Synergistic Effects of Stirring and Aeration Rate on Carotenoid Production in Yeast Rhodotorula toruloides CCT 7815 Envisioning Their Application as Soap Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of and OTR
2.2. Strain and Inoculum
2.3. Production of Carotenoids Using a Stirred-Tank Bioreactor
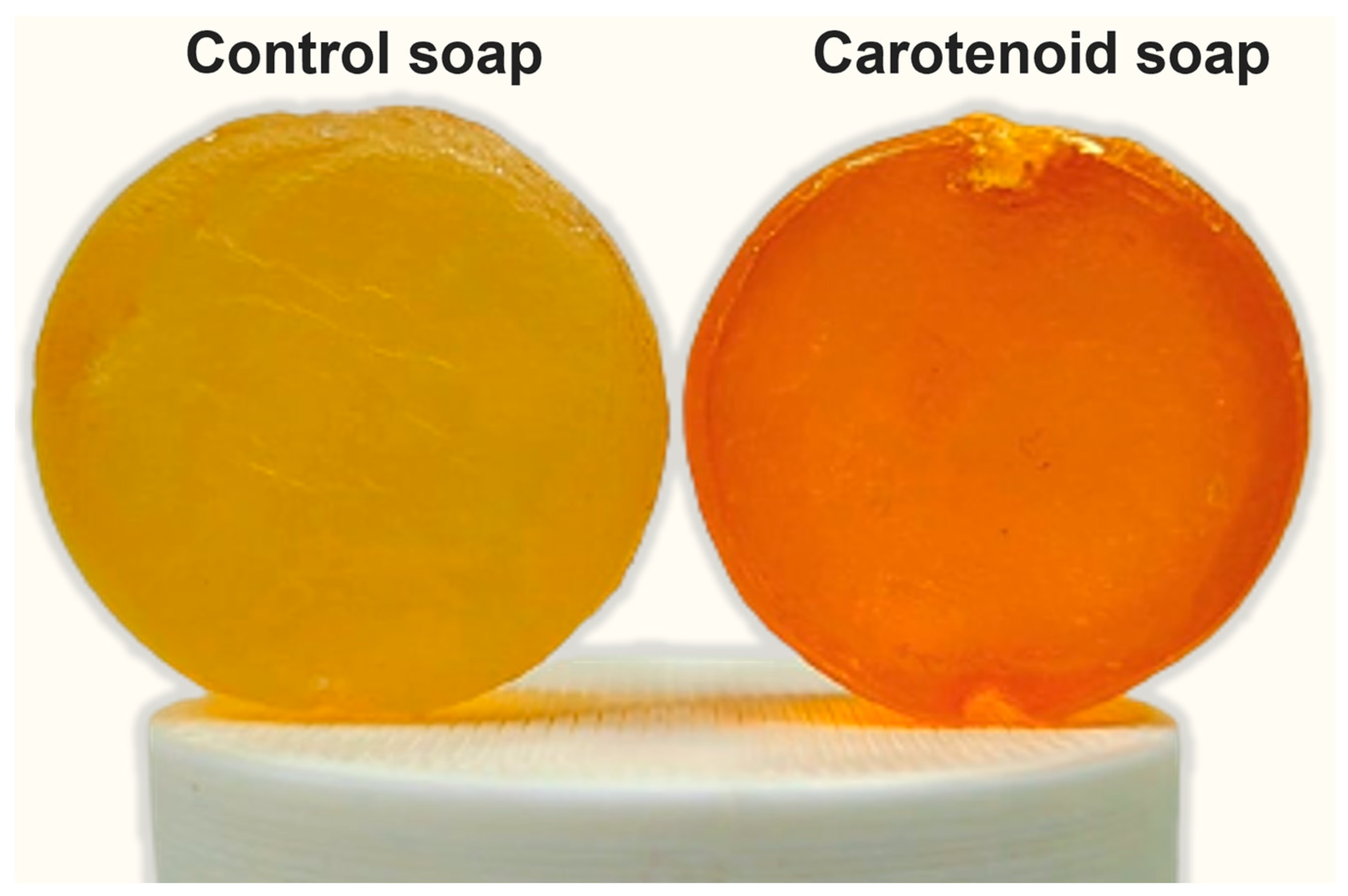
2.4. Carotenoids-Rich Extract as Soap Additive
2.5. Analytical Methods
2.5.1. Measurement of Sugars and Dry Biomass
2.5.2. Extraction and Quantification of Lipids
2.5.3. Quantification of Carotenoids
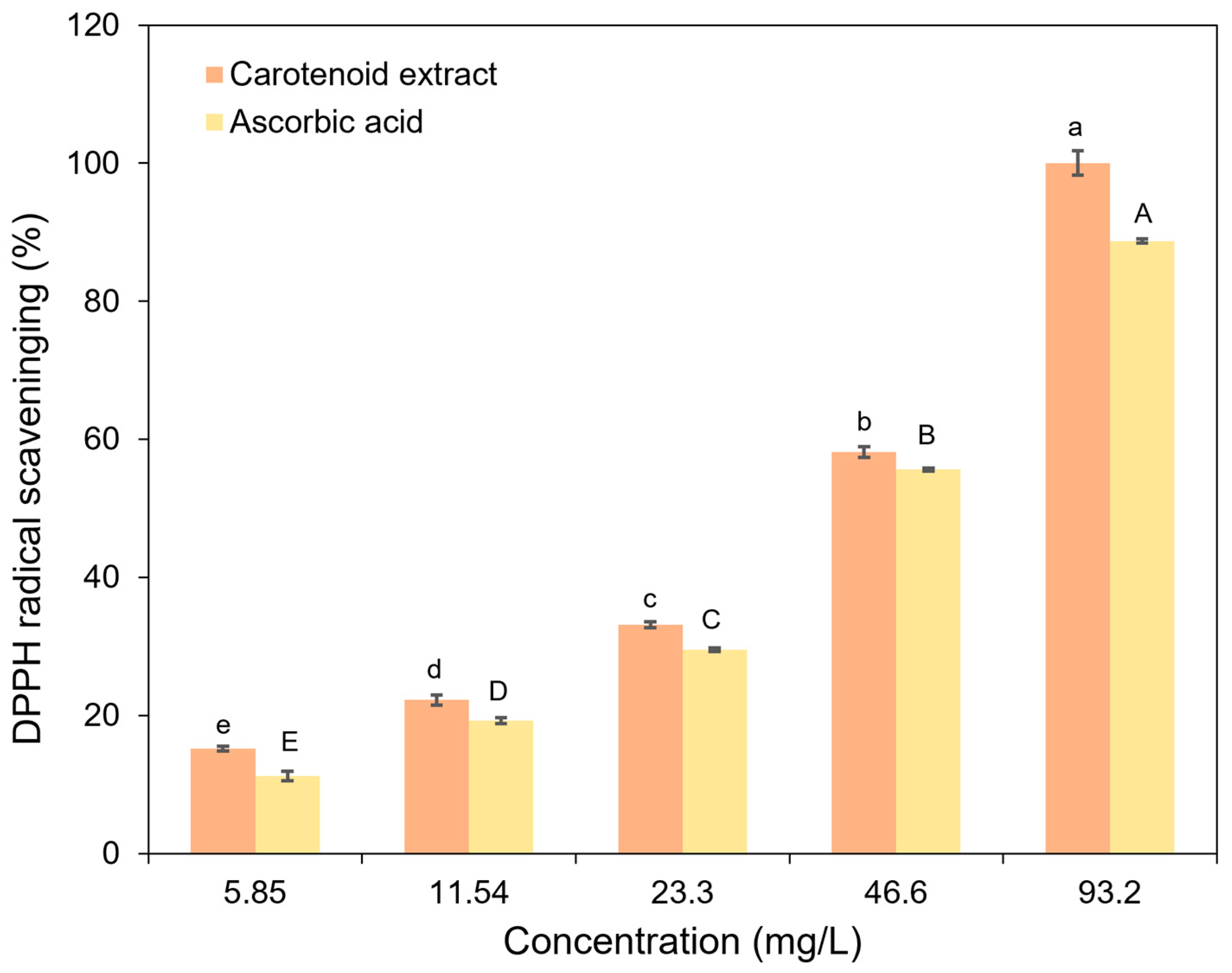
2.5.4. Antioxidant Activity Analysis Using DPPH Scavenging Assay
2.6. Statistical Analysis
3. Results
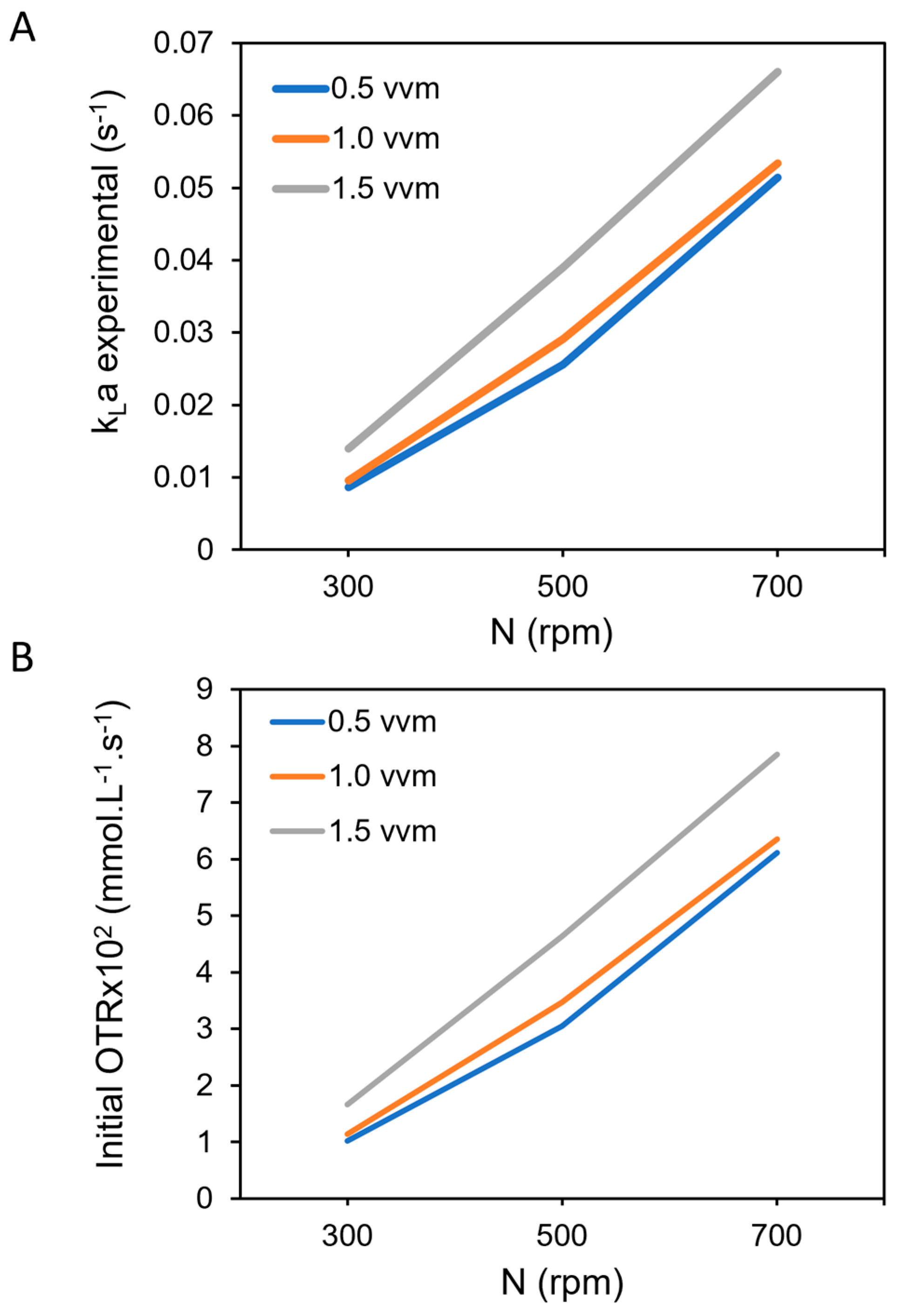
3.1. Influence of Oxygen Transfer
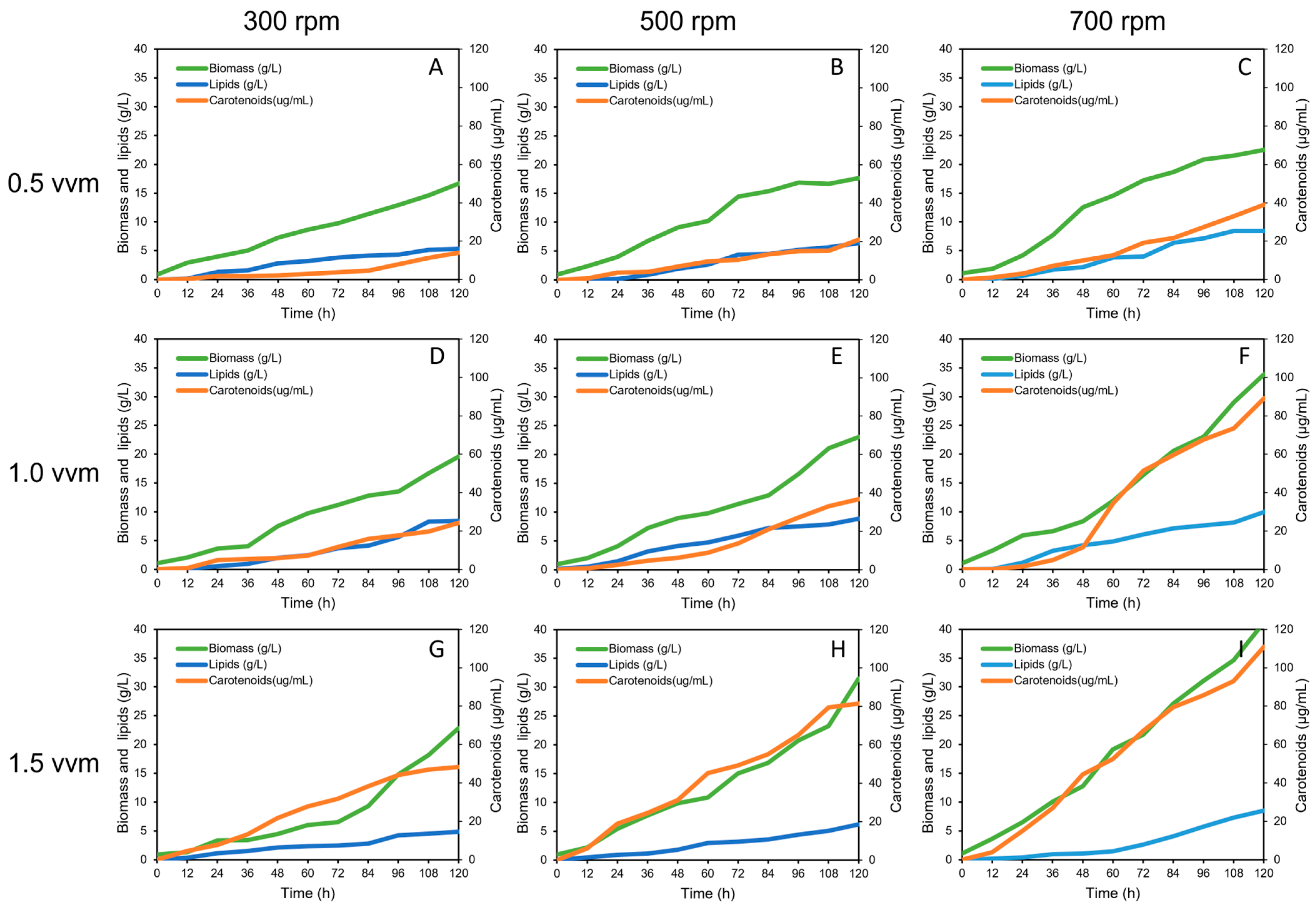
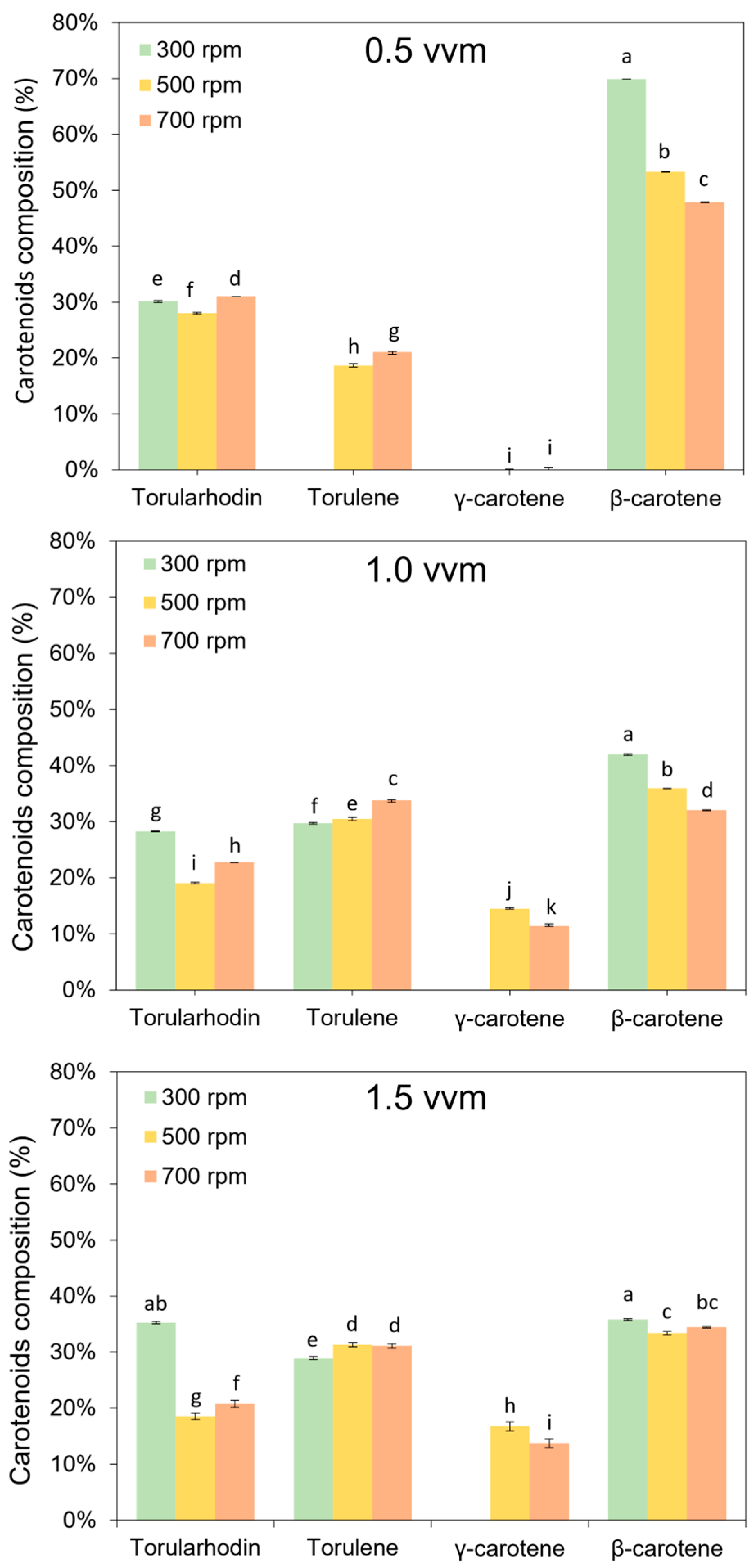
3.2. Effect of Aeration and Agitation Rates on Carotenoids Production
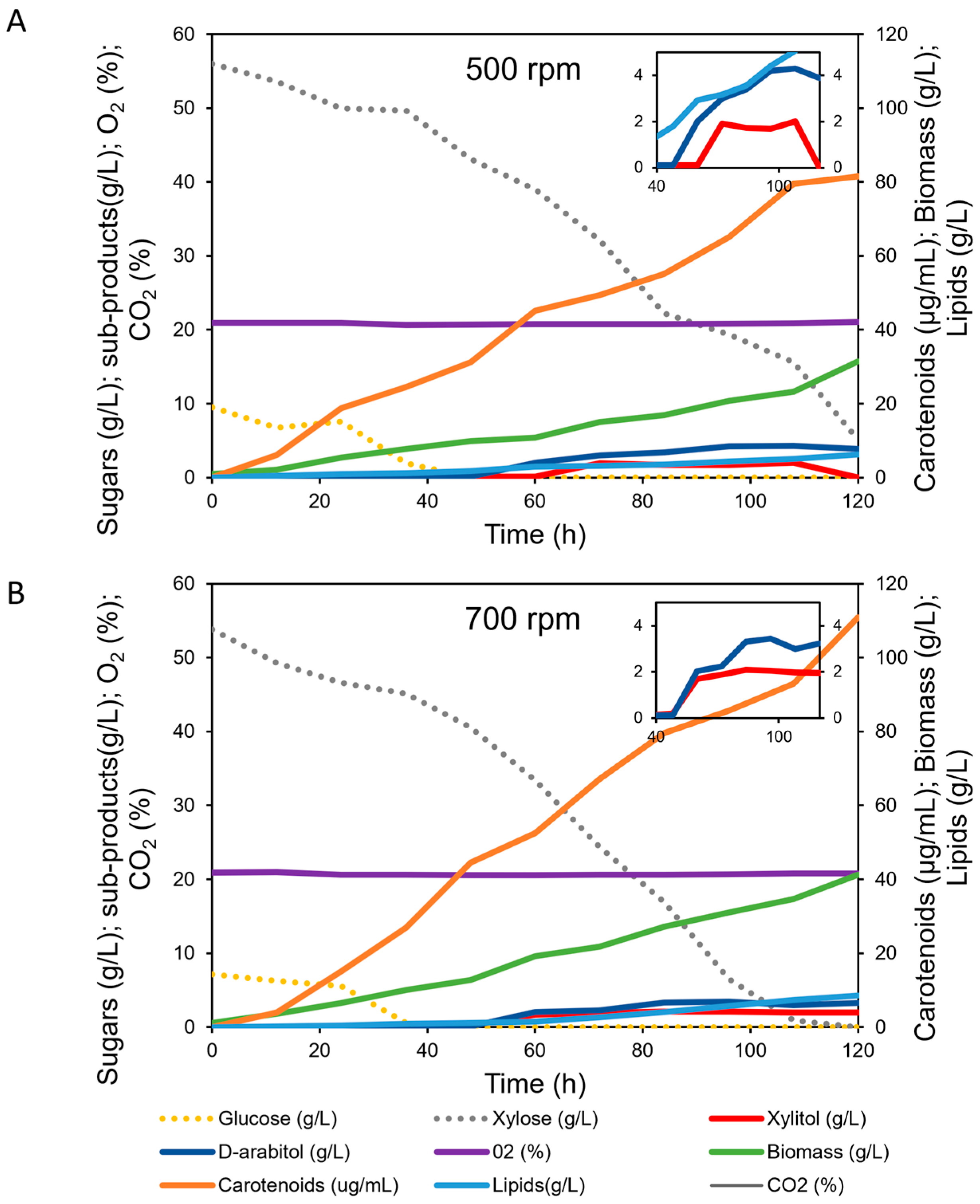
3.3. Kinetics of Carotenoids Bioproduction
3.4. Antioxidant Activities of Ascorbic Acid and Carotenoid Extract
3.5. Carotenoids-Rich Extract as Soap Additive
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inam, A.; Mutaf, T.; Deniz, I. Sustainable Biorefineries for Circular Bioeconomy. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–28. [Google Scholar]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Pereira, J.F.B.; Dufossé, L.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa, A. Advances and Trends in Biotechnological Production of Natural Astaxanthin by Phaffia rhodozyma Yeast. Crit. Rev. Food Sci. Nutr. 2023, 63, 1862–1876. [Google Scholar] [CrossRef]
- Mussagy, C.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and Extraction of Carotenoids Produced by Microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent Advancements in Astaxanthin Production from Microalgae: A Review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Cardoso, L.A.C.; Karp, S.G.; Vendruscolo, F.; Kanno, K.Y.F.; Zoz, L.I.C.; Carvalho, J.C. Biotechnological Production of Carotenoids and Their Applications in Food and Pharmaceutical Products. Carotenoids 2017, 8, 125–141. [Google Scholar]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of Lipids and Carotenoids by Rhodotorula gracilis ATCC 10788 Yeast in a Bioreactor Using Low-Cost Wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Ribeiro, H.F.; Santos-Ebinuma, V.C.; Schuur, B.; Pereira, J.F.B. Rhodotorula Sp.–Based Biorefinery: A Source of Valuable Biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar] [CrossRef]
- Gong, F.; Zhang, C.; Zhang, L.; Liu, J. Changes of Carotenoids Contents and Analysis of Astaxanthin Geometrical Isomerization in Haematococcus pluvialis under Outdoor High Light Conditions. Aquac. Res. 2020, 51, 770–778. [Google Scholar] [CrossRef]
- Mussagy, C.; Guimarães, A.A.C.; Rocha, L.V.F.; Winterburn, J.; Santos-Ebinuma, V.d.C.; Pereira, J.F.B. Improvement of Carotenoids Production from Rhodotorula glutinis CCT-2186. Biochem. Eng. J. 2021, 165, 107827. [Google Scholar] [CrossRef]
- Park, Y.-K.; Nicaud, J.-M.; Ledesma-Amaro, R. The Engineering Potential of Rhodosporidium toruloides as a Workhorse for Biotechnological Applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, B.; Li, J.; Zhang, J. Rhodotorula toruloides: An Ideal Microbial Cell Factory to Produce Oleochemicals, Carotenoids, and Other Products. World J. Microbiol. Biotechnol. 2022, 38, 13. [Google Scholar] [CrossRef]
- Pinheiro, M.J.; Bonturi, N.; Belouah, I.; Miranda, E.A.; Lahtvee, P.-J. Xylose Metabolism and the Effect of Oxidative Stress on Lipid and Carotenoid Production in Rhodotorula toruloides: Insights for Future Biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 1008. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Shen, P.; Hu, R.; Xue, T.; Jiang, X.; Qin, L.; Chen, Y.; Huang, J. Carotenoids and Lipid Production from Rhodosporidium toruloides Cultured in Tea Waste Hydrolysate. Biotechnol. Biofuels. 