Dry Matter Content and Additives with Different Modes of Action Modify the Preservation Characteristics of Grass Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material for Silage Making
2.2. Experimental Treatments and Procedures
2.2.1. Experiment 1—Conventional Silage Additives
- Control I, as a negative treatment without additive.
- Homofermentative LAB Lactobacillus plantarum (DSM 12836; 1k2078; min. 1 × 1011 cfu/g) and Pediococcus pentosaceus (HO; DSM 12834; 1k2103; min. 1 × 1011 cfu/g; Bonsilage, Schaumann Agri International GmbH, Pinneberg, Germany at 1 g/t, resulting in a minimum of 2 × 105 cfu/g of fresh forage).
- Heterofermentative LAB Lactobacillus buchneri (DSM 13573, 1k20733; min. 1 × 1011 cfu/g), combined with homofermentative Lactobacillus plantarum (DSM 3676, 1k20731; min. 0.5 × 1011 cfu/g) and Lactobacillus plantarum (HE; DSM 3677, 1k20732; min. 0.5 × 1011 cfu/g; Feedtech Silage F600, DeLaval, Tumba, Sweden at 1 g/t, resulting in a minimum of 2 × 105 cfu/g of fresh forage).
- Salt-based additive (SA; sodium benzoate, potassium sorbate and sodium nitrite; Safesil Pro, Salinity AB, Göteborg, Sweden at 5 L/t).
- Formic- and propionic-acid-based additive (FPA; formic acid, propionic acid, sodium formate and potassium sorbate; AIV Ässä Na, Eastman, Oulu, Finland at 5 L/t).
2.2.2. Experiment 2—Resin Acids Used as Silage Additives
- Control (C), as a negative treatment without additive.
- Formic- and propionic-acid-based additive (FPA; formic acid, propionic acid, sodium formate and potassium sorbate; AIV Ässä Na, Eastman, Oulu, Finland at 5 L/t).
- Resin acid oil (FOR; Forchem Ltd., Rauma, Finland at 13 L/t).
- FOR (at 26 L/t).
- Resin acid soluble in water (ROS; Forchem Ltd., Rauma, Finland at 13 L/t).
- ROS (at 26 L/t).
2.3. Laboratory Analyses
2.4. Statistical Analyses
3. Results and Discussion
3.1. Raw Material Characteristics
3.2. Fermentation Quality of the Experimental Grass Silages
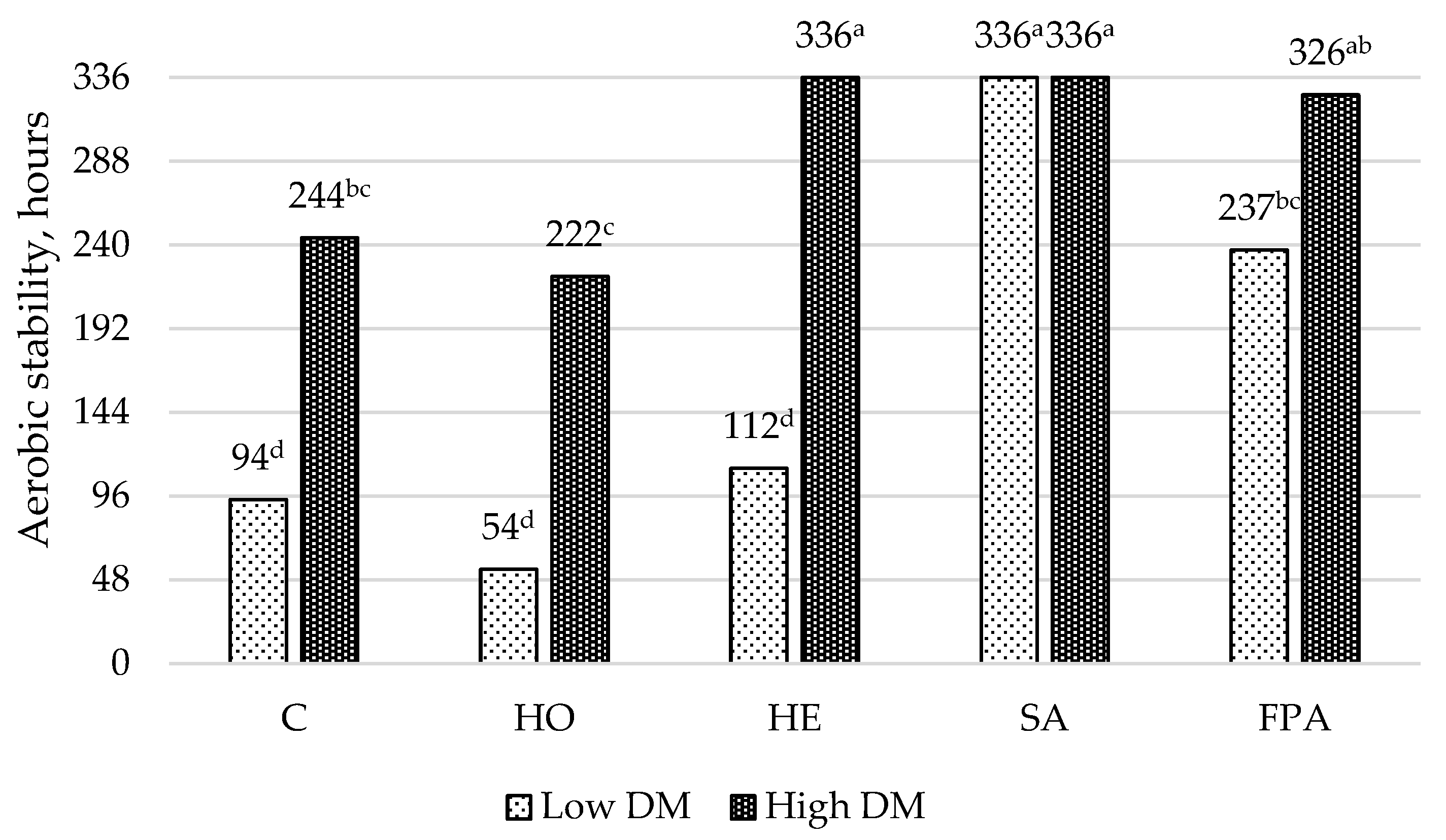
3.2.1. Experiment 1—Conventional Silage Additives
3.2.2. Experiment 2—Resin Acids Used as Silage Additives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, J.M.; Rinne, M. Highlights of Progress in Silage Conservation and Future Perspectives. Grass Forage Sci. 2018, 73, 40–52. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum Additive and Temperature on the Ensiling Quality and Microbial Community Dynamics of Cauliflower Leaf Silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Wright, D.A.; Gordon, F.J.; Steen, R.W.J.; Patterson, D.C. Factors Influencing the Response in Intake of Silage and Animal Performance after Wilting of Grass before Ensiling: A Review. Grass Forage Sci. 2000, 55, 1–13. [Google Scholar] [CrossRef]
- Seppälä, A.; Heikkilä, T.; Mäki, M.; Rinne, M. Effects of Additives on the Fermentation and Aerobic Stability of Grass Silages and Total Mixed Rations. Grass Forage Sci. 2016, 71, 458–471. [Google Scholar] [CrossRef]
