Fermentative Production of L-Theanine in Escherichia coli via the Construction of an Adenosine Triphosphate Regeneration System
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Cultivation Conditions
2.3. Homology Modeling and Molecular Docking
2.4. Analytical Methods
3. Results and Discussion
3.1. Construction of a PPK-Based ATP Regeneration System for Enhancing the Production of L-Theanine in E. coli
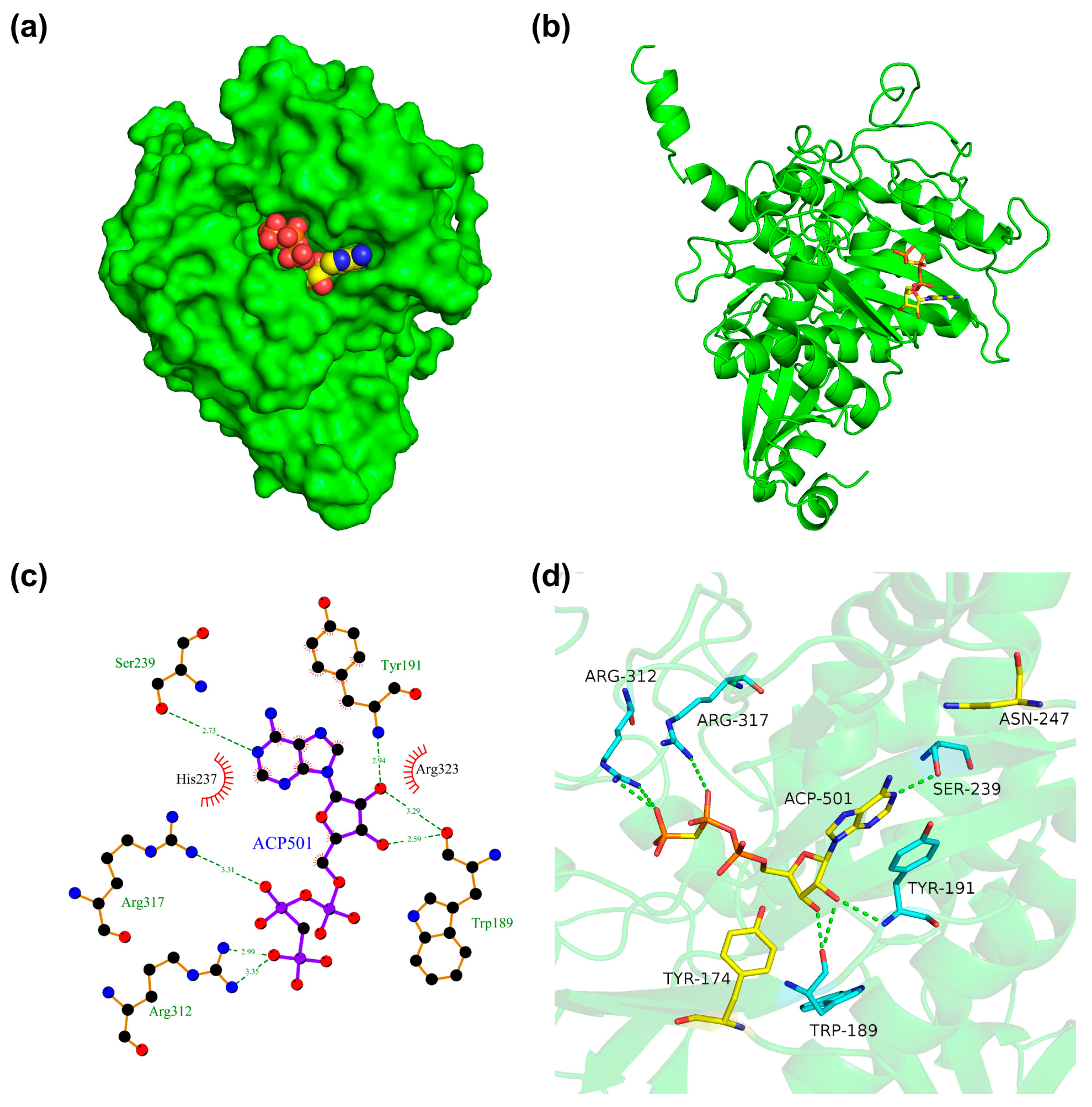
3.2. Molecular Docking Studies
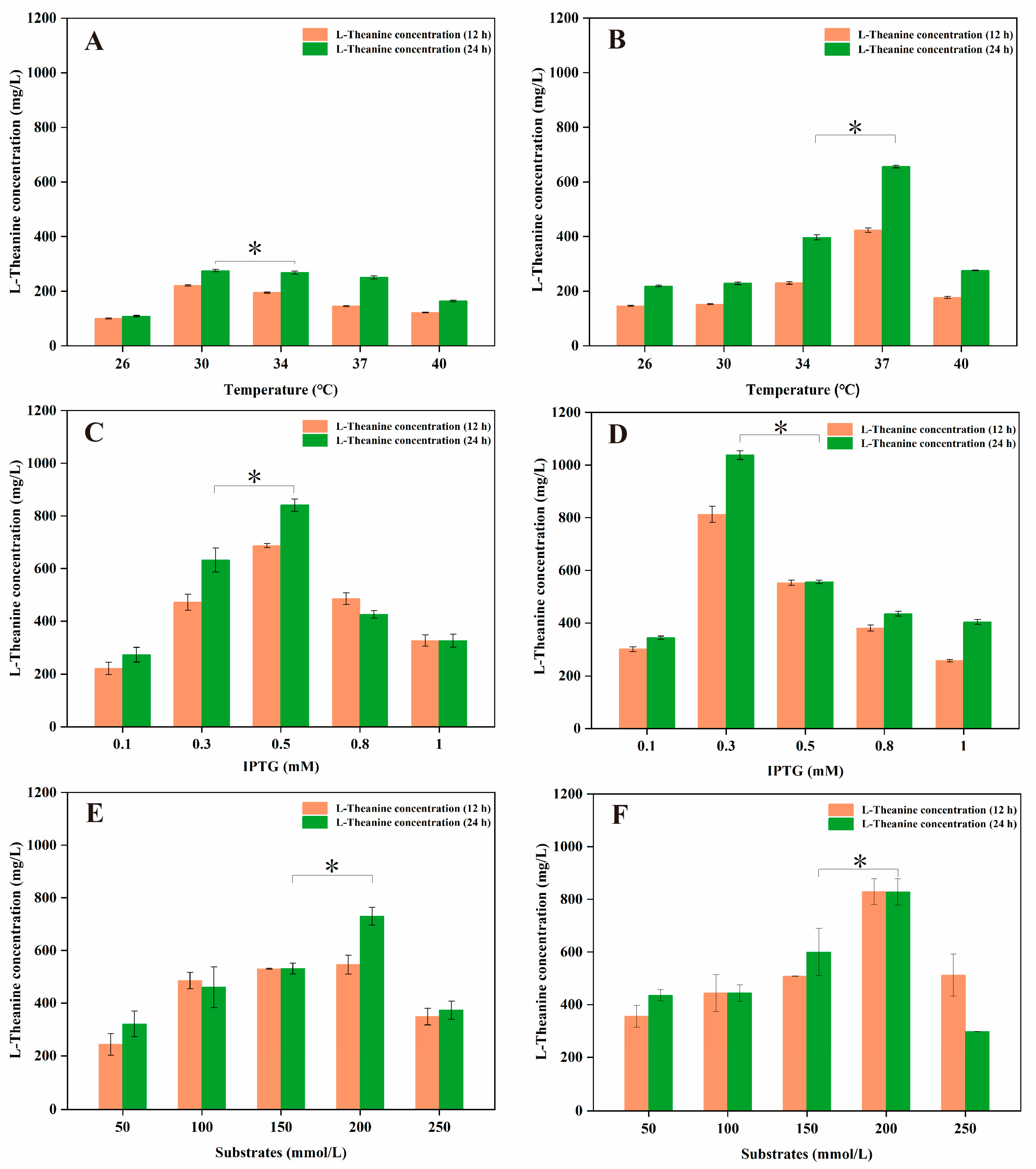
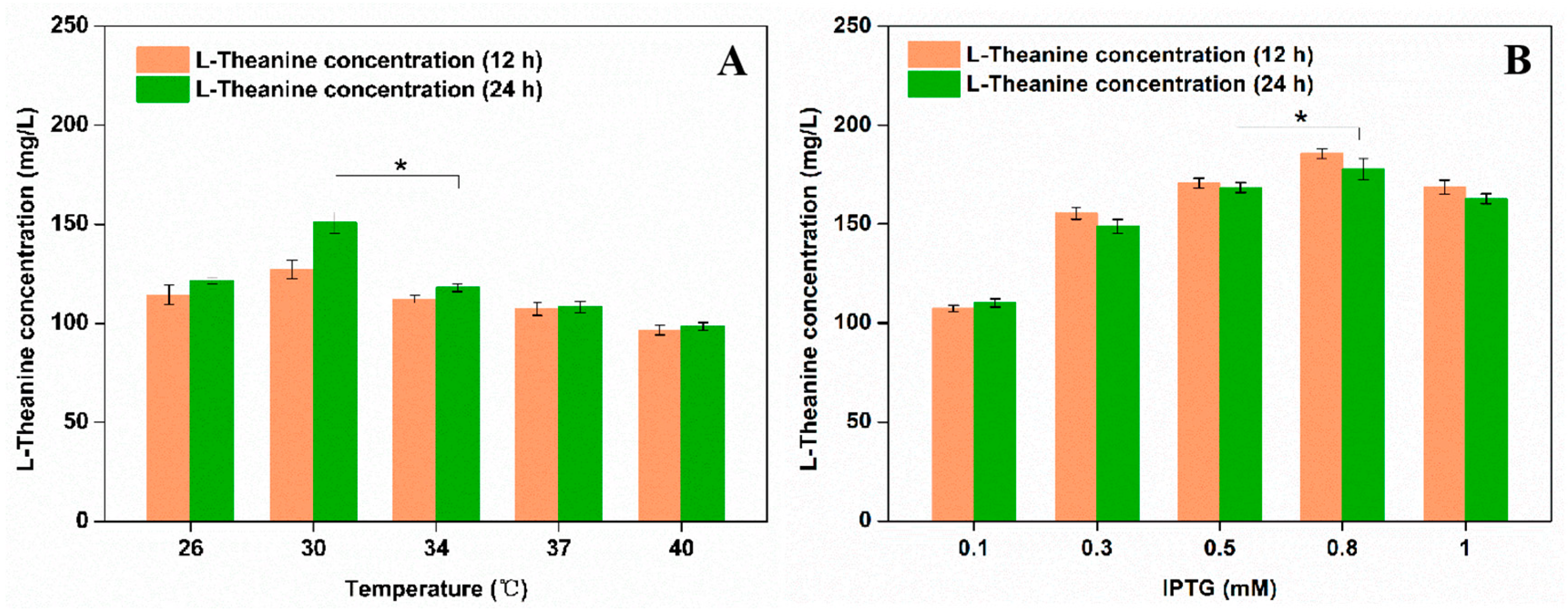
3.3. Whole-Cell Catalytic Synthesis of L-Theanine in Engineered E. coli FD01 and FD02
3.4. De Novo Biosynthesis of L-Theanine via Engineered E. coli FD03
3.5. The Enhancement of L-Theanine Production via the Overexpression of a Glutamine Permease
3.6. Production of L-Theanine from Glucose via Recombinant E. coli FD04 in a 1 L Bioreactor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Zhang, X.; Wu, J.; Li, X.; Li, W.; Sun, X.; Wang, J.; Yan, Y.; Shen, X.; Yuan, Q. Targeting cofactors regeneration in methylation and hydroxylation for high level production of Ferulic acid. Metab. Eng. 2022, 73, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shan, X.; Lyv, Y.; Zhou, J. Bioproduction of quercetin using recombinant thermostable glycosidases from Dictyoglomus thermophilum. Bioresour. Bioprocess. 2022, 9, 48–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zeng, W.; Xu, S.; Li, D.; Yu, S.; Zhou, J. De novo biosynthesis of carminic acid in Saccharomyces cerevisiae. Metab. Eng. 2023, 76, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Rohles, C.; Pauli, S.; Giesselmann, G.; Kohlstedt, M.; Becker, J.; Wittmann, C. Systems metabolic engineering of Corynebacterium glutamicum eliminates all by-products for selective and high-yield production of the platform chemical 5-aminovalerate. Metab. Eng. 2022, 73, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Prell, C.; Vonderbank, S.-A.; Meyer, F.; Pérez-García, F.; Wendisch, V.F. Metabolic engineering of Corynebacterium glutamicum for de novo production of 3-hydroxycadaverine. Curr. Res. Biotechnol. 2022, 4, 32–46. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Y.; Kang, Z.; Cheng, J.; Xiong, X.; Hu, C.Y.; Meng, Y. Engineering a Feruloyl-Coenzyme A Synthase for Bioconversion of Phenylpropanoid Acids into High-Value Aromatic Aldehydes. J. Agric. Food Chem. 2022, 70, 9948–9960. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, W.; Guo, X.; Liu, Y.; Hu, C.; Guo, S.; Kang, Z.; Xu, X.; Ma, C.; Gao, C.; et al. A D-2-hydroxyglutarate biosensor based on specific transcriptional regulator DhdR. Nat. Commun. 2021, 12, 7108. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.J.; Kang, M.; Baritugo, K.-A.; Son, J.; Kang, K.H.; Ryu, M.-H.; Lee, S.; Sohn, M.; Jung, Y.J.; Park, K.; et al. Fermentative High-Level Production of 5-Hydroxyvaleric Acid by Metabolically Engineered Corynebacterium glutamicum. ACS Sustain. Chem. Eng. 2021, 9, 2523–2533. [Google Scholar] [CrossRef]
- Liu, S.-H.; Li, J.; Huang, J.-A.; Liu, Z.-H.; Xiong, L.-G. New advances in genetic engineering for l-theanine biosynthesis. Trends Food Sci. Technol. 2021, 114, 540–551. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans-A comprehensive review. Biomed. Pharm. 2017, 95, 1260–1275. [Google Scholar] [CrossRef]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Yuan, H.; He, N. From Tea Leaves to Factories: A Review of Research Progress in l-Theanine Biosynthesis and Production. J. Agric. Food Chem. 2021, 69, 1187–1196. [Google Scholar] [CrossRef]
- Jang, H.S.; Jung, J.Y.; Jang, I.S.; Jang, K.H.; Kim, S.H.; Ha, J.H.; Suk, K.; Lee, M.G. L-theanine partially counteracts caffeine-induced sleep disturbances in rats. Pharm. Biochem. Behav. 2012, 101, 217–221. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.H.; Roach, P.D. Preparation of decaffeinated and high caffeine powders from green tea. Powder Technol. 2013, 233, 169–175. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, L.; Zhou, L.; Xiang, L.; Jiang, B.; Zhang, T.; Chen, J. Characterization of alkaline Bacillus amyloliquefaciens γ-glutamyltranspeptidase expressed in Bacillus subtilis and its application in enzymatic synthesis of L-Theanine. Process Biochem. 2023, 131, 125–132. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Mao, B.; Hu, C.; Tang, D. The Current Situation and Prospect of the Utilization of Theanine. Chinatea 2021, 43, 6–10. [Google Scholar]
- Shuai, Y.; Shen, Y.; Huang, H.; Li, J. Applications and regulation of functional ingredient L-Theanine. China Food Addit. 2013, S1, 181–185. [Google Scholar]
