Effects of Fermentation Period on the Non-Volatile Metabolites of Chinese Ultra-Long-Term Solid Fermented Kohlrabi Based on Non-Targeted Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Ultra-Long-Term Solid Fermented Kohlrabi
2.2. Extraction of Metabolites from Kohlrabi
2.3. Detection of Non-Volatile Metabolites by LC-MS/MS
2.4. Data Analysis
3. Results and Discussion
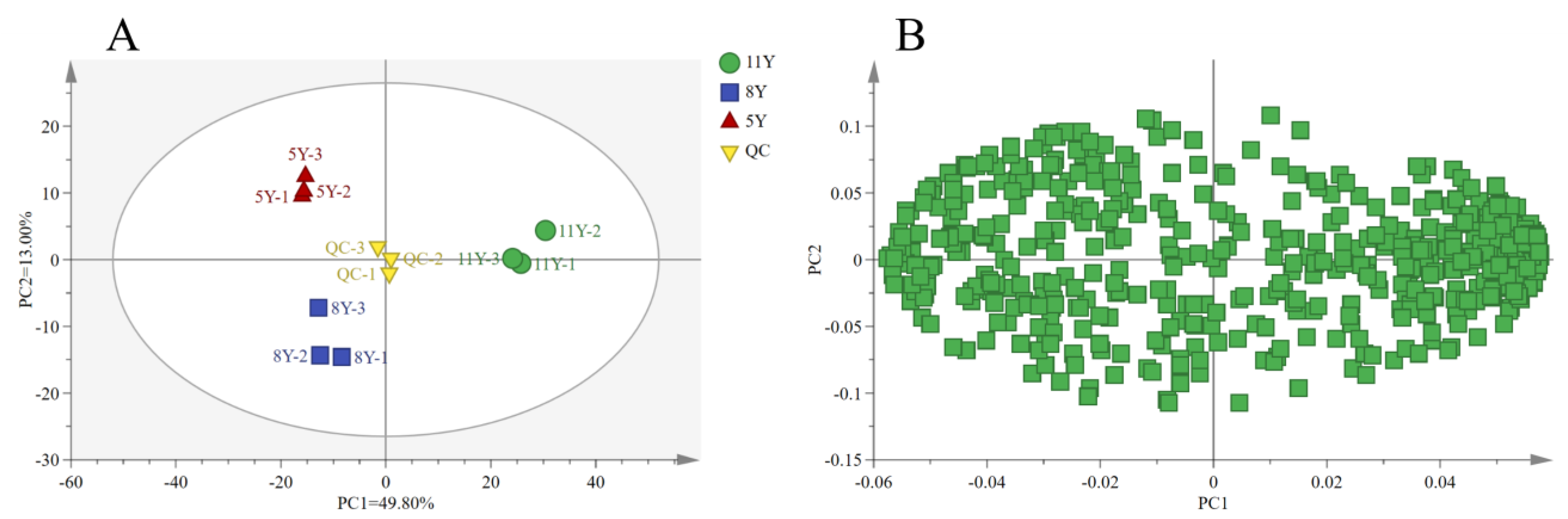
3.1. PCA Analysis for Metabolomics of Kohlrabi
3.2. OPLS-DA Analysis of Non-Volatile Metabolites from Kohlrabi by LC-MS/MS
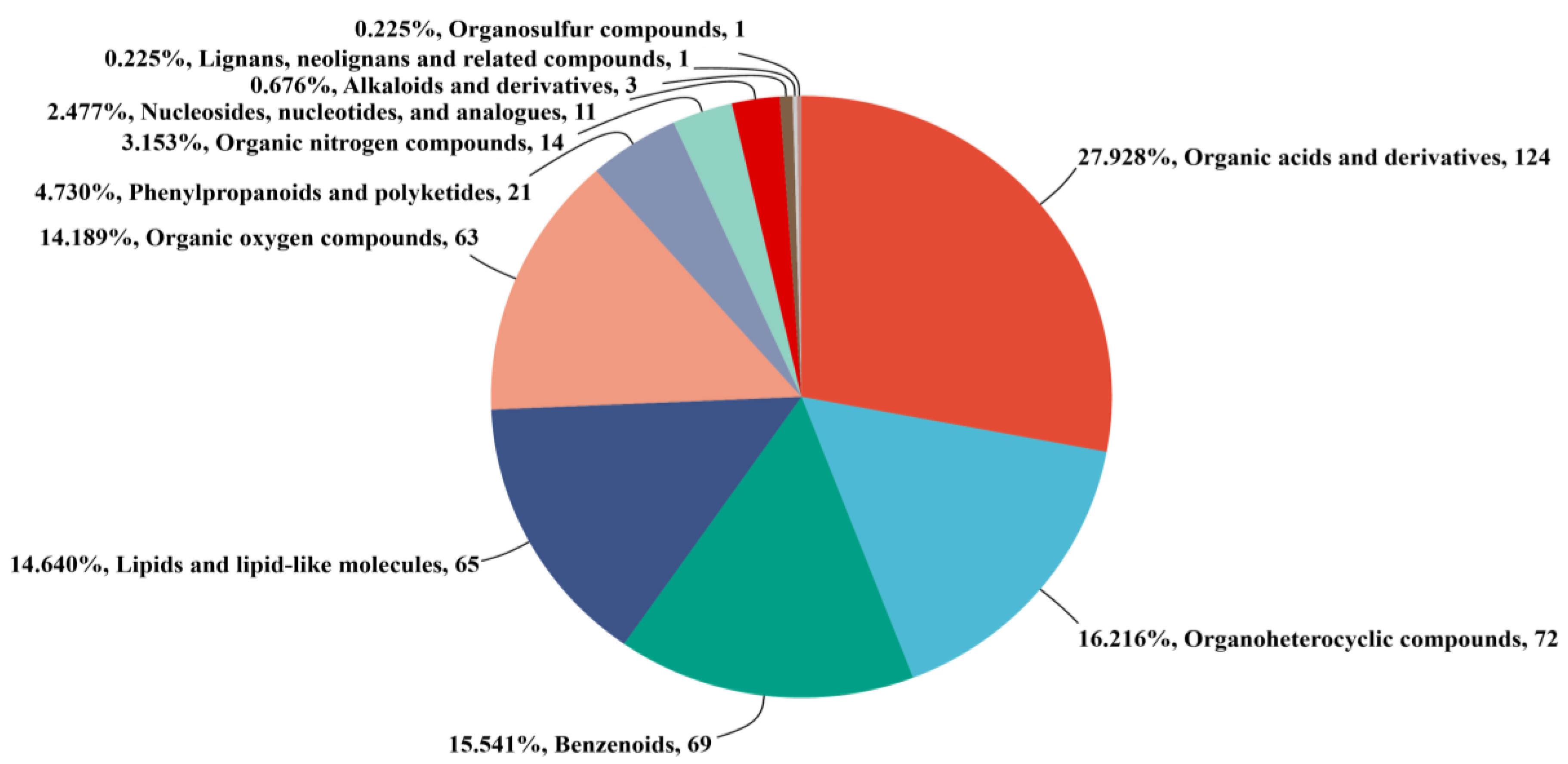
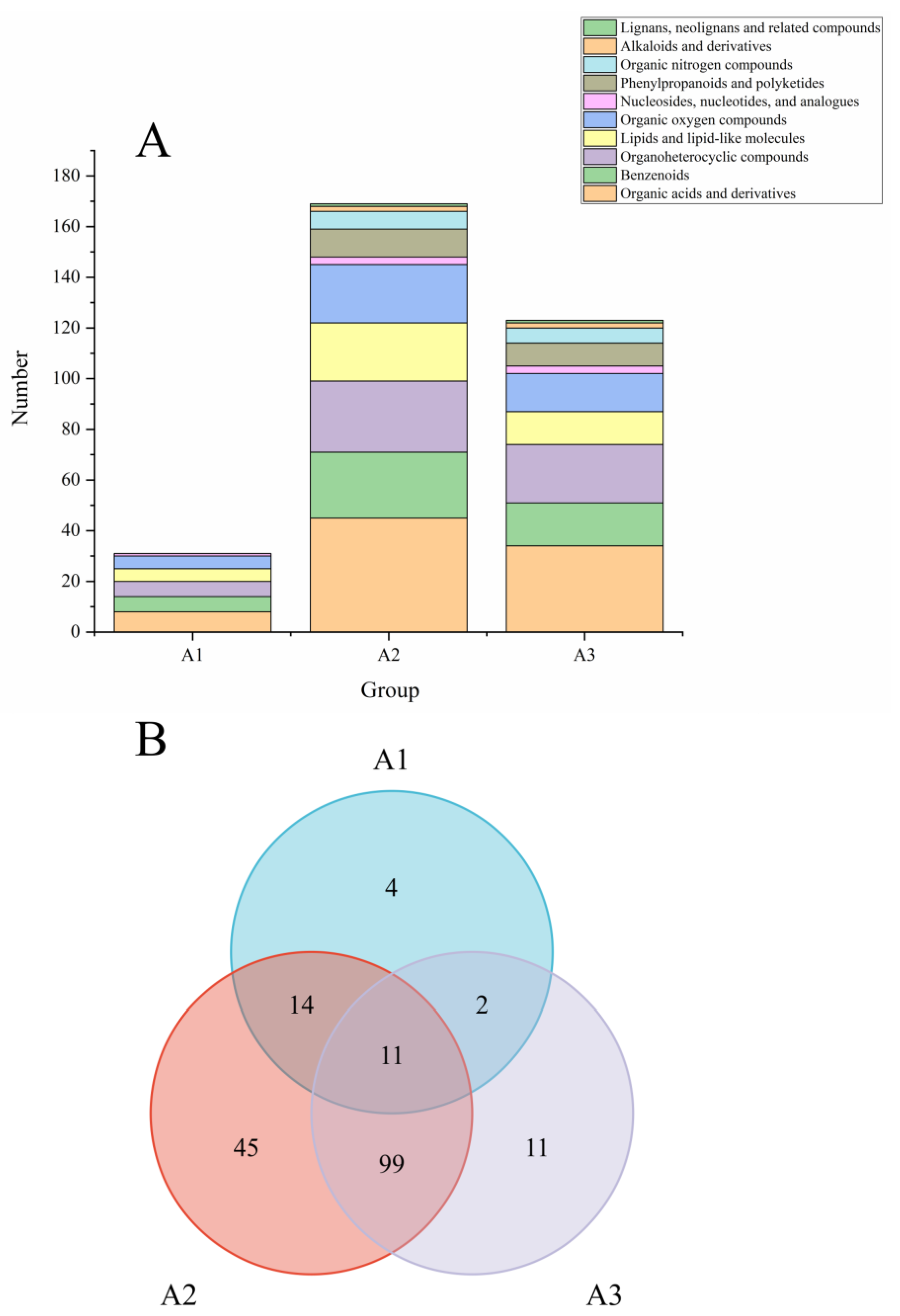
3.3. Screening of Differential Non-Volatile Metabolites in Kohlrabi
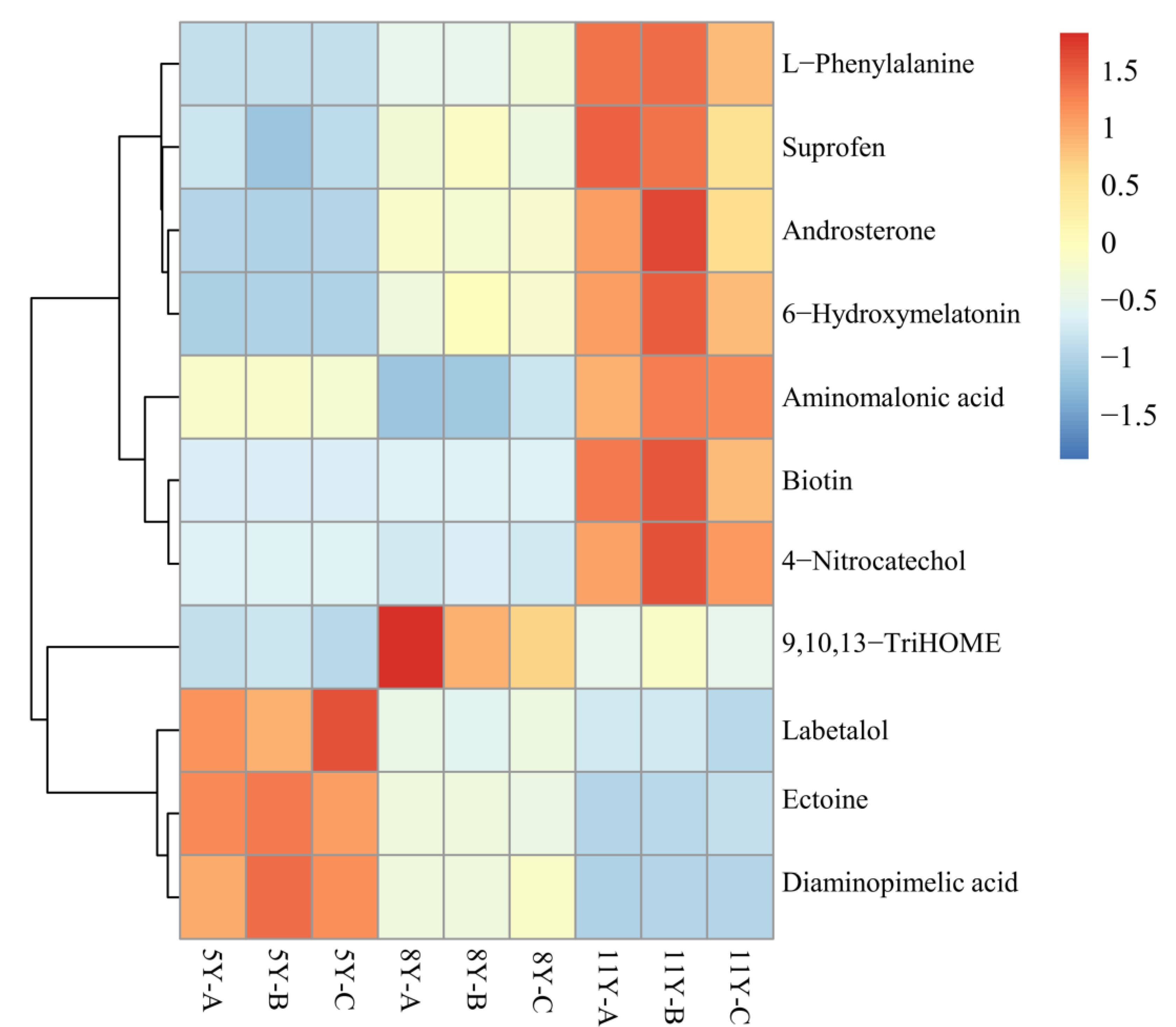
3.4. The Common Differential Metabolites in the Three Groups of Kohlrabi
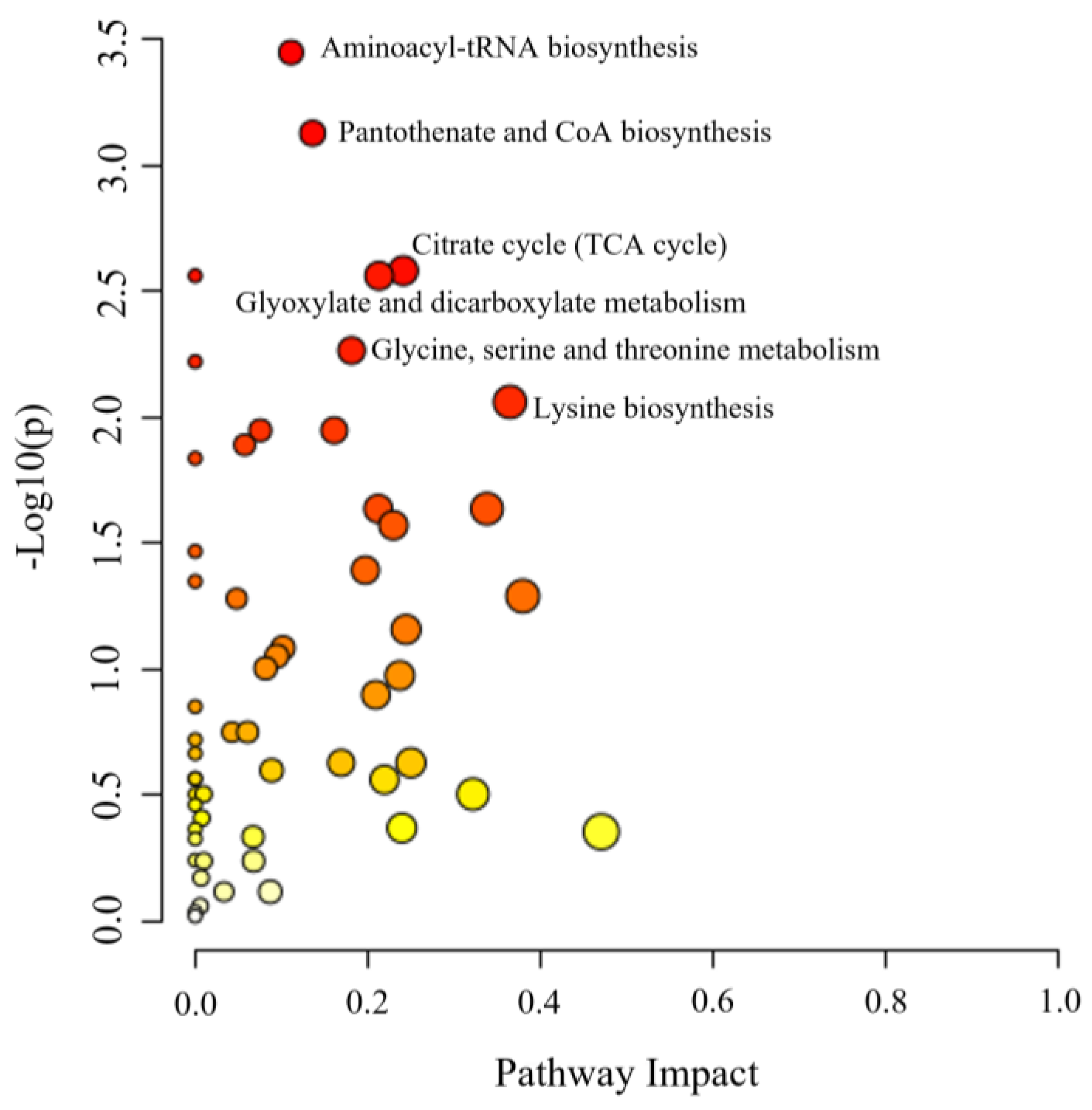
3.5. Metabolic Pathway Analysis for Differential Metabolites in Kohlrabi
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, B.; Zeng, X.; Li, A.; Yang, R.; Jiang, H. Effect of lactic acid bacterial fermentation on the quality of kohlrabi. Food Sci. 2016, 37, 147–153. [Google Scholar] [CrossRef]
- Dun, L. The Development status and prospect of turnip industry in China. China Fruit. Veget. 2017, 37, 40–42. [Google Scholar] [CrossRef]
- Pasko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of different light conditions and time of sprouting on harmful and beneficial aspects of rutabaga sprouts in comparison to their roots and seeds. J. Sci. Food Agric. 2018, 99, 302–308. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Bhandari, S.; Bhandari, A.; Shrestha, J. Effect of different doses of triacontanol on growth and yield of kohlrabi (Brassica oleracea L. var. gongylodes). Heliyon 2021, 7, e08242. [Google Scholar] [CrossRef]