2020, 13, 74. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.-J. C/N Ratio and Carbon Source-Dependent Lipid Production Profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef]
- Marques, M.P.C.; Cabral, J.M.S.; Fernandes, P. Bioprocess Scale-up: Quest for the Parameters to Be Used as Criterion to Move from Microreactors to Lab-Scale. J. Chem. Technol. Biotechnol. 2010, 85, 1184–1198. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Miranda, E.A. Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus Urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies 2020, 13, 795. [Google Scholar] [CrossRef]
- Bonturi, N.; Matsakas, L.; Nilsson, R.; Christakopoulos, P.; Miranda, E.; Berglund, K.; Rova, U. Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction. Energies 2015, 8, 5040–5052. [Google Scholar] [CrossRef]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S.; et al. Effect of Light on Carotenoid and Lipid Production in the Oleaginous Yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.C.; Palma, M.; Angelov, A.; Nevoigt, E.; Liebl, W.; Sá-Correia, I. Complete Utilization of the Major Carbon Sources Present in Sugar Beet Pulp Hydrolysates by the Oleaginous Red Yeasts Rhodotorula toruloides and R. Mucilaginosa. J. Fungi 2021, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential Use of Oleaginous Red Yeast Rhodotorula glutinis for the Bioconversion of Crude Glycerol from Biodiesel Plant to Lipids and Carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Cerri, M.O.; Nordi Esperança, M.; Colli Badino, A.; Perencin de Arruda Ribeiro, M. A New Approach for kLa Determination by Gassing-out Method in Pneumatic Bioreactors. J. Chem. Technol. Biotechnol. 2016, 91, 3061–3069. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Farias, F.O.; Santos-Ebinuma, V.C.; Pereira, J.F.B.; Pessoa, A. Sustainable One-Pot Platform for the Green Recovery of Carotenoids from Phaffia rhodozyma Yeast and Their Use as Natural Additives in Soap Formulation. Environ. Technol. Innov. 2023, 29, 103029. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Kurnia, K.A.; Dias, A.C.R.V.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa, A., Jr. An Eco-Friendly Approach for the Recovery of Astaxanthin and β-Carotene from Phaffia rhodozyma Biomass Using Bio-Based Solvents. Bioresour. Technol. 2022, 345, 126555. [Google Scholar] [CrossRef]
- Canaan, J.M.M.; Brasil, G.S.P.; de Barros, N.R.; Mussagy, C.U.; Guerra, N.B.; Herculano, R.D. Soybean Processing Wastes and Their Potential in the Generation of High Value Added Products. Food Chem. 2022, 373, 131476. [Google Scholar] [CrossRef]
