Effects of Streptococcus thermophilus Fermentation on the Flavors and Antioxidant Properties of Barley Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Fermented Barley Juice Preparation
2.3. Microbiological and Physicochemical Analysis
2.3.1. Determination of Viable Bacterial Counts and pH
2.3.2. Determination of Total Sugars and Titratable Acidity
2.3.3. Determination of Total Phenolic Content
2.3.4. Determination Analysis of Total Flavonoid Content
2.4. Analysis of the Volatile Compounds
2.5. Analysis of the Non-Volatile Compounds
2.6. Antioxidant Activity Analyses
2.6.1. ABTS Radical Scavenging Ability
2.6.2. DPPH Radical Scavenging Ability
2.6.3. O2− Radical Scavenging Ability
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Dynamic Changes in Viable Cell Counts and pH of Barley Juice during Fermentation
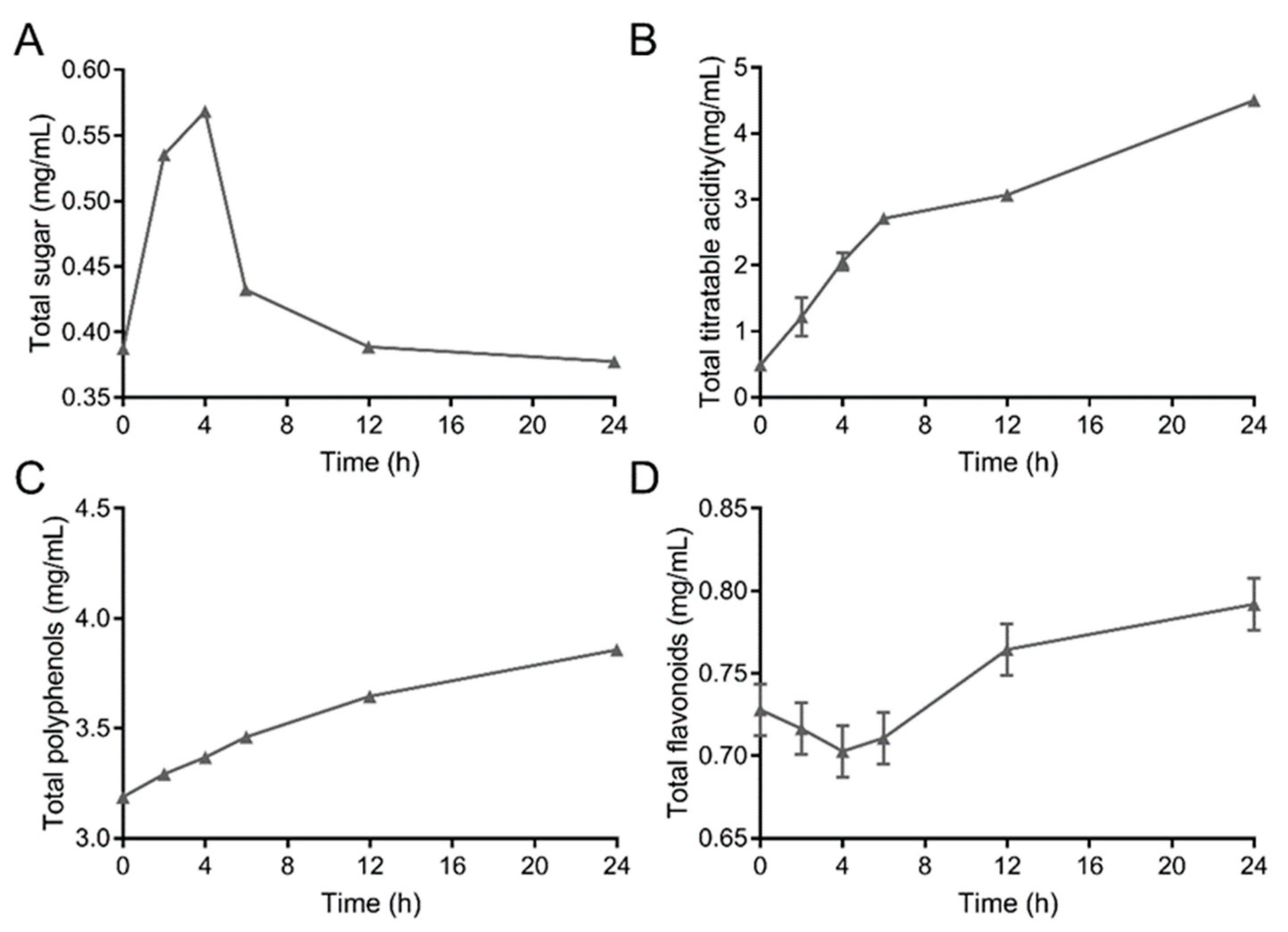
3.2. Dynamic Changes in Total Sugars, Titratable Acidity, Phenols, and Flavonoids in Barley Juice during Fermentation
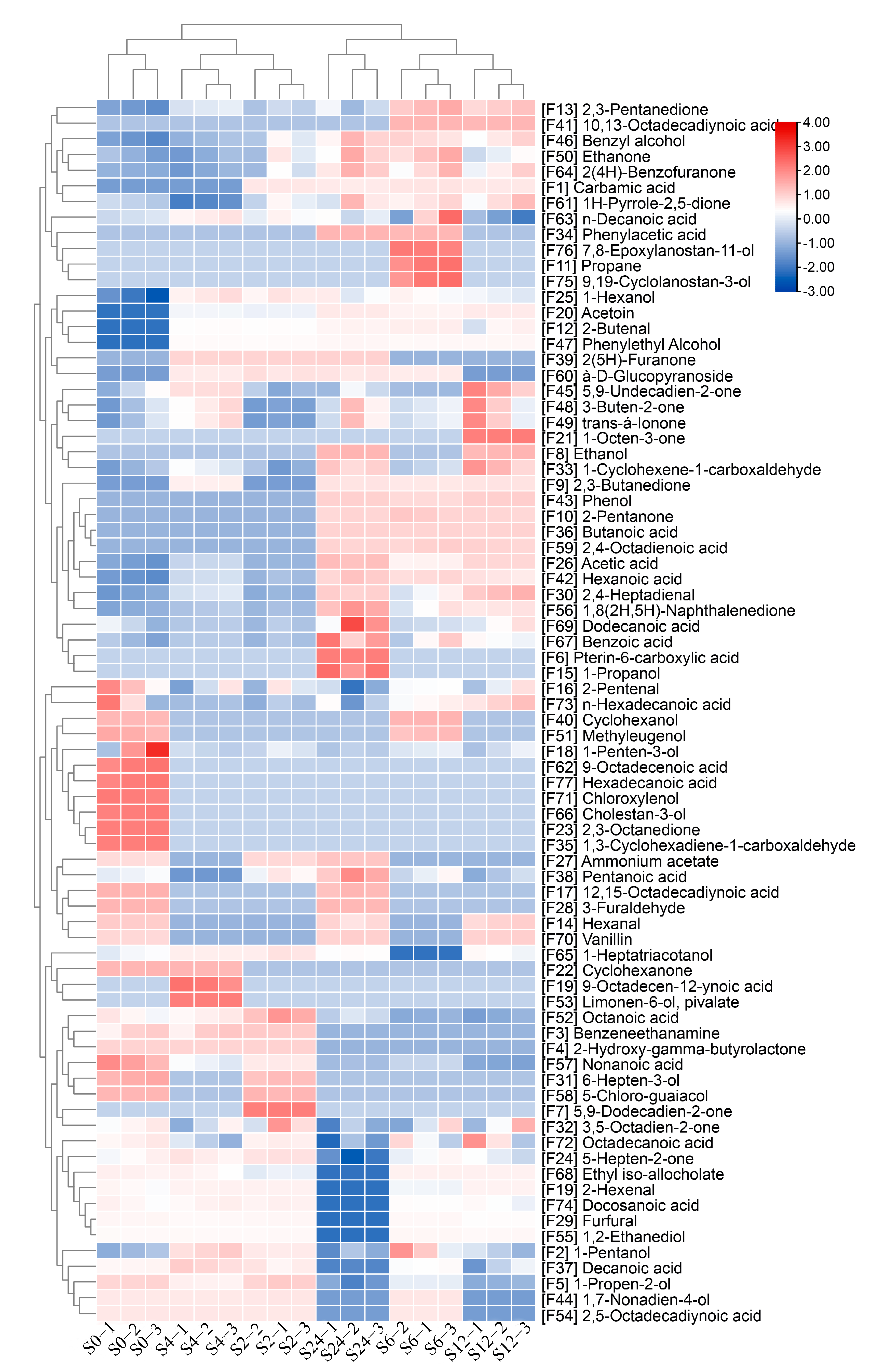
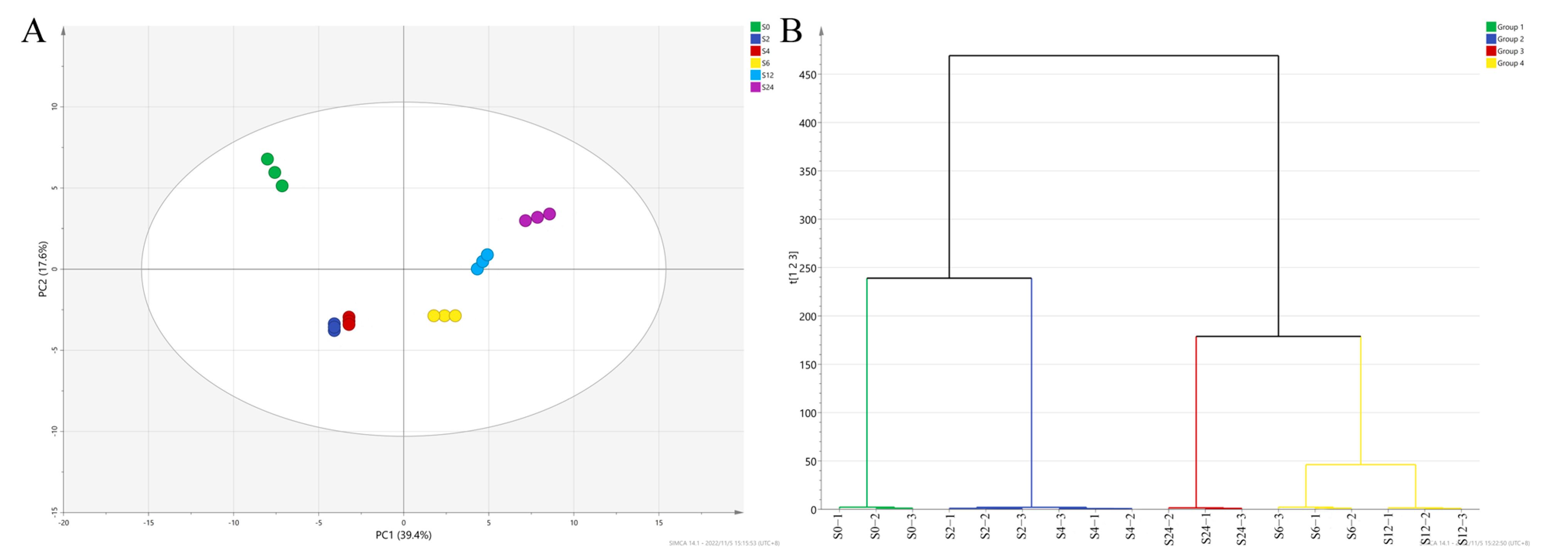
3.3. Volatile Compound Profiles of Unfermented and Fermented Barley Juices
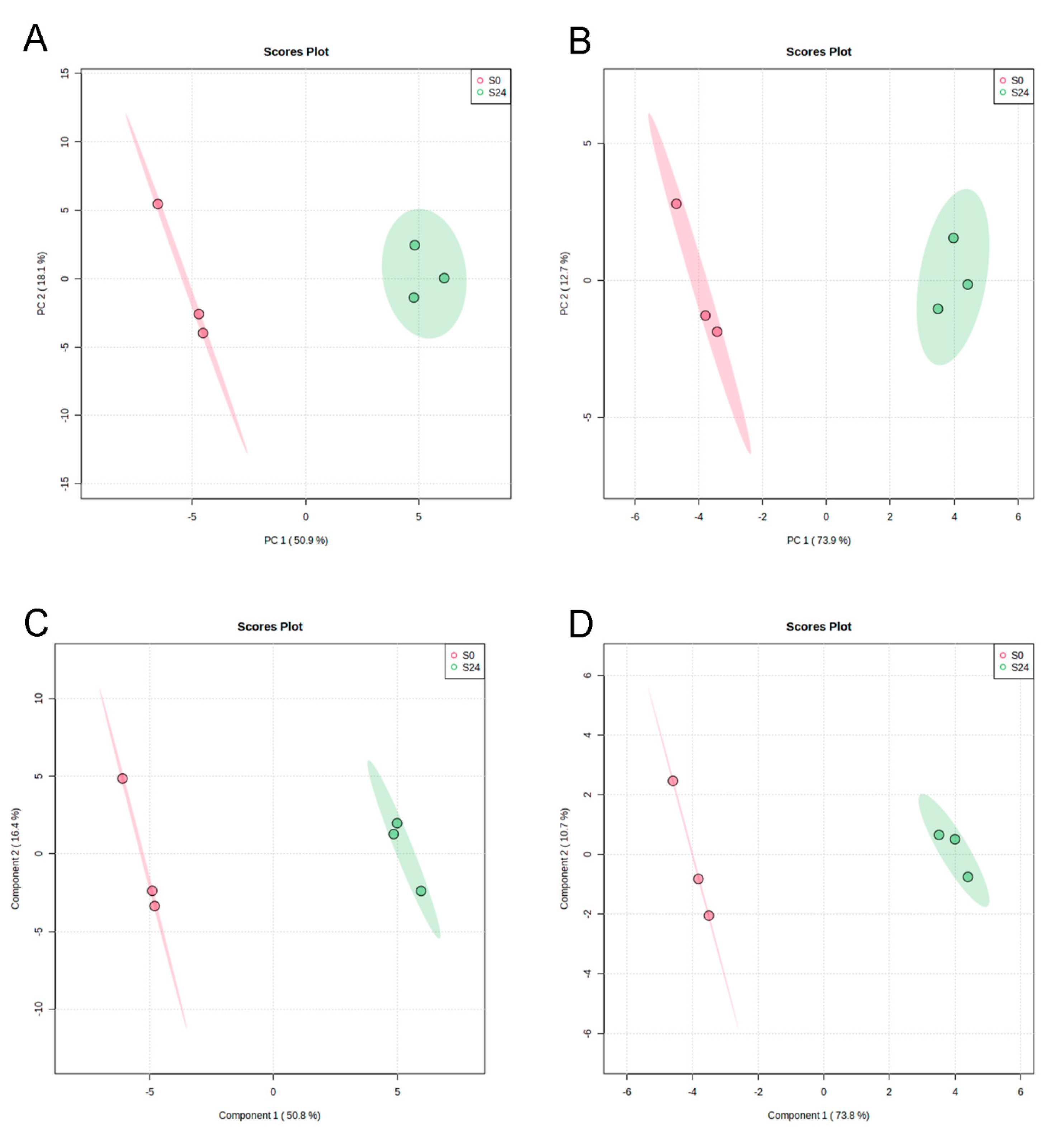
3.4. Non-Volatile Compound Profiles of Unfermented and Fermented Barley Juices
3.5. Antioxidant Activity of Unfermented and Fermented Barley Juices
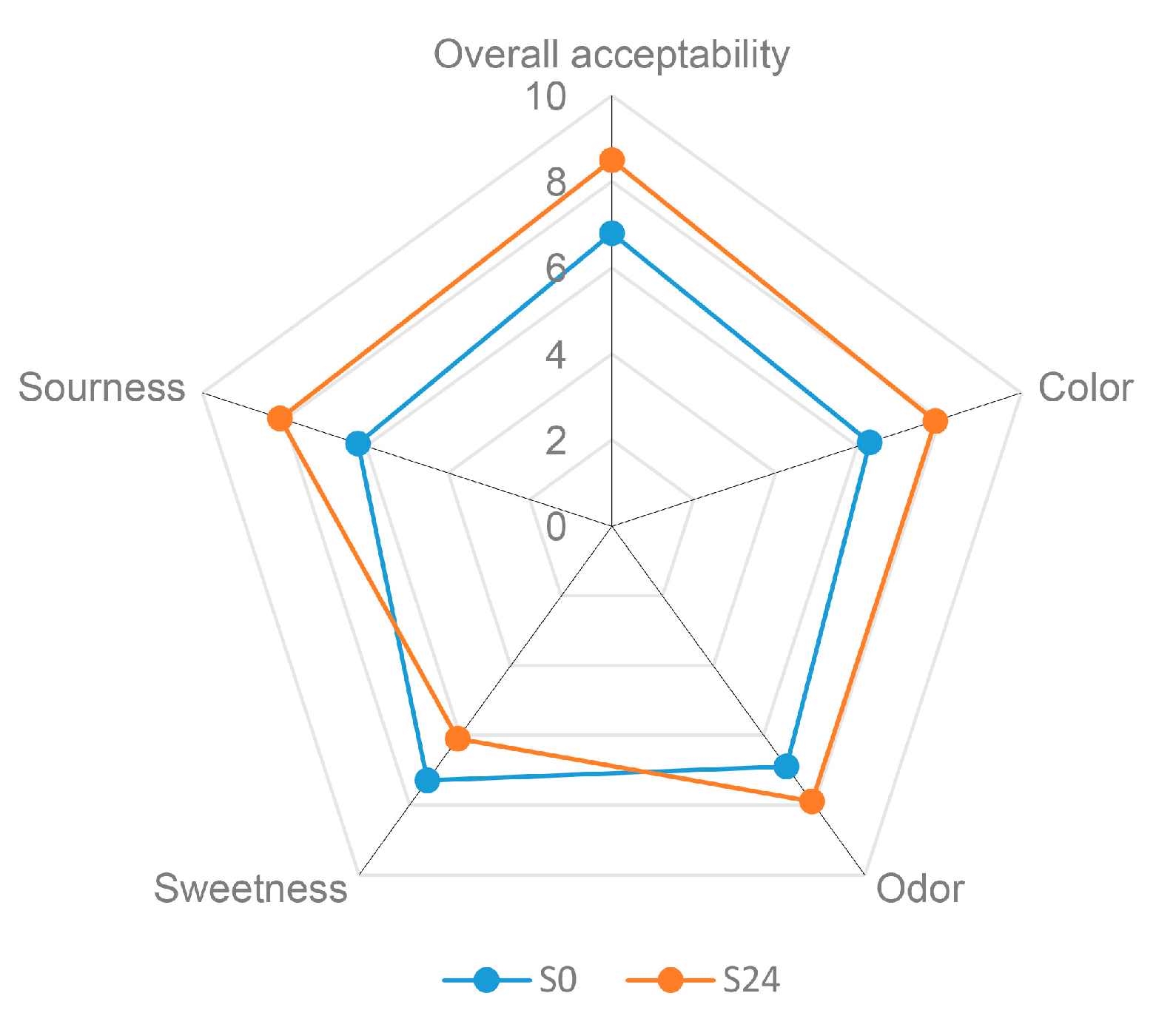
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, J.K.; Chen, T.T.; Wang, Z.W.; Wang, C.; Liu, C.; Li, L. Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chem. 2022, 389, 133083. [Google Scholar] [PubMed]