- Jagim, A.R.; Harty, P.S.; Tinsley, G.M.; Kerksick, C.M.; Gonzalez, A.M.; Kreider, R.B.; Arent, S.M.; Jager, R.; Smith-Ryan, A.E.; Stout, J.R.; et al. International society of sports nutrition position stand: Energy drinks and energy shots. J. Int. Soc. Sport. Nutr. 2023, 20, 2171314. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, T.; Jiang, B. An overview of biological production of L-theanine. Biotechnol. Adv. 2015, 33, 335–342. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, S.-F.; Gong, G.-M.; Qiu, Z.-C. Comparison of Determination of Theanine in Different Tea Species. Food Sci. 2008, 29, 335–337. [Google Scholar]
- Xiao, S.; Qian, R.; Hu, S.; Fu, Z.; Bai, T.; Wang, W.; Cheng, J.; Zhang, J. Advances in the Production of Theanine by Plants and Microorganisms. Fermentation 2023, 9, 543. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, M.; Zheng, N.; Deng, Y.; Wang, Q.; Osire, T.; Xia, X. Redesign of gamma-glutamyl transpeptidase from Bacillus subtilis for high-level production of L-theanine by cavity topology engineering. Appl. Microbiol. Biotechnol. 2023, 107, 3551–3564. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, R.; Ohno, S.; Hayashi, M.; Tabata, K.; Endo, H. Production of L-Theanine by Escherichia coli in the Absence of Supplemental Ethylamine. Appl. Environ. Microbiol. 2021, 87, e00031-21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, B.; Huang, Z.; Shi, Z. Preparative Isolation and Purification of L-Theanine by HPLC. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 875–884. [Google Scholar] [CrossRef]
- Gu, H.; Jiang, Y.; Wang, J. A Practical Synthesis of Ethyl L-Glutamine (L-Theanine). Org. Prep. Proced. Int. 2004, 36, 182–185. [Google Scholar] [CrossRef]
- Feng, J.C.; Yang, C.; Zhao, Z.H.; Xu, J.J.; Li, J.; Li, P. Application of Cell-Free Protein Synthesis System for the Biosynthesis of L-Theanine. ACS Synth. Biol. 2021, 10, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Wen-Xian, Z.; Xing-Hui, L.I.; Li-Yuan, W.; Wan-Ping, F.; Hao, C. Construction of E. coli Recombinant Engineered Strain for Theanine Biosynthesis with GS Gene Embedded. J. Tea Sci. 2008, 28, 242–248. [Google Scholar]
- Tachiki, T.; Yamada, T.; Mizuno, K.; Ueda, M.; Shiode, J.-I.; Fukami, H. γ-Glutamyl Transfer Reactions by Glutaminase from Pseudomonas nitroreducens IFO 12694 and Their Application for the Syntheses of Theanine and γ-Glutamylmethylamide. Biosci. Biotechnol. Biochem. 1998, 62, 1279–1283. [Google Scholar] [CrossRef]
