Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
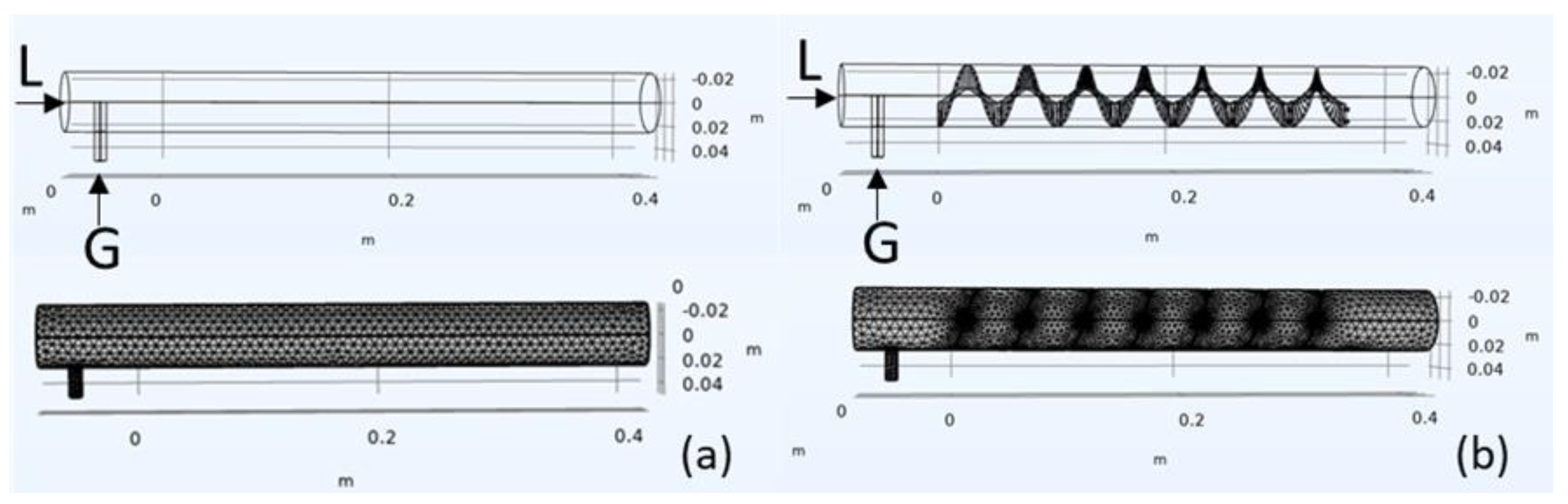
2.1. Computational Model
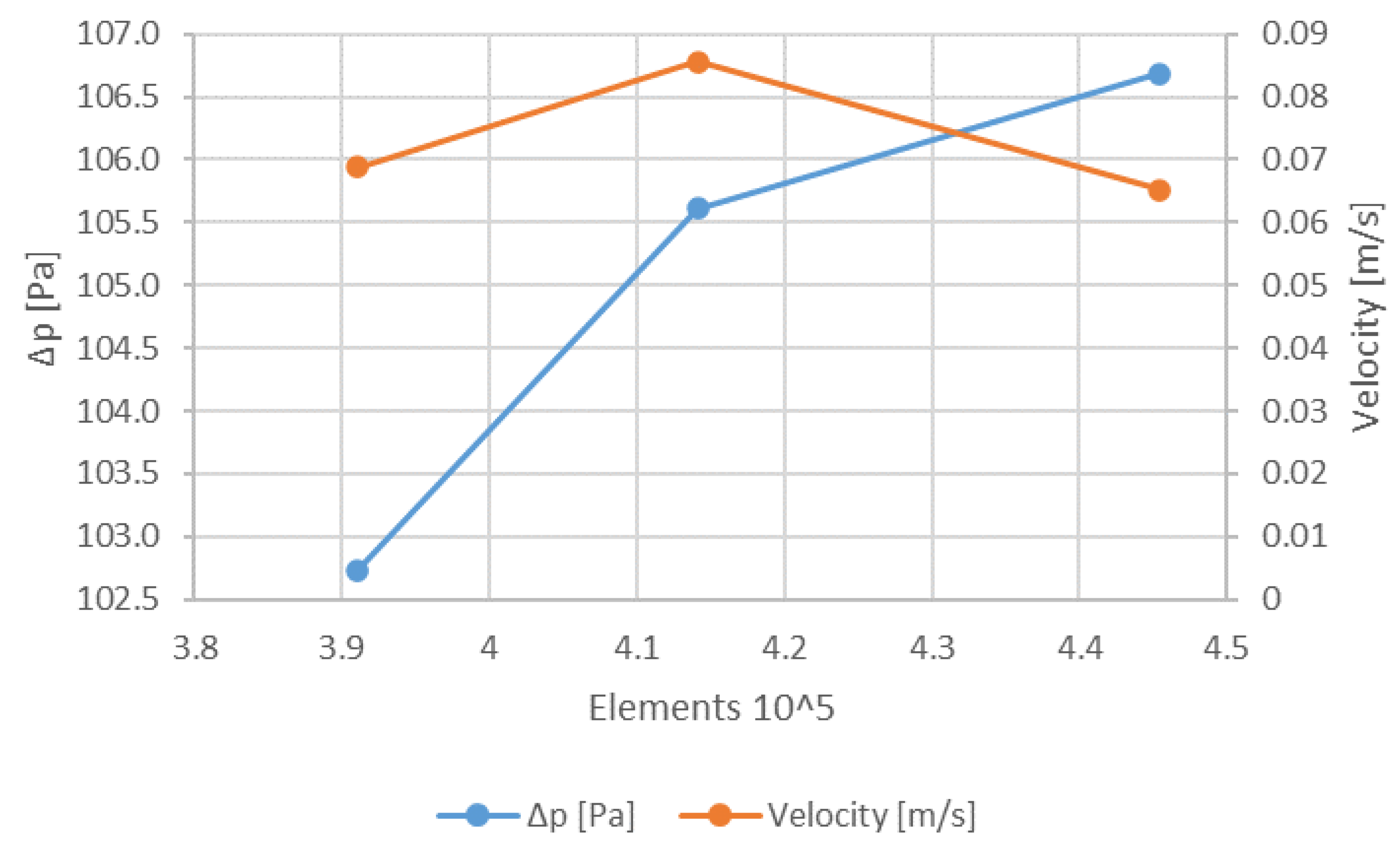