- Zhang, J.; Wang, P.; Tan, C.; Zhao, Y.; Zhu, Y.; Bai, J.; Xiao, X.; Zhang, L.; Teng, D.; Tian, J.; et al. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Tu, Z.; Zhang, C.; Wang, L. Highland barley improves lipid metabolism, liver injury, antioxidant capacities and liver functions in high-fat/cholesterol diet mice based on gut microbiota and LC-MS metabonomics. Food Biosci. 2022, 50, 102094. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Feng, S.; Ma, X.; Wang, S.; Zhang, Y. Correlation between microbe, physicochemical properties of Jiuqu in different plateau areas and volatile flavor compounds of highland barley alcoholic drink. Food Biosci. 2023, 51, 102276. [Google Scholar] [CrossRef]
- Guo, S.; Wu, T.; Peng, C.; Wang, J.; Sun, T.; Zhang, H. Metabolic footprint analysis of volatile metabolites by gas chromatography-ion mobility spectrometry to discriminate between different fermentation temperatures during Streptococcus thermophilus milk fermentation. J. Dairy Sci. 2021, 104, 8541–8553. [Google Scholar] [CrossRef]
- Lecomte, X.; Gagnaire, V.; Lortal, S.; Dary, A.; Genay, M. Streptococcus thermophilus, an emerging and promising tool for heterologous expression: Advantages and future trends. Food Microbiol. 2016, 53, 2–9. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L.; Kerényi, Z. Relationship between total cell counts and exopolysaccharide production of Streptococcus thermophilus T9 in reconstituted skim milk. LWT 2021, 148, 111775. [Google Scholar] [CrossRef]
- Wang, K.; Qi, J.; Jin, Y.; Li, F.; Wang, J.; Xu, H. Influence of fruit maturity and lactic fermentation on physicochemical properties, phenolics, volatiles, and sensory of mulberry juice. Food Biosci. 2022, 48, 101782. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Pan, X.; Lao, F.; Liao, X.; Shi, Y.; Wu, J. Improvement of antioxidant properties of jujube puree by biotransformation of polyphenols via Streptococcus thermophilus fermentation. Food Chem. X 2022, 13, 100214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Guo, W.; Chen, M.