- Deng, J.; Li, P. Changes in Volatiles during pickling of root mustard (Brassica juncea Coss. var. megarrhiza Tsen et Lee). Food Sci. 2013, 34, 225–229. [Google Scholar] [CrossRef]
- Eweys, A.S.; Zhao, Y.S.; Darwesh, O.M. Improving the antioxidant and anticancer potential of Cinnamomum cassia via fermentation with Lactobacillus plantarum. Biotechnol. Rep. 2022, 36, e00768. [Google Scholar] [CrossRef]
- Renz, M.; Dekker, M.; Rohn, S.; Hanschen, F.S. Plant matrix concentration and redox status influence thermal glucosinolate stability and formation of nitriles in selected Brassica vegetable broths. Food Chem. 2023, 404, 134594. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, X.; Yang, J.; Wang, H.; Liu, F.; Xu, X.; Pan, S. Effects of high hydrostatic pressure and thermal treatment on texture properties of pickled kohlrabi. LWT 2022, 157, 113078. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Ascrizzi, R.; Chiboub, W.; Cheikh Mhamed, A.; ElAyeb, A.; Skhiri, F.; Tounsi Saidani, M.; Mastouri, M.; Flamini, G. Volatiles, phenolic compounds, antioxidant and antibacterial properties of kohlrabi leaves. Nat. Prod. Res. 2021, 36, 3143–3148. [Google Scholar] [CrossRef]
- Ye, L.; Li, Y.; Wang, R.; Liu, C.; Jiang, L.; Deng, F.; Zhou, H. Progress in research on the diversity of lactic acid bacteria in traditional Chinese fermented vegetables. Food Sci. 2018, 39, 296–301. [Google Scholar] [CrossRef]
- Chen, H.; Nie, X.; Peng, T.; Xiang, L.; Liu, D.; Luo, H.; Zhao, Z. Effects of low-temperature and low-salt fermentation on the physicochemical properties and volatile flavor substances of Chinese kohlrabi using gas chromatography–ion mobility spectrometry. Fermentation 2023, 9, 146. [Google Scholar] [CrossRef]