- Franco, M.; Tapio, I.; Rinne, M. Preservation Characteristics and Bacterial Communities of Crimped Ensiled Barley Grains Modulated by Moisture Content and Additive Application. Front. Microbiol. 2022, 13, 1092062. [Google Scholar] [CrossRef]
- Guo, X.S.; Ke, W.C.; Ding, W.R.; Ding, L.M.; Xu, D.M.; Wang, W.W.; Zhang, P.; Yang, F.Y. Profiling of Metabolome and Bacterial Community Dynamics in Ensiled Medicago sativa Inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 2018, 8, 357. [Google Scholar] [CrossRef]
- Muck, R.E.; Kung, L., Jr. Effects of Silage Additives on Ensiling. In Proceedings of the Silage: Field to Feedbunk, Northeast Regional Agricultural Engineering Service (NRAES), Hershey, PA, USA, 11–13 February 1997; NRAES-99. pp. 187–199. [Google Scholar]
- Keeling, C.I.; Bohlmann, J. Diterpene Resin Acids in Conifers. Phytochemistry 2006, 67, 2415–2423. [Google Scholar] [CrossRef]
- Kairenius, P.; Qin, N.; Tapio, I.; Mäntysaari, P.; Franco, M.; Lidauer, P.; Stefański, T.; Lidauer, M.H.; Junnikkala, S.; Niku, M.; et al. The Effects of Dietary Resin Acid Inclusion on Productive, Physiological and Rumen Microbiome Responses of Dairy Cows during Early Lactation. Livest. Sci. 2022, 255, 104798. [Google Scholar] [CrossRef]
- Knický, M.; Spörndly, R. Short Communication: Use of a Mixture of Sodium Nitrite, Sodium Benzoate, and Potassium Sorbate in Aerobically Challenged Silages. J. Dairy Sci. 2015, 98, 5729–5734. [Google Scholar] [CrossRef]
- Huida, L.; Väätäinen, H.; Lampila, M. Comparison of Dry Matter Contents in Grass Silage as Determined by Oven Drying and Gas Chromatographic Water Analysis. Ann. Agric. Fenn. 1986, 25, 215–230. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Somogyi, M. A New Reagent for the Determination of Sugars. J. Biol. Chem. 1945, 160, 61–68. [Google Scholar] [CrossRef]
- Huhtanen, P. Effects of Intraruminal Infusions of Sucrose and Xylose on Nitrogen and Fibre Digestion in the Rumen and Intestines of Cattle Receiving Diets of Grass Silage and Barley. J. Agric. Sci. Finl. 1987, 59, 405–424. [Google Scholar] [CrossRef]
- Haacker, K.; Block, H.-J.; Weissbach, F. Zur Kolorimetrischen Milchsäurebestimmung in Silagen Mit P-Hydroxydiphenyl. Arch. Für. Tierernaehrung 1983, 33, 505–512. [Google Scholar] [CrossRef]
- McCullough, H. The Determination of Ammonia in Whole Blood by a Direct Colorimetric Method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Nousiainen, J.; Rinne, M.; Hellämäki, M.; Huhtanen, P. Prediction of the Digestibility of the Primary Growth of Grass Silages Harvested at Different Stages of Maturity from Chemical Composition and Pepsin-Cellulase Solubility. Anim. Feed. Sci. Technol. 2003, 103, 97–111. [Google Scholar] [CrossRef]
