Abstract
This study reports the results of an evaluation of the techno-economic feasibility of a biorefinery with an annual lactic acid production capacity of 100,000 metric tons using lignocellulosic biomass. Corn stover and miscanthus were considered as model feedstocks, and three different fermentation pathways involving bacteria, fungi, and yeast were compared with respect to their ability to convert biomass feedstocks to lactic acid. Equipment, raw materials, utilities and labor requirements, and lactic acid production costs were estimated. The minimum selling price (at a 10% internal rate of return) per metric ton of lactic acid produced from different feedstocks for lactic acid bacteria, fungi, and yeast-based pathways were in the range of USD 1243–1390, USD 1250–1392, and USD 993–1123, respectively, with lower costs for miscanthus. Lactic acid production using genetically engineered yeast strains can eliminate the need for the simultaneous neutralization and recovery of lactic acid, resulting in lower equipment, chemical, and utility requirements and lower lactic acid production costs. Lactic acid production costs were highly sensitive to the conversion rates of sugars into lactic acid, feedstock cost, production plant size, operation hours, and acid hydrolysis reactor costs. Improvements in process conditions and efficiencies and lower costs of equipment and consumables are necessary to utilize lignocellulosic biomass for lactic acid production at lower costs while remaining cost-competitive with respect to first-generation and petroleum-based feedstocks.
1. Introduction
Lactic acid (2-hydroxypropanoic acid; CH3–CH(OH)–COOH) is an organic acid found in many organisms and natural products [1]. Lactic acid is one of the most important acids in the industry due to its widespread application as a flavoring agent, bacterial inhibitor, and acidulant and its capacity to be converted to other useful products, such as esters, bio-solvents, and polymers [2,3]. It has been widely used in the food, pharmaceutical, cosmetic, and leather industries for several decades [4]. Lactic acid has also been identified as an important platform chemical that can be further converted into other important chemicals [5]. In recent years, the production of lactic-acid-derived biodegradable poly-lactic acid and the environmentally benign solvent ethyl lactate has given rise to numerous potential applications for lactic acid, constituting an industry with a projected growth of more than 400% by 2025 compared to 2016 [6,7,8]. The U.S. Department of Energy’s National Renewable Energy Laboratory (NREL) and Pacific Northwest National Laboratory (PNNL) identified a few promising platform chemicals for biorefinery applications, one of which was lactic acid [9,10].
Lactic acid can be produced either through the chemical synthesis of petroleum derivatives or the biotechnological conversion of biobased feedstocks [11]. Lactic acid can be produced from petrochemical sources following the hydrolysis of lactonitrile with a strong acid. The lactic acid and byproduct mixture is then esterified using methanol to produce methyl lactate, which is hydrolyzed to obtain lactic acid [2,12,13]. However, this petroleum-based route can only produce a racemic mixture of L- and D-lactic acid, constituting a major disadvantage of this approach. Optically pure L- or D-lactic acid can produce poly-lactic acid with higher crystallinity and a higher melting point than the racemic mixture [14,15]. Furthermore, the production of lactic acid using the petroleum-based pathway requires pure forms of lactonitrile and sulfuric acid, thus substantially increasing the production cost of the resulting lactic acid [6,16]. In addition, the limited reserves of fossil fuels that must be drawn upon to employ this pathway render it an unsustainable approach for lactic acid production.
In recent years, lactic acid production via the fermentation of renewable raw materials containing sugars has gained more attention from the research community and the lactic acid industry [11,17,18]. Compared to the petroleum route, the microbial lactic acid fermentation route utilizes renewable biomass, requires low production temperatures and lower amounts of energy, and produces single-isomer lactic acid at high purity [19]. The ability to produce pure L-lactic acid is important for the food and pharmaceutical industries because the human body can only incorporate L-lactic acid and D-lactic acid can be harmful to humans [12,17,20]. Presently, more than 90% of lactic acid produced globally is manufactured through the microbial fermentation pathway [13]. Biobased lactic acid is one of the few chemicals that holds great potential to be utilized for a variety of applications.
Lactic acid can be produced from different biomass sources, which include starch and sugar-based feedstocks such as corn grain, sugarcane, and lignocellulosic feedstocks including corn stover and miscanthus. Lactic acid production pathways differ for different biomass feedstocks. Lignocellulosic feedstock requires pretreatment and enzymatic hydrolysis to release the sugars prior to fermentation. In addition, different microorganisms, including lactic acid bacteria, fungi, or yeast strains, can be used for the fermentation of the sugars into lactic acid [5]. The lactic acid yield varies depending on the types of microorganisms and sugar used for fermentation [12]. Also, differences exist in the techniques used for the purification and recovery of lactic acid based on the microorganisms used for fermentation.
Many experimental studies [21,22,23,24,25] have shown that lactic acid can be produced from lignocellulosic feedstocks. However, there are only a few studies [26,27] on the techno-economic feasibility of this process and the potential of lignocellulosic feedstocks to be converted into lactic acid using different fermentation microorganisms. The corresponding lactic acid production costs and economic feasibility depend on the cost of the feedstocks, the pretreatment approach employed, the fermentation organisms used, and the feedstock-to-lactic-acid conversion yields. Prior research on the techno-economic feasibility of producing lactic acid from sugarcane bagasse indicated that cellulose-based processes lead to higher rates of lactic acid production and, consequently, lower costs compared to hemicellulose-based processes, while a yeast-based pathway in which the production of gypsum was avoided had the lowest cost [26]. Since lactic acid has enormous growth potential, exploring the techno-economic feasibility of different pathways could contribute to cost-effective lactic acid production from different biobased feedstocks. Incorporating multiple feedstock sources in a biorefinery would reduce the risks associated with the shortage of the supply of feedstocks for biorefineries in the future. Thus, the objective of this study was to evaluate the techno-economic feasibility of lactic acid production using a lignocellulosic feedstock. Corn stover and miscanthus were selected as the feedstocks for techno-economic modelling due to their potential in terms of being the main lignocellulosic feedstock for biobased industries in the U.S. in the near future [28]. This study considered three fermentation pathways using one of the three different lactic acid producing microorganisms: (1) bacteria, (2) fungi, or (3) yeast.
2. Materials and Methods
2.1. System Overview
The annual capacity of the existing petroleum- and starch-based lactic acid production facilities vary from a few thousand to about 140,000 t [29]. Larger lactic acid production facilities with production capacities above 75,000 t have been established in recent years [30,31], and the lactic acid market is expected to grow substantially in the future [8]. Thus, a lignocellulosic-feedstock-based lactic acid biorefinery with an annual production capacity of 100,000 t, which is on the higher end of the capacity scale for existing petroleum- and starch-based biorefineries, was selected for this analysis.
The two feedstocks considered for this analysis were corn stover and miscanthus. These feedstocks were selected based on their availability and potential to be utilized as lignocellulosic feedstocks for lactic acid biorefinery in current and future scenarios according to the Billion Ton Report and other studies [21,22,24,25,28]. The cellulose, hemi-cellulose, and lignin compositions of corn stover and miscanthus considered for this analysis are provided in Table 1.