- Pasko, P.; Bozena, M.; Katarzyna Sulkowska, Z.; Pawel, Z. Serotonin, melatonin, and certain indole derivatives profiles in rutabaga and kohlrabi seeds, sprouts, bulbs, and roots. Food Sci. Technol. 2014, 59, 740–745. [Google Scholar] [CrossRef]
- Šalić, A.; Šamec, D. Changes in the content of glucosinolates, polyphenols and carotenoids during lactic-acid fermentation of cruciferous vegetables: A mini review. Food Chem. X 2022, 16, 100457. [Google Scholar] [CrossRef]
- Nie, X.; Chen, H.; Xiang, L.; Zhang, Y.; Liu, D.; Zhao, Z. GC-TOF-MS-based non-targeted metabolomic analysis of differential metabolites in Chinese ultra-long-term industrially fermented kohlrabi and their associated metabolic pathways. Metabolites 2022, 12, 991. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhao, Z.; Wang, W.; Nie, X.; Cheng, J.; Zhang, Y.; Liu, D.; Xu, Y.; Luo, H. Analysis of differential metabolites and related metabolism pathways in long-term fermented kohlrabi fermented for different periods. Food Sci. 2022, 43, 192–198. [Google Scholar] [CrossRef]
- Świder, O.; Wójcicki, M.; Bujak, M.; Juszczuk-Kubiak, E.; Szczepańska, M.; Roszko, M. Time evolution of microbial composition and metabolic profile for biogenic amines and free amino acids in a model cucumber fermentation system brined with 0.5% to 5.0% sodium chloride. Molecules 2021, 26, 5796. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, J.; Li, D.