Abstract
The fermentation of syngas is an attractive technology that can be integrated with gasification of lignocellulosic biomass. The coupling of these two technologies allows for treating a great variety of raw materials. Lignin usually hinders microbial fermentations; thus, the thermal decomposition of the whole material into small molecules allows for the production of fuels and other types of molecules using syngas as substrate, a process performed at mild conditions. Syngas contains mainly hydrogen, carbon monoxide, and carbon dioxide in varying proportions. These gases have a low volumetric energy density, resulting in a more interesting conversion into higher energy density molecules. Syngas can be transformed by microorganisms, thus avoiding the use of expensive catalysts, which may be subject to poisoning. However, the fermentation is not free of suffering from inhibitory problems. The presence of trace components in syngas may cause a decrease in fermentation yields or cause a complete cessation of bacteria growth. The presence of tar and hydrogen cyanide are just examples of this fermentation’s challenges. Syngas cleaning impairs significant restrictions in technology deployment. The technology may seem promising, but it is still far from large-scale application due to several aspects that still need to find a practical solution.
1. Introduction
Syngas fermentation is a technology that uses specific biomass to transform small gaseous molecules into other gaseous compounds such as methane, but also other compounds such as ethanol, butanol, and hexanol may be obtained. Alcohols are released into the liquid phase, thus requiring additional separation stages. The main advantages of the process are the mild conditions applied to the fermentation (low temperature and pressure) [1] to achieve the conversion of hydrogen and carbon monoxide (CO)/carbon dioxide (CO2), in an equivalent way as in the Fischer–Tropsch synthesis. Current interest in reducing the use of fossil sources for energy production and attaining a circular economy model in Europe to decouple economic growth and resource use [2] have led to the search for technologies capable of reusing materials and reducing the demand for virgin raw materials.
The circular economy concept attempts to reduce waste generation by keeping materials active in the economic cycle for longer periods, thus reducing mass and energy losses in production balances [3]. This concept is a promising approach to attaining sustainability in economic activity [4]. A circular economy strategy can aid in mitigating global climate change by increasing the reuse of materials, valorizing waste streams, and increasing the share of energy production from renewable sources and wastes [5]. However, not all aspects of this circular conceptualization result in advantages. Integrating wastes into the production chain needs to consider the material quality, dispersion, transportation costs, and pre-treatments required to recover valuable components [6,7], which, as an intrinsic disadvantage, has an increase in energy demand. Other aspects involve market behavior and consumer perception, which may favor or hinder the introduction of recycling materials into the production chain. Although the public accepts recycled plastics and paper, organic materials do not benefit from a good public perception. In addition, concerns regarding contaminants and health risks limit the reintroduction of organic wastes into the production chain. Energy and mass balances must also be carefully evaluated; thus, the conversion of biomass and the development of circular cycles should consider energetic constraints [8]. Absolute circularity is not achievable due to restrictions dictated by the second thermodynamic law since some energy is unavoidably lost [9].
The thermal treatment of biomass involves different processes, such as direct energy production from combustion, transformation to produce energy-containing molecules by pyrolysis/gasification [10,11,12], or processing in the presence of water under high temperature and pressure to generate a stable carbonaceous product [13,14]. This latter process is called hydrothermal carbonization and is currently considered a suitable alternative (currently at the experimental level) to treat biomass material with high moisture content. Thermal hydrolysis and decarboxylation of biomass occur, forming a char solid product and water-soluble compounds [15,16].
In the case of pyrolysis and gasification, the increase in temperature produces the thermal cracking of complex molecules. On the one hand, pyrolysis is carried out in the absence of air, resulting in a solid carbonaceous product (char), liquid and gaseous compounds with varying yields depending upon pyrolysis temperature and heating rate [17,18]. Pyrolysis processes are classified as slow, fast, and flash pyrolysis. The first one takes place at temperatures around 300–500 °C and low heating rates. Usually, the increase in process temperature and heating ramp marks the difference between fast and flash pyrolysis, with the latter having heating rates greater than 1000 k/s [19,20]. A particularly slow pyrolysis process is torrefaction. This slow thermal treatment is intended as a biomass pre-treatment method to increase energy density characteristics, facilitating the storage and transport of biomass to be later used as a primary fuel [21]. Gasification, on the other hand, is a process that involves a first pyrolysis stage. However, in this case, a gaseous agent, which can be air, steam, oxygen, or carbon dioxide, is introduced into the thermal reactor, mediating the conversion of heavy hydrocarbons into light molecules (CO and H2) [22].
Syngas is produced from pyrolysis/gasification using different materials as inputs. The great advantage of thermal processing is its versatility, being capable of treating fossil sources such as oil sludge, a viscous residue derived from crude extraction and processing, heavy oil, coal, plastics and also renewable sources such as biomass, manures, and organic wastes in general [23,24,25,26,27,28]. The gas produced has a low volumetric energy density mainly due to H2 and CO. When air is used in gasification, nitrogen reduces the calorific value even more due to its high content in the incoming air.
