Light Induction of Seed Culture Accelerates Lutein Accumulation in Heterotrophic Fermentation of Chlorella protothecoides CS-41
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.1.1. Optimization of Light Intensity
2.1.2. Optimization of Glucose Concentration
2.1.3. Seed Induction under Different Light Conditions
2.1.4. Seed Induction before Fed-Batch Cultivation
2.2. Determination of Biomass, Glucose Concentration and Urea Concentration
2.3. Determination of Lutein, Total Carotenoids and Total Chlorophylls
2.4. Quantification of Protein, Lipid and Carbohydrate Concentration
2.5. Quantification of Maximum Quantum Yield
2.6. RNA Extraction and Analysis of Differentially Expressed Genes
2.7. Metabolite Extraction and Analysis
2.8. Statistical Analysis and Equations
3. Results
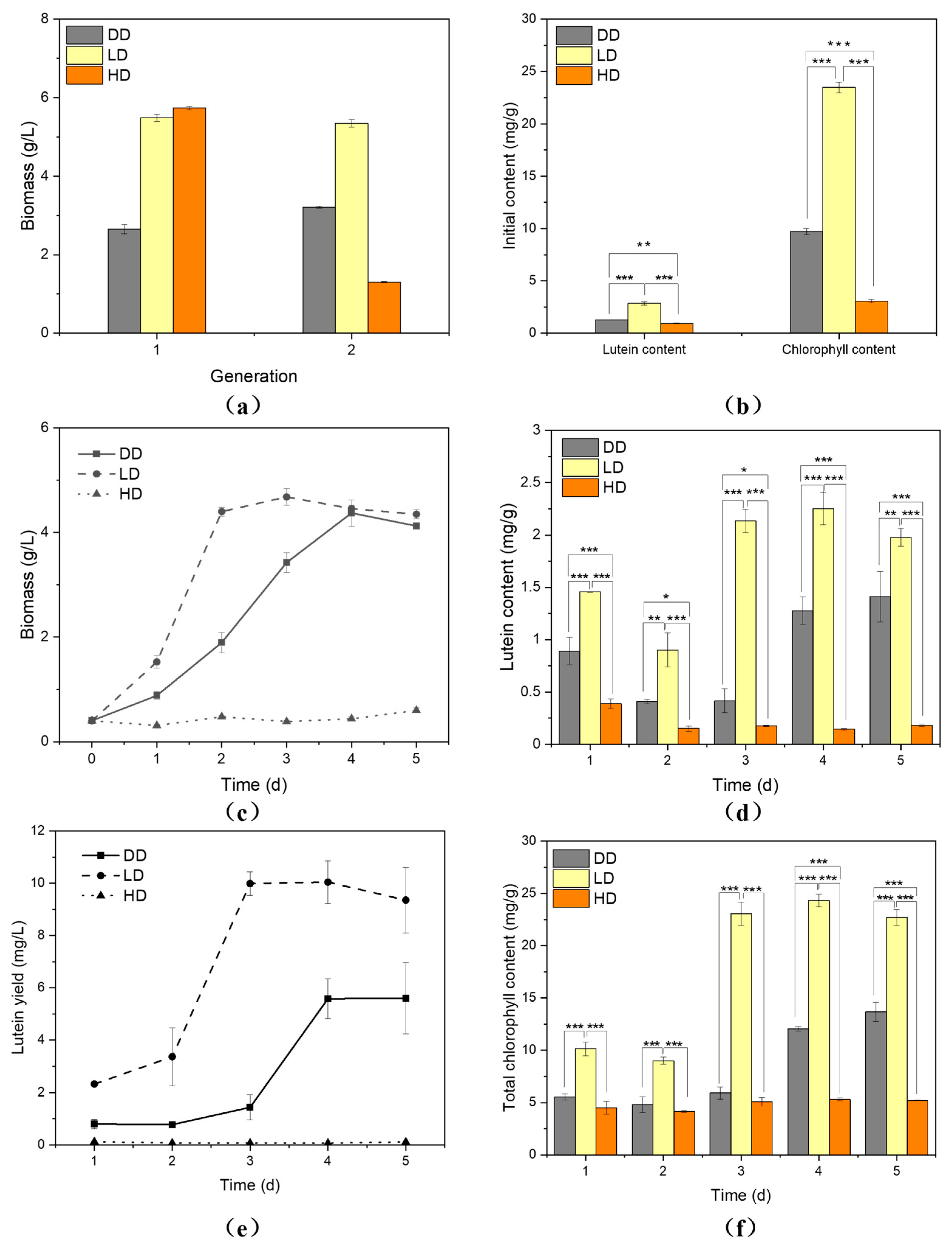
3.1. Low-Light Intensity Contributed to Lutein Biosynthesis
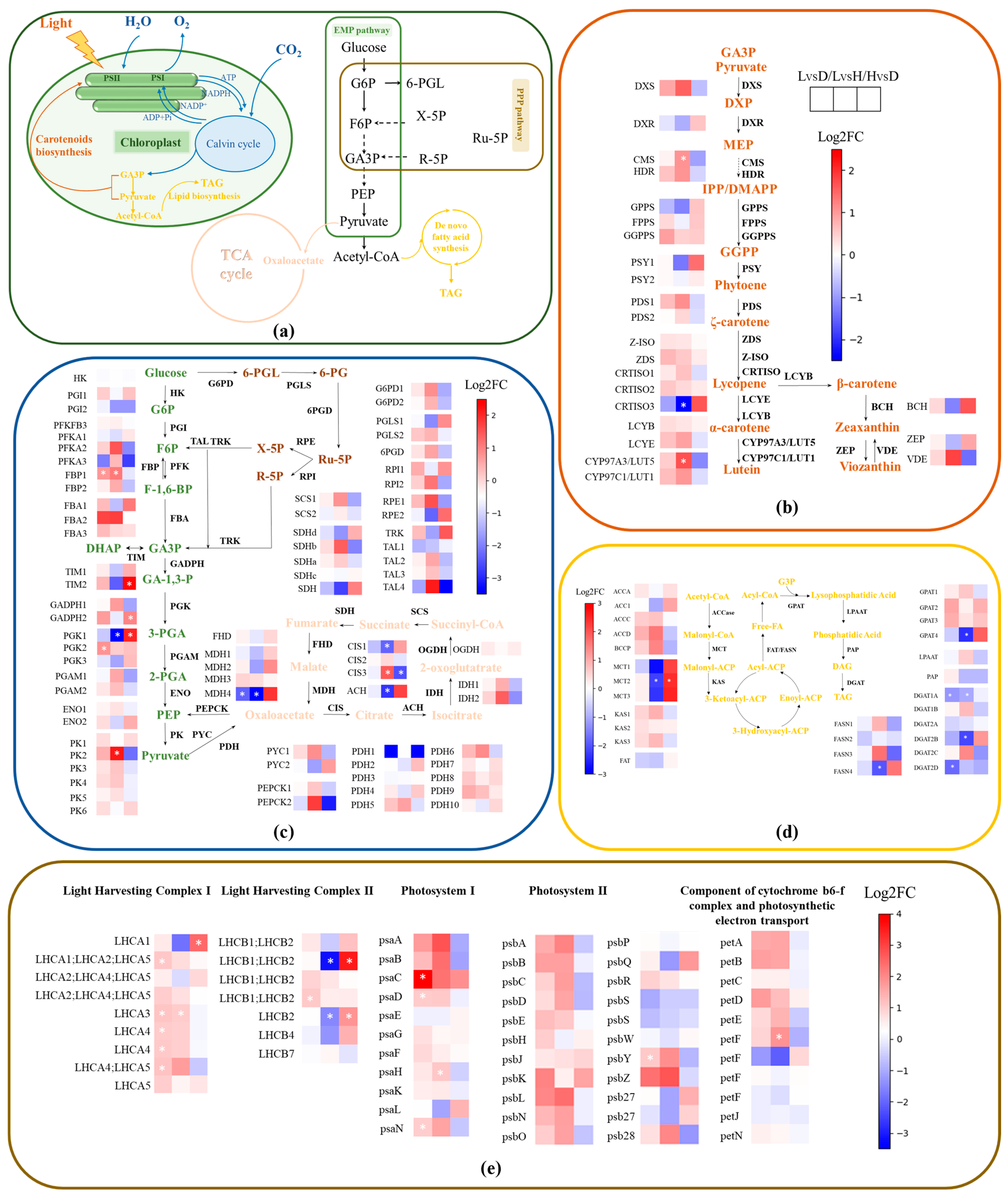
3.2. Transcriptome Data within the Metabolic Network of C. protothecoides CS-41
3.3. Seed Induction under Low Light Accelerate Lutein Accumulation in Heterotrophic Cultivation
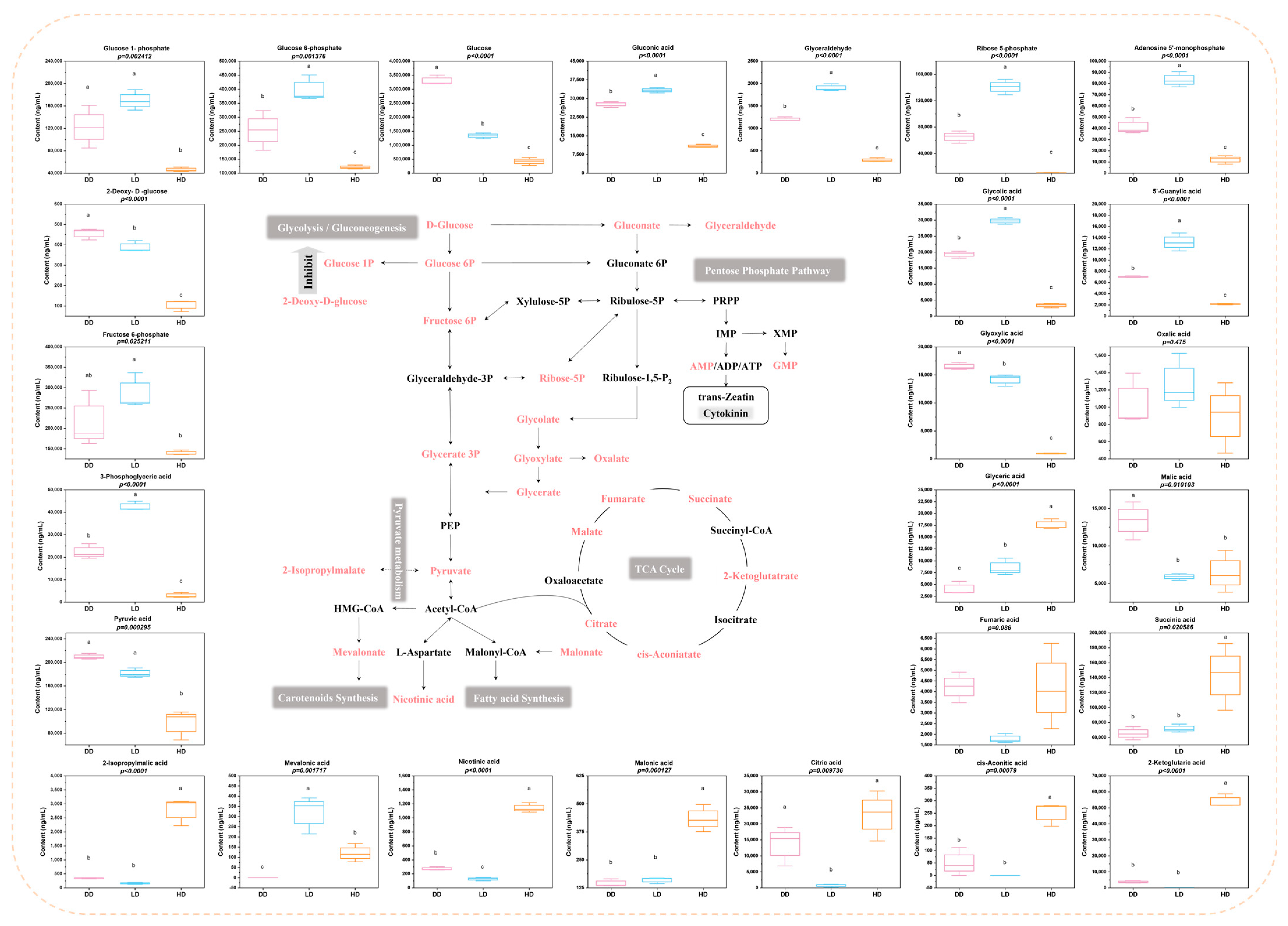
3.4. Metabolites Involved in Central Carbon Metabolism under Different Seed Cultivation Conditions
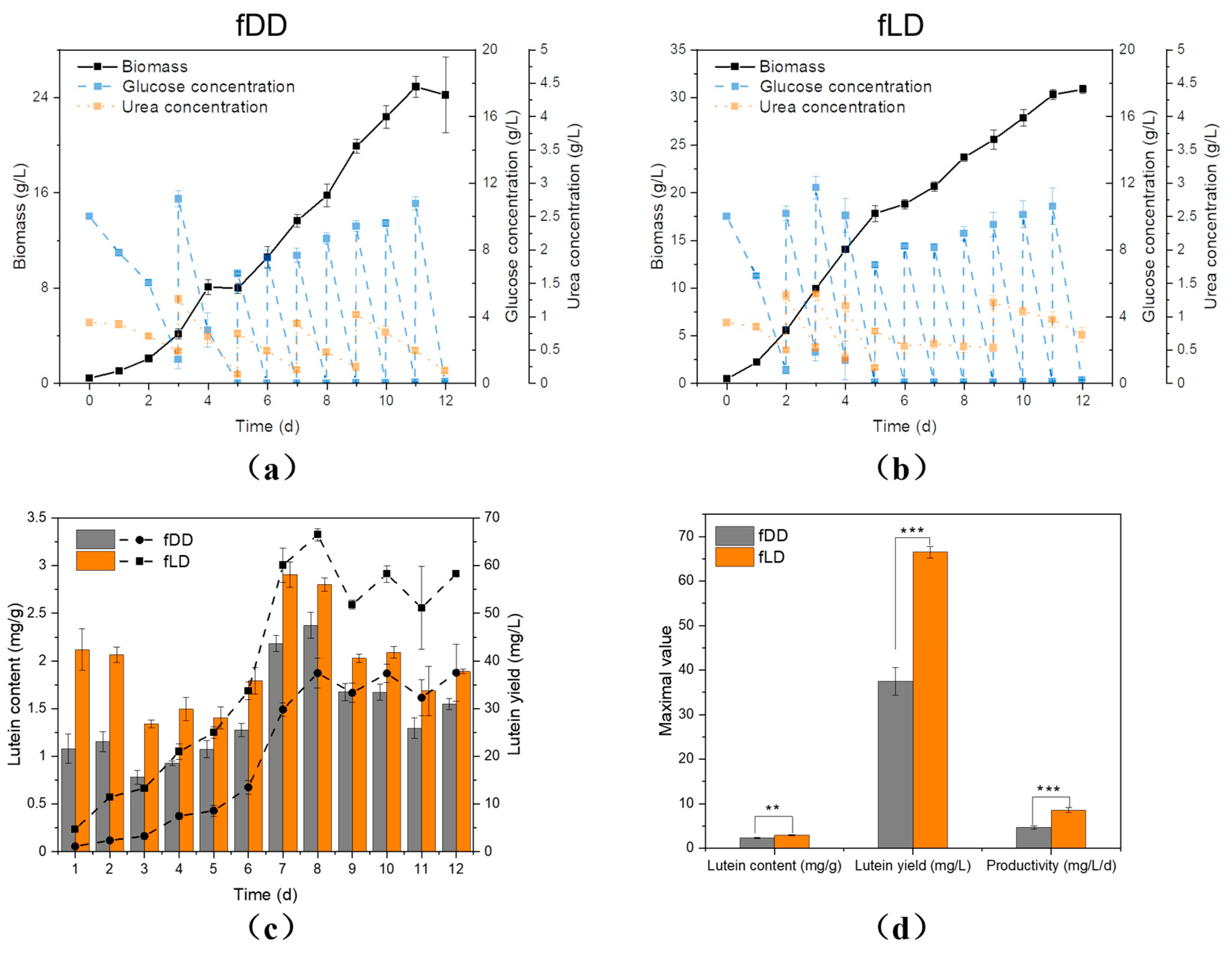
3.5. Fed Batch Cultivation of C. protothecoides CS-41 with Seed Cultivation under Low Light