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Metabolomics approaches for the comprehensive evaluation of fermented foods: A review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhang, J.; Xin, X.; Liao, L. Metagenomics reveals the formation mechanism of flavor metabolites during the spontaneous fermentation of potherb mustard (Brassica juncea var. multiceps). Food Res. Int. 2021, 148, 110622. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Zaunmüller, T.; Eichert, M.; Richter, H.; Unden, G. Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. Appl. Microbiol. Biotechnol. 2006, 72, 421–429. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.; Zhang, H.; Zhou, T.; Sun, T. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy. Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Guo, L.; Cutright, T.J. Effect of citric acid and bacteria on metal uptake in reeds grown in a synthetic acid mine drainage solution. J. Environ. 2015, 150, 235–242. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, C.; Wang, M.; Xu, B.; Du, L. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene treatment promotes umami taste-active amino acids accumulation of Torreya grandis nuts post-harvest by comparative chemical and transcript analyses. Food Chem. 2023, 408, 135214. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Barba, F.J.; Nemati, Z.; Sohrabi Shokofti, S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Ahituv, H.; Henry, A.G. An initial key of starch grains from edible plants of the Eastern Mediterranean for use in identifying archaeological starches. J. Archaeol. Sci. Rep. 2022, 42, 103396. [Google Scholar] [CrossRef]