The fermentation of gaseous components conforming syngas has awakened great interest. The concerns regarding the use of fossil fuels, future availability, and climatic impacts have caused the revival of several forgotten technologies previously considered unfeasible. These technologies are now considered suitable and capable of aiding in decarbonizing the energy and transport sector and increasing the recycling rate of raw materials by valorizing waste streams. Thus, concepts such as power to liquid (PtL) and waste to energy (WtE) have become common in the scientific and technical literature. In the first case, energy is used for producing small molecules to be later transformed into liquid components, mainly using the Fischer–Tropsch synthesis to obtain saturated hydrocarbons or synthetic liquid fuels from other catalyzed processes [29,30,31]. In the WtE case, waste streams, having low quality and high production volume, are used as raw materials. In this sense, municipal solid wastes, sewage sludge, and manures have been considered suitable inputs for energy production either under thermal processes or biological ones such as anaerobic digestion [32,33,34].
The biological conversion of hydrogen and CO/CO2 is possible by the use of specific microflora, which can be obtained from mixed cultures or using specific strains under anaerobic conditions. Anaerobic microorganisms can use C1 substrates as carbon sources, producing short-chain acids, alcohols, or methane. However, the use of syngas requires special cleaning procedures since this gas carries ash particles, nitrogen/sulfur gaseous compounds (H2S, SO2, COS, NH3, HCN, NOx), oxygen, condensable organics, and tar, which can inhibit the activity of microorganisms [35,36,37]. Since the fermentation needs to be coupled to a syngas-producing process, the integration of biomass gasification and subsequent biological syngas conversion seems the logical sequence. However, pyrolysis and gasification processes are full of challenges due to their technical complexity and low profitability associated with high installation costs [38]. Therefore, the coupling with a fermentation stage intended to assimilate gaseous substrates will have to overcome their own constraints associated with mass transfer limitations, inhibitory conditions, and reactor conversion efficiency [39] and those associated with the thermal treatment of raw materials.
The present review aims to analyze the current state of gasification technology and syngas fermentation in an attempt to identify the feasibility of implementing this technology as one of the pathways to increase the share of renewables in the energy sector and aid in attaining full decarbonization of the economy. This review was performed by using the following main keywords for selecting relevant manuscripts: “Biomass pyrolysis” OR “Biomass gasification”, “Syngas fermentation” AND “Lignocellulosic material” OR “Agricultural waste” OR “Animal manure” OR “Sewage sludge”. Preference in selecting scientific material was given to experimental work reported during the last 10 years. However, manuscripts containing basic knowledge associated with the subject were also considered, although they had been published previously. A search associated with commercial companies was also carried out since this information is not usually found in scientific journals.
2. Gasification/Pyrolysis Technologies
The gasification process is a proven technology used to produce syngas from coal for electricity generation in gas turbines. Synthesis of syngas molecules is carried out by catalytic processes such as Fischer–Tropsch, producing liquid hydrocarbons, generating methanol from the Basf process, or methane from the Sabatier reaction [40,41,42]. The use of syngas dates back to 1812 when the Westminster and London Gas Light and Coke Co. was provided with a royal grant to light London streets in 1812 [43]. Although the gasification process has been widely studied, the technology deployment has suffered several drawbacks, still being at a demonstration scale or needing government subsidies to make plant installation feasible.
The integrated gasification combined cycle (IGCC) concept counted with four demonstration plants. Two were located in the United States (Wabash River Coal Gasification Repowering Project and the Tampa Electric Integrated Gasification Combined-Cycle Project), and the other two were located in Europe (Willem Alexander IGCC Plant in Buggenum, near Roermond (Latitude 51.222980, Longitude 5.971580) The Netherlands and ELCOGAS IGCC Plant in Puertollano, Spain) [44]. These plants were either shut down due to air pollutant constraints or retired due to finalizing project activities [45,46]. These large-scale demonstration projects allowed the development of the technology to reach a status close to commercial scale, although high installation costs are still a significant burden. However, experience has been gained regarding syngas composition and direct valorization in turbines for energy production, which is a valuable feature for the deployment of the technology when using other fuels.
The gasification technologies currently available are based on fixed-bed downdraft systems, up-draft, bubbling bed, circulating fluidized bed or dual configurations to increase process efficiency (see Figure 1). It is worth mentioning that any technology should comply with current environmental legislation. Therefore, gasification/pyrolysis is not an exception. The technology must meet restrictions regarding volatile compounds production, NOx, sulfur compounds, and particulate matter (PM2.5). Configurations exploring the feasibility of treating mixtures of coal and biomass capable of complying with emission standards were studied by Ward et al. [47] as part of the Alaska Syngas Project using a gasifier designed by Hamilton Maurer International, Inc. Large-scale configurations capable of producing a significant amount of energy also required high input rates, for this reason the combined use of coal and biomass allows adapting scale and materials availability in addition to reducing the costs of gas cleaning technologies.
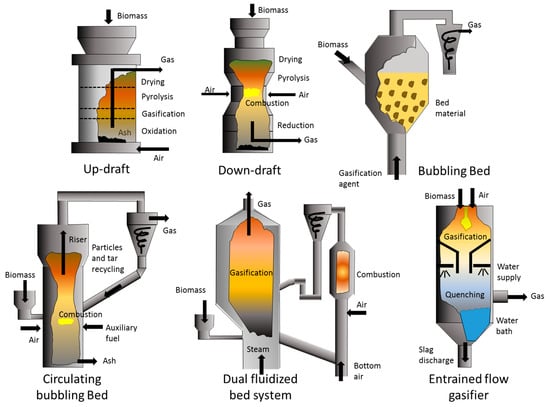
Figure 1.
Schematization of different gasification technologies.