- Miyake, K.; Kakita, S. A novel catalytic ability of gamma-glutamylcysteine synthetase of Escherichia coli and its application in theanine production. Biosci. Biotechnol. Biochem. 2009, 73, 2677–2683. [Google Scholar] [CrossRef]
- Yang, S.Y.; Han, Y.H.; Park, Y.L.; Park, J.Y.; No, S.Y.; Jeong, D.; Park, S.; Park, H.Y.; Kim, W.; Seo, S.O.; et al. Production of L-Theanine Using Escherichia coli Whole-Cell Overexpressing gamma-Glutamylmethylamide Synthetase with Bakers Yeast. J. Microbiol. Biotechnol. 2020, 30, 785–792. [Google Scholar] [CrossRef]
- Fan, X.G.; Zhang, T.; Ji, Y.Q.; Li, J.; Long, K.Y.; Yuan, Y.; Li, Y.J.; Xu, Q.Y.; Chen, N.; Xie, X.X. Pathway engineering of Escherichia coli for one-step fermentative production of L-theanine from sugars and ethylamine. Metab. Eng. Commun. 2020, 11, e00151. [Google Scholar] [CrossRef]
- Yao, J.; Li, J.; Xiong, D.; Qiu, Y.; Shi, G.; Jin, J.M.; Tao, Y.; Tang, S.Y. Development of a highly efficient and specific L-theanine synthase. Appl. Microbiol. Biotechnol. 2020, 104, 3417–3431. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yu, J.; Du, Q.; Zeng, S.; Liu, J.; Jiao, Q.; Zhang, H. Efficient synthesis of γ-glutamyl compounds by co-expression of γ-glutamylmethylamide synthetase and polyphosphate kinase in engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2020, 47, 573–583. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, R.; Liu, Y.; Li, J.; Gao, H.; Hu, N. Gamma-Glutamyltranspeptidase from Bacillus amyloliquefaciens: Transpeptidation activity enhancement and L-theanine production. Enzym. Microb. Technol. 2020, 140, 109644. [Google Scholar] [CrossRef]
- Yang, T.; Liu, S.; Liu, H.; Long, M.; Chen, P.; Zhang, X.; Xu, M.; Rao, Z. Semi-quantitative activity assays for high-throughput screening of higher activity gamma glutamyl transferase and enzyme immobilization to efficiently synthesize L-theanine. J. Biotechnol. 2021, 330, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chang, M.; Li, H.; Zhang, Z.; Chen, Q.; Chen, Y.; Yao, Y.; Pan, A.; Shi, C.; Wang, C.; et al. Endophytic Bacteria as Contributors to Theanine Production in Camellia sinensis. J. Agric. Food Chem. 2019, 67, 10685–10693. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.C.; Lin, M.G.; Huang, Y.F.; Chen, Y.Y.; Wang, T.F.; Lin, L.L. Enzymatic synthesis of L-theanine from L-glutamine and ethylamine by Bacillus licheniformis gamma-glutamyltranspeptidase and its mutants specialized in transpeptidase activity. Biocatal. Agric. Biotechnol. 2019, 22, 101393. [Google Scholar] [CrossRef]