This study analyzed three pathways for fermentation using (1) lactic acid bacteria (LAB), (2) fungi, or (3) yeast. Traditionally, lactic acid has been produced from bacteria belonging to the genera Lactobacillus, Lactococcus, Streptococcus, Bacillus, and Enterococcus. The optimal production conditions for bacterial lactic acid fermentation include a pH in the range of 5 to 7 and a temperature in the 30–45 °C range [12,20]. The lactic acid yields and productivity of the LAB-based process are usually higher compared to those associated with fungal production (Table 1). However, this fermentation pathway (using bacteria for lactic acid production) has limitations. LAB require more nutrients, an organic source of nitrogen, and sterile conditions for operation [32]. Also, the lactic acid produced from the process needs to be neutralized in order to maintain the pH levels required for effective fermentation and recovery in the downstream processes [12,33,34]. Fungal species of the Rhizopus genus can also be used to convert the sugars in biomass to produce lactic acid. The use of fungi during fermentation offers several advantages compared to the use of LAB, such as their amylolytic characteristics enabling them to ferment both hexose and pentose sugars and low nutrient requirements resulting in minimal recovery and purification steps [35]. Studies have indicated that Rhizopus strains present better growth under nutrient-limited environments than LAB [36,37]. However, the corresponding lactic acid yield is usually lower, as other products, such as ethanol and fumaric acid, are also formed during fermentation [38]. In addition, the fermentation broth also needs to be aerated adequately to achieve higher lactic acid yields. The final fermentation pathway considered for this analysis consisted of the use of yeast for the fermentation of feedstock into lactic acid. Cargill Inc. has identified a yeast strain that can tolerate low pH during lactic acid fermentation [31]. This eliminates the need to neutralize the lactic acid as it is produced and discards the recovery step. Furthermore, the yeast strains selected using targeted engineering have higher lactic acid yields (Table 1) [31].
2.2. Discrete Production Processes, Sections, and Data Sources
The process model included all the unit operations required to convert the lignocellulosic feedstocks (corn stover and miscanthus) into lactic acid, which are grouped into multiple discrete sections: feedstock preparation, pretreatment, fermentation, product recovery, and stillage utilization (Figure 1). The different assumptions, distinct sections, and processes considered for this study are discussed in the following sections (Table 1).
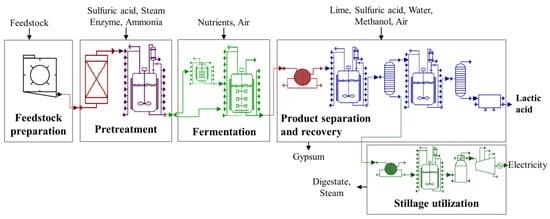
Figure 1.
Overview of steps in the conversion of lignocellulosic feedstock into lactic acid.

Table 1.
Technical parameters considered for lactic acid production and their ranges of values used for sensitivity analysis.
Table 1.
Technical parameters considered for lactic acid production and their ranges of values used for sensitivity analysis.
| Parameters | Unit | Average Value | Pessimistic Value | Optimistic Value |
|---|---|---|---|---|
| Plant size | t/year | 100,000 | 80,000 | 120,000 |
| Annual operation hours | h | 7920 | 7560 | 8280 |
| Feedstock cost (corn stover) [39,40] | USD/t | 119 | 150 | 87 |
| Feedstock cost (miscanthus) [41,42] | USD/t | 89 | 116 | 66 |
| Feedstock moisture content | % | 20 | 25 | 15 |
| Cellulose content in corn stover [43,44] | % | 38 | 35 | 40 |
| Cellulose content in miscanthus [45,46,47,48,49] | % | 46.7 | 35.1 | 52.2 |
| Hemi-cellulose content in corn stover [43,44] | % | 30 | 17 | 35 |
| Hemi-cellulose content in miscanthus [45,46,47,48,49] | % | 29.3 | 19.2 | 34.0 |
| Lignin content in corn stover [43,44] | % | 18 | 7 | 21 |
| Lignin content in miscanthus [45,46,47,48,49] | % | 11.3 | 9.2 | 16.5 |
| Pretreatment—Acid hydrolysis | ||||
| Cellulose to glucose conversion [50] | % | 9.9 | ||
| Hemicellulose to xylose conversion [50] | % | 90 | ||
| Lignin to soluble lignin [50] | % | 5 | ||
| Sulfuric acid cost [51] | USD/t | 70 | 94 | 57 |
| Acid hydrolysis reactor cost [50] | USD | 19,812,400 | 23,774,880 | 15,849,920 |
| Pretreatment—Enzymatic hydrolysis | ||||
| Residence time for enzymatic hydrolysis [50] | h | 84 | 100 | 60 |
| Enzymatic hydrolysis temperature [50] | °C | 45 | ||
| Total solids loading [50] | % | 20 | ||
| Cellulase loading (mg enzyme protein/g cellulose) [50] | mg | 20 | ||
| Cellulose to glucose conversion [50] | % | 95 | ||
| Hemicellulose to xylose conversion [50] | % | 60 | ||
| Enzyme cost [52] | USD/t | 10.15 | 15 | 5 |
| Ammonia cost [53] | USD/t | 496 | 771 | 385 |
| Enzymatic hydrolysis reactor cost [50] | USD | 837,000 | 1,004,400 | 669,600 |
| Fermentation | ||||
| Fermentation duration [50] | h | 48 | 65 | 30 |
| Glucose to lactic acid conversion using LAB [54,55] | % | 90 | 80 | 95 |
| Xylose to lactic acid conversion using LAB [21,56] | % | 70 | 60 | 85 |
| Glucose to lactic acid conversion using fungi [38,57] | % | 85 | 75 | 92 |
| Xylose to lactic acid conversion using fungi [23,25] | % | 80 | 70 | 88 |
| Glucose to lactic acid conversion using yeast [31,58] | % | 93 | 85 | 95 |
| Xylose to lactic acid conversion using yeast [19] | % | 60 | 46.4 | 69.6 |
| Nutrient use [50] | % | 0.35 | ||
| Nutrient cost [50] | USD/kg | 0.05 | 0.02 | 0.08 |
| Diammonium phosphate fraction [50] | % | 0.6 | ||
| Fermentation reactor cost [50] | USD | 837,000 | 1,004,400 | 669,600 |
| Product recovery | ||||
| Lime cost [59] | USD/t | 110 | 150 | 90 |
| Gypsum use cost * | USD/t | −50 | −100 | 8 |
| Distillation temperature (after esterification) [34] | °C | 101 | ||
| Distillation temperature (after hydrolysis) [34] | °C | 66 | ||
| Drying temperature [34] | °C | 150 | ||
| Methanol cost [60] | USD/t | 442 | 530.4 | 353.6 |
| Stillage utilization | ||||
| Anaerobic digestion temperature [61] | °C | 35 | ||
| Overall heat loss during steam generation [62] | % | 5 | ||
| Multistage turbine efficiencies [62] | ||||
| Stage 1 | % | 67 | ||
| Stage 2 | % | 60 | ||
| Power generation efficiency [62] | % | 90 |
Note: * Gypsum management approach. Gypsum can be either disposed of (shown as negative value) or utilized as a byproduct (shown as positive value) [63,64].