Researchers have studied the gasification of lignocellulosic biomass and wastes as a solution for transforming different materials. Gasification is an interesting option for materials with either a low value or that require high pretreatment costs when attempting other valorization pathways, such as fermentation for producing ethanol, butanol, and methane, among others [48,49,50]. Applying pretreatments for releasing cellulose and subsequent transformation into sugars impairs high energy demand and operating costs, translating into difficulties when reaching profitability [51,52,53]. The production of inhibitory compounds released during pretreatments should also be added, adversely affecting the subsequent fermentation stages [54,55].
Most of the research on gasification and pyrolysis has been carried out at an experimental scale with a wide variety of substrates being evaluated [56,57,58,59,60]. The translation of these abundant results into an increase in scale and technology deployment has not been attempted at the same pace. Most of these works evaluate product yields and composition. However, several aspects regarding the practical implementation of a technology are usually missed when performing small pilot-scale studies. Ash melting behavior, energy recovery, gas cleaning to remove acids, toxic compounds and trace contaminants, wastewater production, and the presence of aromatic compounds in char are just a few issues that can endanger plant operability.
Table 1 lists different studies performed under laboratory and pilot-scale reactors. Other thermal processes, such as hydrothermal carbonization, were not considered in this table to set the focus on those technologies producing syngas. Thermal processes have the advantage of cracking complex molecules; thus, organic pollutants initially present in sludge may be eliminated. In the case of pyrolysis, the char produced is almost free from the presence of compounds that may cause concerns, such as hormones, antibiotics, microplastics, per- and polyfluoroalkyl substances (PFAS) [61]. Although metals may be concentrated in the char, the stability of these species increases with the increase in pyrolysis temperature, reducing its propensity to produce metal lixiviates [62,63]. Even though scientific literature considers the process feasible when using low-grade materials with high ash content, small commercial plants currently treat high-quality wood chip material, and only large-scale plants incorporating complex post-treatment equipment can treat a wide-quality range of input materials.

Table 1.
Gasification and pyrolysis assays carried out at pilot and semi-pilot scale available in the scientific literature using as substrate sewage sludge, manures, and lignocellulosic biomass.
The experience and knowledge obtained with the gasification process in the petroleum industry have been adapted to treat waste and lignocellulosic biomass. Table 2 shows a list of companies with experience in this technology. The complexity of the process regarding emissions and the presence of tar and other contaminants in the gas can be better controlled with an increase in scale. However, when considering sole biomass as input, the amount of material locally available may not be sufficient for producing enough syngas to be efficiently coupled to an electricity production system, as would be the case of combined heat and power (CHP) engines or gas turbines. A solution is to deal with a flexible input; thus, mixtures of municipal solid wastes (MSW), plastics and biomass can serve as raw materials in this type of facility. Other options may be partial decentralization of gasification stages; therefore, biomass can be transformed close to its location, generating a condensed product that is easy to transport. A centralized large-scale unit capable of cleaning and processing syngas would receive the condensed material. This is the idea behind the bioliq® process, where fast pyrolysis was selected as the pretreatment technology of biomass in a decentralized configuration. The oil obtained can be transported and transformed in a large-scale plant where syngas cleaning is effective. Poisoning components can be removed prior to catalytic conversion to dimethyl ether (DME) and then to biogasoline [92,93], which is compatible with current engine fuels [94]. The increase in scale allows for a decrease in Levelized unitary costs. Therefore, it becomes feasible to use pure oxygen or oxygen-enriched air to obtain syngas with higher calorific value.

Table 2.
Description of different gasification processes developed by commercial companies. Processes presented in this table are ready for commercialization or close to commercial stage.
Small-scale gasification technologies are currently available in the market for producing electricity or thermal energy. Syngas cleaning is simplified in this case since burning syngas will focus on reducing NOx and sulfur emissions. When internal combustion engines are used, a cooling stage is necessary, requiring careful control of tar formation to avoid precipitation on valves and engine components [113]. Patuzzi et al. [114] reviewed the performance of small gasification units installed in South Tyrol (Northern Italy) thanks to a very favorable feed-in tariff. Small gasification units resulted in profitability with the increase in electricity tariff, but all these units had as a requirement the use of very high-quality biomass with low ash content. Therefore, the feasibility of applying decentralized gasification for valorizing crop or livestock wastes is currently null, although researchers have set the focus on valorizing low-grade lignocellulosic materials and wastes. Another fact that deserves special attention from their study is that the available technologies showed high efficiency in producing gaseous products with low char yields. However, the generated char presented high metal and PAH (polycyclic aromatic hydrocarbons) content; therefore, its direct use as a soil amendment is not recommended.
Thermal analysis is a widely used technique for establishing reaction kinetics and understanding sample behavior under different atmospheres. Pyrolysis, combustion, and gasification of biomass have been studied by different authors, determining best fits to different degradation models and kinetics parameters based on curves profiles derived from thermal analysis using isothermal and non-isothermal methods [115,116,117,118,119]. However, there is a lack of correlation between kinetic parameters and gasifier/pyrolyzer performance. Fernandez-López et al. [120] studied the gasification of manure samples using thermal analysis. In this study, samples were initially pyrolyzed and then submitted to gasification under CO2 at 800–900 °C. Temperature affected the rate of conversion and the amount of residual ash obtained. Lower temperatures caused a significant decrease in reaction rates but enhanced the final conversion, resulting in a greater mass loss. Gil et al. [121] studied the effect of biomass characteristics on gasification performance using an atmospheric pressure bubbling fluidized bed reactor. The H/O ratio in biomass affected syngas composition with higher values, resulting in higher H2 content. Mineral content also affected biomass reactivity, with the presence of K2O in the ash leading to better conversion efficiency, whereas CaO showed an adverse effect.