- Huhtanen, P.; Nousiainen, J.; Rinne, M. Recent Developments in Forage Evaluation with Special Reference to Practical Applications. Agric. Food Sci. 2006, 15, 293–323. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Weissbach, F.; Schmidt, L.; Hein, E. Method of Anticipation of the Run of Fermentation in Silage Making Based on the Chemical Composition of Green Fodder. In Proceedings of the 12th International Grassland Congress, Moscow, Russia, 8–20 June 1974; pp. 663–673. [Google Scholar]
- Pahlow, G.; Rammer, C.; Slottner, D.; Tuori, M. Ensiling of Legumes, Proceedings of an International Workshop Supported by the EU and Held in Braunschweig—Legume Silages for Animal Production-LEGSIL, Braunschweig, Germany, 8–9 July 2001; Wilkins, R., Paul, C., Eds.; Landbauforsch Völk Sonderheft: Brauschweig, Germany, 2002; pp. 27–31. [Google Scholar]
- Höglind, M.; Hanslin, H.M.; Van Oijen, M. Timothy Regrowth, Tillering and Leaf Area Dynamics Following Spring Harvest at Two Growth Stages. Field Crops Res. 2005, 93, 51–63. [Google Scholar] [CrossRef]
- Salo, T.; Eurola, M.; Rinne, M.; Seppälä, A.; Kaseva, J.; Kousa, T. The Effect of Nitrogen and Phosphorus Concentrations on Nutrient Balances of Cereals and Grass Silage; MTT Report 147; MTT Agrifood Research Finland: Jokioinen, Finland, 2014; ISBN 9789524875417. [Google Scholar]
- Weissbach, F. New Developments in Crop Conservation, Proceedings of the 11th International Silage Conference, Aberystwyth, UK, 8–11 September 1996; Jones, D.I.H., Dewhurst, R., Merry, R., Haigh, P.M., Eds.; IGER Publ. Section: Aberystwyth, UK, 1996; pp. 11–25. [Google Scholar]
- Knicky, M.; Spörndly, R. The Ensiling Capability of a Mixture of Sodium Benzoate, Potassium Sorbate, and Sodium Nitrite. J. Dairy Sci. 2011, 94, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Jalava, T.; Stefanski, T.; Kuoppala, K.; Timonen, P.; Winquist, E.; Siika-aho, M.; Rinne, M. Grass Silage for Biorefinery—Effect of Additives on Silage Quality and Liquid Solid Separation of Timothy and Red Clover Silages, Proceedings of the 9th Nordic Feed Science Conference, Uppsala, Sweden, 12–13 June 2018; Udén, P., Eriksson, T., Spörndly, R., Rustas, B.-O., Liljeholm, M., Eds.; Swedish University of Agricultural Sciences (SLU): Uppsala, Sweden, 2018; pp. 49–54. [Google Scholar]
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Sloth, K.H.; Højberg, O.; Spliid, N.H.; Jensen, C.; Thøgersen, R. Effects of Microbial Inoculants on Corn Silage Fermentation, Microbial Contents, Aerobic Stability, and Milk Production under Field Conditions. J. Dairy Sci. 2010, 93, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Whiter, A.G.; Kung, L. The Effect of a Dry or Liquid Application of Lactobacillus plantarum MTD1 on the Fermentation of Alfalfa Silage. J. Dairy Sci. 2001, 84, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, S.; Rinne, M.; Heikkilä, T.; Toivonen, V.; Huhtanen, P. Effects of Restriction of Silage Fermentation with Formic Acid on Milk Production. Agric. Food Sci. 2006, 15, 200–218. [Google Scholar] [CrossRef]