- Ribeiro, R.M.M.G.P.; Esperança, M.N.; Sousa, A.P.A.; Neto, Á.B.; Cerri, M.O. Individual Effect of Shear Rate and Oxygen Transfer on Clavulanic Acid Production by Streptomyces clavuligerus. Bioprocess. Biosyst. Eng. 2021, 44, 1721–1732. [Google Scholar] [CrossRef]
- Picão, B.W.; Gonçalves, D.O.; Ribeiro, R.M.M.G.P.; Esperança, M.N.; Peixoto, G.; Cerri, M.O. Oxygen transfer and gas holdup in airlift bioreactors assembled with helical flow promoters. Bioprocess. Biosyst. Eng. 2023, 46, 681–692. [Google Scholar] [CrossRef]
- Davoli, P.; Mierau, V.; Weber, R.W.S. Carotenoids and Fatty Acids in Red Yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004, 40, 392–397. [Google Scholar] [CrossRef]
- Malisorn, C.; Suntornsuk, W. Improved β-carotene production of Rhodotorula glutinis in fermented radish brine by continuous cultivation. Biochem. Eng. J. 2009, 43, 27–32. [Google Scholar] [CrossRef]
- Yamane, Y.; Higashida, K.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Influence of Oxygen and Glucose on Primary Metabolism and Astaxanthin Production by Phaffia rhodozyma in Batch and Fed-Batch Cultures: Kinetic and Stoichiometric Analysis. Appl. Environ. Microbiol. 1997, 63, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Gonzalez, M.; Santos-Ebinuma, V.; Pereira, J.F. Microbial torularhodin—A comprehensive review. Crit. Rev. Biotechnol. 