Similar studies can be cited regarding this subject; however, the major drawbacks of the gasification technology are associated with gas cleaning and tar removal. No matter what the final use of syngas is, removing poisoning compounds is needed if a chemical synthesis is the valorization choice for syngas or if fermentation would be the technology selected. Given the wide experimental work regarding gasification and experience gained in biomass gasification under laboratory and industrial scale, the technology can be considered mature, but syngas cleaning is the next challenging step. Tar seems unavoidable unless excessive high-temperature conditions are applied, or catalysts are added to the gasification unit to favor tar cracking [122].
Co-gasification with fossil sources is a widely studied option, starting with coal and mixtures with organic wastes [123,124,125,126,127], and following with the treatment of heavy hydrocarbons, which presents challenges [128,129]. Bottom sludge from the petrochemical industry was gasified by Ongen et al. [130] in an updraft fixed bed gasifier with a recirculation zone to reduce tar formation. These authors added chicken manure in different proportions as a complementary organic input material. The final gas output was affected by the proportion of the mixture, and the best operation was found to be with 25% manure content. The high input rate of thermal processes makes the volume of residues to be locally treated unable to satisfy the demand for raw materials unless a flexible feeding strategy is assumed. However, this option may not be perceived as environmentally friendly for the public as it would mean the use of lignocellulosic biomass as a single feeding material. Treating mixtures of different wastes is a suitable option for attaining a desired power output but brings as a disadvantage a complexity in gas cleaning procedures and by-product disposal. As in any other process, the scale of the plant highly affects the profitability. Thus, levelized unit costs decrease with the increase in plant size, and although co-gasification units may require a more complex cleaning system, the larger scale allows for affording this complexity.
Pattanayak et al. [131] studied the profitability of gasification in India by considering a downdraft gasifier in conjunction with electricity production by a combined heat and power engine (500–1000 kW). The proposal was reported as feasible, with electricity cost production prices much lower than the current tariff from conventional technologies. A similar study was previously performed by Porcu et al. [132], also intended for electricity production and indicating the economic feasibility of the approach. However, this study did not consider any adverse effect on plant performance when changing biomass from high-quality material to low-grade waste biomass with high ash content, thus skipping difficulties that may arise with tar condensation.
The gasification process may seem feasible if only considering product yields, but the technology necessary to comply with environmental regulations or to avoid problems in subsequent valorization stages, such as the presence of filters to remove ash and tar components, catalysts to reduce tar formation, and adsorbents to eliminate inhibitory compounds increases the capital investments and maintenance costs, thus requiring substantial subsidies to become feasible [113]. The experience gained with small-scale biomass gasification and energy production by CHP units may serve as a first step to moving forward to syngas fermentation since common requirements are established, such as syngas cooling and tar/particle removal.
3. Syngas Fermentation
Fermentation is an environmentally friendly technology due to its low energy demand compared to other technologies that operate under high pressure and temperature conditions. Several microorganisms have been studied for their capability of transforming small carbon molecules into organic acids and alcohols. Among the common species studied are Clostridium ljungdahlii, C. autoethanogenum, Acetobacterium woodii, C. carboxidivorans, and Butyribacterium methylotrophicum [133,134,135,136,137]. However, the fermentation process must deal with mass transfer limitations and culture contamination by undesired microflora or the presence of phage. Thus, the feasibility of the process may be compromised due to low titter production and high operating costs.
Demonstration plants have been built in different projects with the technology developed by companies such as INEOS Bio, Coskata Inc. and LanzaTech. Fermentation of syngas derived from biomass gasification was attempted in the bioenergy demonstration plant in Vero Beach, FL, USA [138]. However, inhibitory conditions experienced in the fermentation stage due to the presence of hydrogen cyanide led to unsuccessful performance after spending several millions of governmental subsidies [139]. Removing trace contaminants was not an easy task; thus, the approach of Coskata Inc. by coupling the fermentation process with the technology of Westinghouse Plasma (a subsidiary of Alter NRG), which allows plasma gasification obtaining a free-tar syngas, seemed to facilitate fermentation operation by eliminating the tar production [140]. Although, the approach could not be demonstrated, the Mihama-Mikata plasma gasification plant is clear proof of the significant advantages plasma technology offers [141,142].
Coskata Inc. was out of business, and its technology was acquired by Synata Bio [143] and then by True North Venture Partners (TNVP) [144], with no further demonstration plants being constructed. LanzaTech, on the other hand, has been able to keep working in demonstration plants thanks to the Steelanol project (financed under Horizon 2020), in conjunction with ArcelorMittal [145]. Process gases derived from the blast furnace located in Ghent, Belgium, will be transformed by biological means into ethanol [145]. Commercial facilities of LanzaTech are located in China (Shougang LanzaTech, Shougnag JiYuan and NingXia Binze) [146], with the plant of Shougang LanzaTech receiving a carbon label certification from the China Electronic Energy Saving Technology Association for its bioethanol products in 2023 [147]. However, information regarding ethanol yields, product concentration and energy demand associated with the distillation process is not easily available.