- Sun, A.; Chen, L.; Wu, W.; Soladoye, O.P.; Zhang, Y.; Fu, Y. The potential meat flavoring generated from Maillard reaction products of wheat gluten protein hydrolysates-xylose: Impacts of different thermal treatment temperatures on flavor. Food Res. Int. 2023, 165, 112512. [Google Scholar] [CrossRef]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- McMahon, R.J. Biotin in metabolism and molecular biology. Annu. Rev. Nutr. 2002, 22, 221–239. [Google Scholar] [CrossRef]
- Zhang, L.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Wang, Y.; Granato, D. Multivariate effects of Chinese keemun black tea grades (Camellia sinensis var. sinensis) on the phenolic composition, antioxidant, antihemolytic and cytotoxic/cytoprotection activities. Food Res. Int. 2019, 125, 108516. [Google Scholar] [CrossRef]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Parua, S.; Vágvölgyi, C.; Das Mohapatra, P.K.; Pati, B.R.; et al. Role of probiotic Lactobacillus fermentum KKL1 in the preparation of a rice based fermented beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Eweys, A.S.; Zhang, J.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or facilitators? Roles of lipids in plant root–microbe interactions. Trends Plant. Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef]
- Phan, Q.; Tomasino, E. Untargeted lipidomic approach in studying pinot noir wine lipids and predicting wine origin. Food Chem. 2021, 355, 129409. [Google Scholar] [CrossRef]
- Liang, N.; Tang, K.; Curtis, J.M.; Gänzle, M.G. Identification and quantitation of hydroxy fatty acids in fermented sausage samples. J. Agric. Food Chem. 2020, 68, 8648–8657. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kishino, S.; Park, S.B.; Hirata, A.; Kitamura, N.; Saika, A.; Ogawa, J. Efficient enzymatic production of hydroxy fatty acids by linoleic acid Δ9 hydratase from Lactobacillus plantarum AKU 1009a. J. Appl. Microbiol. 2016, 120, 1282–1288. [Google Scholar] [CrossRef]
