Abstract
In this study, we aimed to isolate and phenotypically characterize non-Saccharomyces yeast strains (NSYSs) from the skin of aromatic (Italy) and non-aromatic (Negra Criolla) grapes from vineyards in Moquegua, Peru, typically used for the production of pisco. Our second objective was to characterize the volatile compounds and sensory attributes of pisco made from these grapes. Pichia terricola (56%), Metschnikowia pulcherrima (31%), and Naganishia vaughanmartiniae (13%) were the main NSYSs isolated from the skin of aromatic Italy grapes and identified; meanwhile, Vishniacozyma carnescens (50%), Vishniacozyma heimaeyensis (30%), and Aureobasidium pullulans (20%) were identified on the skin of the non-aromatic Negra Criolla grapes. These NSYSs showed different capacities in terms of carbohydrate fermentation, polygalacturonase activity, ethanol tolerance, sulphite production, and nitrogen consumption. Moreover, the pisco resulting from these varieties of grape had different volatile profiles. Terpene alcohols such as citronellol, geraniol, linalool, and nerol were found in pisco made from Italy grapes, while higher contents of 2-phenylacetate and ethyl esters were found in Negra Criolla Pisco. Intermediate levels of both 1-hexanol and bencyl alcohol were also found in all the pisco. Sensory analysis performed by a trained pisco tasting panel showed that citric, floral, alcohol, and syrup descriptors were more marked in Italy Pisco, while nuts, syrup, alcohol, and floral were the most intense attributes of Negra Criolla Pisco. These results will contribute to determining the potential of indigenous grape yeasts from the Moquegua region as fermentation starters to improve the typical sensory qualities of the pisco produced in this region, which deserves further study.
1. Introduction
Pisco is a spirit that is typically not aged and is in high demand both domestically within Peru and internationally. It is obtained by distilling wine made from different varieties of aromatic (Albilla, Italy, Moscatel, and Torontel) and non-aromatic (Mollar, Negra Criolla, Quebranta, and Uvina) grapes, produced in certain areas along the Peruvian coast [1,2]. Grape variety has a large influence on the sensory properties of the resultant pisco, such as its characteristic fruity and flowery descriptors, including banana and passion fruit, as well as chocolate and black raisins [1,2].
In addition to the quality and characteristics of the grape variety, the sensory attributes of pisco also depend on the vitivinicola technique adopted and distillation process [2]. Moreover, many factors such as weather, soil characteristics, and geographical location [2,3,4] influence the wild microbiota involved in the spontaneous fermentation of the grapes [5,6]. Wine chemistry is the result of the contribution of the entire microbiota, including selected Saccharomyces cerevisiae [5,7] and non-Saccharomyces yeast strains (NSYSs) that are generally present at the beginning of the fermentation process and contribute to the aromatic profile [8]. Wine with different aromatic characteristics has thus been produced by simultaneous or sequential inoculation of specific NSYSs isolated from grapes from different regions [9]. Several NSYSs involved in wine aromas have been isolated from the skin of grapes, including Torulaspora delbrueckii, Metschnikowia pulcherrima, Pichia kluyveri, Lachancea thermotolerans, Schizosaccharomyces pombe, Candida railenensis, Hanseniasporas genus, and Debaryomyces hansenii, among others [10,11,12]. However, the particular characteristics of NSYS microbiota depend on many factors such as the weather, soil characteristics, and geographical location mentioned above, as well as the grape variety, among others [13]. In fact, the same grape variety growing in different regions or vineyards might produce wine with highly distinct sensory characteristics [14,15].
Few studies have addressed the identification of the grape microbiota typically involved in making pisco. Therefore, the aims of the present study were, on the one hand, to isolate and characterize phenotypically NSYSs from the skin of aromatic Italy (I) [1] and non-aromatic Negra Criolla (NC) [2] grapes collected systematically from vineyards in the Moquegua region of Peru. This is one of the five regions with a protected designation of origin for pisco. On the other hand, our goal was to characterize the volatile compounds and sensory attributes of pisco elaborated industrially using the mentioned varieties of grape. To this end, we engaged a trained national pisco tasting panel. Our study is focused on strategies for improving the sensory properties of pisco using isolated NSYSs as starter cultures in the fermentation process.
2. Material and Methods
2.1. Samples
Grape samples were collected from three zones located in the Moquegua Valley and included in the protected designation of origin for pisco (Figure S1). Eighteen samples of the Italy (I) variety grapes and 18 samples of the Negra Criolla (NC) variety grapes were aseptically transferred to sterile 100 mL plastic containers and stored at −20 °C until analysis. Twenty kilograms of I and NC grapes were collected for the production of pisco by replicates.
2.2. Non-Saccharomyces Yeast from Italy and Negra Criolla Grape Varieties
2.2.1. Isolation and Culture
The frozen grape samples were thawed at 25 °C for 30 min and a total of 10 g of each grape variety (I and NC) was randomly selected and put in a sterile jar by triplicates. Then, 90 mL of potato dextrose broth (Laboratorio Conda, Madrid, Spain) was added to each jar and the jars were incubated at 25 °C while being rotated at 130 rpm for 24 h (Sanyo Incubator–Model Mir 154) to promote the growth of the microbiota from the surface of the grapes. A sample was taken from each jar with a seeding loop and yeast isolation was carried out on plates of Sabouraud chloramphenicol dextrose (SCD) agar and dichloran rose bengal chloramphenicol (DRBC) agar (Laboratorio Conda, Madrid–Spain) at 25 °C for 72 h. From these plates, 34 colonies (24 from I and 10 from NC varieties) were randomly selected based on color and morphological differences, and re-isolated on SDC agar at 25 °C for 72 h. An isolated colony was then taken from each of the plates and inoculated in sterile 25 mL tubes containing potato dextrose broth and incubated at 25 °C while being rotated at 150 rpm for 96 h. Finally, 10 mL of the incubation broth was collected and frozen at −20 °C for 24 h to cause physical lysis of cell membranes and facilitate DNA extraction from the isolated yeast.
2.2.2. DNA Extraction, Amplification, and Sequencing
DNA was extracted from isolated yeast strains (24 from I and 10 from NC varieties) using DNA extraction kit number 78870 (Thermo Scientific, Hanover Park, IL, USA) following the manufacturer’s instructions. Yeast cells suspended in potato dextrose broth agar medium (10 mL) were centrifuged at 5 min at 5000× g at room temperature. The pellet obtained (~70 mg) was resuspended in 560 µL of yeast protein extraction reagent and the mixture was incubated at 65 °C for 10 min, then centrifuged at 13,000× g for 5 min. The supernatant was discarded and 400 µL of DNA releasing reagents A and B was added to the pellet. The mixture was homogenized and then incubated at 65 °C for 10 min. Then, 200 μL of protein removal reagent was added and the mixture was agitated then centrifuged at 13,000× g for 5 min. The supernatant was transferred to new sterile 1.5 mL tubes. DNA precipitation was induced by the addition of 600 μL of isopropyl alcohol, mixing by inversion, and centrifuged at 13,000× g for 10 min. The supernatant was then eliminated by aspiration and the extracted DNA was washed via the addition of 1.5 mL of 70% v/v ethanol to the pellet and centrifuged at 13,000× g for 1 min. The pellet containing DNA was dried in a vacuum centrifuge for 5 min (Speed Vac–SPD 111V, Savant Instruments from Thermo Scientific, Midland, MI, USA) and then it was resuspended in 50 µL of buffered peptone water (PanReac Química S.L.U., Barcelona, Spain) by homogenization for 5 min. The concentration of DNA at 260 nm and its purity according to the ratio A260nm/A280nm were measured using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The suspension containing the DNA was stored at −20 °C until use.