3.1. Fundamentals of Syngas Fermentation
Alcohols and organic acids can be produced by acetogens using the acetyl-CoA reductive pathway, which is their main unifying feature given their wide genetic diversity. Ethanol and acetate production has been extensively studied, but C4 to C8 chemicals can also be produced from this pathway using different types of clostridium organisms [148]. This pathway is known as Wood–Ljungdahl and allows for reducing CO/CO2 using H2, obtaining energy, and assimilating carbon dioxide to produce cell carbon [149]. The pathway contains two branches, the methyl and carbonyl branch. In the methyl branch, the methyl group obtained from the reduction of CO/CO2 is transformed into formate by formate dehydrogenase and by a sequence of multiple steps forms methyl radicles that are further assimilated along with CO, forming acetyl-CoA [37]. CO can also be oxidized by biological water gas shift reaction into CO2, and the reducing equivalents derived from the reaction are conserved as reduced ferredoxin [150,151]. The production of acetyl-CoA is mediated by acetyl-CoA synthase (ACS) and carbon monoxide dehydrogenase complex (CODH) [1]. The bifunctional CO dehydrogenase enzyme reduces CO2 into CO, thus serving as the carbonyl group. In contrast, the methyl group is derived by a multistep reduction of CO2 where formyl, methenyl, methylene, and methyl intermediates are bound to a pterin cofactor [152]. The carbonyl branch is exclusive of anaerobic microorganisms.
Acetate is formed from acetyl-CoA to recover energy, and ethanol is produced from further reduction of acetate [152]. Hydrogenases are inhibited by the presence of CO, causing a decrease in hydrogen uptake, but as CO levels decrease, hydrogen uptake increases again [153]. Some acetyl-CoA is used to produce complex organic cell components, carbohydrates, proteins, and lipids [154]. Figure 2 shows a schematized simplification of the Wood–Ljungdahl pathway. Detailed descriptions of this pathway are available at Bengelsdorf et al. [155], Sahoo et al. [156], and Gencic et al. [157].
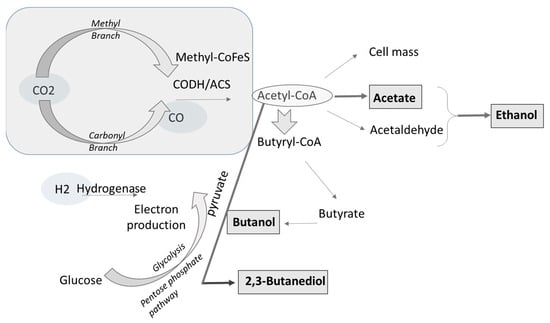
Figure 2.
Simplified schematization of the Wood–Ljungdahl pathway. Figure adapted from Ahuja et al. [37], Daniell et al. [153], and Phillips et al. [154].
Ethanol and acetic acid are produced from syngas by the following equations [158].
6CO + 3H2O → C2H5OH + 4CO2
6H2 + 2CO2 → C2H5OH + 3H2O
4CO + 2H2O → CH3COOH + 2CO2
Ethanol production from syngas is usually carried out by using Clostridium ljungdahlii strains. The fermentation requires a change in conditions to promote solventogenesis, transforming organic acids into alcohol. Thus, ethanol production should be initially favored by biomass growth along with organic acid production (acetate) and, by shifting conditions, ethanol is produced in a subsequent stage [159]. Martin et al. [160] compared the performance of three different clostridium strains under batch conditions, reporting that Clostridium ljungdahlii showed a higher ethanol production rate (0.301 g/L h), whereas Clostridium autoethanogenum showed a poor performance when solventogenesis phase was induced. However, ethanol yields only reach a concentration of 1.9%. Klasson et al. [161] reported the highest ethanol concentration ever recorded (48 g/L) using C. ljungdahlii at a pH of 4.0–4.5 in a continuously stirred tank reactor (CSTR) under nutrient-limited conditions during 560 h of fermentation.
Keeping high ethanol productivity under continuous conditions is still a challenge due to the loss of culture viability and low levels of metabolic activity, as demonstrated by Mohammadi et al. [162], who reported that recovery stages and pH adjustments are needed to keep active microflora. These authors obtained an ethanol concentration of 6.5 g/L, but this value was just a peak concentration attained during a short period. The fermentation showed irregular performance for the 30-day operation period due to changes in biomass activity and pH, with ethanol evolution initially increasing until reaching peak values, suffering a decrease and posterior recovery afterward.
Liu et al. [163] also evaluated fermentation performance under continuous conditions, retaining biomass by cell recycling and using corn-steep liquor to reduce fermenting medium costs. These authors used as a fermenting organism a pure culture of Alkalibaculum bacchi strain CP15, although contamination was reported during continuous operation of the system. The ethanol concentration achieved was 6 g/L, whereas the contamination experienced caused a positive effect, allowing the coupled production of propanol and butanol. The acid production phase and subsequent transformation into alcohols must be clearly understood to control fermentation performance, either under continuous or batch-sequential conditions. Low availability of CO due to mass transfer limitations, pH values, and acetate concentration seem to be determinants in promoting metabolic changes in the microflora to activate the solventogenesis phase to counteract the inhibitory effect of undissociated acetic acid [164].