2.2.1. Feedstock Collection and Preparation
This study considered feedstocks with 20% average moisture content that are intended to be delivered to the production facility in bale format. The costs of the biorefinery gate-delivered feedstocks (Table 1) are estimated by considering the size of the lactic acid biorefinery established in the Midwestern U.S. and the costs of different production, harvest, and post-harvest operations, obtained from the literature, for delivering the feedstocks to a lactic acid biorefinery of the selected size. The potential yields of feedstocks are also considered to estimate the feedstock delivery costs. After the feedstocks are unloaded in the biorefinery, the corn stover and miscanthus feedstocks are stored in a biorefinery yard. The feedstocks are then ground to a particle size less than 6 mm using a hammer mill, which reduces the crystallinity of cellulose and improves thee feedstocks’ digestibility during the hydrolysis process [50]. This study assumed that the cellulose, hemicellulose, lignin, and ash fractions of the feedstock were within the ranges reported for corn stover and miscanthus (Table 1) [43,44,45,46,65].
2.2.2. Pretreatment
The feedstocks considered in this study are composed of complex carbohydrates that need to be broken down to their monomeric sugars before they can be fermented into lactic acid. Pretreatment breaks the lignin barriers and the polymeric bonds in the carbohydrates present in the feedstocks and renders the sugars readily available for fermentation. Among the different pretreatment methods available, the dilute acid pretreatment process is widely used in commercial production, mainly due to its lower cost and high sugar yields [66,67]; thus, it was considered for this study. Sulfuric acid concentrations of 0.5–2 wt.% were used for pretreatment of biomass based on different literature [68,69,70,71]. However, a sulfuric acid concentration of 1 wt.% and a solid loading ratio of 30 wt.% were considered for this study based on the recommendations from studies conducted by NREL [50,66]. The process conditions and conversion rates for cellulose and hemicellulose to glucose and xylose for acid hydrolysis were obtained from data on pretreatment and hydrolysis of biomass feedstocks from recent research [50,66,72] and are summarized in Table 1.
The pretreated slurry was then conditioned in a reactor for 30 min, where it was treated with ammonia to increase its pH to 5–6, which is a suitable range for enzymatic hydrolysis [50]. Despite its considerably higher cost, ammonia is preferred over lime for the conditioning of the hydrolysate slurry because it is more effective at conditioning due to its higher miscibility, its reduced propensity to degrade sugars (1–2% sugar loss) in the slurry compared to liming (up to 13% sugar loss), and the fact that is eliminates the separation step that would be required for removing gypsum produced while conditioning with lime [50].
The conditioned slurry includes complex carbohydrates, which are then hydrolyzed into soluble sugars using enzymes; subsequently, these sugars can be readily fermented by different microorganisms. Cellulases and hemicellulases are two general categories of enzymes used for enzymatic hydrolysis. A mixture of cellulases and hemicellulases are considered for effective hydrolysis leading to decreased hydrolysis times and process costs [73,74]. Sugar conversion rates during enzymatic hydrolysis (Table 1) were obtained from recent studies [50].
2.2.3. Fermentation
The hydrolysate obtained after the hydrolysis of lignocellulosic biomass includes a mixture of cellulose and hemicellulose-derived sugars and traces of furfural, acetic acid, and 5-hydroxymethyl furfural, which are fermented in presence of either bacteria, fungi, or yeasts to produce lactic acid. As lactic acid is formed during fermentation, the pH of the fermentation slurry progressively decreases, which can potentially affect the lactic acid yield depending on the type of microorganism used. Depending on the microorganism used and its tolerance to the low pH conditions present during fermentation, this study evaluated three pathways of fermentation using LAB, fungi, and yeast. The fermentation yields for different pathways are provided in Table 1.
2.2.4. Lactic Acid Separation and Recovery
Lactic acid is separated and purified before it can be used for other applications. Among the few routes suggested for lactic acid recovery and purification, the lactic acid separation process in which lime (calcium hydroxide) is used to neutralize the fermentation broth, followed by the recovery of lactic acid, is preferred for commercial scale production due to its low overall cost [34]; thus, it was considered for this study. Sulfuric acid is then added to the broth including calcium lactate to recover the lactic acid while simultaneously producing gypsum. However, lactic acid produced from genetically engineered yeast does not need to be neutralized and precipitated, as discussed above, and usually undergoes filtration. The recovered lactic acid also contains other impurities, such as other organic acids and residual sugars, and thus requires further purification [34,75]. Purification processes such as esterification, hydrolysis, and distillation have been selected for all three pathways [34,76]. Lactic acid is esterified using methanol to produce methyl lactate. The impurities in the methyl lactate broth are separated using distillation and used as stillage, whereas the distilled methyl lactate is hydrolyzed to produce lactic acid of higher purity.
2.2.5. Stillage Utilization
Commercial cellulosic lactic acid production produces large quantities of stillage, which includes wastewater, unutilized sugars, and lignin obtained during different conversion steps during lactic acid production. The quantities of wastewater, residual sugars, and lignin are based on the sugar conversion rates during pretreatment and fermentation and the requirements of different chemicals and their conversion rates during pretreatment, fermentation, and product recovery steps. This study considered stillage utilization via the anaerobic digestion of stillage followed by the combustion of biogas to produce steam. In this process, the stillage stream obtained from the purification and recovery unit is separated into solid and liquid fractions using a pressure filter [50]. The solid fraction along with lignin is sent to the boiler, and the liquid fraction is sent to an anaerobic digestion unit, where the sugars and wastewater are converted to biogas, which is sent to the boiler. The steam from the boiler is sent to a multistage turbine, which produces electricity and process steam. The electricity and steam produced are utilized in different processes.
2.3. Techno-Economic Modeling Overview
2.3.1. Process Modeling
The lactic acid production process for different pathways and feedstocks were modelled using SuperPro Designer v10. The assumptions made in this study were based on information from the literature. This study considered a lactic acid biorefinery operating 24 h/day and 330 days/year. The main input parameters for the techno-economic model included performance parameters (efficiencies and productivity of different processes and the equipment, consumables, and energy/fuel requirements for each process), temporal parameters (feedstock loading/unloading and preparation time; process residence times; and heating and cooling times), and quality parameters (conversion rates and yields for different conversion steps). Equipment types and size, labor and utilities requirements, and their unit costs were considered for each process in the model. The process model results included the details of materials requirements and flow through different processes and their utilities requirements.
2.3.2. Economic Analysis
The lactic acid production costs include total capital investments and annual operating costs. The total capital investment cost includes direct fixed cost (DFC), working capital, and start-up cost. The direct fixed cost includes direct costs incurred for total equipment purchases (PCs) and installation in a facility (Table 2). Equipment size and cost considered for this study were based on existing biochemical plants [50], which were adjusted for the analysis year 2022 to account for suitable equipment capacities and inflation (Supplementary Materials, Table S1). In addition, this study also estimated indirect costs such as construction and engineering costs, contractor’s fees, and contingency. This study assumed a working capital for one month of operation. Working capital includes costs for raw materials, consumables, labor, and utilities and ensures that a biorefinery can continue its operations for a short-term. This study also considered start-up cost, which includes expenses incurred to establish and start a new biorefinery. Start-up cost is a one-time cost that covers registration and salaries/wages while developing the facility, and it is assumed to be 5% of direct fixed capital cost.

Table 2.
Economic parameters for lactic acid production facility.