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein production from microalgae: A review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-S.; Lee, D.-J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016, 36, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Research, M.M. Lutein Market–Global Industry Analysis and Forecast (2022–2029); Maximize Market Research PVT. LTD.: Chicago, IL, USA, 2022. [Google Scholar]
- Zhao, W.; Sun, H.; Ren, Y.; Wu, T.; He, Y.; Chen, F. Chlorella zofingiensis as a promising strain in wastewater treatment. Bioresour. Technol. 2018, 268, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Gu, Z.; Mou, H.; Sun, H. Stimulating carbon and nitrogen metabolism of Chlorella pyrenoidosa to treat aquaculture wastewater and produce high-quality protein in plate photobioreactors. Sci. Total Environ. 2023, 878, 163061. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Yang, S.; Fan, Y.; Cao, Y.; Wang, Y.; Mou, H.; Sun, H. Technological readiness of commercial microalgae species for foods. Crit. Rev. Food Sci. 2023, 1–25. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. 2022, 1–17. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, J.; Yang, T.; Xiong, P.; Zheng, X.; Lu, Y.; Jing, K. Exploring engineering reduced graphene oxide-titanium dioxide (RGO-TiO2) nanoparticles treatment to effectively enhance lutein biosynthesis with Chlorella sorokiniana F31 under different light intensity. Bioresour. Technol. 2022, 348, 126816. [Google Scholar] [CrossRef]