The number of scientific publications regarding syngas fermentation has increased exponentially in recent years [165]. Alcohols with longer chains than ethanol can also be obtained from syngas fermentation. Clostridium carboxidivorans and C. kluyveri have been proposed as organisms suitable for producing elongated alcohols and fatty acids in co-cultures or mixed microbial cultures [166,167,168]. Hexanol is produced along with butanol and ethanol, with the latter being the predominant product [169]. In this type of syngas fermentation, butanol and hexanol concentrations hardly surpass the threshold of 1 g/L, thus setting great difficulties in subsequent alcohol recovery stages. Fernández-Naveira et al. [170] reported higher hexanol values, but the concentration of this alcohol just reached 2.66 g/L. The decrease in fermentation temperature from 37 to 25 °C allows for the reduction of acid crash. It results beneficial to hexanol and butanol production, but although a significant improvement is attained, the hexanol concentration reported by Ramió-Pujol et al. [171] using this strategy was just 8.21 mM (0.84 g/L).
Reported titters in the scientific literature seem extremely low compared to conventional ethanol fermentation and even with fermentation of pre-treated lignocellulosic biomass, which can reach values around 100 to 140 g/L [172,173]. Table 3 shows a list of experimental studies regarding syngas fermentation and media optimization. Most experimental studies deal with laboratory conditions using reactors with a volume of a few liters, showing low productivity and low product concentration. This fact is striking if compared with the aim of the Steelanol project, where a plant of 64,000 t ethanol/year will be built using a similar fermentation system [174].

Table 3.
Experimental studies available in the scientific literature reporting on syngas fermentation.
3.2. Experimental Work and Integration with Biomass Gasification
The experimental work available in the scientific literature deals mainly with small-batch scale reactors using synthetic syngas free of inhibitory components. Data obtained from large-scale experiences are scarce and even more if the use of syngas derived from biomass gasifiers is considered. Kundiyana et al. [201] evaluated a Clostridium strain P11T in a semi-pilot scale system. These authors tested a biomass gasifier and a 100 L reactor to ferment the resulting syngas. The main fermentation products were ethanol, acetic acid, and 2-propanol. Ethanol concentration reached a value of 25.3 g/L. The fermentation was tested under batch conditions, and cell concentration in the reactor was kept low, at about 0.84 g/L. Ethanol showed an increasing trend during the whole operation period (about two months), indicating promising future performance on a larger scale. However, the presence of trace contaminants resulted in severe fermentation failure. Rückel et al. [189] tested a culture of Clostridium carboxidivorans to produce short and medium-chain alcohols. These authors reported a beneficial effect under the presence of ammonia and hydrogen sulfide, but strong inhibition was caused by nitrogen oxides (added in the form of sodium nitrate or nitrite).
Gasification of low-quality biomass tends to produce more tars and inhibitors, thus demanding specific cleaning operations to remove contaminants, increasing cleaning-associated costs and adversely affecting process feasibility. Ramachandriya et al. [202] reviewed the effect of contaminants such as HCN, NOx, sulfide compounds, oxygen, and tars, among others, highlighting the relevance of producing clean syngas to integrate the two processes safely. Given the difficulties found in attaining stable performance when coupling small-scale biomass gasification and CHP energy production, it is expected that syngas fermentation can be linked to large-scale gasification units, which can stand a costly syngas upgrading process and afford additional equipment needed for controlling and removing contaminants.
Liakakou et al. [203] tested Clostridium ljungdahlii in a fermenting system coupled to a biomass gasifier. These authors studied the inhibitory effect of trace substances and tar components, evaluating the efficiency of the syngas cleaning processes developed to remove benzene, toluene, and xylene (BTX) mixtures using a specific oil absorber. The cleaning system also included a hydrodesulfurization and a bed adsorption unit for removing H2S and COS. The high efficiency of the cleaning process allowed for running a fermentation free of inhibitory conditions. The final ethanol concentration attained when feeding the clean gas was 2.2 g/L, using a 1.5 L CSTR.
Sharma et al. [204] alerted on the difficulties of producing a clean syngas stream. Cleaning technologies usually involve a wet scrubber to remove contaminants, causing syngas to cool down, affecting process efficiency and creating a wastewater disposal problem. On the contrary, removing contaminants at high temperatures seems more efficient, although extreme environments create other technical challenges when eliminating tars and particulate contaminants [205]. The use of oil instead of water is a concept tested by the OLGA technology, where heavy and light tar components are efficiently recovered using a two-stage absorber. Tar removed is then recycled back into the gasifier, reducing waste disposal costs and recovering energy from these undesirable by-products [206].
Other cleaning technologies developed include catalytic decomposition, BTX absorbers, removal of acid gases using adsorbents, and removal of nitrogen species [92,207]. A new cleaning process was tested recently by Frilund et al. [208] using low-cost adsorption systems and organic solvent-free removal concepts to reduce costs and complexity of operation. On the contrary, Calì et al. [209] tested a water scrubber along with a chemical and physical wastewater management unit containing an oil skimmer and an activated carbon adsorption filter, attaining a significant reduction in the water demanded by the cleaning stage. The use of an innovative cleaning system was tested by Hai et al. [210], proposing a mop fan with water spry, obtaining a tar removal efficiency of 89.6%.
Not all components act as toxic inhibitors; some may even have a beneficial effect at specific concentrations. Oliveira et al. [211] studied the addition of sodium sulfide in a culture of Clostridium ragsdalei, observing an increase in ethanol concentration to 7.67 g/L. In a recent study, Oliveira and co-workers [212] tested different cultures of C. autoethanogenum, C. ljungdahlii, and C. ragsdalei, with C. ljungdahlii showing the highest tolerance to the presence of ammonium and nitrate whereas C. ragsdalei showed a positive effect under the presence of 0.1 g/L of H2S. Table 4 lists studies that evaluate the effect of contaminants in syngas fermentation.