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Dynamics changes in physicochemical properties, volatile metabolites, non-volatile metabolites, and physiological functions of barley juice during Bifidobacterium infantis fermentation. Food Chem. 2023, 407, 135201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, Z.; Zhang, L.; Jiang, Y.; Zhu, J. Dynamic changes of quality and flavor characterization of Zhejiang rosy vinegar during fermentation and aging based on untargeted metabolomics. Food Chem. 2023, 404, 134702. [Google Scholar] [CrossRef]
- Guo, W.; Chen, M.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Effects of Lacticaseibacillus casei fermentation on the bioactive compounds, volatile and non-volatile compounds, and physiological properties of barley beverage. Food Biosci. 2023, 53, 102695. [Google Scholar] [CrossRef]
- Wu, T.; Sakamoto, M.; Phacharapan, S.; Inoue, N.; Kamitani, Y. Antioxidant characteristic changes, sensory evaluation, processing and storage of functional water modified juice. Food Biosci. 2023, 52, 102468. [Google Scholar] [CrossRef]
- Isas, A.S.; Mariotti Celis, M.S.; Pérez Correa, J.R.; Fuentes, E.; Rodríguez, L.; Palomo, I.; Mozzi, F.; Van Nieuwenhove, C. Functional fermented cherimoya (Annona cherimola Mill.) juice using autochthonous lactic acid bacteria. Food Res. Inter. 2020, 138, 109729. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Mushtaq, B.S.; Mahdi, A.A.; Al-Maqtari, Q.A.; Al-Adeeb, A.; Ahmed, A.; Fan, M.; Li, Y.; Qian, H.; Jinxin, L.; et al. Molecular structure, morphological, and physicochemical properties of highlands barley starch as affected by natural fermentation. Food Chem. 2021, 356, 129665. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Li, F.; Jiao, X.; Ma, D.; Zhang, L.; Yang, B.; Zhao, J.; Han, J.; Li, Q. Effects of lactic acid bacteria fermentation on chemical compounds, antioxidant capacities and hypoglycemic properties of pumpkin juice. Food Biosci. 2022, 50, 102126. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Han, M.; Liang, J.; Zhang, M.; Bai, X.; Yue, T.; Gao, Z. Evaluating the changes in phytochemical composition, hypoglycemic effect, and influence on mice intestinal microbiota of fermented apple juice. Food Res. Inter. 2022, 155, 110998. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; de las Rivas, B.; Muñoz, R. Aryl glycosidases from Lactobacillus plantarum increase antioxidant activity of phenolic compounds. J. Funct. Foods 2014, 7, 322–329. [Google Scholar] [CrossRef]
- Oh, B.T.; Jeong, S.Y.; Velmurugan, P.; Park, J.H.; Jeong, D.Y. Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J. Biosci. Bioeng. 2017, 124, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Huang, H.; Wang, J.; Liu, N.; Chen, X.; Jiang, T.; Xu, H.; Lei, H. Insights into the improvement of bioactive phytochemicals, antioxidant activities and flavor profiles in Chinese wolfberry juice by select lactic acid bacteria. Food Biosci. 2021, 43, 101264. [Google Scholar] [CrossRef]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2023, 51, 102337. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, L.; Liu, H.; Liu, H.; Zhou, Y.; Li, M.; Gao, R. Analysis of the changes of volatile flavor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Sci. Hum. Well. 2023, 12, 173–182. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Asanuma, M.; Mizobe, H.; Kojima, K.; Nagai, T.; Beppu, F.; Gotoh, N. Characterization of cis- and trans-octadecenoic acid positional isomers in edible fat and oil using gas chromatography–flame ionisation detector equipped with highly polar ionic liquid capillary column. Food Chem. 2014, 160, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, G.S.; Hwang, S.; Kim, B.W.; Lim, J.H.; Lee, J.C.; Kim, H.C.; Kim, W.K.; Kim, Y.S. Methyleugenol reduces cerebral ischemic injury by suppression of oxidative injury and inflammation. Free Radical Res. 2010, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Abe, K.; Morishita, Y.; Yamashita, S.; Segawa, R.; Dong, J.; Moriya, T.; Hiratsuka, M.; Hirasawa, N. Pentanoic acid induces thymic stromal lymphopoietin production through Gq/11 and Rho-associated protein kinase signaling pathway in keratinocytes. Int. Immunopharmacol. 2017, 50, 216–223. [Google Scholar] [CrossRef]
- Shao, T.; Song, X.; Jiang, Y.; Wang, C.; Li, P.; Sun, S.; Wang, D.; Wei, W. Vanillin-Catalyzed highly sensitive luminol chemiluminescence and its application in food detection. Spectrochim. Acta A 2023, 294, 122535. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Vitale, D.; Letizia, C.S.; Api, A.M. Fragrance material review on benzyl alcohol. Food Chem. Toxicol. 2012, 50, S140–S160. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Shen, J.; Manickam, S.; Li, S.; Tao, Y.; Li, D.; Liu, D.; Han, Y. Investigation of blueberry juice fermentation by mixed probiotic strains: Regression modeling, machine learning optimization and comparison with fermentation by single strain in the phenolic and volatile profiles. Food Chem. 2023, 405, 134982. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.W.; Zhu, D.; Sng, J.