- Heo, J.; Shin, D.-S.; Cho, K.; Cho, D.-H.; Lee, Y.J.; Kim, H.-S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Vadrale, A.P.; Dong, C.-D.; Haldar, D.; Wu, C.-H.; Chen, C.-W.; Singhania, R.R.; Patel, A.K. Bioprocess development to enhance biomass and lutein production from Chlorella sorokiniana Kh12. Bioresour. Technol. 2023, 370, 128583. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Z.; Ho, S.-H.; Ruan, C.; Li, J.; Xie, Y.; Shi, X.; Liu, L.; Chen, J. Two-stage bioprocess for hyper-production of lutein from microalga Chlorella sorokiniana FZU60: Effects of temperature, light intensity, and operation strategies. Algal Res. 2020, 52, 102119. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Ma, R.; Liu, X.; Miao, M.; Ho, S.-H.; Chen, J.; Leong, Y.K.; Chang, J.-S. High-cell-density heterotrophic cultivation of microalga Chlorella sorokiniana FZU60 for achieving ultra-high lutein production efficiency. Bioresour. Technol. 2022, 365, 128130. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chen, C.-Y.; Chang, J.-S. Lutein production with wild-type and mutant strains of Chlorella sorokiniana MB-1 under mixotrophic growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 66–73. [Google Scholar] [CrossRef]
- Shi, X.-M.; Liu, H.-J.; Zhang, X.-W.; Chen, F. Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem. 1999, 34, 341–347. [Google Scholar] [CrossRef]