The annual operating cost includes the costs associated with the facility, raw materials, consumables, utilities, labor, quality control, and waste management. The costs related to the facility include those related to the maintenance of the facility itself, equipment maintenance, and other costs such as insurance, taxes, and overhead expenses. The costs of feedstocks, consumables, and utilities were obtained from the literature and are summarized in Table 1 and Table 2. The labor rate includes the basic rate, benefits, and administration. The economic lifetime of a lactic acid production facility is assumed to be 30 years for this analysis. An internal rate of return (IRR) of 10%, assumed to ensure some profitability of the production facility, and discounted cash flow analysis were adopted and employed, respectively, to estimate the minimum selling price of the lactic acid [50]. Considering the same 10% IRR, financial analysis was conducted to estimate the net present value (NPV), return on investment (ROI), payback period, and gross margin.
2.4. Sensitivity Analysis
The lactic acid production cost for the base case scenario was estimated using average values of different input parameters. Sensitivity analysis was performed to evaluate the effect of the most pessimistic and optimistic values of different input parameters on lactic acid production cost (Table 1). Sensitivity analysis was performed for a corn-stover-based lactic acid production facility, assuming similar trends for miscanthus based lactic acid production facility. This study considered a plant with an annual lactic acid production capacity of 100,000 t. However, variation in plant size could be possible depending on the production plant’s location, availability of feedstock, and product demand. Thus, pessimistic and optimistic values for plant size were assumed to be −20% and +20% of the plant size for the base case, respectively. Variation in annual operation hours could be due to maintenance requirements, feedstock availability, and/or market demand. The delivery cost of feedstock can vary significantly depending on the availability of biomass, the location of the production facility, and the selected feedstock logistics systems. The moisture content of the delivered feedstock can vary from approximately 15 to 25%, depending on the time at which the biomass is harvested, the weather conditions during harvest, and storage conditions [78]. The cost of equipment and consumables such as enzymes, sulfuric acid, ammonia, nutrients, lime, and methanol can vary depending on their availability, market demand, and the location of the production facility. The gypsum produced in bacterial and fungal based pathways can be considered as a byproduct or waste, depending on the management practice, and can be sold [63] or disposed of. However, gypsum disposal adds to the lactic acid production costs [64].
3. Results and Discussion
3.1. Material Requirement
Depending on the material balances for the different conversion steps of lactic acid production (Figure 2), lactic acid production pathways using yeast for fermentation required higher quantities for both the corn stover and miscanthus feedstocks. Although the glucose-to-lactic-acid yield is high for the fermentation pathway using yeast, the xylose-to-lactic-acid yield is much lower compared to the fermentation pathways using bacteria and fungi. Thus, the overall feedstock requirements for lactic acid production using the yeast pathway were higher compared to the bacterial and fungal pathways. For all fermentation pathways, the feedstock requirements for miscanthus were lower compared to corn stover. The acid, ammonia, and enzyme requirements during feedstock pretreatment for all three fermentation pathways using both feedstocks were based on the quantity of the feedstocks and the sugars obtained from the feedstocks (Figure 2) and were similar to the requirements reported by previous studies based on corn stover feedstock [50,66,79]. The bacteria- and fungi-based fermentation pathways required similar quantities of lime (30% calcium hydroxide) and sulfuric acid to neutralize and recover the lactic acid and produced similar quantities of gypsum, as the lactic acid production was considered the same. As the neutralization of lactic acid was not necessary for the fermentation pathway using yeast tolerant to low pH conditions, lime and sulfuric acid were not required. Previous studies [26,34] also discussed and reported similar requirements for a neutralizing agent and acid for the two lactic acid fermentation pathways. For all pathways, the same quantity of methanol was used for lactic acid esterification. The water required for different conversion processes (Figure 2) varied and corresponded to the water required to maintain the solid loadings and concentrations for chemicals used for different processes [50]. Compared to corn stover, miscanthus had higher cellulose and hemicellulose content, resulting in higher sugar content. Thus, lower quantities of miscanthus feedstock than corn stover were required to produce the same quantity of lactic acid from a production facility. The miscanthus feedstock also produced less stillage than corn stover, as the miscanthus feedstock had a higher fraction of fermentable sugars and less of the remaining fraction was transferred as stillage. This resulted in lower amounts of biogas, steam, and electricity production for lactic acid production.
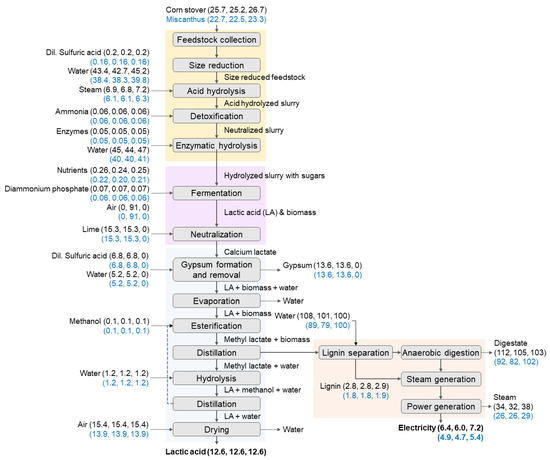
Figure 2.
Material flow for lactic acid production using corn stover and miscanthus feedstocks for three pathways using bacteria, fungi, and yeast for fermentation of sugars into lactic acid. (Note: The material flows are presented in t/h; electricity production is presented in megawatts; the material flows for each component for different feedstocks and fermentation pathways are presented as follows: Line 1—corn stover–bacteria, corn stover–fungi, and corn stover–yeast; Line 2—miscanthus–bacteria, miscanthus–fungi, and miscanthus–yeast.)
3.2. Equipment, Utilities, and Labor Requirements
All three pathways required similar amounts and sizes of equipment for feedstock preparation, pretreatment (acid and enzymatic hydrolysis), and fermentation, as the feedstock and material flows were not significantly different. For the fungi-based pathway, an air compressor and a filtration unit were also required to provide aeration during fermentation. For the yeast-based pathway, lactic acid was not neutralized during fermentation; thus, equipment for the formation of gypsum from calcium lactate and the removal of gypsum from the mixture were not necessary. For the two feedstocks, there were differences in equipment sizes based on the material flow.
The fungi-based pathway had the highest external electrical energy requirements due to the additional electrical power requirements of the air compressor and filtration unit required for fermentation (Table 3). The electricity requirements were higher for the fermentation and stillage processes used to operate large bioreactors, which required electricity to maintain temperature and mix the slurries. The yeast-based pathway was associated with the lowest amount of electricity consumed, as the electricity required for the reactors used for gypsum formation and the filtering unit for gypsum removal were not necessary. The steam and cooling water requirements were the lowest for the yeast-based pathway as a result of lower overall material flow during distillation. The recovery and purification units required the highest quantities of steam and cooling water for the distillation process conducted to recover the lactic acid. Lactic acid production using miscanthus had relatively lower utilities requirements compared to the corn-stover-based lactic acid production system. The lower feedstock requirements resulted in lower demand for water and other chemicals for different processes during lactic acid production, thereby reducing the demand for utilities.

Table 3.
Utilities requirements for the selected lactic acid production pathways and feedstocks.
The yeast-based pathway had the lowest labor requirements; this was mainly due to a reduction in the labor hours required to operate the reactors used for lactic acid neutralization and recovery (Table 4). The labor requirements for the fungi-based pathway were higher due to additional labor hours required to operate and oversee the air compressor and filtration units during the fermentation process. For all pathways, the labor requirements for fermentation and purification and recovery units were higher, as these processes required more equipment and frequent monitoring. The labor requirements for the production facility based on miscanthus were lower than those for corn stover due to a lower level of biomass handling.

Table 4.