Ribosomal internal transcribed spacers (ITSs) in the DNA were amplified by polymerase chain reaction (PCR) using the ITS1-F (5′TCCGTAGGTGAACCTGCGG) and ITS4-B (5′-TCCTCCGTCTATTGATATGC) primers, which are specific for fungi and basidiomycetes [16]. The PCR conditions were: 96 °C for 150 s, followed by 40 cycles at 60 °C for 1 min, then 72 °C for 2 min, and a final extension at 72 °C for 1 min. The PCR was performed in duplicate in a volume of 20 μL with commercial PerfeCTa SYBR Green FastMix Low ROX concentrate (Quanta Biosciences Laboratory Cat. No. 95074-012), 2× concentrate containing the necessary components, with the exception of the primer whose concentration was 0.5 µM. The amplified DNA was purified using an ExoCleanUp kit (VWE-Life Science, Flanders, Belgium) according to the manufacturer’s instruction. The DNA concentration and purity were measured using the Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
The DNA was sequenced using 100–150 ng DNA (500 and 1000 bp) and the same primers (5 pmol) together with a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Waltham, MA, USA) in a 3730xl DNA Analyzer (Applied Biosystems, Waltham, MA, USA). Prior to the DNA sequencing, the reaction products were purified to eliminate dideoxynucleotide triphosphates (ddNTPS) and free primers using Optima DTR 96-well plates (EdgeBio, San Jose, CA, USA). The sequence similarities were obtained using the Basic Local Alignment Search Tool (BLAST) from the National Center for Biotechnology Information, U.S. National Library of Medicine (http://www.ncbi.nlm.nih.gov/blast/, accessed on 20 April 2022). Identification was considered correct when the identity of gene sequences was 98% or higher.
2.2.3. Phenotypic Characterization
Carbohydrate Fermentation
The isolated and identified NSYSs were analyzed for their capacity to ferment different carbohydrates such as fructose, melibiose, saccharose, glucose, maltose, mannose, raffinose, and lactose [17]. A cell loop was inoculated into test tubes containing sterile culture medium by filtration: reducing sugar (5–10 g), meat extract (1 g), peptone (10 g), sodium chloride (5 g), and bromocresol purple (0.015 g) into 1 L of distilled water at pH 6.6. A sterile inverted Durham tube was immersed in each test tube. Each substrate tested was considered to give a positive fermentation result for the samples, which produced gas and induced acid formation as shown by changes in the color of the indicator. The tests were performed in duplicate and readings were taken after 48 h.
Polygalacturonase Activity
Prior to screening for polygalacturonase activity, the isolated and identified NSYSs were grown for 24 h at 30 °C in yeast malt (YM) broth with shaking at 200 rpm (for yeast) supplemented with 1.5% w/v citrus pectin (Spectrum Chemical, Gardena, CA, USA) to induce the production of pectinolytic enzymes [18]. A total of 5 µL of each yeast, constituting approximately 5 × 106 cells, was inoculated into the wells of the diffusion assay plate containing YM agar supplemented with pectin. This agar without an inoculate was used as a negative control for the assay. The diffusion assay plates were incubated overnight at 30 °C, and the diameters of the clear circular zones were measured to evaluate the capacity to hydrolyze polygalacturonic acid.
Ethanol Tolerance
Ethanol tolerance analysis was conducted in YPD broth (glucose 20 g/L, peptone 10 g/L, yeast extract 5 g/L, NaCl 0.5 g/L, pH 5.6) with specific quantities of ethanol (6%, 12%, and 16% v/v) [19]. The isolated and identified NSYS cell loops were inoculated in the YPD broth and incubated at 30 °C; growth was qualitatively monitored by turbidimetry at 600 nm in a Genesys 10S spectrophotometer (Thermo Scientific, Madison, WI, USA) [20].
Sulphite Production
A colorimetric assay based on malachite green with some modifications was used to measure sulphite production [21]. The isolated and identified NSYS cell loops were inoculated in the carbohydrate solution (5 mL) and I or NC grape must (5 mL) by filtration and incubated for 48 h under anaerobiosis. Then, 0.5 mL of malachite green solution (25%) was added to the solutions and mixed for 2 min; absorbance was measured at 560 nm using the Genesys 10S spectrophotometer (Thermo Scientific, Madison, WI, USA). The sulphite produced was estimated from a calibration curve for sodium sulphite (20–100 mg/L; Y = −0.0029X + 0.304, R2 = 0.995).
Nitrogen Consumption
The nitrogen consumed by the isolated and identified NSYS was measured in terms of ammonium using the Nessler assay [22]. The isolated yeasts were inoculated in YPD broth (yeast extract 1% p/v, peptone 2% p/v, glucose 2% p/v) and incubated at 28 °C for 48 h while being rotated at 120 rpm. Five-hundred microliters of aliquot was centrifuged at 10,000× g for 5 min and 250 µL was transferred to a 96-well plate and mixed with 10 µL of Nessler’s reagent (0.66 g NaI, 1 g HgI2 and 0.42 g KCl dissolved in 100 mL of 3M NaOH) and incubated until it developed a yellow-to-brown color, which was measured at 405 nm in the Genesys 10S spectrophotometer (Thermo Scientific, Madison, WI, USA). A standard curve for ammonia chloride (0.1–400 mg/L; Y = 0.001X + 0.0156; R2 = 0.9982) was used to estimate ammonia content. Nitrogen consumption was estimated as a percentage from the ammonium concentration in the inoculated medium and pure medium.
2.2.4. Pisco from Italy and Negra Criolla Grape Varieties
Production
Italy or Negra Criolla grape varieties were destemmed and crushed by hand and the must obtained was macerated for 24 h. Then, the grape pomace was eliminated and the must was transferred into glass containers and fermented at 22 ± 2 °C for 12 days. The must was then distilled in a 20 L capacity copper still, the head was cut at 0.8% of total volume of the must, and the pisco was cut using 42% final alcohol grade. The pisco obtained was transferred to a glass container for analysis of volatile compounds and for sensory assessment.
