Mathematical Modeling of Nitrification in Mixed Cultures: Insights into Nitrite-Oxidizing Bacteria Growth and Ammonia Starvation Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Enrichment of Nitrifying Microorganisms
2.2. Analytical Techniques
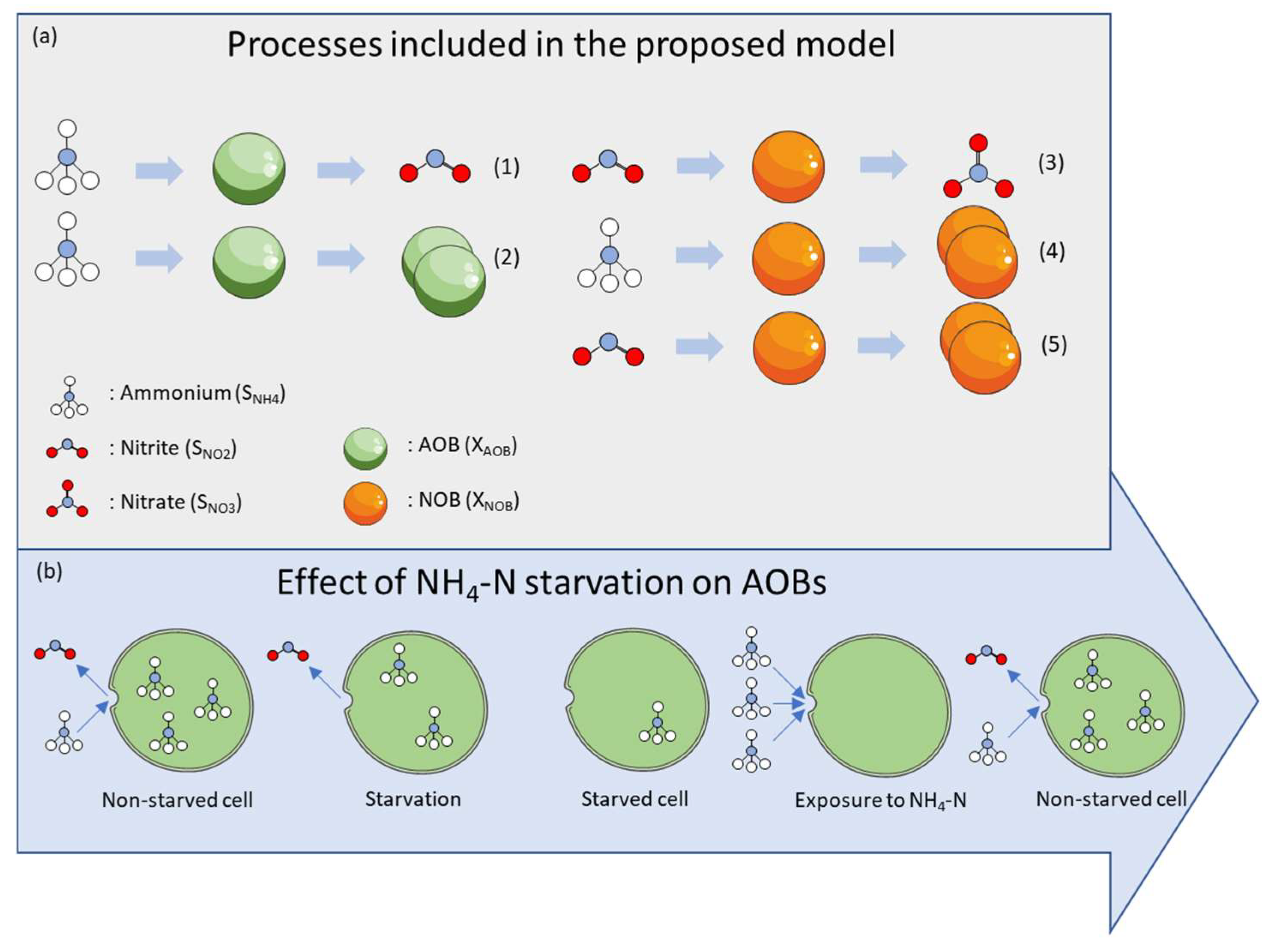
2.3. Model Development
2.4. Numerical Methods
3. Results
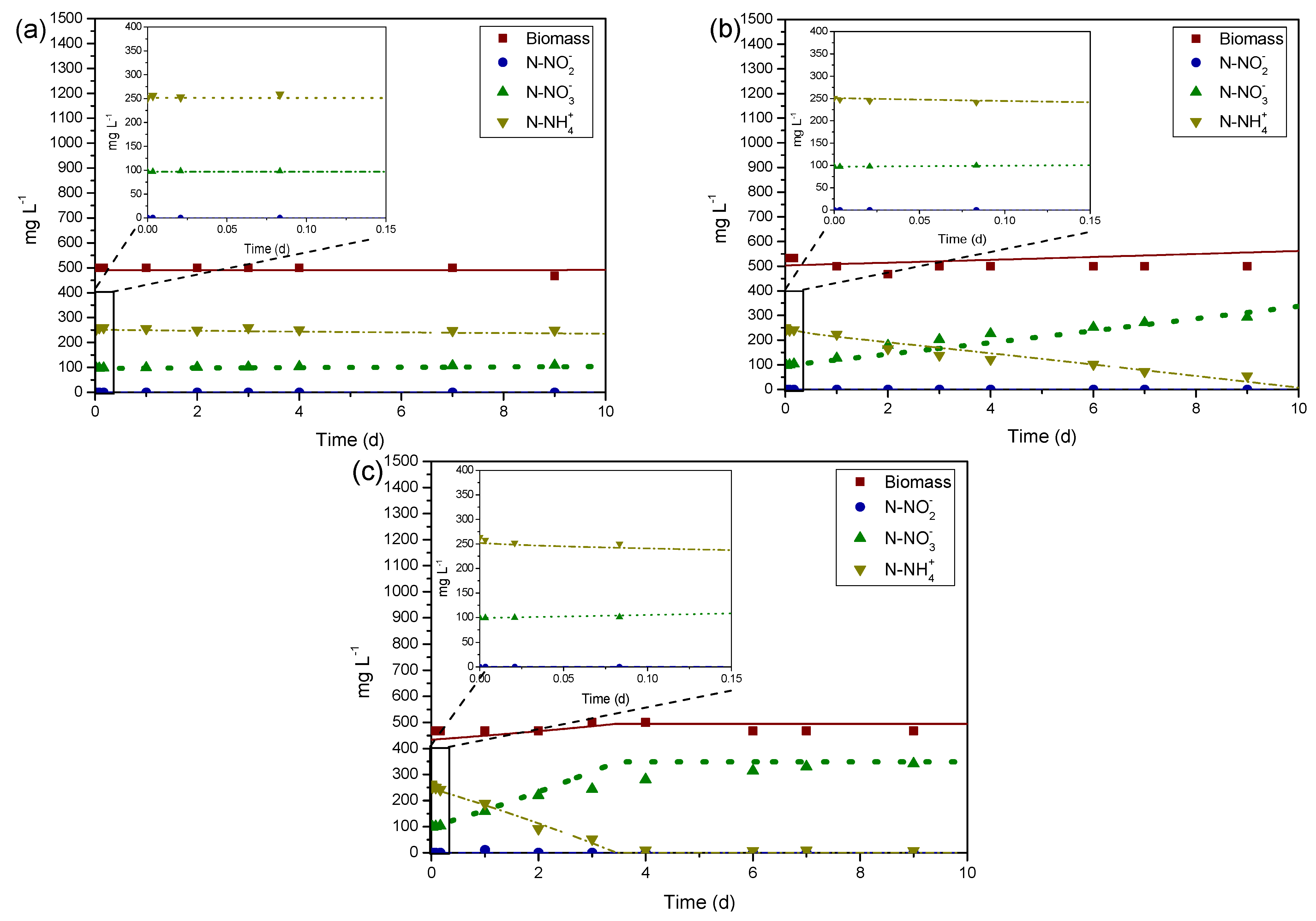
3.1. Determination of Kinetic Parameters—Non-Starved Cultures
3.2. Determination of Kinetic Parameters—Starved Cultures
3.3. Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen Cycling during Wastewater Treatment. Adv. Appl. Microbiol. 2019, 106, 113–192. [Google Scholar]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of Human Modification of the Global Nitrogen Cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef]
- Law, Y.; Ye, L.; Pan, Y.; Yuan, Z. Nitrous Oxide Emissions from Wastewater Treatment Processes. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1265–1277. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W.; Park, J.; Kim, M. Substrate Uptake, Loss, and Reserve in Ammonia-Oxidizing Bacteria (AOB) under Different Substrate Availabilities. Process Biochem. 2020, 91, 303–310. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, D.-C. Nitrite Oxidizing Bacteria (NOB) Dominating in Nitrifying Community in Full-Scale Biological Nutrient Removal Wastewater Treatment Plants. AMB Express 2017, 7, 25. [Google Scholar] [CrossRef]
- Hayden, C.J.; Beman, J.M. High Abundances of Potentially Active Ammonia-Oxidizing Bacteria and Archaea in Oligotrophic, High-Altitude Lakes of the Sierra Nevada, California, USA. PLoS ONE 2014, 9, e111560. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A Model for Molecular Microbial Ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef]
- Mullan, G.D.O.; Ward, B.B. Relationship of Temporal and Spatial Variabilities of Ammonia-Oxidizing Bacteria to Nitrification Rates in Monterey Bay, California. Appl. Environ. Microbiol. 2005, 71, 697–705. [Google Scholar] [CrossRef]