2.2. Mesh Independence Test and Model Validation
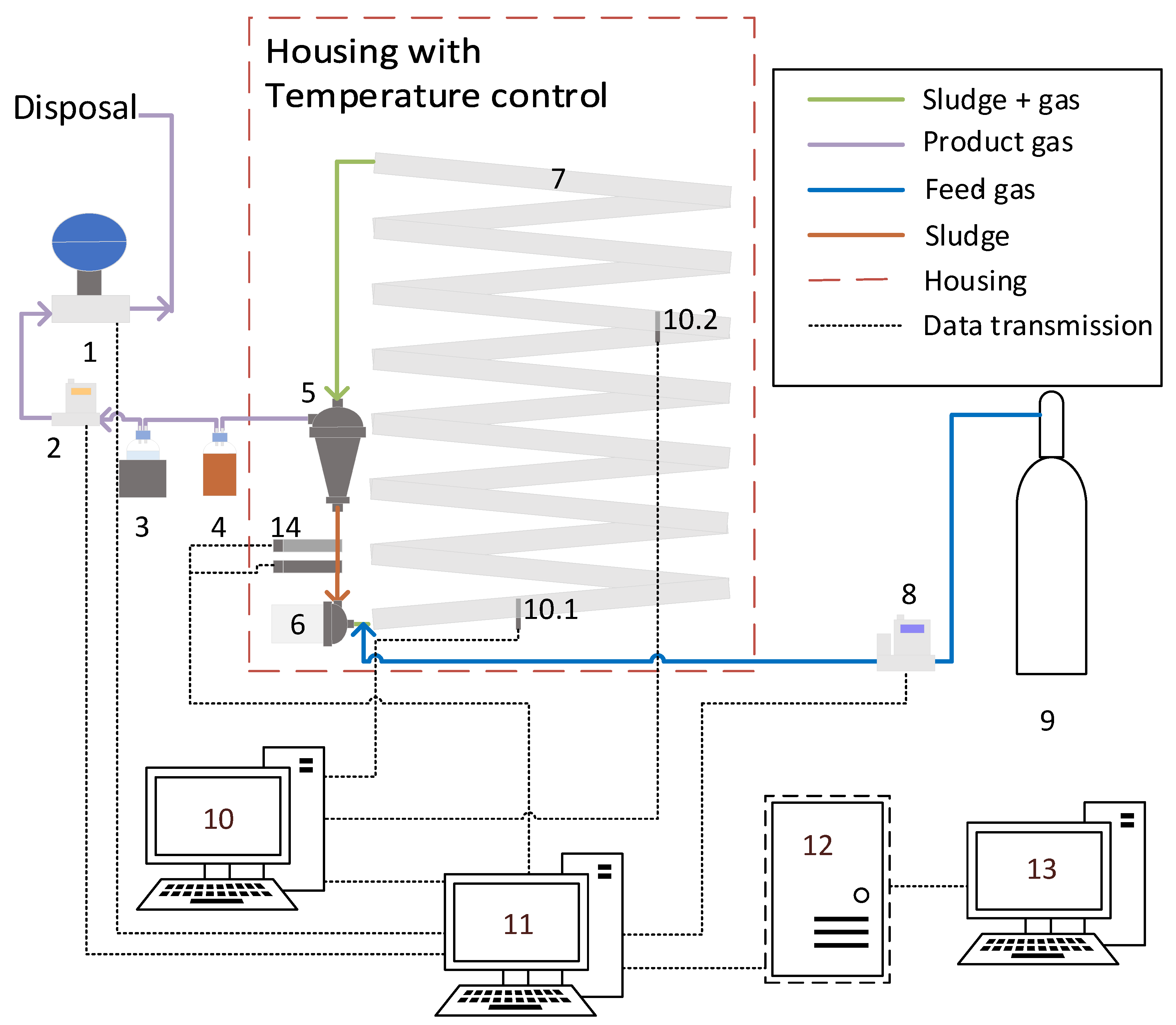
2.3. Experimental Setup
2.4. Construction of the Meandering Column Reactor