- Ma, H.K.; Fan, X.G.; Cai, N.Y.; Zhang, D.Z.; Zhao, G.H.; Wang, T.; Su, R.; Yuan, M.; Ma, Q.; Zhang, C.L.; et al. Efficient fermentative production of l-theanine by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2020, 104, 119–130. [Google Scholar] [CrossRef]
- Yamamoto, S.; Wakayama, M.; Tachiki, T. Cloning and expression of Pseudomonas taetrolens Y-30 gene encoding glutamine synthetase: An enzyme available for theanine production by coupled fermentation with energy transfer. Biosci. Biotechnol. Biochem. 2006, 70, 500–507. [Google Scholar] [CrossRef][Green Version]
- Sharma, E.; Lal, M.K.; Gulati, A.; Gulati, A. Biochemical Characterization of gamma-Glutamyl Transpeptidase from Bacillus altitudinis IHB B1644 and Its Application in the Synthesis of l-Theanine. J. Agric. Food Chem. 2023, 71, 5592–5599. [Google Scholar] [CrossRef]
- Hu, B.; Yu, H.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Lee, S.Y.; Zhao, X. Whole-Cell P450 Biocatalysis Using Engineered Escherichia coli with Fine-Tuned Heme Biosynthesis. Adv. Sci. 2023, 10, 2205580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Tu, W.; Luo, Z.; Gou, X.; Li, Q.; Wang, D.; Zhou, J. A High-Efficiency Artificial Synthetic Pathway for 5-Aminovalerate Production From Biobased L-Lysine in Escherichia coli. Front Bioeng. Biotechnol. 2021, 9, 633028. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, Y.; Mi, L.; Chen, W.; Wang, D.; Wang, Q. An economically and environmentally acceptable synthesis of chiral drug intermediate l-pipecolic acid from biomass-derived lysine via artificially engineered microbes. J. Ind. Microbiol. Biotechnol. 2018, 45, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.W.; Muir, D.D. Properties of a thermostable β-galactosidase from a thermophilic Bacillus: Comparison of the enzyme activity of whole cells, purified enzyme and immobilised whole cells. J. Sci. Food Agric. 1978, 29, 753–761. [Google Scholar] [CrossRef]
- Pan, X.R.; Liu, J.Z.; Zhang, H.J.; Jiao, Q.C. Synthesis of L-Theanine by Whole Cell Catalyst Co-expressing PPK and GMAS. Fine Chem. 2019, 36, 1827–1832. [Google Scholar] [CrossRef]