- Cao, H.; Hong, Y.; Li, M.; Gu, J.; Cao, H.; Hong, Y.; Li, M.; Gu, J. Lower Abundance of Ammonia-Oxidizing Archaea than Ammonia-Oxidizing Bacteria Detected in the Subsurface Sediments of the Northern South China Sea. Geomicrobiol. J. 2012, 29, 332–339. [Google Scholar] [CrossRef]
- Pedrouso, A.; Tocco, G.; Val del Río, A.; Carucci, A.; Morales, N.; Campos, J.L.; Milia, S.; Mosquera-Corral, A. Digested Blackwater Treatment in a Partial Nitritation-Anammox Reactor under Repeated Starvation and Reactivation Periods. J. Clean. Prod. 2020, 244, 118733. [Google Scholar] [CrossRef]
- Bollmann, A.; Bär-Gilissen, M.J.; Laanbroek, H.J. Growth at Low Ammonium Concentrations and Starvation Response as Potential Factors Involved in Niche Differentiation among Ammonia-Oxidizing Bacteria. Appl. Environ. Microbiol. 2002, 68, 4751–4757. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Ammonia-Oxidizing Bacteria (AOB): Opportunities and Applications—A Review. Rev. Environ. Sci. Biotechnol. 2018, 17, 285–321. [Google Scholar] [CrossRef]
- Geets, J.; Boon, N.; Verstraete, W. Strategies of Aerobic Ammonia-Oxidizing Bacteria for Coping with Nutrient and Oxygen Fluctuations. FEMS Microbiol. Ecol. 2006, 58, 1–13. [Google Scholar] [CrossRef]
- Bollmann, A.; Schmidt, I.; Saunders, A.M.; Nicolaisen, M.H. Influence of Starvation on Potential Ammonia-Oxidizing Activity and AmoA MRNA Levels of Nitrosospira Briensis. Appl. Environ. Microbiol. 2005, 71, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- French, E.; Bollmann, A. Freshwater Ammonia-Oxidizing Archaea Retain AmoA MRNA and 16S RRNA during Ammonia Starvation. Life 2015, 5, 1396–1404. [Google Scholar] [CrossRef]
- Laanbroek, H.J.; Bär-Gilissen, M.J. Weakened Activity of Starved Ammonia-Oxidizing Bacteria by the Presence of Pre-Activated Nitrobacter Winogradskyi. Microbes Environ. 2002, 17, 122–127. [Google Scholar] [CrossRef]
- Tappe, W.; Laverman, A.; Bohland, M.; Braster, M.; Rittershaus, S.; Groeneweg, J.; Van Verseveld, H.W. Maintenance Energy Demand and Starvation Recovery Dynamics of Nitrosomonas Europaea and Nitrobacter Winogradskyi Cultivated in a Retentostat with Complete Biomass Retention. Appl. Environ. Microbiol. 1999, 65, 2471–2477. [Google Scholar] [CrossRef]