- Xiao, M.; Xiong, T.; Peng, Z.; Liu, C.; Huang, T.; Yu, H.; Xie, M. Correlation between microbiota and flavours in fermentation of Chinese Sichuan Paocai. Food Res. Int. 2018, 114, 123–132. [Google Scholar] [CrossRef]
- Şen, D.; Gökmen, V. Kinetic modeling of Maillard and caramelization reactions in sucrose-rich and low moisture foods applied for roasted nuts and seeds. Food Chem. 2022, 395, 133583. [Google Scholar] [CrossRef]
- Pastoriza, S.; Quesada, J.; Rufián Henares, J. Lactose and oligosaccharides: Maillard reaction. Ref. Modul. Food Sci. 2018. [Google Scholar] [CrossRef]
- Chatterjee, B.K.; Mondal, D.; Bera, S. Diaminopimelic acid and its analogues: Synthesis and biological perspective. Tetrahedron 2021, 100, 132403. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Shi, M.; Jiang, M.; Li, L.; Zheng, Y. Microbial production of ectoine and hydroxyectoine as high-value chemicals. Microb. Cell. Factories 2021, 20, 76. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, Y.; Geng, Z.; Zhang, M.; Sun, C.; Bian, H.; Wang, D.; Xu, W. Progress in research on hydroxyoctadecaenoic acids as oxidation products of linoleic acid. Food Sci. 2018, 39, 278–284. [Google Scholar] [CrossRef]
- Yang, J.; Tian, D.; Yin, Y.; Jiang, L.; Li, L.; Zhang, E.; Hu, F.; Cheng, P. Optimization and application of ultra-high performance liquid chromatography-high resolution mass spectrometry for determination of the bitter compound trihydroxyoctadecenoic acid in Chinese Baijiu. Food Sci. 2023, 44, 352–359. [Google Scholar] [CrossRef]