Table 4.
List of experimental studies evaluating the effect of inhibitory compounds in syngas fermentation.
It should be noted that CO can also exert a toxic effect on hydrogenase enzymes, thus making the CO route favorable for reducing power and decreasing ethanol production efficiency [202]. This effect explains the results obtained by Lanzillo et al. [137] and Kim et al. [195]. However, results reported by Hurstand and Lewis [158] indicated a better performance when increasing the gas reactor pressure from 0.35 to 2.0 atm, reporting almost nil ethanol production at the lowest pressure tested and reaching an ethanol concentration up to 2.5 g/L using Clostridium carboxidivorans P7T. Experiments performed at an even higher pressure, on the contrary, confirm the decrease experienced in product yield.
Stoll et al. [219] reported an initial increase in acetic acid production with pressure increase when assessing the performance of Clostridium ljungdahlii DSM13528 in a CSTR with a continuous supply of gas and operating under batch culture mode. The increase in pressure led to disappointing results. At 7 bar, the culture was unstable, showing significantly lower gas consumption and cell growth. There should be an equilibrium between enhancing gaseous substrate mass transfer to the liquid media and avoiding excessive CO levels to prevent inhibitory conditions.
CSTR are the most widely used laboratory reactors tested due to the simplified operation of the system at a small scale. However, other configurations offer outstanding performance when attempting an increase in mass transfer. Factors that affect the mass transfer phenomenon of gaseous species into the liquid phase include impeller configuration, agitation speed, partial pressure, temperature, the surface area available for mass transfer, mean bubble size, bubble size distribution, and bubble residence time [220,221,222]. In the present case, the inhibitory effect caused by CO sets limits to the amount of substrate safely transferred from the gas phase.
Munasinghe and Khanal [223] tested different reactors and diffuser types, indicating that gas lift reactors with bulb diffusers showed better performance regarding mass transfer, surpassing the performance of hollow fiber membrane modules. Impeller configuration also has a great effect on mass transfer. The propensity to gas flooding is greatly reduced if impellers are changed from standard Rushton turbines to concave-shaped turbines. However, impellers are not convenient when large-volume reactors are considered due to the high increase in the energy demand experimented when scale increases [224]. Figure 3 shows different reactor configurations suitable for fermenting gaseous substrates.
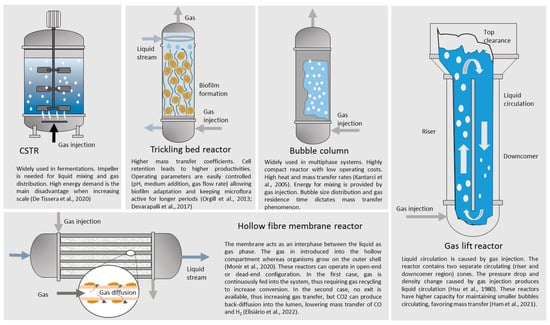
Figure 3.
Schematization of different reactor configurations suitable for the fermentation of gaseous substrates. References: Monir et al. (2020) [216], De Tissera et al. (2020) [225], Orgill et al. (2013) [226], Devarapalli et al. (2017) [227], Kantarci et al. (2005) [228], Hsu et al. (1980) [229], Ham et al. (2021) [230], Elisiário et al. (2022) [231].
It must be kept in mind that the fermentation is not a final stage of a global process. Separation units are necessary to obtain the desired product, and energy demand is highly dependent on the fermentation yield attained. In addition, sterilization requirements to keep a pure culture free of external microbial contamination increase the process energy demand. Syngas fermentation has similarities with acetone-butanol-ethanol (ABE) fermentation, a process that, after experiencing an industrial boom, suffered from high energy prices, bacteriophage infection, and more efficient process competitors [232,233,234], being relegated to experimental studies and waiting for a new opportunity. Configurations capable of overcoming inhibitory conditions and achieving higher titer and separation efficiency may make this fermentation a viable alternative to petroleum-based processes.
The capabilities of clostridium species to be integrated into a biorefinery context have been reviewed by Liberato et al. [235], considering these species the ideal candidates due to their versatility in using different types of substrates and operating under anaerobic conditions. Although the variety of species and products generated by different strains include alcohols and organic acids, the process of utilizing the conversion of simple sugars, lignocellulosic biomass, or gaseous streams remains to become feasible at an industrial scale. Efforts to reduce fermentation costs have been attempted by using low-cost fermenting media and supplements [194,196,236]. However, a simple solution is yet to be found due to the several complexities associated with fermentation stability and those added when coupling with biomass gasification.
The discovery of new strains capable of producing higher product concentrations opens a promising route. However, several deficiencies must be solved for a profitable process scaling. Lee et al. [237] reported on a novel strain (Clostridium sp. AWRP), which was isolated and studied using small batch serum bottles. The ethanol concentration attained was 119 mM (5.47 g/L), an interesting value compared with the previous ones reported in Table 3, but remains extremely far from those obtained by the conventional fermentation processes.