2023, 43, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Silva, P.G.P.; Amantino, C.F.; Burkert, J.F.M.; Primo, F.L.; Pessoa, A.; Santos-Ebinuma, V.C. Production of natural astaxanthin by Phaffia rhodozyma and its potential application in textile dyeing. Biochem. Eng. J. 2022, 187, 108658. [Google Scholar] [CrossRef]
- Jagtap, S.S.; Rao, C.V. Production of d-arabitol from d-xylose by the oleaginous yeast Rhodosporidium toruloides IFO0880. Appl. Microbiol. Biotechnol. 2018, 102, 143–151. [Google Scholar] [CrossRef]
- Umai, R.D.; Jacob, S.; Kumar, V. Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium toruloides NCIM 3547. Fermentation 2022, 8, 591. [Google Scholar] [CrossRef]
- Hu, C.-C.; Lin, J.-T.; Lu, F.-J.; Chou, F.-P.; Yang, D.-J. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008, 109, 439–446. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.M.M.G.P.; Picão, B.W.; Gonçalves, D.O.; Scontri, M.; Mazziero, V.T.; Mussagy, C.U.; Raghavan, V.; Astudillo-Castro, C.; Córdova, A.; Cerri, M.O.; et al. Synergistic Effects of Stirring and Aeration Rate on Carotenoid Production in Yeast Rhodotorula toruloides CCT 7815 Envisioning Their Application as Soap Additives. Fermentation 2023, 9, 828. https://doi.org/10.3390/fermentation9090828