Analysis of Volatile Compounds
The volatile compounds in the pisco obtained from I and NC grape varieties were analyzed by gas chromatography–mass spectrometry (GC-MS) using the procedure described by Cacho et al. (2012) [1] with some modifications. To extract the volatile compounds from the pisco, solid phase extraction (SPE) was performed in a VacMaster 10 (Biotage, Charlotte, NC, USA) using LiChrolut EN cartridges, which were initially conditioned with dichloromethane (4 mL), methanol (4 mL), and ethanol 12% v/v (4 mL). The pisco was diluted with Milli-Q water to obtain samples containing 12% ethanol v/v and β-damascenone was added as an internal standard (200 mg/L). Then, each sample was passed through a conditioned LiChrolut EN cartridge, which together with the retained compounds was dried under vacuum (−0.6 bar for 20 min) and eluted with 1.6 mL of dichloromethane. The eluted sample was mixed with the internal standards 4-hydroxy-4-methyl-2-pentanone and 2-octanol dissolved in dichloromethane (400 mg/L). One microliter of the aromatic pisco extract was injected using a CTC autosampler in a Bruker 436 GC coupled to a Bruker EVOQ GC-TQ MS detector (Bruker, Billerica, MA, USA). The injector temperature was maintained at 250 °C. The chromatographic oven was held at 40 °C for 5 min and then raised to 136 °C at 4 °C/min and 250 °C at 6 °C/min. The carrier gas was helium at a constant flow of 1 mL/min. The capillary column used was a Restek Rxi-5Sil MS (30 m × 0.25 mm i.d. × 0.25 μm film thickness) (Restek, Bellefonte, PA, USA). The MS parameters were: MS transfer line at 250 °C and chamber ionization temperature 200 °C with an electron energy of 70 eV. A 35–350 m/z range was recorded in full scan mode. Volatile compounds were identified by comparing the retention times and mass spectra of reference compounds injected under the same chromatographic conditions with those available in the MS library (NIST 2.0). Standard curves of volatile compounds were prepared in dichloromethane and injected under the same conditions to carry out the quantitative analysis (Table S1).
Sensory Analysis
Sensory analysis of the pisco was performed according to the procedure described by Rabitti et al. (2022) [23] with an experienced trained tasting panel formed of 10 individuals (30–50 years old: 4 women and 6 men) from the Asociación Nacional de Catadores Oficiales- Pisco. The panelists were asked to avoid smoking, eating, and drinking anything for at least one hour before the sensory analysis. They were informed about the nature of the analysis and all of them gave their written consent to participate.
The sensory analysis consisted of two phases. In the first phase, the panel selected 12 aromatic descriptors from a list of 23 descriptors: chemical, fruit, citric, aniseed, floral, roses, herbs, spicy, oily, sulphurous, vinegar, empyreumatic, caramel, butter, syrup, alcohol, cheese, onion, phenolic, olive, nuts, vegetables, and cooked vegetables. In the second phase, the panel identified and evaluated the intensity of the descriptors in a pisco sample. To do this, each panelist received 25 mL of Italy and Negra Criolla piscos in AFNOR cups, which were coded with three-digit numbers and covered with a Petri dish to avoid loss of volatile substances. The intensity of each attribute was evaluated in duplicate, using a five-point hedonic scale under white light and the samples were served at 22 °C in a random order over two sessions for each pisco. Between each sensory analysis, the panelists rinsed their mouths using mineral water and ate crackers to avoid carry-over effects.
2.2.5. Statistical Analysis
ANOVA, Tukey’s comparison test, and linear regression analysis were carried out using Prism GraphPad software version 6.1 (GraphPad Software Inc., San Diego, CA, USA) and principal component analysis of the volatile compounds and sensory analysis were performed using Minitab 17 software (Minitab Inc., Bellefonte, PA, USA).
3. Results and Discussion
3.1. Isolation and Molecular Identification of Yeast Strains from the Skin Surface of Italy and Negra Criolla Grapes
SCD and DRBC media were used to isolate yeast from the skin surface of the I and NC grape varieties collected from Moquegua, a region in the south of Peru, based on color and morphological characteristics. The identification was further confirmed by molecular procedures. The abundance and type of the yeasts isolated depended on the grape variety. Different NSYSs were found in the I and NC grape varieties. Pichia terricola (56%), Metschnikowia pulcherrima (31%), and Naganishia vaughanmartiniae (13%) all with Per Ident = 100% were isolated and identified from I grapes (Table 1 and Figure 1A). From NC grapes, a variety which is typical in the Moquegua region, three NSYSs, namely Vishniacozyma carnescens (50%), Vishniacozyma heimaeyensis (30%), and Aureobasidium pullulans (20%) with Per Ident = 99.81%, 98.48%, and 99.49%, respectively, were the most abundant (Table 1 and Figure 1A). NSYSs are generally present at the beginning of wine fermentation and contribute to the wine aroma through direct biosynthesis of volatile aromatic compounds including terpenoids, esters, and acetaldehyde, among others, or through an indirect effect that releases volatile compounds from grape glucosidic precursors [24]. Thus, wines with different aromatic characteristics can be produced by simultaneous or sequential inoculation of NSYSs isolated from grapes from different regions [8]. Different NSYSs isolated from grape skin and implicated in the aromatic profile of wines might include Torulaspora delbrueckii, Metschnikowia pulcherrima, Pichia kluyveri, Lachancea thermotolerans, Schizosaccharomyces pombe, Candida railenensis, Hanseniasporas genus, and Debaryomyces hansenii, among others [10,11,12,25]. Some enzymatic activities found in the NSYSs, such as β-glucosidase, β-xylosidase, proteolytic, and xylanolytic activities, have also been associated with the aromatic composition of wines [26]. In general, grape skin microbiota is dependent on the grape variety, geographic region, climate, vineyard location, and topography, which explains the different types and abundances of NSYSs found in I and NC grape varieties [5,7,27]. In the present study, Metschnikowia pulcherrima was isolated from I grape skins from the Moquegua region (Table 1 and Figure 1A). M. pulcherrima and native S. cerevisiae have been used as biocontrol agents and wine aroma enhancers (ethyl butyrate, ethyl hexanoate, isoamyl acetate, and β-phenyl ethanol) of wines with a low SO2 content [28]. Metschnikowia spp. have been associated with the formation of terpenes, volatile thiols, higher alcohols, and esters; but with a low content of volatile phenols [8]. Moreover, Pichia genus co-inoculated with S. cerevisiae enhanced the aromatic attributes of wines [29] and P. terricola, formerly Issatchenkia terricola, has been shown to increase the volatile content in Cabernet Sauvignon wine, which has been associated with its β-glucosidase activity [30]. Meanwhile, Naganishia genus appears as the most widespread yeast genus within the core microbiome of vineyard soils worldwide [31]; however, to the best of our knowledge, this is the first time that it has been identified on the skins of I grapes (Table 1 and Figure 1A). Naganishia genus has showed β-glucosidase activity [30] and therefore it could play a role in mediating the volatile compounds in wines. In relation to Negra Criolla grapes, Vishniacozyma genus was found to be the most predominant yeast strain (80%). This yeast has been reported to be one of the major yeasts (representing around 69%) in Vidal grapes used in ice wine fermentation processes [32]. Meanwhile, Vishniacozyma victoriae has been recognized as being of great interest due to its biological control activity against some pathogens of table grapes [33]. Aureobasidium pullulans was also isolated from NC grapes. This yeast showed very high pectinase activity (Figure 1). A. pullulans is a well-known yeast strain with high pectinase activity that had previously been isolated from Argentine Bonarda grapes from the Mendoza region [34]. Finally, in the present study, Saccharomyces cerevisiae was not isolated from either of the two grape varieties examined, which could be associated with the youth of the Moquegua vineyard from which they were collected, thereby highlighting the importance of vine age for yeast colonization [35].

Table 1.
Molecular identification of non-Saccharomyces yeast strains isolated from Italy and Negra Criolla grape varieties.