- Fu, X.M.; Liao, Y.Y.; Cheng, S.H.; Deng, R.F.; Yang, Z.Y. Stable Isotope-Labeled Precursor Tracing Reveals that L-Alanine is Converted to L-Theanine via L-Glutamate not Ethylamine in Tea Plants In Vivo. J. Agric. Food Chem. 2021, 69, 15354–15361. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, Y.; Zhang, T.; Xie, H.; Dong, D.; Chen, N.; Fan, X. Production technology of L—Theanine and its application prospect. Food Ferment. Ind. 2022, 48, 303–311. [Google Scholar] [CrossRef]
- Benninghaus, L.; Walter, T.; Mindt, M.; Risse, J.M.; Wendisch, V.F. Metabolic Engineering of Pseudomonas putida for Fermentative Production of L-Theanine. J. Agric. Food Chem. 2021, 69, 9849–9858. [Google Scholar] [CrossRef]
- Dong, C.X.; Li, F.; Yang, T.Y.; Feng, L.; Zhang, S.P.; Li, F.D.; Li, W.H.; Xu, G.H.; Bao, S.L.; Wan, X.C.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef]
- Liu, K.; Peng, Y.; Lin, L.; Gong, Z.; Xiao, W.; Li, Y. L-Theanine Regulates the Abundance of Amino Acid Transporters in Mice Duodenum and Jejunum via the mTOR Signaling Pathway. Nutrients 2023, 15, 142. [Google Scholar] [CrossRef]




| Host | Engineered Strategy | L-Theanine Titer (g/L) | L-Theanine Yield (g/g) | Substrate | Reference |
|---|---|---|---|---|---|
| E. coli | Enzyme-catalyzed reaction | 33.0 | 0.95 | Glutamine | [15] |
| E. coli | Whole-cell catalytic reaction | 16.5 | 0.66 | Glutamate | [30] |
| E. coli | Engineering of a one-step fermentation pathway from sugar and ethylamine | 70.6 | 0.42 | Glucose | [31] |
| E. coli | Optimization of PPK to reduce ATP consumption | 30.4 | 0.871 | Sodium glutamate | [32] |
| E. coli | Point mutation, pure enzyme catalysis | 26.1 | 0.83 | Glutamine | [34] |
| B. subtlis | Random mutagenesis and high-throughput screening | 70.6 | 0.67 | Glutamine | [35] |
| E. coli | Enzyme-catalyzed reaction | 78.3 | 0.94 | Glutamine | [37] |
| C. glutamicum | Batch make-up fermentation strategy | 42.0 | 0.196 | Glucose | [38] |
| E. coli | Expression of GS | 31.35 | 0.6 | Sodium glutamate | [39] |
| B. altitudinis | Enzyme-catalyzed reaction | 2.2 | 0.6–0.65 | Glutamine | [40] |
| P. nitroreducens | Enzyme-catalyzed reaction | 47.0 | 0.458 | Glutamine | [28] |
| Strains or Plasmids | Description | Sources |
|---|---|---|
| Strains | ||
| BL21(DE3) | Wild type | Novagen |
| FD01 | E. coli BL21(DE3) harboring plasmid pETDuet-1-gmas | This study |
| FD02 | E. coli BL21(DE3) harboring plasmid pETDuet-1-gmas-ppk | This study |
| FD03 | E. coli BL21(DE3) harboring plasmid pETDuet-1-gmas-ppk-bsAld-csAlaDC | This study |
