plant Signaling Molecules; Woodhead Publishing: Cambridge, UK, 2019; pp. 417–436. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts. Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Méndez, S.J.; Urbano-Gálvez, A.; Mahouachi, J. Mitigation of salt stress damages in Carica papaya L. seedlings through exogenous pretreatments of gibberellic acid and proline. Chil. J. Agric. Res. 2022, 82, 167–176. [Google Scholar] [CrossRef]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Dwelle, R.B. Photosynthesis and photoassimilate partitioning. In Potato Physiology; Academic Press Inc.: Palm Bay, FL, USA, 1985; pp. 35–58. [Google Scholar]
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kardile, H.B. Key players associated with tuberization in potato: Potential candidates for genetic engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Kolachevskaya, O.O.; Lomin, S.N.; Arkhipov, D.V.; Romanov, G.A. Auxins in potato: Molecular aspects and emerging roles in tuber formation and stress resistance. Plant Cell Rep. 2019, 38, 681–698. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Mubushar, M. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Miyawaki, K.; Matsumoto-Kitano, M.; Kakimoto, T. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 2004, 37, 128–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).