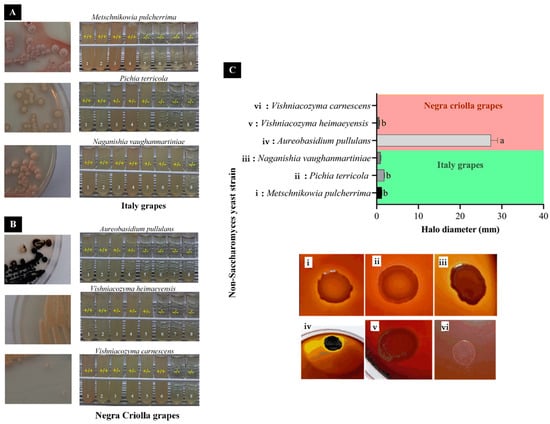
Figure 1.
Carbohydrate fermentation (A,B) and polygalacturonase activity (C) of isolated non-Saccharomyces yeast strains from Italy (Metschnikowia pulcherrima, Pichia terricola, and Naganishia vaughanmartiniae) and Negra Criolla (Aureobasidium pullulans, Vishniacozyma heimaeyensis, and Vishniacozyma carnescens) grapes from the Moquegua region. +/+ = change in color of bromocresol indicator/gas production, +/− = change in color of bromocresol indicator/no gas production, and −/− = no change in color of bromocresol indicator/no gas production. 1: fructose, 2: melibiose, 3: saccharose, 4: glucose, 5: maltose, 6: mannose, 7: raffinose, and 8: lactose. Different letter in each column means statistical difference using Tukey test (p < 0.05).
3.2. Phenotypic Characterization of NSYSs Isolated from Italy and Negra Criolla Grape Varieties
3.2.1. Carbohydrate Fermentation
All the NSYSs isolated from Italy grapes were able to metabolize fructose and melibiose, reducing the pH and increasing gas production (Figure 1A). M. pulcherrima and N. vaughanmartiniae metabolized saccharose and glucose, which is reflected by pH changes and gas production, whereas P. terricola only produced a decrease of pH (Figure 1A). Yeasts are physiologically classified with respect to the type of energy-generating process involved in their sugar metabolism, namely non-, facultative- or obligate fermentative yeasts [36]. Regarding this sugar metabolism, M. pulcherrima has been characterized as a strain that follows a respiratory metabolic pathway with slower fermentation yields; therefore, it can be used to reduce the final ethanol concentration in wine [37]. A respiratory metabolic pathway involves tricarboxylic acid (TCA), which can produce different acids that in turn reduce the pH [38], as shown in the present study (Figure 1A,B). Additionally, P. terricola, formerly Issatchenkia terricola [30], has been described as a NSYS with β-glucosidase activity that is tolerant to glucose and ethanol with the potential to rapidly release aromatic compounds in both white and red wine [39]. Similar to M. pulcherrima, P. terricola metabolizes glucose and fructose via TCA, thereby acidifying the medium (Figure 1A) but without gas production. P. terricola and N. vaughanmartiniae also produced pH changes in the presence of maltose (Figure 1A). Interestingly, N. vaughanmartiniae changed the pH when mannose and raffinose were added to the culture medium (Figure 1A); although this yeast strain has been isolated from plant leaves and soil, its enological contribution has not yet been characterized [40]. None of the NSYSs metabolized lactose, since no pH change or gas production was observed (Figure 1A).
All the NSYSs isolated from NC grapes were able to metabolize fructose and melibiose, as reflected in pH decreases (increases in the level of acid) and gas production (Figure 1B). Aureobasidium pullulans, also called black yeast, is an ascomycetous dimorphic fungus: a common yeast-like species. It has been isolated from different grape cultivars that have suffered physical damage [41] and can be used as an effective biocontrol agent in agriculture against several of the major post-harvest pathogens [42]. However, A. pullulans has been recognized as a major grape-skin resident that in co-culture with S. cerevisiae can accelerate alcoholic fermentation [43]. A plausible explanation of this is the polygalacturonase activity observed in A. pullulans [44], which could increase carbon availability from grape-skin materials such as the cell wall and cuticles [43], thereby improving the color and reducing filtration time and turbidity [34]. The enzymatic activity of A. pullulans was very high, compared to other NSYSs isolated from I and NC grape varieties, including M. pulcherrima (Figure 1C). In addition, A. pullulans can hydrolyze grape glycosides and displays β-D-glucosidase, α-L-arabinofuranosidase, and α-L-rhamnosidase activities [45]. In contrast, Vishniacozyma carnescens is a yeast associated with wild grapes and used as a promising biocontrol agent against fungal grape pathogens [46]. This yeast has been found in withered grape berries of the Nosiola, Corvina and Garganega varieties [47], and also in Cabernet Sauvignon berries [48]. However, the technological relevance of V. carnescens for winemaking has not been reported and deserves further research, taking into account that it represents about 50% of the NSYSs isolated from I and NC grape varieties. Similarly, V. heimaeyensis has been found in the microbiota of grape berries from cv. Vinhão and Loureiro grapes, although its technological relevance has not been reported [49]. Moreover, A. pullulans, V. carnescens, and V. heimaeyensis were also able to metabolize saccharose and glucose, reducing the pH but without gas production (Figure 1B). Both these yeasts were also able to metabolize maltose, similarly reducing the pH but without gas production (Figure 1B). In contrast, V. heimaeyensis metabolized mannose, producing a pH decrease and gas production (Figure 1B). None of the NSYSs metabolized raffinose or lactose (Figure 1A).
3.2.2. Polygalacturonase Activity
A. pullulans isolated from NC grapes showed much higher polygalacturonase activity (PGA) than the other NSYSs we isolated from Moquegua grapes, except for M. pulcherrima, which had a similar PGA (Figure 1C). In a previous study, Aureobasidium pullulans, Metschnikowia pulcherrima, and Metschnikowia fructicola showed PGA, which can affect filterability and turbidity and increase the color intensity, and the anthocyanin and polyphenol contents of wines when fermented in combination with S. cerevisiae [44]. In particular, it has been demonstrated that during pre-fermentative cold maceration A. pullulans influences the red quality, thereby improving color and the production of desirable volatile compounds, such as esters and norisoprenoids, which have been associated with pectinolytic activity [34,50].
3.2.3. Ethanol Tolerance
All the NSYSs isolated from I and NC grapes showed medium to low growth rates when at 8–12% v/v ethanol (Table 2). The ethanol tolerance of M. pulcherrima depends on the strain and it ranged from 6% to 12% v/v [51]. In the present study, this yeast showed a medium growth rate at 16% v/v ethanol. Pichia terricola isolated from I grapes showed a slower growth at 16% v/v ethanol concentration than that reported for Pichia kudriavzevii, which was capable of growing at a similar ethanol concentration [52]. A. pullulans was also capable of growth, although at a slower rate, in a medium containing up to 12% v/v ethanol, which agrees with the fact that most A. pullulans isolated from south Australian vineyards tolerated up to 10% ethanol before showing a marked decrease in viability [53]. Similarly, N. vaughanmartiniae, V. heimaeyensis, and V. carnescens only showed growth at 8 and 10% v/v of ethanol (Table 2). NSYSs generally present low fermentation performances throughout the entire fermentation process due to their weak ethanol tolerance [8,54]. A previous study demonstrated that some NSYSs, such as R. mucilaginosa, D. pseudopolymorphus, and Brettanomyces spp. also showed moderate tolerance of a 14–15% v/v ethanol environment, in which they maintain 40–60% of maximum activity [55]. The different levels of ethanol tolerance among yeasts correlate with their lipid membrane composition, which defines their response to the disrupting action of ethanol [56].