- Jones, R.D.; Morita, R.Y. Survival of a Marine Ammonium Oxidizer Under Energy-Source Deprivation. Mar. Ecol. 1985, 26, 175–179. [Google Scholar] [CrossRef]
- Johnstone, B.H.; Jones, R.D. Recovery of a Marine Chemolithotrophic Ammonium-Oxidizing Bacterium from Long-Term Energy-Source Deprivation. Can. J. Microbiol. 1988, 34, 1347–1350. [Google Scholar] [CrossRef]
- Johnstone, B.; Jones, R. Physiological Effects of Long-Term Energy-Source Deprivation on the Survival of a Marine Chemolithotrophic Ammonium-Oxidizing Bacterium. Mar. Ecol. Prog. Ser. 1988, 49, 295–303. [Google Scholar] [CrossRef]
- Bollmann, A.; Laanbroek, H.J. Continuous Culture Enrichments of Ammonia-Oxidizing Bacteria at Low Ammonium Concentrations. FEMS Microbiol. Ecol. 2001, 37, 211–221. [Google Scholar] [CrossRef]
- Hastings, R.C.; Saunders, J.R.; Hall, G.H.; Pickup, R.W.; McCarthy, A.J. Application of Molecular Biological Techniques to a Seasonal Study of Ammonia Oxidation in a Eutrophic Freshwater Lake. Appl. Environ. Microbiol. 1998, 64, 3674–3682. [Google Scholar] [CrossRef]
- Speksnijder, A.G.C.L.; Kowalchuk, G.A.; Roest, K.; Laanbroek, H.J. Recovery of a Nitrosomonas-like 16S RDNA Sequence Group from Freshwater Habitats. Syst. Appl. Microbiol. 1998, 21, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, L.; Koutra, E.; Tsigkou, K.; Gaspari, M.; Kougias, P.G.; Kornaros, M. Nitrification upon Nitrogen Starvation and Recovery: Effect of Stress Period, Substrate Concentration and PH on Ammonia Oxidizers’ Performance. Fermentation 2022, 8, 387. [Google Scholar] [CrossRef]
- Gujer, W. Nitrification and Me—A Subjective Review. Water Res. 2010, 44, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-J.; Ruscalleda, M.; Pellicer-Nacher, C.; Smets, B.F. Modeling Nitrous Oxide Production during Biological Nitrogen Removal via Nitrification and Denitrification: Extensions to the General ASM Models. Environ. Sci. Technol. 2011, 45, 7768–7776. [Google Scholar] [CrossRef]