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Characterisation of calamansi (Citrus microcarpa). Part II: Volatiles, physicochemical properties and non-volatiles in the juice. Food Chem. 2012, 134, 696–703. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wei, X.; Wang, Z.; Wang, H.; Chen, J.; Li, J.; Liu, J. Utilization of high-K+-cane molasses for enhanced S-Adenosylmethionine production by manipulation of a K+ transport channel in Saccharomyces cerevisiae. Biochem. Eng. J. 2023, 193, 108846. [Google Scholar] [CrossRef]
- Chang, C.J.; Dai, R.Y.; Leu, Y.L.; Tsai, T.Y. Effects of the melanogenic inhibitor, uracil, derived from Lactobacillus plantarum TWK10-fermented soy milk on anti-melanogenesis in B16F0 mouse melanoma cells. J. Funct. Foods 2015, 17, 314–327. [Google Scholar] [CrossRef]
- Garg, D.; Singh, M.; Verma, N.; Monika. Review on recent advances in fabrication of enzymatic and chemical sensors for hypoxanthine. Food Chem. 2022, 375, 131839. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Teng, J.; Huang, L.; Wei, B.; Xia, N. Determination of the variations in the metabolic profile and sensory quality of Liupao tea during fermentation through UHPLC–HR–MS metabolomics. Food Chem. 2023, 404, 134773. [Google Scholar] [CrossRef]
- Su, X.; Cui, W.; Zhang, Z.; Zhang, J.; Zhou, H.; Zhou, K.; Xu, Y.; Wang, Z.; Xu, B. Effects of L-lysine and L-arginine on the structure and gel properties of konjac glucomannan. Food Hydrocoll. 2023, 137, 108404. [Google Scholar] [CrossRef]
- Yamamoto, E.; Watanabe, R.; Tooyama, E.; Kimura, K. Effect of fumaric acid on the growth of Lactobacillus delbrueckii ssp. bulgaricus during yogurt fermentation. J. Dairy Sci. 2021, 104, 9617–9626. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Li, X.; Gao, J.; Simal-Gandara, J.; Wang, X.; Caprioli, G.; Mi, S.; Sang, Y. Effect of fermentation by Lactobacillus acidophilus CH-2 on the enzymatic browning of pear juice. LWT 2021, 147, 111489. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Li, C.; Yu, Q.; Xie, J.; Dong, R.; Xie, Y.; Li, B.; Tian, J.; Chen, Y. Natural variation on free, esterified, glycosylated and insoluble-bound phenolics of Rubus chingii Hu: Correlation between phenolic constituents and antioxidant activities. Food Res. Int. 2022, 162, 112043. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, M.; Shimada, A.; Matsuzaki, A.; Eguchi, A.; Tominaga, M. Chemical composition and sensory properties of fermented citrus juice using probiotic lactic acid bacteria. Food Biosci. 2021, 39, 100810. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef] [PubMed]



| No. | m/z | Chemical Formula | Compound Name | S24 vs. S0 |
|---|---|---|---|---|
| ESI+ | ||||
| 1 | 399.144 | C15H22N6O5S | S-Adenosylmethionine | up |
| 2 | 200.032 | C4H10NO6P | Iminoerythrose-4-phosphate | up |
| 3 | 279.170 | C15H22N2O3 | Tolycaine | up |
| 4 | 156.115 | C9H16O2 | Nonane-4,6-dione | up |
| 5 | 258.228 | C10H15N3O4 | 5-Methyldeoxycytidine | up |
| 6 | 255.247 | C11H14N2O5 | N-Ribosylnicotinamide | up |
| 7 | 112.027 | C4H4N2O2 | Uracil | up |
| 8 | 104.030 | C4H8OS | Tetramethylene sulfoxide | up |
| 9 | 122.048 | C6H6N2O | Nicotinamide | down |
| 10 | 146.106 | C6H14N2O2 | L-Lysine | down |
| 11 | 129.079 | C6H11NO2 | L(-)-Pipecolinic acid | down |
| 12 | 160.100 | C10H12N2 | Anatabine | up |
| 13 | 182.084 | C12H10N2 | Azobenzene | up |
| 14 | 136.039 | C5H4N4O | Hypoxanthine | up |
| 15 | 268.081 | C10H12N4O5 | Inosine | up |
| ESI− | ||||
| 1 | 165.055 | C9H10O3 | D(+)-Phenyllactic acid | up |
| 2 | 89.024 | C3H6O3 | DL-Lactic acid | up |
| 3 | 267.072 | C10H12N4O5 | Inosine | up |