Labor requirements (h/year) for different lactic acid production pathways and feedstocks.
3.3. Capital Costs
The capital investments for lactic acid production facilities with a production capacity of 100,000 t/year were in the range of USD 241–268 million, USD 244–266 million, and USD 236–257 million for the bacteria-, fungi-, and yeast-based pathways, respectively (Figure 3). The pathway using yeast for fermentation had the lowest capital investment as lactic acid neutralization and recovery processes were not required in this pathway, thus reducing the costs associated with the purchase and installation of the equipment needed for these processes. The fungi-based pathway required higher capital investment since the fermentation process employing fungi required a continuous supply of air to be effective. This required additional air filters and compressor units, thus increasing the capital investment. The cost of different types of equipment and engineering operations were based on the shares of different components, as discussed in Table 2. For the facility using corn stover as a feedstock and an LAB-based fermentation pathway, the pretreatment stage contributed the most to the total equipment costs as it required acid-resistant reactors to hydrolyze the corn stover. The impact of the pretreatment process on the overall costs was also highlighted by a previous study [80]. The working capital for the yeast-based pathway was lower due to a reduction in the use of utilities, chemicals, and labor for the additional steps for lactic acid neutralization and recovery (Figure 3). For all three fermentation pathways, the miscanthus-based facility had slightly lower feedstock and other resources requirements; thus, it had lower equipment sizes and overall capital costs (USD 236–244 million) than the corn-stover-based facility (USD 257–268 million). The startup capital followed a trend similar to that of the direct fixed capital costs, as it was estimated as 5% of the direct fixed capital.
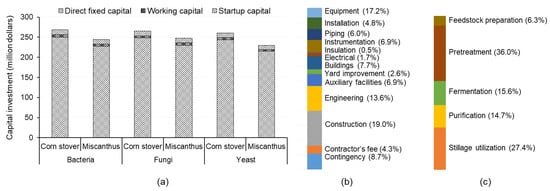
Figure 3.
Capital investments for the facility producing 100,000 t/year of lactic acid from different fermentation pathways using corn stover and miscanthus feedstocks (a); share of different components of direct fixed capital for the facility using corn stover feedstock and the LAB-based pathway (b); contribution of different conversion steps to the facility costs (c).
3.4. Lactic Acid Production Costs
For a lactic acid production facility with an annual production capacity of 100,000 t/year, the per metric ton costs of lactic acid production were in the range of USD 1136–1281, USD 1137–1279, and USD 894–1033 for the bacteria-, fungi-, and yeast-based pathways, respectively (Figure 4). The production costs in this study were similar to the lactic acid production cost of USD 1.25/kg using ultra-filtered whey presented in a prior study [81]. The lactic acid production costs for the facility using miscanthus were lower than those for the facility using corn stover due to the higher lactic acid yields from miscanthus. Raw materials and facility-dependent costs were the major contributors to the total production costs of lactic acid. Raw materials accounted for 27–34% of the total lactic acid production costs for different production pathways using corn stover feedstock and 22–32% using miscanthus feedstock. The yeast-based pathway did not require the use of lime to neutralize the lactic acid in the fermentation broth nor sulfuric acid and water for the recovery of lactic acid from the neutralized mixture; thus, it had the lowest raw materials cost. The facility-dependent costs amounted to 37–44% and 37–47% of the total lactic acid production costs for different production pathways using corn stover and miscanthus feedstocks, respectively. The fungi-based pathway had higher facility-dependent costs due to the addition of air filter and compressor units required for fermentation. The yeast-based pathway did not require the use of reactors to produce and remove gypsum and thus had the lowest facility-dependent costs. Labor costs accounted for 4–5% of the lactic acid production cost and had a trend similar to that of the facility-dependent costs. Utilities contributed between 21 and 26% of the total lactic acid production costs using both feedstocks. The utilities costs were the lowest for the yeast-based pathway due to the elimination of the processes of producing and removing gypsum, which reduced the utilities requirements. The bacteria- and fungi-based pathways had higher waste treatment costs than the yeast-based pathway due to the additional gypsum removal process. Several studies have evaluated the technoeconomic feasibility of lactic acid production from different lignocellulosic feedstocks such as corn stover, sugarcane bagasse, leaves, and macroalgae [26,82,83]. Similar results were also reported in multiple studies evaluating lactic acid production from lignocellulosic feedstocks [26,83].
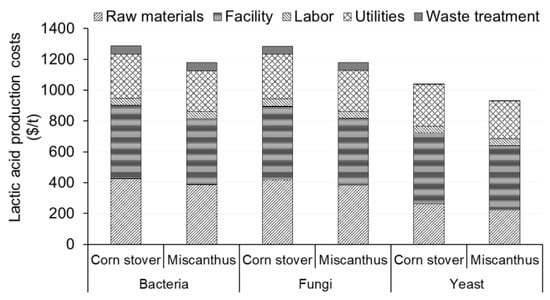
Figure 4.
Lactic acid production costs for the facility producing 100,000 t/year of lactic acid from corn stover and miscanthus feedstocks and different pathways using bacteria, fungi, and yeast for fermentation.
The feedstock preparation steps contributed to 17–23% of the corn-stover- and 14–18% of the miscanthus-based lactic acid production costs. Purification and recovery had the highest shares of lactic acid production costs, amounting to 25–30% for the corn-stover-based and 27–32% for the miscanthus-based lactic acid production costs. Higher purification and recovery costs were due to higher costs for equipment and utilities required for distillation. Acid and enzymatic hydrolyses steps amounted to 21–28% of the total lactic acid production costs, which were mainly due to reactor, utilities, and chemical costs. The fermentation step amounted to 10–20% of the corn-stover-based and 11–22% of the miscanthus-based lactic acid production costs. The costs of the fermentation reactor, utilities, and nutrient contributed to the fermentation costs. Similarly, stillage utilization amounted to 11–12% of the total costs for lactic acid production from corn stover and miscanthus.
3.5. Financial Analysis
The unit lactic acid production costs (USD/t) for the bacteria-, fungi-, and yeast-based pathways were between USD 1136–1281, USD 1137–1279, and USD 894–1033, respectively. Additional revenues from selling the electricity produced while producing a ton of lactic acid were between USD 54–70, USD 53–67, and USD 60–79 for the bacteria-, fungi-, and yeast-based pathways, respectively (Table 5). The yeast-based pathway had lower conversion rates for sugar fermentation; thus, the remaining organic matter was utilized in anaerobic digestion, which increased biogas, steam, and electricity production, resulting in higher revenues from selling electricity. Considering the production costs, revenues, and 10% IRR (after tax), the minimum selling prices per ton of lactic acid were USD 1243–1390 for the bacteria-based pathway, USD 1250–1392 for the fungi-based pathway, and USD 993–1123 for the yeast-based pathway, respectively. Based on the financial analysis of all three pathways (Table 5), the pathway utilizing yeast as a fermentation medium was the most economically beneficial pathway for producing lactic acid using both corn stover and miscanthus feedstocks. The lactic acid produced using miscanthus had a lower minimum selling price than that when using corn stover as feedstock.

Table 5.
Financial analysis of different pathways to produce lactic acid using corn stover and miscanthus feedstocks.