- Selen, E.S.; Rodriguez, S.; Cavagnini, K.S.; Kim, H.B.; Na, C.H.; Wolfgang, M.J. Requirement of hepatic pyruvate carboxylase during fasting, high fat, and ketogenic diet. J. Biol. Chem. 2022, 298, 102648. [Google Scholar] [CrossRef]
- Esen Marti, M.; Zeidan, H. Using eco-friendly alternatives for the recovery of pyruvic acid by reactive extraction. Sep. Purif. 2023, 312, 123309. [Google Scholar] [CrossRef]
- Ahanger, M.; Gul, F.; Ahmad, P.; Aisha, N. Environmental stresses and metabolomics—Deciphering the role of stress responsive metabolites. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–67. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.R.; Moradi, P.; MohseniFard, E.; Shekari, F.; Kompany-Zareh, M. Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant. Physiol. Biochem. 2018, 132, 391–399. [Google Scholar] [CrossRef]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant. Sci. 2014, 5, 552. [Google Scholar] [CrossRef]
- Patel, M.; Pandey, D.S.; Tanna, B.; Alkan, N.; Mishra, A. Comparative metabolomics unveils the role of metabolites and metabolic pathways in the adaptive mechanisms of shrubby halophytes. Environ. Exp. Bot. 2022, 202, 105030. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Elango, D.; Liu, H.; Jin, D.; Wang, X.; Wu, Y. Metabolome and transcriptome analysis reveals molecular mechanisms of watermelon under salt stress. Environ. Exp. Bot. 2023, 206, 105200. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Naggar, K.; Taha, A.E.; Khafaga, A.F.; Madkour, M.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T.; et al. Betaine and related compounds: Chemistry, metabolism and role in mitigating heat stress in poultry. J. Therm. Biol. 2022, 104, 103168. [Google Scholar] [CrossRef] [PubMed]