- Rinne, M.; Jalava, T.; Stefanski, T.; Kuoppala, K.; Timonen, P.; Winquist, E.; Siika-aho, M. Optimizing Grass Silage Quality for Green Biorefineries. In Proceedings of the 27th General Meeting of the European Grassland Federation, Cork, Ireland, 18–21 June 2018; Horan, B., Hennessy, D., O’Donovan, M., Kennedy, E., McCarthy, B., O’Brien, B., Eds.; Grassland Science in Europe. pp. 820–822. [Google Scholar]
- Franco, M.; Tapio, I.; Pirttiniemi, J.; Stefański, T.; Jalava, T.; Huuskonen, A.; Rinne, M. Fermentation Quality and Bacterial Ecology of Grass Silage Modulated by Additive Treatments, Extent of Compaction and Soil Contamination. Fermentation 2022, 8, 156. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Silage, 6th ed.; Chalcombe Publications: Marlow, UK, 1990. [Google Scholar]
- Pahlow, G.; Muck, R.; Driehuis, F.; Oude Elferink, S.; Spoelstra, S.F. Microbiology of Ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; Volume 42, pp. 31–93. ISBN 0891181512. [Google Scholar]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage Review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Huhtanen, P.; Nousiainen, J.; Rinne, M. Prediction of Silage Composition and Organic Matter Digestibility from Herbage Composition and Pepsin-Cellulase Solubility. Agric. Food Sci. 2005, 14, 154–165. [Google Scholar] [CrossRef]
- Huhtanen, P.; Rinne, M.; Nousiainen, J. Evaluation of the Factors Affecting Silage Intake of Dairy Cows: A Revision of the Relative Silage Dry-Matter Intake Index. Animal 2007, 1, 758–770. [Google Scholar] [CrossRef]
- Kung, L.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J.L. The Effect of Preservatives Based on Propionic Acid on the Fermentation and Aerobic Stability of Corn Silage and a Total Mixed Ration. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef]
- McEniry, J.; O’Kiely, P.; Clipson, N.; Forristal, P.D.; Doyle, E. Manipulating the Ensilage of Wilted, Unchopped Grass through the Use of Additive Treatments. Ir. J. Agric. Food Res. 2007, 46, 77–91. [Google Scholar]
- Woolford, M.K. Microbiological Screening of the Straight Chain Fatty Acids (C1–C12) as Potential Silage Additives. J. Sci. Food Agric. 1975, 26, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Env. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Tapio, I.; Huuskonen, A.; Rinne, M. Fermentation Quality and Bacterial Ecology of Red Clover Dominated Silage Modulated by Different Management Factors. Front. Anim. Sci. 2022, 3, 1080535. [Google Scholar] [CrossRef]


| Low DM | High DM | |
|---|---|---|
| DM, g/kg | 224 | 534 |
| Buffering capacity, g lactic acid/100 g DM | 5.4 | 5.6 |
| Fermentation coefficient | 39.3 | 74.3 |
| In DM, g/kg | ||
| Ash | 92 | 92 |
| Crude protein | 111 | 107 |
| Water-soluble carbohydrates | 115 | 145 |
| Neutral detergent fibre | 548 | 527 |