- Rausch, T. The Estimation of Micro-Algal Protein Content and Its Meaning to the Evaluation of Algal Biomass I. Comparison of Methods for Extracting Protein. Hydrobiologia 1981, 78, 237–251. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Ostace, G.S.; Cristea, V.M.; Agachi, P.Ş. Extension of Activated Sludge Model No 1 with Two-Step Nitrification and Denitrification Processes for Operation Improvement. Environ. Eng. Manag. J. 2011, 10, 1529–1544. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, Q.; Zu, B.; Bai, C.; Zhang, P. The Influence of Trace NO2 on the Kinetics of Ammonia Oxidation and the Characteristics of Nitrogen Removal from Wastewater. Water Sci. Technol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Giménez, J.B.; Ruano, M.V.; Ferrer, J.; Serralta, J. Effect of PH and Nitrite Concentration on Nitrite Oxidation Rate. Bioresour. Technol. 2011, 102, 8741–8747. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, J.; Yang, R.; Alshammari, J.; Zhu, M.-J.; Sablani, S.; Tang, J. Moisture Content of Bacterial Cells Determines Thermal Resistance of Salmonella Enterica Serotype Enteritidis PT 30. Appl. Environ. Microbiol. 2021, 87, e02194-20. [Google Scholar] [CrossRef] [PubMed]
- Tsafrakidou, P.; Manthos, G.; Zagklis, D.; Mema, J.; Kornaros, M. Assessment of Substrate Load and Process PH for Bioethanol Production–Development of a Kinetic Model. Fuel 2022, 313, 123007. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill Education: New York, NY, USA, 2001; ISBN 1260440591. [Google Scholar]
- Liu, X. Comparing Three Mathematical Models Using Different Substrates for Prediction of Partial Nitrification. Sci. Total Environ. 2020, 749, 141643. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Park, S.; Mo, K.; Lee, W.; Lee, H.; Kim, M. Experimentation and Mathematical Models for Partial Nitrification in Aerobic Granular Sludge Process. KSCE J. Civ. Eng. 2017, 21, 127–133. [Google Scholar] [CrossRef]