- Pardo, Z.; Lara, L.; Nieto, R.; Fernández-Fígares, I.; Seiquer, I. Muscle quality traits and oxidative status of Iberian pigs supplemented with zinc and betaine under heat stress. Meat Sci. 2023, 198, 109119. [Google Scholar] [CrossRef] [PubMed]


| Group | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|
| A1 | 0.728 | 1.000 | 0.984 |
| A2 | 0.751 | 1.000 | 0.986 |
| A3 | 0.678 | 1.000 | 0.969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Chen, H.; Wang, X.; Nie, X.; Xiang, L.; Liu, D.; Zhao, Z. Effects of Fermentation Period on the Non-Volatile Metabolites of Chinese Ultra-Long-Term Solid Fermented Kohlrabi Based on Non-Targeted Metabolomic Analysis. Fermentation 2023, 9, 753. https://doi.org/10.3390/fermentation9080753
Jia X, Chen H, Wang X, Nie X, Xiang L, Liu D, Zhao Z. Effects of Fermentation Period on the Non-Volatile Metabolites of Chinese Ultra-Long-Term Solid Fermented Kohlrabi Based on Non-Targeted Metabolomic Analysis. Fermentation. 2023; 9(8):753. https://doi.org/10.3390/fermentation9080753
Chicago/Turabian StyleJia, Xiaohan, Hongfan Chen, Xinyi Wang, Xin Nie, Lu Xiang, Dayu Liu, and Zhiping Zhao. 2023. "Effects of Fermentation Period on the Non-Volatile Metabolites of Chinese Ultra-Long-Term Solid Fermented Kohlrabi Based on Non-Targeted Metabolomic Analysis" Fermentation 9, no. 8: 753. https://doi.org/10.3390/fermentation9080753
APA StyleJia, X., Chen, H., Wang, X., Nie, X., Xiang, L., Liu, D., & Zhao, Z. (2023). Effects of Fermentation Period on the Non-Volatile Metabolites of Chinese Ultra-Long-Term Solid Fermented Kohlrabi Based on Non-Targeted Metabolomic Analysis. Fermentation, 9(8), 753. https://doi.org/10.3390/fermentation9080753