| Organic matter digestibility, g/g OM | 0.760 | 0.781 |
| Microbial counts, colony-forming units/g | ||
| Yeasts | 2.6 × 105 | 6.2 × 105 |
| Moulds | 1.4 × 105 | 3.9 × 105 |
| Total aerobic bacteria | 4.5 × 107 | 9.9 × 107 |
| Dry Matter (DM) | Low DM | High DM | SEM 2 | p-Value 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Additives 1 (Add) | C | HO | HE | SA | FPA | C | HO | HE | SA | FPA | DM | Add | DM × Add | |
| DM, g/kg | 221 b | 218 b | 223 b | 226 b | 228 b | 526 a | 512 a | 512 a | 513 a | 521 a | 3.9 | <0.001 | 0.104 | 0.231 |
| Chemical composition, g/kg DM | ||||||||||||||
| Water-soluble carbohydrates | 29 d | 30 d | 23 d | 34 d | 32 d | 82 b | 90 b | 62 c | 82 b | 172 a | 3.6 | <0.001 | <0.001 | <0.001 |
| Ethanol | 4.5 b | 4.1 bc | 3.5 bcd | 1.2 e | 7.2 a | 2.0 cde | 1.8 de | 2.0 cde | 1.0 e | 0.7 e | 0.42 | <0.001 | <0.001 | <0.001 |
| Acids, g/kg DM | ||||||||||||||
| Lactic (LA) | 93.5 b | 114.0 a | 97.0 b | 86.6 b | 54.8 d | 52.5 d | 68.7 c | 60.2 cd | 51.5 d | 19.1 e | 2.49 | <0.001 | <0.001 | 0.245 |
| Acetic (AA) | 15.3 d | 12.6 ef | 20.1 b | 17.1 c | 22.0 a | 10.7 g | 8.0 h | 13.8 de | 11.8 fg | 6.3 i | 0.32 | <0.001 | <0.001 | <0.001 |
| Propionic 4 | 0.19 ab | 0.21 ab | 0.25 a | 0.15 bcd | 0 e | 0.08 d | 0.08 d | 0.08 d | 0.10 cd | 0.16 bc | 0.014 | <0.001 | <0.001 | <0.001 |
| Butyric | 0.05 | 0.03 | 0.03 | 0.01 | 0.06 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.010 | 0.013 | 0.398 | 0.180 |
| Total volatile fatty acids | 15.5 d | 12.8 ef | 20.4 b | 17.3 c | 22.1 a | 10.8 g | 8.1 h | 13.9 de | 12.0 fg | 6.5 h | 0.33 | <0.001 | <0.001 | <0.001 |
| Total fermentation acids 5 | 109 bc | 127 a | 117 ab | 104 c | 77 d | 63 e | 77 d | 74 de | 64 e | 26 f | 2.5 | <0.001 | <0.001 | 0.204 |
| Total fermentation products 6 | 114 bc | 131 a | 121 ab | 105 c | 84 d | 65 e | 79 d | 76 de | 65 e | 26 f | 2.5 | <0.001 | <0.001 | 0.029 |
| LA/AA ratio | 6.13 b | 9.11 a | 4.85 bc | 5.06 bc | 2.48 e | 4.92 bc | 8.62 a | 4.36 cd | 4.35 cd | 3.02 de | 0.266 | 0.012 | <0.001 | 0.053 |
| Losses, g/kg initial DM | 9.3 cd | 8.7 cd | 9.5 cd | 6.6 d | 12.3 abcd | 16.3 ab | 14.7 abc | 18.3 a | 16.8 a | 9.9 bcd | 1.32 | <0.001 | 0.262 | 0.001 |
| Microbial counts, cfu 7/g | ||||||||||||||
| Yeasts | 1.5 × 104 | 1.7 × 104 | 4.0 × 103 | 1.0 × 102 | 2.0 × 102 | 4.4 × 104 | 1.0 × 104 | 4.0 × 102 | 9.8 × 103 | 1.6 × 103 | 1.5 × 104 | 0.524 | 0.320 | 0.761 |
| Moulds | 3.8 × 103 a | 1.4 × 103 ab | 1.5 × 103 ab | 2.0 × 102 b | 1.7 × 103 ab | 3.0 × 102 b | 2.0 × 102 b | 2.0 × 102 b | 1.0 × 102 b | 1.0 × 102 b | 6.2 × 102 | <0.001 | 0.080 | 0.136 |
| Additives 1 | C | FPA | FOR | ROS | SEM 2 | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 L/t | 26 L/t | 13 L/t | 26 L/t | Lin FOR | Lin ROS | C vs. Res Acids | FPA vs. Res Acids | FOR vs. ROS | ||||
| Dry matter (DM), g/kg | 222 b | 229 b | 228 b | 245 a | 226 b | 227 b | 2.0 | <0.001 | 0.174 | 0.003 | 0.349 | 0.001 |