- Thalla, A.K.; Bhargava, R.; Kumar, P. Nitrification Kinetics of Activated Sludge-Biofilm System: A Mathematical Model. Bioresour. Technol. 2010, 101, 5827–5835. [Google Scholar] [CrossRef]
- Prosser, J.I. Autotrophic Nitrification in Bacteria. In Advances in Microbial Physiology; Rose, A.H., Tempest, D.W., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 30, pp. 125–181. ISBN 0065-2911. [Google Scholar]




| Parameter | Value | Units |
|---|---|---|
| μmax1,NO2 | 0.158 | d−1 |
| μmax2,NO2 | 0.083 | d−1 |
| μmax,ΝH4 | 0.091 | d−1 |
| qmax,1 | 1.365 | gN-NO3 gbiomass−1 d |
| qmax,2 | 0.788 | gN-NO3 gbiomass−1 d |
| KS,NO2 | 0.003 | gN-NO2 L−1 |
| KS,NH4 | 0.002 | gN-NH4 L−1 |
| YAOB | 0.141 | gbiomass gN-NH4−1 |
| KNH4 | 4 × 10−4 | gN-NH4 L−1 |
| KSS,NH4 | 7.555 | gN-NH4 L−1 |
| A | 6.758 | - |
| K | 7.713 | - |
| KNH3 | 7.819 | gN-NH3 L−1 |
| kN | 25.07 | d−1 |
| kD | 0.099 | gN gN-NH4−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manthos, G.; Abbaszadeh, L.; Zagklis, D.; Kornaros, M. Mathematical Modeling of Nitrification in Mixed Cultures: Insights into Nitrite-Oxidizing Bacteria Growth and Ammonia Starvation Effect. Fermentation 2023, 9, 681. https://doi.org/10.3390/fermentation9070681
Manthos G, Abbaszadeh L, Zagklis D, Kornaros M. Mathematical Modeling of Nitrification in Mixed Cultures: Insights into Nitrite-Oxidizing Bacteria Growth and Ammonia Starvation Effect. Fermentation. 2023; 9(7):681. https://doi.org/10.3390/fermentation9070681
Chicago/Turabian StyleManthos, Georgios, Leila Abbaszadeh, Dimitris Zagklis, and Michael Kornaros. 2023. "Mathematical Modeling of Nitrification in Mixed Cultures: Insights into Nitrite-Oxidizing Bacteria Growth and Ammonia Starvation Effect" Fermentation 9, no. 7: 681. https://doi.org/10.3390/fermentation9070681
APA StyleManthos, G., Abbaszadeh, L., Zagklis, D., & Kornaros, M. (2023). Mathematical Modeling of Nitrification in Mixed Cultures: Insights into Nitrite-Oxidizing Bacteria Growth and Ammonia Starvation Effect. Fermentation, 9(7), 681. https://doi.org/10.3390/fermentation9070681