Ribeiro RMMGP, Picão BW, Gonçalves DO, Scontri M, Mazziero VT, Mussagy CU, Raghavan V, Astudillo-Castro C, Córdova A, Cerri MO, et al. Synergistic Effects of Stirring and Aeration Rate on Carotenoid Production in Yeast Rhodotorula toruloides CCT 7815 Envisioning Their Application as Soap Additives. Fermentation. 2023; 9(9):828. https://doi.org/10.3390/fermentation9090828
Chicago/Turabian StyleRibeiro, Renata M. M. G. P., Bruno W. Picão, Daniele O. Gonçalves, Mateus Scontri, Vitor T. Mazziero, Cassamo U. Mussagy, Vijaya Raghavan, Carolina Astudillo-Castro, Andrés Córdova, Marcel O. Cerri, and et al. 2023. "Synergistic Effects of Stirring and Aeration Rate on Carotenoid Production in Yeast Rhodotorula toruloides CCT 7815 Envisioning Their Application as Soap Additives" Fermentation 9, no. 9: 828. https://doi.org/10.3390/fermentation9090828
APA StyleRibeiro, R. M. M. G. P., Picão, B. W., Gonçalves, D. O., Scontri, M., Mazziero, V. T., Mussagy, C. U., Raghavan, V., Astudillo-Castro, C., Córdova, A., Cerri, M. O., & Tambourgi, E. B. (2023). Synergistic Effects of Stirring and Aeration Rate on Carotenoid Production in Yeast Rhodotorula toruloides CCT 7815 Envisioning Their Application as Soap Additives. Fermentation, 9(9), 828. https://doi.org/10.3390/fermentation9090828