2.5. Step Experiment
3. Results and Discussion
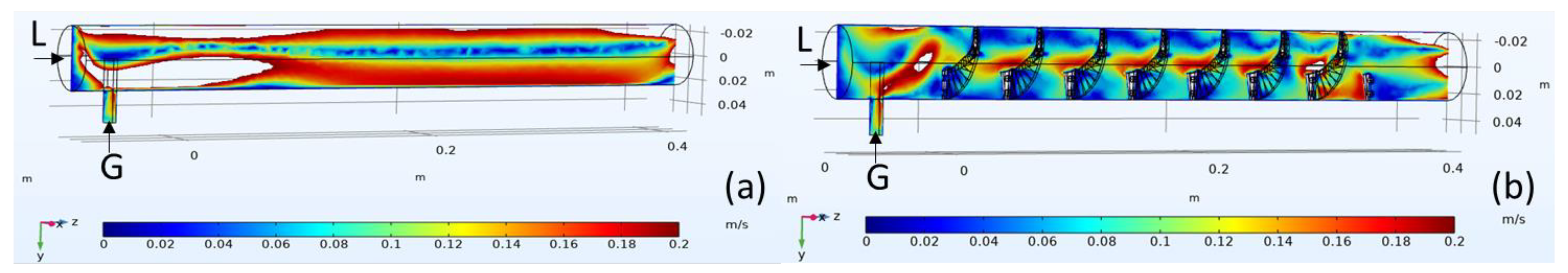
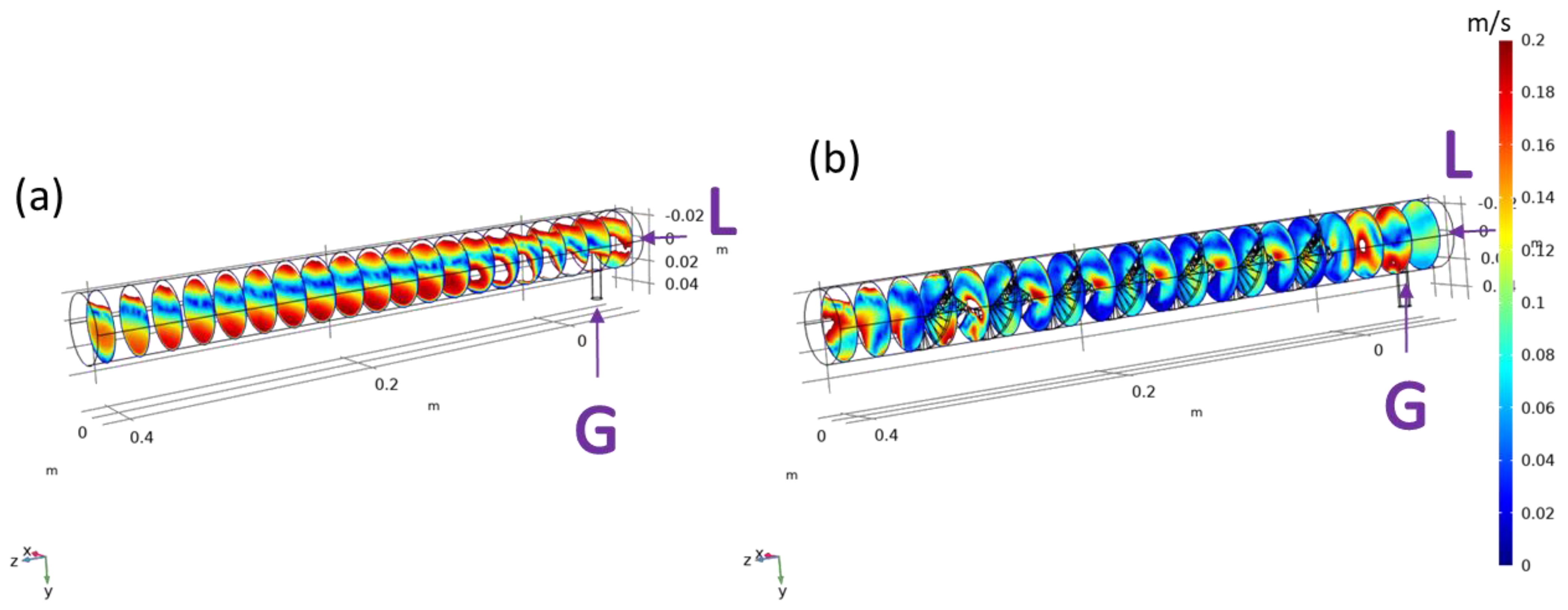