Table 2.
Ethanol tolerance, sulphite production, and nitrogen consumption of non-Saccharomyces yeast strains isolated from Italy and Negra Criolla grape varieties.
3.2.4. Sulphite Production
In general, it is recognized that yeast strains can produce sulphites (SO32−) from sulfate (SO42−) in juice when it is imported into the yeast through a series of enzymatic steps [57,58]. NSYSs isolated from I and NC grapes produced high concentrations of sulphites, ranging from 45.3 to 66.0 mg/L (Table 1), which has not previously been reported. Winemaking yeast strains isolated from aromatic white grapes produce around 20 mg/L of sulphite [57]. In yeast peptone glucose broth, P. terricola, A. pullulans, V. heimaeyensis, and V. carnescens showed higher sulphite production than M. pulcherrima and N. vaughanmartiniae (around 56 mg/L vs. 47 mg/L) (Table 1). However, all the NSYSs showed the same level of sulphite production when grape must (I or NC) was used as the growing medium (Table 1). In practice, winemakers frequently report an increase in fruitiness in red wines fermented without sulphites through the use of NSYSs as bioprotection and an enhancer of the aromatic profile of the wines [59]. For instance, it has been shown that selected strains of M. pulcherrima play a dual role as a biocontroller and wine aroma enhancer [28]. A plausible biocontrol mechanism through which this yeast strain acts could be related to the sulphite production reported in the present study.
3.2.5. Nitrogen Consumption
The NSYSs isolated showed no differences in their nitrogen consumption, which ranged between 90.8 and 95.8% (Table 2) at 28 °C. This is a higher rate than for Saccharomyces cerevisiae yeast which can consume about 66% of assimilable nitrogen expressed as ammonium at 25 °C fermentation [60]. During wine fermentation, NSYSs such as T. delbrueckii, M. pulcherrima, and Metschnikowia fructicola, consumed different nitrogen sources in an order similar to that observed for S. cerevisiae, but not as quickly due to their lower ethanol tolerance [9], which could influence the nitrogen consumption found in the present study. NSYSs preferentially consumed certain nitrogen sources, such as alanine, ammonium, and aspartic acid, among others [9]. They can also consume proline, which cannot be assimilated by S. cerevisiae under anaerobic conditions [6]. The differential uptake of nitrogen among the NSYSs has an impact on the fermentation rate and might also affect the production of volatile compounds; therefore, it influences the efficiency of the process and wine quality [60].
3.3. Aromatic and Sensory Characterization of Pisco Obtained from Italy and Negra Criolla Grape Varieties
3.3.1. Volatile Compounds
Table 3 shows the volatile composition in pisco obtained from I and NC grapes collected from three zones located in the Moquegua Valley (Figure S1). As shown, different types of terpenes, including citronellol (C), geraniol (G), linalool (L), nerol (N), and α-Terpineol (α-T), were identified in pisco from I grapes, which were not detected, except α-T and at a low concentration, in pisco from NC grapes (Table 3). However, the contents of C, G, L, N, and α-T were lower than the values previously found in pisco from I grapes sampled from the Moquegua region (C = 575 µg/L, G = 1451 µg/L, L = 4821 µg/L, and N = 885 µg/L) [1]. This difference in volatile composition could be related to the zone and month of grape collection and differences in the fermentation, distillation, and pisco production process. Nonetheless, our results agree with previous ones which showed that terpenes dominate the volatile profile of piscos produced from the I grape variety. As previously shown, terpenes influence the primary or varietal aroma, and they have an impact on sensory quality despite their very low concentrations [1]. Meanwhile, NC Pisco exhibited a higher concentration of 2-phenylacetate (2-Pa) than I Pisco (Table 3). This compound has a major impact on the sensory properties of pisco; 2-Pa has previously been found in I Pisco at concentrations from 263 to 982 µg/L [1]. Generally, aromatic piscos are characterized by high concentrations of 2-phenylethanol, 2-phenylethyl acetate, and terpenes [1]. Both, I and NC Piscos showed a very high content of 1-hexanol (Table 3) compared to previous commercial piscos produced from I grapes, which was 2.3–3.6 µg/L [1]. It has been shown that 1-hexanol contributes to the floral and green sensory attributes of Peruvian Pisco, although it is at lower concentrations than other distillates [1]. Bencyl alcohol (Ba) was identified for the first time in both I and NC Piscos at low concentrations and phenylethyl alcohol (Pa) was found at very high concentrations in NC Pisco (22.7 mg/L) (Table 3). Ba has also been found in cider and contributes to fruity, floral, and sweet sensory properties [61]. Furthermore, 3-hexen-1-ol (3-H-ol) was also found at much higher concentrations in I Pisco than in NC Pisco (about 30-fold), similar to the levels found in grape distillates [62]. However, the contribution of 3-H-ol to the sensory quality of pisco has not yet been established. In contrast, NC Pisco showed higher contents of ethyl esters (Eb, Ed, Ehx, Ehp, El, Eo, Ep, and Ev) than I Pisco (Table 3). Moreover, the contents of Ehx and Ed found in I Pisco were higher than those reported for commercial I Piscos on the market (Ehx = 36.6 µg/L and Ed = 242 µg/L). Ethyl esters have been found at lower concentrations in other distillates obtained from grape marc [63] and sorghum [64].

Table 3.
Minimum, maximum, mean and standard deviation values for volatile compounds in pisco made from Italia and Negra Criolla grape varieties.
3.3.2. Sensory Analysis
Regarding the sensory descriptors most often associated with pisco, Figure 2 shows that the citric (Ci), floral (Fl), alcohol (Al), and syrup (Sy) descriptors were highly cited for the Italy variety Pisco, while nuts (Un), syrup (Sy), alcohol (Al), and floral (Fl) were highly selected for the Negra Criolla variety Pisco. The Ci sensory descriptor for I Pisco could be associated with its terpene content which, as previously shown (Table 3), includes citronellol (C), geraniol, linalool, nerol, and α-Terpineol [1]. These terms were not associated with NC Pisco, which in previous work has been described as having a neutral aroma with no particular aromatic notes [2]. Nonetheless, in the case of NC Pisco, the 1-hexanol and 2-phenylethyl alcohol content (Table 3) might contribute to the floral and green sensory attributes. In addition, the content of bencyl alcohol found in the piscos (Table 3) could be related to the fruity, floral, and sweet sensory descriptors [61]. The nuts sensory descriptor was markedly found in NC Pisco but it was not found in I Pisco (Figure 3). This is not in agreement with previous work concerning piscos on the market [1], although this sensory descriptor has not been associated with any specific volatile compound. However, the nuts aroma descriptor has been identified in brandy that contains furan compounds such as 2-acetyl-5-methylfuran and 5-methyl-2(3H)-furanone [65]. The alcohol (Al) sensory descriptor has been related with the alcohol content [66]; but in the case of I Pisco, it could be partially masked by the terpene content, which confers a strong aromatic characteristic.