3.6. Sensitivity Analysis
The sensitivity analysis indicated that the conversion rates of glucose and xylose c to lactic acid, feedstock costs and moisture content, production plant capacities, annual operation hours, acid hydrolysis reactor costs, and enzyme costs were the most sensitive parameters affecting the lactic acid production costs for all three pathways (Figure 5). Lower conversion rates of sugar to lactic acid resulted in lower lactic acid production and thus increased the per ton lactic acid production costs, and vice versa. The lactic acid production cost was also highly sensitive to the feedstock costs. An increase in feedstock costs directly increased the costs of input materials and, ultimately, the unit cost of lactic acid production. Changes in the feedstock moisture content affected the wet feedstock quantity required to meet the lactic acid production demand. Using feedstock with lower moisture content reduced the quantity of wet feedstock, thereby reducing the size of equipment required in the production facility. Higher production plant size or biorefinery capacity increased the scale of operation and thus lowered the unit lactic acid production costs due to an economy of scale. An increase in annual operation hours subsequently increased the annual productivity of the biorefinery and thus increased the quantity of lactic acid produced with the same capital investment. Thus, increasing annual operation hours lowered the unit production cost of lactic acid. A higher cost of reactors increased the total costs of equipment and capital investment and, ultimately, the lactic acid production cost. In addition, the lactic acid production costs for the bacteria- and fungi-based pathways were highly sensitive to gypsum use and lime costs, as a substantial quantity of lime was required to maintain the pH conditions during fermentation. Similarly, a substantial quantity of gypsum was produced while recovering the lactic acid in the purification and recovery section. This resulted in sensitivity of lactic acid production costs based on the approach used to manage the gypsum, thus affecting the lactic acid production costs. The sensitivity for parameters is different for miscanthus and corn stover due to the differences in cellulose and xylose content.
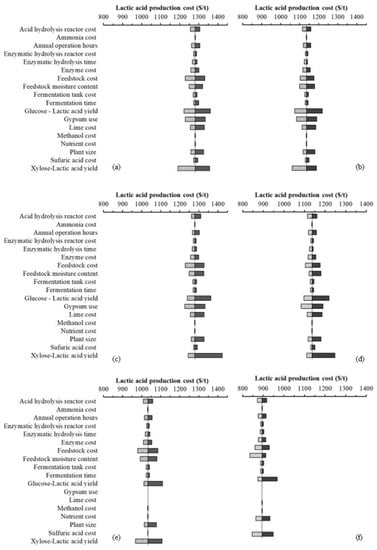
Figure 5.
Sensitivity analyses for lactic acid production via different fermentation pathways and feedstocks: (a) lactic acid bacteria–corn stover, (b) lactic acid bacteria–miscanthus, (c) fungi–corn stover, (d) fungi–miscanthus, (e) yeast–corn stover, and (f) yeast–miscanthus.
4. Conclusions
The cost of the lactic acid produced from the lignocellulosic feedstocks, miscanthus and corn stover, varied between USD 894 and USD 1281 per ton, depending on the three different fermentation pathways using LAB, fungi, and yeast. Lactic acid production using genetically engineered yeast strains can be fermented under lower pH conditions and thus does not require the simultaneous neutralization of lactic acid during fermentation and its recovery at later stages. This reduced the chemical, equipment, and utilities requirements for lactic acid production using yeast compared to that for LAB and fungi and, consequently, resulted in the lowest production costs. Miscanthus had higher sugar yields and lower feedstock production costs and thus lower lactic acid production costs. Raw materials and facilities contributed to more than 70% of the total cost of lactic acid production. For all three pathways, the sugar-into-lactic-acid conversion rates, feedstock costs, production plant capacity, annual operation hours, and acid hydrolysis reactor costs were the most sensitive to lactic acid production costs. The gypsum management approach also highly influenced the lactic acid production costs and the minimum selling price for the pathways using LAB and fungi. This study indicated the possibility of producing lactic acid using different lactic acid fermentation pathways and lignocellulosic feedstocks. Lactic acid production costs can be further reduced by improving process efficiencies, increasing sugar conversion rates, and lowering equipment and chemical costs. The identification of technically feasible and low-cost lactic acid production methods using biobased resources could contribute to a sustainable bioeconomy in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9070641/s1, Table S1: Technical data related to different equipment used for lactic acid production [50,62,84,85].
Author Contributions
Conceptualization, A.M. and A.S.; data collection and analysis, A.M.; writing—original draft preparation, A.M.; review and editing, A.M. and A.S.; supervision, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by United States Department of Agriculture NIFA (Award no. 2019-67019-29310) and United States Department of Agriculture Hatch project (Project no. 1021119). The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the sponsoring agencies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supplementary data is provided with this submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, J. Biodegradable Poly(Lactic Acid); Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-17596-1. [Google Scholar]
- Rodrigues, C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; de Oliveira, J.; Letti, L.A.J.; Soccol, C.R. Production and Application of Lactic Acid. In Current Developments in Biotechnology and Bioengineering—Production, Isolation and Purification of Industrial Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 543–556. ISBN 9780444636621. [Google Scholar] [CrossRef]
- Gao, C.; Ma, C.; Xu, P. Biotechnological Routes Based on Lactic Acid Production from Biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.; Kim, J.; Ryu, H. Biotechnological Production of Lactic Acid and Its Recent Applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Datta, R.; Henry, M. Lactic Acid: Recent Advances in Products, Processes and Technologies—A Review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative Production of Lactic Acid from Renewable Materials: Recent Achievements, Prospects, and Limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- Shiin, H.D.; Guo, X.; Chen, R.R. Biocatalysis for Chiral Synthesis. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 351–371. ISBN 9780444521149. [Google Scholar]
- Grand View Research Lactic Acid Market Size Worth $9.8Bn by 2025 & PLA to Reach $6.5Bn. Available online: www.grandviewresearch.com/press-release/global-lactic-acid-and-poly-lactic-acid-market (accessed on 21 March 2018).
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Biddy, M.J.; Scarlata, C.J.; Kinchin, C.M. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016.