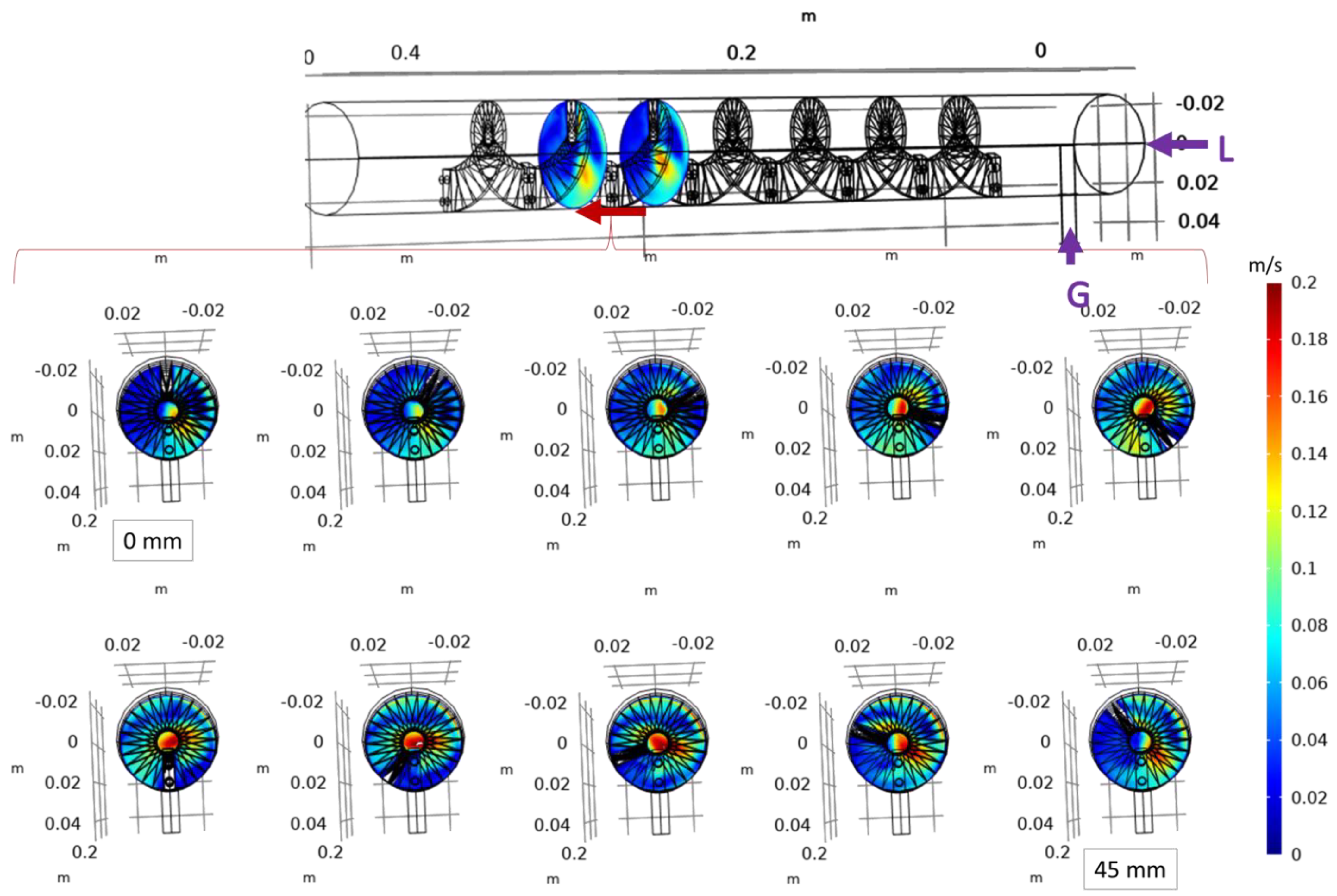
3.1. Computational Model
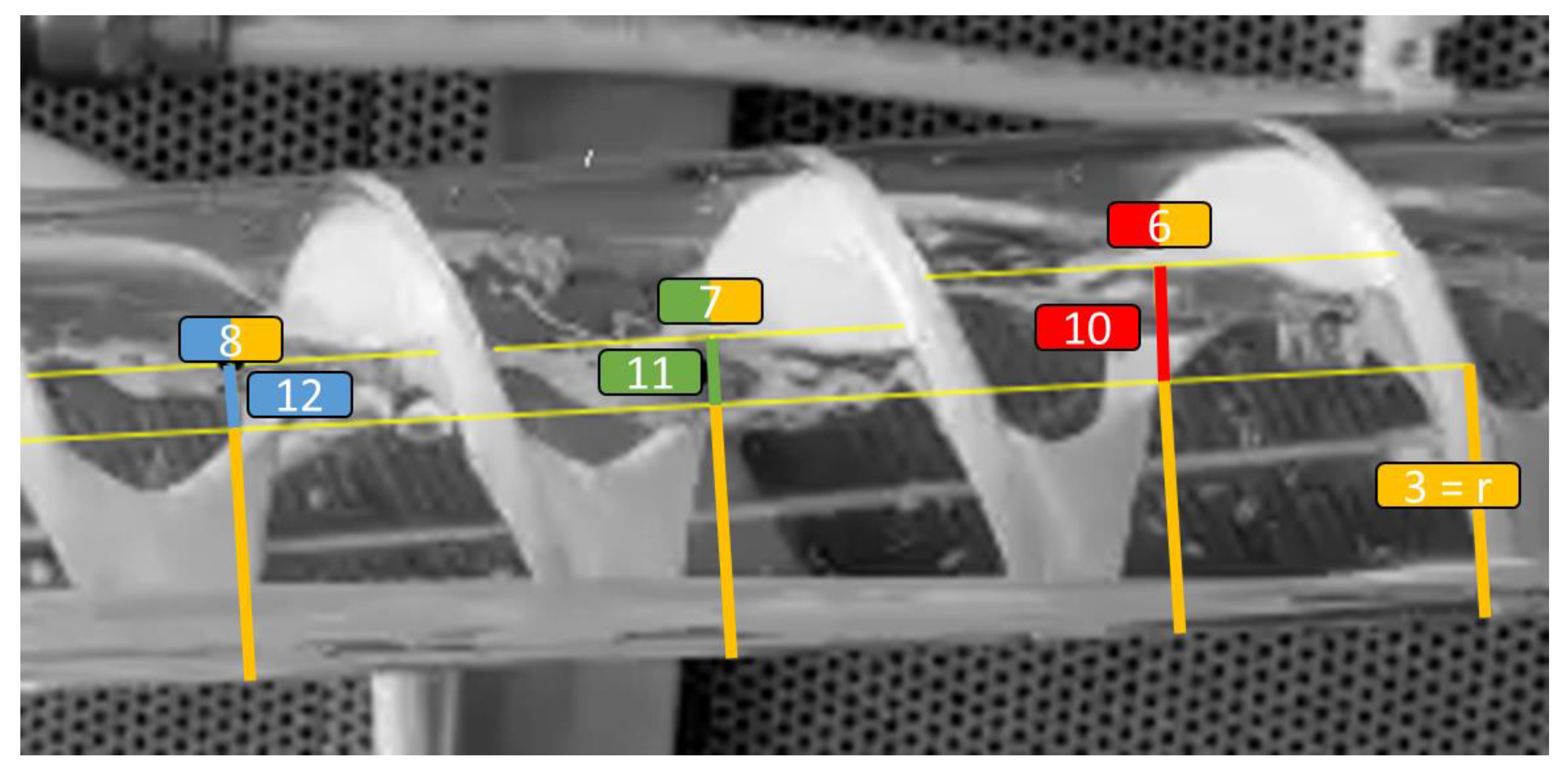
3.2. Experimental Results
3.3. Reactor Setup
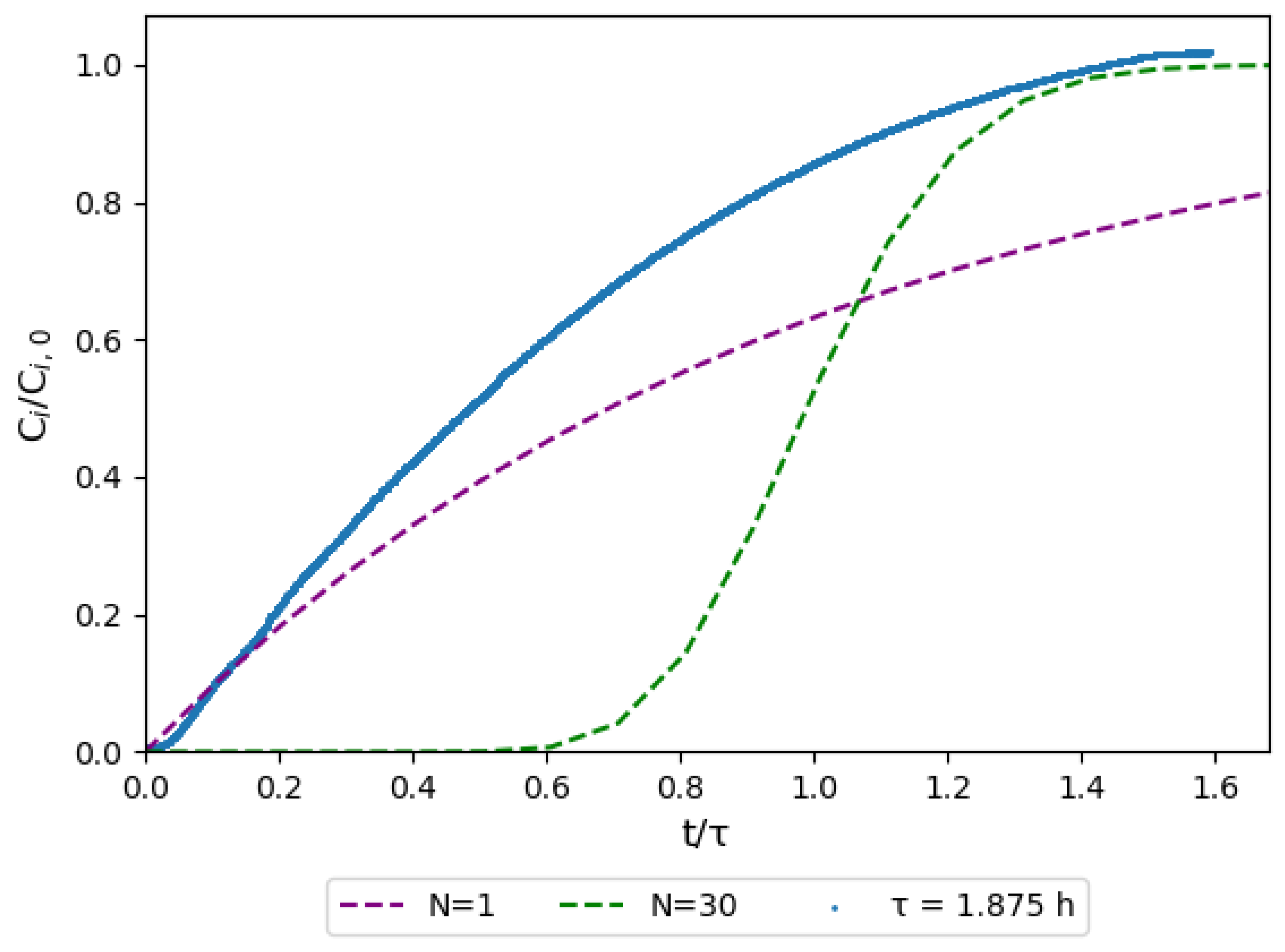
3.4. Step Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Summary for Policymakers. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2022; IPCC: Geneva, Switzerland, 2022; in press.
- International Energy Agency. Electricity Market Report—July 2021; OECD Publishing: Paris, France, 2021.