| 4 | 87.008 | C3H4O3 | Pyruvic acid | up |
| 5 | 135.031 | C5H4N4O | Hypoxanthine | up |
| 6 | 150.041 | C5H5N5O | Guanine | up |
| 7 | 131.071 | C6H12O3 | 6-Hydroxycaproic acid | up |
| 8 | 242.077 | C9H13N3O5 | Cytarabine | up |
| 9 | 313.091 | C4H5N3O2 | 5-Amino-4-imidazolecarboxylate | up |
| 10 | 266.088 | C10H13N5O4 | Deoxyguanosine | up |
| 11 | 133.014 | C4H6O5 | DL-Malic acid | down |
| 12 | 115.003 | C4H4O4 | Fumaric acid | down |
| 13 | 243.061 | C9H12N2O6 | Pseudouridine | up |
| 14 | 134.037 | C6H5N3O | HOBT | down |
| 15 | 145.100 | C6H14N2O2 | (3S)-3,6-Diaminohexanoate | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, B.; Guo, W.; Chen, M.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Effects of Streptococcus thermophilus Fermentation on the Flavors and Antioxidant Properties of Barley Juice. Fermentation 2023, 9, 623. https://doi.org/10.3390/fermentation9070623
Mao B, Guo W, Chen M, Tang X, Zhang Q, Zhao J, Zhang H, Cui S. Effects of Streptococcus thermophilus Fermentation on the Flavors and Antioxidant Properties of Barley Juice. Fermentation. 2023; 9(7):623. https://doi.org/10.3390/fermentation9070623
Chicago/Turabian StyleMao, Bingyong, Weiling Guo, Minxuan Chen, Xin Tang, Qiuxiang Zhang, Jianxin Zhao, Hao Zhang, and Shumao Cui. 2023. "Effects of Streptococcus thermophilus Fermentation on the Flavors and Antioxidant Properties of Barley Juice" Fermentation 9, no. 7: 623. https://doi.org/10.3390/fermentation9070623
APA StyleMao, B., Guo, W., Chen, M., Tang, X., Zhang, Q., Zhao, J., Zhang, H., & Cui, S. (2023). Effects of Streptococcus thermophilus Fermentation on the Flavors and Antioxidant Properties of Barley Juice. Fermentation, 9(7), 623. https://doi.org/10.3390/fermentation9070623