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic Acid Properties, Applications and Production: A Review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn–Hägerdal, B. Factors Affecting the Fermentative Lactic Acid Production from Renewable Resources. Enzym. Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices-A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Taskila, S.; Ojamo, H. The Current Status and Future Expectations in Industrial Production of Lactic Acid by Lactic Acid Bacteria. In Lactic Acid Bacteria-R&D Food, Health Livestock Purposes; IntechOpen: London, UK, 2013; pp. 615–632. [Google Scholar] [CrossRef]
- Lunelli, B.H.; Andrade, R.R.; Atala, D.I.P.; MacIel, M.R.W.; Filho, F.M.; Filho, R.M. Production of Lactic Acid from Sucrose: Strain Selection, Fermentation, and Kinetic Modeling. Appl. Biochem. Biotechnol. 2010, 161, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(Lactic Acid)—Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Vijayakumar, J.; Aravindan, R.; Viruthagiri, T. Recent Trends in the Production, Purification and Application of Lactic Acid. Chem. Biochem. Eng. Q. 2008, 2, 245–264. [Google Scholar]
- Manandhar, A.; Shah, A. Techno-Economic Analysis of Bio-Based Lactic Acid Production Utilizing Corn Grain as Feedstock. Processes 2020, 8, 199. [Google Scholar] [CrossRef]
- Ilmen, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttil, M. Efficient Production of L-Lactic Acid from Xylose by Pichia Stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Åkerberg, C.; Hofvendahl, K.; Zacchi, G.; Hahn-Hägerdal, B. Modelling the Influence of PH, Temperature, Glucose and Lactic Acid Concentrations on the Kinetics of Lactic Acid Production by Lactococcus lactis Ssp. Lactis ATCC 19435 in Whole-Wheat Flour. Appl. Microbiol. Biotechnol. 1998, 49, 682–690. [Google Scholar] [CrossRef]
- Cui, F.; Li, Y.; Wan, C. Lactic Acid Production from Corn Stover Using Mixed Cultures of Lactobacillus Rhamnosus and Lactobacillus Brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lee, Y.Y.; Elander, R.T. Conversion of Aqueous Ammonia-Treated Corn Stover to Lactic Acid by Simultaneous Saccharification and Cofermentation. Appl. Biochem. Biotechnol. 2007, 137–140, 721–738. [Google Scholar] [CrossRef]
- Maas, R.H.W.; Bakker, R.R.; Eggink, G.; Weusthuis, R.A. Lactic Acid Production from Xylose by the Fungus Rhizopus Oryzae. Appl. Microbiol. Biotechnol. 2006, 72, 861–868. [Google Scholar] [CrossRef]
- Shen, X.; Xia, L. Lactic Acid Production from Cellulosic Material by Synergetic Hydrolysis and Fermentation. Appl. Biochem. Biotechnol. 2006, 133, 251–262. [Google Scholar] [CrossRef]
- Bai, D.M.; Li, S.Z.; Liu, Z.L.; Cui, Z.F. Enhanced L-(+)-Lactic Acid Production by an Adapted Strain of Rhizopus Oryzae Using Corncob Hydrolysate. Appl. Biochem. Biotechnol. 2008, 144, 79–85. [Google Scholar] [CrossRef]
- Daful, A.G.; Goergens, J.F. Techno-Economic Analysis and Environmental Impact Assessment of Lignocellulosic Lactic Acid Production. Chem. Eng. Sci. 2017, 162, 53–65. [Google Scholar] [CrossRef]
- Datta, R.; Tsai, S.-P. Lactic Acid Production and Potential Uses: A Technology and Economics Assessment; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 666, pp. 223–236. [Google Scholar]
- Langholtz, M.H.; Stokes, B.J.; Eaton, M.; U.S. Department of Energy. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; ORNL/TM-2016/160; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016. [CrossRef]
- Adom, F.K.; Dunn, J.B. Life Cycle Analysis of Corn-Stover-Derived Polymer-Grade l-Lactic Acid and Ethyl Lactate: Greenhouse Gas Emissions and Fossil Energy Consumption. Biofuels Bioprod. Biorefin. 2017, 11, 258–268. [Google Scholar] [CrossRef]
- Biomass Magazine Total Corbion PLA Starts-up 75,000-Ton-per-Year Bioplastics Plant. Available online: http://biomassmagazine.com/articles/15800/total-corbion-pla-starts-up-75-000-ton-per-year-bioplastics-plant (accessed on 20 December 2018).
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. Industrial Production of Lactic Acid. Compr. Biotechnol. Second Ed. 2011, 3, 179–188. [Google Scholar] [CrossRef]
- Åkerberg, C.; Zacchi, G. An Economic Evaluation of the Fermentative Production of Lactic Acid from Wheat Flour. Bioresour. Technol. 2000, 75, 119–126. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Rocha de Oliveira, J.A.; da Silva Martins, L.H.; Maciel Filho, R. Purification of Lactic Acid Produced by Fermentation: Focus on Non-Traditional Distillation Processes. Sep. Purif. Rev. 2017, 46, 241–254. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative Assessment of Downstream Processing Options for Lactic Acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of Lactic Acid from Renewable Materials by Rhizopus Fungi. Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar] [CrossRef]
- Soccol, C.R.; Stonoga, V.I.; Raimbault, M. Production of L-Lactic Acid by Rhizopus Species. World J. Microbiol. Biotechnol. 1994, 10, 433–435. [Google Scholar] [CrossRef]
- Rosenberg, M.; Krišofíková, L. Physiological Restriction of the L-lactic Acid Production by Rhizopus Arrhizus. Acta Biotechnol. 1995, 15, 367–374. [Google Scholar] [CrossRef]
- Tay, A.; Yang, S.T. Production of L(+)-Lactic Acid from Glucose and Starch by Immobilized Cells of Rhizopus Oryzae in a Rotating Fibrous Bed Bioreactor. Biotechnol. Bioeng. 2002, 80, 1–12. [Google Scholar] [CrossRef]
- Shah, A.; Darr, M. A Techno-Economic Analysis of the Corn Stover Feedstock Supply System for Cellulosic Biorefineries. Biofuels Bioprod. Biorefin. 2016, 10, 542–559. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V.; Tiffany, D.G. Economic and Environmental Analysis for Corn Stover and Switchgrass Supply Logistics. Bioenergy Res. 2015, 8, 1433–1448. [Google Scholar] [CrossRef]
- Khanna, M.; Dhungana, B.; Clifton-Brown, J. Costs of Producing Miscanthus and Switchgrass for Bioenergy in Illinois. Biomass Bioenergy 2008, 32, 482–493. [Google Scholar] [CrossRef]
- Jain, A.K.; Khanna, M.; Erickson, M.; Huang, H. An Integrated Biogeochemical and Economic Analysis of Bioenergy Crops in the Midwestern United States. Glob. Chang. Biol. 2010, 2, 217–234. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Brosse, N.; Dufour, A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A Fast- Growing Crop for Biofuels and Chemicals Production. Biofuels Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Vasco-correa, J.; Li, Y. Solid-State Anaerobic Digestion of Fungal Pretreated Miscanthus Sinensis Harvested in Two Different Seasons. Bioresour. Technol. 2015, 185, 211–217. [Google Scholar] [CrossRef]
- Hodgson, E.M.; Lister, S.J.; Bridgwater, A.V.; Clifton-brown, J.; Donnison, I.S. Genotypic and Environmentally Derived Variation in the Cell Wall Composition of Miscanthus in Relation to Its Use as a Biomass Feedstock. Biomass Bioenergy 2010, 34, 652–660. [Google Scholar] [CrossRef]
- Karp, A.; Shield, I. Bioenergy from Plants and the Sustainable Yield Challenge. 2008, 179, 15–32. New Phytol. 2008, 179, 15–32. [Google Scholar] [CrossRef]
- Byrt, C.S.; Grof, C.P.L.; Furbank, R.T.; Furbank, R.T. C4 Plants as Biofuel Feedstocks: Optimising Biomass Production and Feedstock Quality from a Lignocellulosic Perspective. J. Integr. Plant Biol. 2011, 53, 120–135. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; NREL/TP-5100-47764; National Renewable Energy Lab.: Golden, CO, USA, 2011. [CrossRef]
- ICIS Sulfuric Acid Cost. Available online: https://www.icis.com/explore/commodities/chemicals/channel-info-chemicals-a-z/ (accessed on 17 June 2018).
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The Challenge of Enzyme Cost in the Production of Lignocellulosic Biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef]
- ICIS Cost of Ammonia. Available online: https://www.icis.com/chemicals/channel-info-chemicals-a-z/ (accessed on 22 July 2018).