- Shi, X.-M.; Zhang, X.-W.; Chen, F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym. Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Miranda, L.; Cañizares-Villanueva, R.O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Flores-Ortíz, C.M. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74. [Google Scholar] [CrossRef]
- Gu, N.; Lin, Q.; Li, G.; Qin, G.; Lin, J.; Huang, L. Effect of salinity change on biomass and biochemical composition of Nannochloropsis oculata. J. World Aquacult. Soc. 2012, 43, 97–106. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.; Hiltunen, E. Strategies for lipid production improvement in microalgae as a biodiesel feedstock. BioMed Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef]
- Sun, H.; Mao, X.; Wu, T.; Ren, Y.; Chen, F.; Liu, B. Novel insight of carotenoid and lipid biosynthesis and their roles in storage carbon metabolism in Chlamydomonas reinhardtii. Bioresour. Technol. 2018, 263, 450–457. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Lao, Y.; Li, X.; Chen, F. A novel fed-batch strategy enhances lipid and astaxanthin productivity without compromising biomass of Chromochloris zofingiensis. Bioresour. Technol. 2020, 308, 123306. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wang, Y.; Yang, S.; Wang, J.; Wu, T.; Lu, X.; Chu, Y.; Chen, F. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresour. Technol. 2022, 347, 126401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Gu, Z.; Yang, S.; He, Y.; Mou, H.; Sun, H. Altering autotrophic carbon metabolism of Nitzschia closterium to mixotrophic mode for high-value product improvement. Bioresour. Technol. 2023, 371, 128596. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Yi, L.; Yang, S.; Lu, X.; Liu, B.; Chen, F.; Huang, J.; Cheng, K.; Sun, H.; Wu, X. Light Induction of Seed Culture Accelerates Lutein Accumulation in Heterotrophic Fermentation of Chlorella protothecoides CS-41. Fermentation 2023, 9, 768. https://doi.org/10.3390/fermentation9080768
Fu Y, Yi L, Yang S, Lu X, Liu B, Chen F, Huang J, Cheng K, Sun H, Wu X. Light Induction of Seed Culture Accelerates Lutein Accumulation in Heterotrophic Fermentation of Chlorella protothecoides CS-41. Fermentation. 2023; 9(8):768. https://doi.org/10.3390/fermentation9080768
Chicago/Turabian StyleFu, Yunlei, Lanbo Yi, Shufang Yang, Xue Lu, Bin Liu, Feng Chen, Junchao Huang, Kawing Cheng, Han Sun, and Xiaolei Wu. 2023. "Light Induction of Seed Culture Accelerates Lutein Accumulation in Heterotrophic Fermentation of Chlorella protothecoides CS-41" Fermentation 9, no. 8: 768. https://doi.org/10.3390/fermentation9080768
APA StyleFu, Y., Yi, L., Yang, S., Lu, X., Liu, B., Chen, F., Huang, J., Cheng, K., Sun, H., & Wu, X. (2023). Light Induction of Seed Culture Accelerates Lutein Accumulation in Heterotrophic Fermentation of Chlorella protothecoides CS-41. Fermentation, 9(8), 768. https://doi.org/10.3390/fermentation9080768