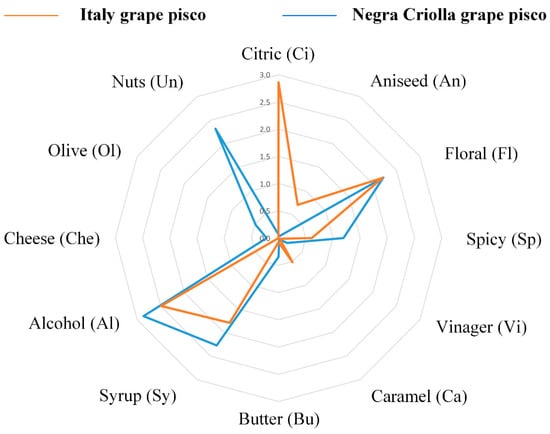
Figure 2.
Sensory analysis of pisco made from Italy and Negra Criolla grape varieties.
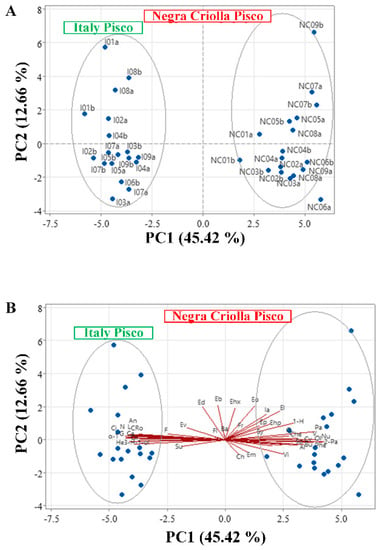
Figure 3.
Principal component analysis of volatile compounds and sensory attributes of the pisco obtained by spontaneous fermentation of Italy and Negra Criolla grape varieties from the Moquegua region. (A) Score plot and (B) Biplot of volatile compounds: 1-hexanol (1−H), 3-hexen-1-ol (3−H −1−ol), 2-phenylethyl acetate (2-Pa), bencyl alcohol (Ba), ethyl butanoate (Eb), citronellol (C), ethyl decanoate (Ed), phenylethyl alcohol (Pa), furfural (F), geraniol (G), ethyl hexanoate (Ehx), ethyl heptanoate (Ehp), isoamyl acetate (Ia), ethyl laurate (ethyl dodecanoate) (El), linalool (L), nerol (N), ethyl octanoate (Eo), ethyl pelargonate (Ep), α-terpineol (α-T), and ethyl valerate (Ev); and sensory attributes: chemical (Ch), fruit (Fr), citric (Ci), aniseed (An), floral (Fl), roses (Ro), herbs (He), spicy (Sp), oily (Oi), sulphurous (Su), vinegar (Vi), empyreumatic (Em), caramel (Ca), butter (Bu), syrup (Sy), alcohol (Al), cheese (Che), onion (O), phenolic (Phe), olive (Ol), nuts (Nu), vegetables (V), and cooked vegetables (Cv).
3.3.3. Principal Component Analysis of Volatile Compounds and Sensory Properties
We performed principal component analysis (PCA) of volatile compound contents and sensory attributes of I and NC Piscos in order to enhance comprehension of the volatile and sensory information by reducing the complexity of the dataset in order to be able to better visualize the correlations between these variables. The PCA score plot showed that I and NC Piscos can be differentiated by their volatile compound contents and sensory attributes via the first two components, which explained more than 58% of the data variability (Figure 3A). The biplot shows that citric (Ci) and rose (Ro) sensory attributes of I Pisco are associated with a high content of geraniol, citronellol, linalool, α-terpineol, and to a lesser degree 3-hexen-1-ol. On the other hand, 2-phenylethyl acetate (2-Pa) content was related with the butter, cooked vegetables, and spicy attributes. Furthermore, the sulphurous (Su), chemical (Ch), empyreumatic (Em), vinegar (Vi), syrup (Sy), onion (O), vegetables (V), cheese (Che), nuts (Nu), phenolic (Phe), and olive (Ol) sensory attributes of the I and NC Piscos were not associated with any of the volatile compounds identified. Finally, the contents of ethyl derivatives were not associated with any of the sensory attributes identified (Figure 3B).
4. Conclusions
Italy and Negra Criolla grapes collected from vineyards in the Moquegua region of Peru showed different non-Saccharomyces yeast strain (NSYS) microbiota. In addition, the NSYSs showed differences in their metabolisms, including carbohydrate fermentation, polygalacturonase activity, ethanol tolerance, sulphite production, and nitrogen consumption. Pisco elaborated from I and NC variety grapes also showed differences in volatile profiles and sensory descriptors.
This work represents an initial approach to the use of NSYSs alone or in combination as starters in sequential fermentation to improve the sensory quality and typicity of Moquegua Pisco, which deserves further study. The NSYSs isolated from I and NC grapes could contribute to the volatile compounds found in the resultant piscos.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9080757/s1, Figure S1: Zones located in the Moquegua Valley from grape samples were collected; Table S1: Standard curves of volatile compounds for gas chromatography analysis.
Author Contributions
Conceptualization, M.Á.P.-B. and L.C.-H.; methodology, C.A.N.-A., C.C., L.C.-H. and M.Á.P.-B.; software, J.M.-H. and L.C.-H.; formal analysis, C.A.N.-A., C.C., J.M.-H., M.S.-J., L.C.-H. and M.Á.P.-B.; investigation, C.A.N.-A., C.C., L.C.-H. and M.Á.P.-B.; resources, J.M.-H., L.C.-H. and M.Á.P.-B.; writing–original draft preparation, C.A.N.-A. and C.C.; writing–review and editing, M.Á.P.-B., L.C.-H., J.M.-H. and M.S.-J.; visualization, C.A.N.-A., C.C. and L.C.-H.; supervision, L.C.-H. and M.Á.P.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Universidad Nacional de Moquegua via the project “New strategies to improve the aromatic quality of piscos produced in the Moquegua region”. Cesar Augusto Napa-Almeyda acknowledges a scholarship from the Concytec-Banco Mundial project (Funds Award Agreement No. 02-2018-FONDECYT-BM), through its executive unit, Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (Fondecyt).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Technical assistance from E. Berciano of the “Unidad de Técnicas Analíticas y Bioanalíticas” (BAT) at CIAL is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cacho, J.; Moncayo, L.; Palma, J.C.; Ferreira, V.; Culleré, L. Characterization of the aromatic profile of the Italia variety of Peruvian pisco by gas chromatography-olfactometry and gas chromatography coupled with flame ionization and mass spectrometry detection systems. Food Res. Int. 2012, 49, 117–125. [Google Scholar] [CrossRef]
- Cacho, J.; Moncayo, L.; Palma, J.C.; Ferreira, V.; Culleré, L. The impact of grape variety on the aromatic chemical composition of non-aromatic Peruvian pisco. Food Res. Int. 2013, 54, 373–381. [Google Scholar] [CrossRef]
- Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.Q.; Gao, X.T.; Lu, H.C.; Peng, W.T.; Chen, W.; Li, S.D.; Li, S.P.; Duan, C.Q.; Wang, J. Influence of attenuated reflected solar radiation from the vineyard floor on volatile compounds in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Res. Int. 2020, 137, 109688. [Google Scholar] [CrossRef] [PubMed]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous yeasts: Emerging trends and challenges in winemaking. Curr. Opin. Food Sci. 2020, 32, 133–143. [Google Scholar] [CrossRef]
- Englezos, V.; Jolly, N.P.; Di Gianvito, P.; Rantsiou, K.; Cocolin, L. Microbial interactions in winemaking: Ecological aspects and effect on wine quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Song, Y.; Zang, X.; Wang, G.; Pei, Y.; Song, Y.; Qin, Y.; Liu, Y. Yeast Diversity during Spontaneous Fermentations and Oenological Characterisation of Indigenous Saccharomyces cerevisiae for Potential as Wine Starter Cultures. Microorganisms 2022, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Comitini, F.; Agarbati, A.; Canonico, L.; Ciani, M. Yeast Interactions and Molecular Mechanisms in Wine Fermentation: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7754. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F.; Zhang, X. Selection of non-Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef]