- Suskovic, J.; Novak, S.; Maric, V.; Matosic, S. Lactic Acid Fermentation Kinetics on Different Carbon Sources. Prehrambeno—Tehnol. Biotehnol. Rev. 1991, 29, 155–158. [Google Scholar]
- Trontel, A.; Bar, V.; Slavica, A.; Novak, S. Modelling the Effect of Different Substrates and Temperature on the Growth and Lactic Acid Production by Lactobacillus Amylovorus DSM 20531T in Batch Process. Food Technol. Biotechnol. 2010, 48, 352–361. [Google Scholar]
- Guo, W.; Jia, W.; Li, Y.; Chen, S. Performances of Lactobacillus Brevis for Producing Lactic Acid from Hydrolysate of Lignocellulosics. Appl. Biochem. Biotechnol. 2010, 161, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Kosakai, Y.; Park, Y.S.; Okabe, M. Enhancement of L(+)-Lactic Acid Production Using Mycelial Flocs of Rhizopus Oryzae. Biotechnol. Bioeng. 1997, 55, 461–470. [Google Scholar] [CrossRef]
- Valli, M.; Sauer, M.; Branduardi, P.; Borth, N.; Porro, D.; Mattanovich, D. Improvement of Lactic Acid Production in Saccharomyces Cerevisiae by Cell Sorting for High Intracellular PH. Appl. Environ. Microbiol. 2006, 72, 5492–5499. [Google Scholar] [CrossRef]
- Index Mundi Lime Prices in the United States. Available online: https://www.indexmundi.com/en/commodities/minerals/lime/lime_t5.html (accessed on 10 November 2018).
- Painuly, G. Methanol Prices Skyrocket by 180% Over Last Year. Available online: www.costinsights.com/methanol-prices-skyrocket (accessed on 12 December 2018).
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic Digestion for Bioenergy Production: Global Status, Environmental and Techno-Economic Implications, and Government Policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef]
- Intelligen Inc. SuperPro Designer Software Version 13. Available online: https://www.intelligen.com/superpro_overview.html (accessed on 11 March 2020).
- Statista Average Price of Crude Gypsum on a Free-on Board (FOB) Mine Basis in the U.S. from 2007 to 2017 (in U.S. Dollars per Metric Ton). Available online: www.statista.com/statistics/219363/wallboard-products-crude-price-in-the-us/ (accessed on 21 December 2018).
- Laquatra, J.; Pierce, M.R. Waste Management at the Construction Site. Cornell Coop. Ext. 2002, 1, 281–300. [Google Scholar]
- Belyea, R.L.; Rausch, K.D.; Tumbleson, M.E. Composition of Corn and Distillers Dried Grains with Solubles from Dry Grind Ethanol Processing. Bioresour. Technol. 2004, 94, 293–298. [Google Scholar] [CrossRef]
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons; NREL/TP-5100-60223; National Renewable Energy Lab.: Golden, CO, USA, 2013.
- Moreno, A.D.; Olsson, L. Pretreatment of Lignocellulosic Feedstocks. In Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy; Sani, R.K., Krishnaraj, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 31–52. ISBN 978-3-319-54684-1. [Google Scholar]
- Uppugundla, N.; da Costa, S.L.; Chundawat, S.P.S.; Yu, X.R.; Simmons, B.; Singh, S.; Gao, X.D.; Kumar, R.; Wyman, C.E.; Dale, B.E.; et al. A Comparative Study of Ethanol Production Using Dilute Acid, Ionic Liquid and AFEX Pretreated Corn Stover. Biotechnol. Biofuels 2014, 7, 72. [Google Scholar] [CrossRef]
- Lloyd, T.A.; Wyman, C.E. Combined Sugar Yields for Dilute Sulfuric Acid Pretreatment of Corn Stover Followed by Enzymatic Hydrolysis of the Remaining Solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Z.-H.; Li, B.-Z.; Dale, B.E.; Yuan, Y.-J. Mass Balance and Transformation of Corn Stover by Pretreatment with Different Dilute Organic Acids. Bioresour. Technol. 2012, 112, 319–326. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-Economic Comparison of Process Technologies for Biochemical Ethanol Production from Corn Stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Comparative Techno-Economic Analysis of Steam Explosion, Dilute Sulfuric Acid, Ammonia Fiber Explosion and Biological Pretreatments of Corn Stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Qi, W.; He, Z. Enhanced Enzymatic Hydrolysis of Lignocellulose by Optimizing Enzyme Complexes. Appl. Biochem. Biotechnol. 2010, 160, 1407–1414. [Google Scholar] [CrossRef]
- Lin, Z.X.; Zhang, H.M.; Ji, X.J.; Chen, J.W.; Huang, H. Hydrolytic Enzyme of Cellulose for Complex Formulation Applied Research. Appl. Biochem. Biotechnol. 2011, 164, 23–33. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic Acid Production from Lignocellulose-Derived Sugars Using Lactic Acid Bacteria: Overview and Limits. J. Biotechnol. 2010, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Filachione, E.M.; Fisher, C.H. Purification of Lactic Acid. Ind. Eng. Chem. 1946, 38, 228–232. [Google Scholar] [CrossRef]
- Trading Economics United State Inflation Rate. Available online: https://tradingeconomics.com/united-states/inflation-cpi (accessed on 12 December 2018).
- Shah, A.; Darr, M.J.; Webster, K.; Hoffman, C. Outdoor Storage Characteristics of Single-Pass Large Square Corn Stover Bales in Iowa. Energies 2011, 4, 1687–1695. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Techno-Economic Analysis of Cellulosic Butanol Production from Corn Stover through Acetone-Butanol-Ethanol Fermentation. Energy Fuels 2016, 30, 5779–5790. [Google Scholar] [CrossRef]
- Munagala, M.; Shastri, Y.; Nalawade, K.; Konde, K.; Patil, S. Life Cycle and Economic Assessment of Sugarcane Bagasse Valorization to Lactic Acid. Waste Manag. 2021, 126, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.I.; Alvarez, S.; Riera, F.; Alvarez, R. Economic Evaluation of an Integrated Process for Lactic Acid Production from Ultrafiltered Whey. J. Food Eng. 2007, 80, 553–561. [Google Scholar] [CrossRef]
- Marchesan, A.N.; Leal Silva, J.F.; Maciel Filho, R.; Wolf Maciel, M.R. Techno-Economic Analysis of Alternative Designs for Low-PH Lactic Acid Production. ACS Sustain. Chem. Eng. 2021, 9, 12120–12131. [Google Scholar] [CrossRef]
- Li, Y.; Bhagwat, S.S.; Cortés-Peña, Y.R.; Ki, D.; Rao, C.V.; Jin, Y.-S.; Guest, J.S. Sustainable Lactic Acid Production from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 1341–1351. [Google Scholar] [CrossRef]
- Wright, M.M.; Satrio, J.; Brown, R.C.; Daugaard, D.E. Techno-Economic Analysis of Biomass Fast Pyrolysis to Transportation Fuels; Technical Report NREL/TP-6A20-46586; National Renewable Energy Laboratory: Golden, CO, USA, 2010.
- Jones, S.; Meyer, P.; Snowden-Swan, L.; Padmaperuma, A.; Tan, E.; Dutta, A.; Jacobson, J.; Cafferty, K. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydro-Carbon Fuels: Fast pyrolysis and Hydrotreating Bio-Oil Pathway; Technical Report PNNL-23053\NREL/TP-5100-61178; OSTI: Albuquerque, NM, USA, 2013.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).