- Johnson, S.C.; Rhodes, J.D.; Webber, M.E. Understanding the Impact of Non-Synchronous Wind and Solar Generation on Grid Stability and Identifying Mitigation Pathways. Appl. Energy 2020, 262, 114492. [Google Scholar] [CrossRef]
- Maurer, F.; Rieke, C.; Schemm, R.; Stollenwerk, D. Analysis of an Urban Grid with High Photovoltaic and E-Mobility Penetration. Energies 2023, 16, 3380. [Google Scholar] [CrossRef]
- Zhang, M.; Millar, M.-A.; Yu, Z.; Yu, J. An Assessment of the Impacts of Heat Electrification on the Electric Grid in the UK. Energy Rep. 2022, 8, 14934–14946. [Google Scholar] [CrossRef]
- Rekioua, D. Energy Storage Systems for Photovoltaic and Wind Systems: A Review. Energies 2023, 16, 3893. [Google Scholar] [CrossRef]
- Hossain, E.; Faruque, H.; Sunny, M.; Mohammad, N.; Nawar, N. A Comprehensive Review on Energy Storage Systems: Types, Comparison, Current Scenario, Applications, Barriers, and Potential Solutions, Policies, and Future Prospects. Energies 2020, 13, 3651. [Google Scholar] [CrossRef]
- Zakeri, B.; Gissey, G.C.; Dodds, P.E.; Subkhankulova, D. Centralized vs. Distributed Energy Storage—Benefits for Residential Users. Energy 2021, 236, 121443. [Google Scholar] [CrossRef]
- Sterner, M.; Stadler, I. (Eds.) Energiespeicher—Bedarf, Technologien, Integration; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-48892-8. [Google Scholar]
- Ueckerdt, F.; Bauer, C.; Dirnaichner, A.; Everall, J.; Sacchi, R.; Luderer, G. Potential and Risks of Hydrogen-Based e-Fuels in Climate Change Mitigation. Nat. Clim. Chang. 2021, 11, 384–393. [Google Scholar] [CrossRef]
- Ogden, J.; Jaffe, A.M.; Scheitrum, D.; McDonald, Z.; Miller, M. Natural Gas as a Bridge to Hydrogen Transportation Fuel: Insights from the Literature. Energy Policy 2018, 115, 317–329. [Google Scholar] [CrossRef]
- You, Y.; Kim, S.; Lee, J.C. Comparative Study on Ammonia and Liquid Hydrogen Transportation Costs in Comparison to LNG. Int. J. Nav. Archit. Ocean Eng. 2023, 15, 100523. [Google Scholar] [CrossRef]
- Evans, P.N.; Boyd, J.A.; Leu, A.O.; Woodcroft, B.J.; Parks, D.H.; Hugenholtz, P.; Tyson, G.W. An Evolving View of Methane Metabolism in the Archaea. Nat. Rev. Microbiol. 2019, 17, 219–232. [Google Scholar] [CrossRef]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 Ratio and H2 Supply Fluctuation on Methane Content and Microbial Community Composition during in-Situ Biological Biogas Upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef]
- Logroño, W.; Kleinsteuber, S.; Kretzschmar, J.; Harnisch, F.; De Vrieze, J.; Nikolausz, M. The Microbiology of Power-to-X Applications. FEMS Microbiol. Rev. 2023, 47, fuad013. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Horn, S.J. Impact of Operational Conditions on Methane Yield and Microbial Community Composition during Biological Methanation in in Situ and Hybrid Reactor Systems. Biotechnol. Biofuels 2021, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, W.; Bockrath, R.; De Wever, H. Effects of Moderately Elevated Pressure on Gas Fermentation Processes. Bioresour. Technol. 2019, 293, 122129. [Google Scholar] [CrossRef] [PubMed]
- Ale Enriquez, F.; Ahring, B.K. Strategies to Overcome Mass Transfer Limitations of Hydrogen during Anaerobic Gaseous Fermentations: A Comprehensive Review. Bioresour. Technol. 2023, 377, 128948. [Google Scholar] [CrossRef] [PubMed]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological Methanation of CO2 in a Novel Biofilm Plug-Flow Reactor: A High Rate and Low Parasitic Energy Process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Rusmanis, D.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Biological Hydrogen Methanation Systems—An Overview of Design and Efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef]
- Jensen, M.B.; Ottosen, L.D.M.; Kofoed, M.V.W. H2 Gas-Liquid Mass Transfer: A Key Element in Biological Power-to-Gas Methanation. Renew. Sustain. Energy Rev. 2021, 147, 111209. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Savari, C. Determination of Interfacial Area and Overall Volumetric Mass-Transfer Coefficient in a Novel Type of Two Impinging Streams Reactor by Chemical Method. Ind. Eng. Chem. Res. 2011, 50, 6426–6435. [Google Scholar] [CrossRef]
- Logroño, W.; Popp, D.; Nikolausz, M.; Kluge, P.; Harms, H.; Kleinsteuber, S. Microbial Communities in Flexible Biomethanation of Hydrogen Are Functionally Resilient Upon Starvation. Front. Microbiol. 2021, 12, 619632. [Google Scholar] [CrossRef]