- Ge, Q.; Guo, C.; Yan, Y.; Sun, X.; Ma, T.; Zhang, J.; Li, C.; Gou, C.; Yue, T.; Yuan, Y. Contribution of non-Saccharomyces yeasts to aroma-active compound production, phenolic composition and sensory profile in Chinese Vidal icewine. Food Biosci. 2022, 46, 101152. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The Sensory Quality Improvement of Citrus Wine through Co-Fermentations with Selected Non-Saccharomyces Yeast Strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, J.; Xiao, J.; Cheng, W.; Zheng, X.; Wang, B.; Shi, X. Microbial community composition on grape surface controlled by geographical factors of different wine regions in Xinjiang, China. Food Res. Int. 2019, 122, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Qiu, S.; Liu, C.; Zhang, L.; Wu, X.; Ma, L.; Li, J. Abiotic factors play important roles in complexity and characterization of aroma precursors in Vidal blanc grape. Food Res. Int. 2022, 162, 112015. [Google Scholar] [CrossRef]
- Keller, M. Managing grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Garrity, G.; Berner, D.; Kreig, N.; Staley, J. Bergey’s Manual of Systematic Bacteriology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, Part B. [Google Scholar]
- Golomb, B.L.; Morales, V.; Jung, A.; Yau, B.; Boundy-Mills, K.L.; Marco, M.L. Effects of pectinolytic yeast on the microbial composition and spoilage of olive fermentations. Food Microbiol. 2013, 33, 97–106. [Google Scholar] [CrossRef]
- De la Torre-González, F.J.; Narváez-Zapata, J.A.; López-y-López, V.E.; Larralde-Corona, C.P. Ethanol tolerance is decreased by fructose in Saccharomyces and non-Saccharomyces yeasts. LWT Food Sci. Technol. 2016, 67, 1–7. [Google Scholar] [CrossRef]
- Sturm, M.E.; Arroyo-Lopez, F.N.; Garrido-Fernandez, A.; Querol, A.; Mercado, L.A.; Ramirez, M.L.; Combina, M. Probabilistic model for the spoilage wine yeast Dekkera bruxellensis as a function of pH, ethanol and free SO2 using time as a dummy variable. Int. J. Food Microbiol. 2014, 170, 83–90. [Google Scholar] [CrossRef]
- Sullivan, J.; Hollingworth, T.; Wekel, M.; Newton, R.; Larose, J. Determination of Sulfite in Food by Flow Injection Analysis. J. Assoc. Off. Anal. Chem. 1986, 69, 542–546. [Google Scholar]
- Fernandez-San Millan, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Rabitti, N.S.; Cattaneo, C.; Appiani, M.; Proserpio, C.; Laureati, M. Describing the Sensory Complexity of Italian Wines: Application of the Rate-All-That-Apply (RATA) Method. Foods 2022, 11, 2417. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Delac Salopek, D.; Horvat, I.; Hranilovic, A.; Plavsa, T.; Radeka, S.; Paskovic, I.; Lukic, I. Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation. Foods 2022, 11, 3088. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, J.; Chen, Y. Characterization of tolerance and multi-enzyme activities in non-Saccharomyces yeasts isolated from Vidal blanc icewine fermentation. J. Food Biochem. 2019, 43, e13027. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, I.; Walker, M.E.; Vallejo-Pascual, M.E.; Naharro-Carrasco, G.; Jiranek, V. Capturing yeast associated with grapes and spontaneous fermentations of the Negro Sauri minority variety from an experimental vineyard near Leon. Sci. Rep. 2021, 11, 3748. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as biocontrol agent and wine aroma enhancer in combination with a native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Scansani, S.; van Wyk, N.; Nader, K.B.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pasch, L.; Pretorius, I.S.; von Wallbrunn, C.; et al. The film-forming Pichia spp. in a winemaker’s toolbox: A simple isolation procedure and their performance in a mixed-culture fermentation of Vitis vinifera L. cv. Gewurztraminer must. Int. J. Food Microbiol. 2022, 365, 109549. [Google Scholar] [CrossRef]
- Bezus, B.; de Ovalle, S.; González-Pombo, P.; Cavalitto, S.; Cavello, I. Production and characterization of a novel cold-active ß-glucosidase and its influence on aromatic precursors of Muscat wine. Food Biosci. 2023, 53, 102572. [Google Scholar] [CrossRef]
- Vicente, J.; Ruiz, J.; Tomasi, S.; de Celis, M.; Ruiz-de-Villa, C.; Gombau, J.; Rozes, N.; Zamora, F.; Santos, A.; Marquina, D.; et al. Impact of rare yeasts in Saccharomyces cerevisiae wine fermentation performance: Population prevalence and growth phenotype of Cyberlindnera fabianii, Kazachstania unispora, and Naganishia globosa. Food Microbiol. 2023, 110, 104189. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Yi, H.; Wang, B.; Xiao, J.; Zhou, X.; Jiankun, X.; Jiang, L.; Shi, X. Microbial community composition and its role in volatile compound formation during the spontaneous fermentation of ice wine made from Vidal grapes. Process Biochem. 2020, 92, 365–377. [Google Scholar] [CrossRef]
- Perpetuini, G.; Rossetti, A.P.; Battistelli, N.; Zulli, C.; Cichelli, A.; Arfelli, G.; Tofalo, R. Impact of vineyard management on grape fungal community and Montepulciano d’Abruzzo wine quality. Food Res. Int. 2022, 158, 111577. [Google Scholar] [CrossRef] [PubMed]
- Merin, M.G.; Morata de Ambrosini, V.I. Kinetic and metabolic behaviour of the pectinolytic strain Aureobasidium pullulans GM-R-22 during pre-fermentative cold maceration and its effect on red wine quality. Int. J. Food Microbiol. 2018, 285, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nadai, C.; Vendramini, C.; Carlot, M.; Andrighetto, C.; Giacomini, A.; Corich, V. Dynamics of Saccharomyces cerevisiae Strains Isolated from Vine Bark in Vineyard: Influence of Plant Age and Strain Presence during Grape must Spontaneous Fermentations. Fermentation 2019, 5, 62. [Google Scholar] [CrossRef]
- Rodrigues, F.; Ludovico, P.; Leao, C. Sugar Metabolism in Yeasts: An Overview of Aerobic and Anaerobic Glucose Catabolism. In Biodiversity and Ecophysiology of Yeasts; The Yeast Handbook; Springer: Berlin/Heidelberg, Germany, 2006; Chapter 6; pp. 101–121. [Google Scholar]