| pH | 3.89 | 3.92 | 3.88 | 3.86 | 3.89 | 3.89 | 0.020 | 0.257 | 0.907 | 0.658 | 0.098 | 0.332 |
| Ammonia-N, g/kg N | 59 a | 34 c | 58 a | 47 b | 55 ab | 56 ab | 2.2 | 0.003 | 0.390 | 0.064 | <0.001 | 0.205 |
| Chemical composition, g/kg DM | ||||||||||||
| Water-soluble carbohydrates | 32 a | 17 b | 27 a | 27 a | 34 a | 27 a | 1.8 | 0.141 | 0.125 | 0.221 | <0.001 | 0.095 |
| Ethanol | 9.3 bc | 5.6 c | 10.4 abc | 8.2 bc | 15.2 ab | 16.9 a | 1.53 | 0.603 | 0.006 | 0.079 | 0.002 | 0.001 |
| Acids, g/kg DM | ||||||||||||
| Lactic (LA) | 99.5 a | 65.1 b | 94.9 a | 94.8 a | 95.0 a | 94.7 a | 3.36 | 0.345 | 0.340 | 0.245 | <0.001 | 0.984 |
| Acetic (AA) | 19.9 b | 26.3 a | 20.7 b | 18.2 b | 18.0 b | 17.8 b | 0.89 | 0.198 | 0.128 | 0.240 | <0.001 | 0.120 |
| Propionic 4 | 0.16 bc | 0 c | 0.17 bc | 0.18 bc | 0.40 ab | 0.57 a | 0.075 | 0.879 | 0.003 | 0.075 | 0.003 | 0.002 |
| Butyric | 0 | 0.03 | 0 | 0.01 | 0 | 0 | 0.008 | 0.290 | 1.000 | 0.731 | 0.033 | 0.448 |
| Total volatile fatty acids | 20.1 b | 26.5 a | 20.9 b | 18.4 b | 18.4 b | 18.4 b | 0.95 | 0.239 | 0.239 | 0.341 | <0.001 | 0.221 |
| Total fermentation acids 5 | 120 a | 92 b | 116 a | 113 a | 113 a | 113 a | 3.64 | 0.242 | 0.239 | 0.191 | <0.001 | 0.755 |
| Total fermentation products 6 | 129 a | 97 b | 126 a | 121 a | 129 a | 130 a | 3.99 | 0.210 | 0.843 | 0.608 | <0.001 | 0.190 |
| LA/AA ratio | 5.03 a | 2.47 b | 4.59 a | 5.23 a | 5.33 a | 5.34 a | 0.268 | 0.626 | 0.433 | 0.776 | <0.001 | 0.139 |
| Aerobic Stability (2 °C), hours | 40 b | 313 a | 36 b | 58 b | 40 b | 37 b | 15.7 | 0.434 | 0.903 | 0.875 | <0.001 | 0.592 |
| Microbial counts, cfu 7/g | ||||||||||||
| Yeasts | 1.2 × 105 | 2.0 × 102 | 2.0 × 105 | 2.4 × 105 | 1.9 × 105 | 2.0 × 105 | 6.5 × 104 | 0.251 | 0.434 | 0.269 | 0.017 | 0.725 |
| Moulds | 5.1 × 102 | 2.0 × 102 | 1.0 × 102 | 1.0 × 102 | 1.0 × 102 | 3.0 × 102 | 1.6 × 102 | 0.108 | 0.384 | 0.078 | 0.792 | 0.558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, M.; Rinne, M. Dry Matter Content and Additives with Different Modes of Action Modify the Preservation Characteristics of Grass Silage. Fermentation 2023, 9, 640. https://doi.org/10.3390/fermentation9070640
Franco M, Rinne M. Dry Matter Content and Additives with Different Modes of Action Modify the Preservation Characteristics of Grass Silage. Fermentation. 2023; 9(7):640. https://doi.org/10.3390/fermentation9070640
Chicago/Turabian StyleFranco, Marcia, and Marketta Rinne. 2023. "Dry Matter Content and Additives with Different Modes of Action Modify the Preservation Characteristics of Grass Silage" Fermentation 9, no. 7: 640. https://doi.org/10.3390/fermentation9070640
APA StyleFranco, M., & Rinne, M. (2023). Dry Matter Content and Additives with Different Modes of Action Modify the Preservation Characteristics of Grass Silage. Fermentation, 9(7), 640. https://doi.org/10.3390/fermentation9070640