- Jønson, B.D.; Tsapekos, P.; Tahir Ashraf, M.; Jeppesen, M.; Ejbye Schmidt, J.; Bastidas-Oyanedel, J.-R. Pilot-Scale Study of Biomethanation in Biological Trickle Bed Reactors Converting Impure CO2 from a Full-Scale Biogas Plant. Bioresour. Technol. 2022, 365, 128160. [Google Scholar] [CrossRef] [PubMed]
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Anaerobic Thermophilic Trickle Bed Reactor as a Promising Technology for Flexible and Demand-Oriented H2/CO2 Biomethanation. Appl. Energy 2018, 232, 543–554. [Google Scholar] [CrossRef]
- Youssef, A.A.; Al-Dahhan, M.H.; Dudukovic, M.P. Bubble Columns with Internals: A Review. Int. J. Chem. React. Eng. 2013, 11, 169–223. [Google Scholar] [CrossRef]
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble Column Reactors. Process Biochem. 2005, 40, 2263–2283. [Google Scholar] [CrossRef]
- Nandagopal, N.S. Chemical Engineering Principles and Applications; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-27878-5. [Google Scholar]
- Valdés, J.P.; Kahouadji, L.; Matar, O.K. Current Advances in Liquid–Liquid Mixing in Static Mixers: A Review. Chem. Eng. Res. Des. 2022, 177, 694–731. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bahadori, A. Single-Phase and Multiphase Flow in Natural Gas Production Systems. In Natural Gas Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 59–150. ISBN 978-0-08-099971-5. [Google Scholar]
- Shin, W.-S. Application of Scale-Up Criterion of Constant Oxygen Mass Transfer Coefficient (KLa) for Production of Itaconic Acid in a 50 L Pilot-Scale Fermentor by Fungal Cells of Aspergillus Terreus. J. Microbiol. Biotechnol. 2013, 23, 1445–1453. [Google Scholar] [CrossRef]
- Tardy, P.M.; Flamant, N.C.; Lac, E.; Parry, A.; Sutama, C.S.; Almagro, S.P. New Generation 3D Simulator Predicts Realistic Mud Displacement in Highly Deviated and Horizontal Wells. In Proceedings of the Day 2 Wed, The Hague, The Netherlands, 14–15 March 2017; p. D021S011R004. [Google Scholar]
- Kockmann, N. Entwurf und Betrieb eines Rohrreaktors mit enger Verweilzeitverteilung. Chem. Ing. Tech. 2020, 92, 685–691. [Google Scholar] [CrossRef]
- Grühn, J.; Behr, A.S.; Eroglu, T.H.; Trögel, V.; Rosenthal, K.; Kockmann, N. From Coiled Flow Inverter to Stirred Tank Reactor—Bioprocess Development and Ontology Design. Chem. Ing. Tech. 2022, 94, 852–863. [Google Scholar] [CrossRef]
- Hobbs, D.M.; Muzzio, F.J. Reynolds Number Effects on Laminar Mixing in the Kenics Static Mixer. Chem. Eng. J. 1998, 70, 93–104. [Google Scholar] [CrossRef]
- Meng, H.; Jiang, X.; Yu, Y.; Wang, Z.; Wu, J. Laminar Flow and Chaotic Advection Mixing Performance in a Static Mixer with Perforated Helical Segments. Korean J. Chem. Eng. 2017, 34, 1328–1336. [Google Scholar] [CrossRef]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2021, 12, 5259–5282. [Google Scholar] [CrossRef]
- Petersen, L.A.H.; Villadsen, J.; Jørgensen, S.B.; Gernaey, K.V. Mixing and Mass Transfer in a Pilot Scale U-Loop Bioreactor: Mixing and Mass Transfer in a Pilot Scale U-Loop Bioreactor. Biotechnol. Bioeng. 2017, 114, 344–354. [Google Scholar] [CrossRef] [PubMed]


| Properties and Settings | Boundary Conditions | ||
|---|---|---|---|
| Variable | Value | Boundary | Velocity (V) |
| Temperature | T = 313.15 K | Inlet A (liquid) | = 0.02 m/s |
| Liquid density | ρ1 = 992.63 kg/m3 | Inlet B (gas) | = 0.1 m/s |
| Liquid dynamic viscosity | μ1 = 6.66 × 10−4 Pa·s | Wall | No slip condition, = 0 |
| Gas density | ρ2 = 0.441 kg/m3 | ||
| Gas dynamic viscosity | μ2= 1.05 × 10−5 Pa·s | ||
| Gravity (y-component) | g = −9.806 m/s2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffstadt, K.; Cheenakula, D.; Nikolausz, M.; Krafft, S.; Harms, H.; Kuperjans, I. Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen. Fermentation 2023, 9, 774. https://doi.org/10.3390/fermentation9080774
Hoffstadt K, Cheenakula D, Nikolausz M, Krafft S, Harms H, Kuperjans I. Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen. Fermentation. 2023; 9(8):774. https://doi.org/10.3390/fermentation9080774
Chicago/Turabian StyleHoffstadt, Kevin, Dheeraja Cheenakula, Marcell Nikolausz, Simone Krafft, Hauke Harms, and Isabel Kuperjans. 2023. "Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen" Fermentation 9, no. 8: 774. https://doi.org/10.3390/fermentation9080774
APA StyleHoffstadt, K., Cheenakula, D., Nikolausz, M., Krafft, S., Harms, H., & Kuperjans, I. (2023). Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen. Fermentation, 9(8), 774. https://doi.org/10.3390/fermentation9080774