| FD04 | E. coli BL21(DE3) harboring plasmid pETDuet-1-gmas-ppk-bsAld-csAlaDC-gnp1 | This study |
| Plasmids | ||
| pETDuet-1 | Empty plasmid, AmpR | [41] |
| pETDuet-1-gmas | pETDuet-1 carries a γ-glutamylmethylamide synthetase (GMAS) gene from Methyloversatilis universalis, AmpR | This study |
| pETDuet-1-gmas-ppk | pETDuet-1 carries a γ-glutamylmethylamide synthetase (GMAS) gene from Methyloversatilis universalis and a polyphosphate kinase (PPK) gene from E. coli, AmpR | This study |
| pETDuet-1-gmas-ppk-bsAld-csAlaDC | pETDuet-1 carries a γ-glutamylmethylamide synthetase (GMAS) gene from Methyloversatilis universalis, a polyphosphate kinase (PPK) gene from E. coli, an alanine transaminase gene from Bacillus subtilis (bsAld), and an alanine decarboxylase gene from Camellia sinensis (csAlaDC), AmpR | This study |
| pETDuet-1-gmas-ppk-bsAld-csAlaDC-gnp1 | pETDuet-1 carries a γ-glutamylmethylamide synthetase (GMAS) gene from Methyloversatilis universalis, a polyphosphate kinase (PPK) gene from E. coli, an alanine transaminase gene from Bacillus subtilis (bsAld), an alanine decarboxylase gene (AlaDC) from Camellia sinensis, and a glutamine permease gene from Saccharomyces cereviside (gnp1), AmpR | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, R.; Hu, S.; Lu, Y.; Wang, W.; Fu, Z.; Cheng, J. Fermentative Production of L-Theanine in Escherichia coli via the Construction of an Adenosine Triphosphate Regeneration System. Fermentation 2023, 9, 875. https://doi.org/10.3390/fermentation9100875
Cao R, Hu S, Lu Y, Wang W, Fu Z, Cheng J. Fermentative Production of L-Theanine in Escherichia coli via the Construction of an Adenosine Triphosphate Regeneration System. Fermentation. 2023; 9(10):875. https://doi.org/10.3390/fermentation9100875
Chicago/Turabian StyleCao, Ruiqi, Shunyang Hu, Yao Lu, Wei Wang, Zhongdan Fu, and Jie Cheng. 2023. "Fermentative Production of L-Theanine in Escherichia coli via the Construction of an Adenosine Triphosphate Regeneration System" Fermentation 9, no. 10: 875. https://doi.org/10.3390/fermentation9100875
APA StyleCao, R., Hu, S., Lu, Y., Wang, W., Fu, Z., & Cheng, J. (2023). Fermentative Production of L-Theanine in Escherichia coli via the Construction of an Adenosine Triphosphate Regeneration System. Fermentation, 9(10), 875. https://doi.org/10.3390/fermentation9100875