- Castrillo, D.; Blanco, P. Characterization of Indigenous Non-Saccharomyces Yeast Strains with Potential Use in Winemaking. Front. Biosci. 2023, 15, 1. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Shin, H.D.; Du, G.; Liu, L.; Chen, J. Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: Advances and prospects. Biotechnol. Adv. 2015, 33, 830–841. [Google Scholar] [CrossRef]
- James, A.; Yao, T.; Ke, H.; Wang, Y. Microbiota for production of wine with enhanced functional components. Food Sci. Hum. Wellness 2023, 12, 1481–1492. [Google Scholar] [CrossRef]
- Li, A.H.; Yuan, F.X.; Groenewald, M.; Bensch, K.; Yurkov, A.M.; Li, K.; Han, P.J.; Guo, L.D.; Aime, M.C.; Sampaio, J.P.; et al. Diversity and phylogeny of basidiomycetous yeasts from plant leaves and soil: Proposal of two new orders, three new families, eight new genera and one hundred and seven new species. Stud. Mycol. 2020, 96, 17–140. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Onetto, C.A.; Borneman, A.R.; Schmidt, S.A. Investigating the effects of Aureobasidium pullulans on grape juice composition and fermentation. Food Microbiol. 2020, 90, 103451. [Google Scholar] [CrossRef]
- Watanabe, D.; Hashimoto, W. Accelerated Alcoholic Fermentation of Intact Grapes by Saccharomyces Cerevisiae in Symbiosis with Microbial Community Inhabiting Grape-skin. Commun. Biol. 2021; submitted. [Google Scholar] [CrossRef]
- Belda, I.; Conchillo, L.B.; Ruiz, J.; Navascues, E.; Marquina, D.; Santos, A. Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. [Google Scholar] [CrossRef]
- Graf, F.M.R.; Weber, H.E.; Buchhaupt, M. Investigation of non-Saccharomyces yeasts with intracellular beta-glycosidase activity for wine aroma modification. J. Food Sci. 2022, 87, 4868–4877. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Mangieri, N.; Maghradze, D.; Foschino, R.; Valdetara, F.; Cantoral, J.M.; Vigentini, I. Wild Grape-Associated Yeasts as Promising Biocontrol Agents against Vitis vinifera Fungal Pathogens. Front. Microbiol. 2017, 8, 2025. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Zapparoli, G. Yeast-like fungi and yeasts in withered grape carposphere: Characterization of Aureobasidium pullulans population and species diversity. Int. J. Food Microbiol. 2019, 289, 223–230. [Google Scholar] [CrossRef]
- Wang, X.; Schlatter, D.C.; Glawe, D.A.; Edwards, C.G.; Weller, D.M.; Paulitz, T.C.; Abatzoglou, J.T.; Okubara, P.A. Native yeast and non-yeast fungal communities of Cabernet Sauvignon berries from two Washington State vineyards, and persistence in spontaneous fermentation. Int. J. Food Microbiol. 2021, 350, 109225. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Costa, L.; Soares, R.; Ayogu, P.; Teixeira, A.; Gerós, H. A catalogue of cultivable yeasts from the microbiota of grape berries cv. Vinhão and Loureiro. OENO One 2022, 56, 247–260. [Google Scholar] [CrossRef]
- Merin, M.G.; Martin, M.C.; Rantsiou, K.; Cocolin, L.; de Ambrosini, V.I. Characterization of pectinase activity for enology from yeasts occurring in Argentine Bonarda grape. Braz. J. Microbiol. 2015, 46, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Nieto-Sarabia, V.L.; Ballinas-Cesatti, C.B.; Melgar-Lalanne, G.; Cristiani-Urbina, E.; Morales-Barrera, L. Isolation, identification, and kinetic and thermodynamic characterization of a Pichia kudriavzevii yeast strain capable of fermentation. Food Bioprod. Process. 2022, 131, 109–124. [Google Scholar] [CrossRef]
- Lin, M.M.; Boss, P.K.; Walker, M.E.; Sumby, K.M.; Grbin, P.R.; Jiranek, V. Evaluation of indigenous non-Saccharomyces yeasts isolated from a South Australian vineyard for their potential as wine starter cultures. Int. J. Food Microbiol. 2020, 312, 108373. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: A mini-review. Food Microbiol. 2021, 100, 103859. [Google Scholar] [CrossRef]
- Pina, C.; Santos, C.; Couto, J.A.; Hogg, T. Ethanol tolerance of five non-Saccharomyces wine yeasts in comparison with a strain of Saccharomyces cerevisiae—Influence of different culture conditions. Food Microbiol. 2004, 21, 439–447. [Google Scholar] [CrossRef]
- Dahabieh, M.; Swanson, J.; Kinti, E.; Husnik, J. Hydrogen sulfide production by yeast during alcoholic fermentation: Mechanisms and mitigation. Wine Vitic. J. 2015, 30, 23–28. [Google Scholar]
- Donalies, U.E.; Stahl, U. Increasing sulphite formation in Saccharomyces cerevisiae by overexpression of MET14 and SSU1. Yeast 2002, 19, 475–484. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.-C.; Coulon, J.; Thibon, C.; Masneuf-Pomarède, I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT 2021, 149, 111781. [Google Scholar] [CrossRef]
- Beltran, G.; Rozès, N.; Mas, A.; Guillamón, J.M. Effect of low-temperature fermentation on yeast nitrogen metabolism. World J. Microbiol. Biotechnol. 2006, 23, 809–815. [Google Scholar] [CrossRef]
- Anton-Diaz, M.J.; Suarez Valles, B.; Mangas-Alonso, J.J.; Fernandez-Garcia, O.; Picinelli-Lobo, A. Impact of different techniques involving contact with lees on the volatile composition of cider. Food Chem. 2016, 190, 1116–1122. [Google Scholar] [CrossRef]
- Lukic, I.; Banovic, M.; Persuric, D.; Radeka, S.; Sladonja, B. Determination of volatile compounds in grape distillates by solid-phase extraction and gas chromatography. J. Chromatogr. A 2006, 1101, 238–244. [Google Scholar] [CrossRef]
- Cortés, S.; Salgado, J.M.; Rodríguez, N.; Domínguez, J.M. The storage of grape marc: Limiting factor in the quality of the distillate. Food Control 2010, 21, 1545–1549. [Google Scholar] [CrossRef]
- Szambelan, K.; Nowak, J.; Szwengiel, A.; Jeleń, H. Quantitative and qualitative analysis of volatile compounds in sorghum distillates obtained under various hydrolysis and fermentation conditions. Ind. Crops Prod. 2020, 155, 112782. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, J.; Zhang, B.; Shen, C.; Yu, L.; Gong, C.; Xu, Y.; Tang, K. Identification, quantitation and organoleptic contributions of furan compounds in brandy. Food Chem. 2023, 412, 135543. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, S.J.; Lim, J.H.; Kim, B.K.; Park, K.J. Chemical and sensory profiles of makgeolli, Korean commercial rice wine, from descriptive, chemical, and volatile compound analyses. Food Chem. 2014, 152, 624–632. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).