Cleaning syngas is essential for avoiding the cessation of microbial growth during fermentation. The type of gasifier employed, gasification conditions, and biomass quality greatly influence the type of impurities and syngas cleaning requirements. Therefore, a global optimization of the process is needed [238] to attain low levels of contaminants without the requirement of costly cleaning processes. Although efforts are being made with successful performance, as demonstrated by Hai et al. [210], Frilund et al. [208], and Straczewski et al. [239]. Industrial application of the whole integrated approach still seems far away due to other alternatives having better performance or demonstrating to be much closer to a commercial scale, as it would be integrating gasification and energy production by the use of solid oxide fuel cells [240,241], gas turbines [242,243], CHP engines, and even its fermentation homologous for the production of methane, since this small molecule can be used as fuel, having greater energetic density than carbon monoxide and hydrogen and does not need a phase separation, as is the case of producing acids of alcohols.
H2 and CO/CO2 can be converted into methane by a sequence or reactions involving water-gas shift reaction, acetogenesis, hydrogenotrophic methanation, carboxydotrophic methanation, and acetoclastic methanation [244]. Multiple reviews on this subject highlight the various benefits of this fermentation [222,245,246], and several studies report on high conversion rates when using membrane reactors, tricking beds, and high-pressure systems capable of attaining high cell concentration and productivity [247,248,249].
A final remark worth mentioning is the scarce information regarding ethanol recovery from low-concentrated streams. This stage is fundamental to attaining process feasibility since the ethanol concentration greatly affects the performance of recovery stages and energy demand [250]. However, this fermentation suffers from product inhibition. Thus, simultaneous removal of products during the fermentation stage may become an option for increasing reactor productivity, as already proposed for ABE fermentation [251,252,253]. Technical feasibility evaluated by Michailos et al. [254] clearly summarized the disadvantages of this technology, indicating that conventional ethanol biorefineries have a better performance in terms of sustainability. The challenges to be overcome have been widely exposed: an increase in ethanol yields and mass transfer improvement of the gaseous substrate without causing metabolic shifts would benefit the mass and energy balance of the fermentation system. The integration with a biomass gasification stage requires solving the well-known tar formation problem along with removing specific toxic trace contaminants at a low cost.
4. Conclusions
This manuscript examines the key factors that impact the successful integration of biomass gasification and the subsequent conversion of syngas into alcohols. The fermentation process is an exciting technology, but unfortunately, the process suffers from several inhibitory effects when syngas derived from biomass gasification is used as substrate. Several studies in the scientific literature report on fermentation yields and the effect of contaminants. There is a lack of fermentation data obtained from pilot scale systems that consider the whole integration of the process. Significant technical advances have been made in the development of efficient gasifiers. However, research must continue to allow the development of high-rate fermenters capable of converting syngas without suffering from substrate or product inhibition. A global optimization is necessary, considering also the alcohol recovery stage to allow comparison with other types of fermentations and energy-producing processes.
Author Contributions
Conceptualization, X.G.; methodology, X.G. and M.V.G.; formal analysis, M.V.G.; investigation, X.G. and M.E.; resources, X.G.; data curation, M.E.; writing—original draft preparation, X.G.; writing—review and editing, M.V.G.; visualization, M.E.; supervision, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- European Commission: Circular Economy. The EU Aims to Transition to a Circular Economy to Make Europe Cleaner and More Competitive. Available online: https://environment.ec.europa.eu/topics/circular-economy_en (accessed on 8 August 2023).
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Hazen, B.T.; Russo, I.; Confente, I.; Pellathy, D. Supply chain management for circular economy: Conceptual framework and research agenda. Int. J. Logist. Manag. 2021, 32, 510–537. [Google Scholar] [CrossRef]
- Koval, V.; Arsawan, I.W.; Suryantini, N.P.; Kovbasenko, S.; Fisunenko, N.; Aloshyna, T. Circular economy and sustainability-oriented innovation: Conceptual framework and energy future avenue. Energies 2022, 16, 243. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Steinmann, Z.J.N.; Huijbregts, M.A.J.; Reijnders, L. How to define the quality of materials in a circular economy? Resour. Conserv. Recycl. 2019, 141, 362–363. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Herwig, H. How to Teach Heat Transfer More Systematically by Involving Entropy. Entropy 2018, 20, 791. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Elorf, A.; Kandasamy, J.; Belandria, V.; Bostyn, S.; Sarh, B.; Gökalp, I. Heating rate effects on pyrolysis, gasification and combustion of olive waste. Biofuels 2021, 12, 1157–1164. [Google Scholar] [CrossRef]
- Panepinto, D.; Tedesco, V.; Brizio, E.; Genon, G. Environmental Performances and Energy Efficiency for MSW Gasification Treatment. Waste Biomass Valoriz. 2015, 6, 123–135. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Soroush, S.; Ronsse, F.; Park, J.; Heynderickx, P.M. Comparison Study on the Water-to-Biomass Ratio in Hydrothermal Carbonization of Fresh Seaweed. Processes 2023, 11, 1123. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefin. 2013, 3, 113–126. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Golonka, M.; Scholz, M.; Białowiec, A. Opportunities and Challenges of High-Pressure Fast Pyrolysis of Biomass: A Review. Energies 2021, 14, 5426. [Google Scholar] [CrossRef]
- Chun, Y.; Lee, S.K.; Yoo, H.Y.; Kim, S.W. Recent advancements in biochar production according to feedstock classification, pyrolysis conditions, and applications: A review. BioResources 2021, 16, 6512–6547. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Martínez, O.; Gómez, X.; Morán, A. Pyrolysis of mixtures of sewage sludge and manure: A comparison of the results obtained in the laboratory (semi-pilot) and in a pilot plant. Waste Manag. 2007, 27, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Che, Y.; Wang, Z.; Qiao, Y.; Tian, Y. Coupling Process of Heavy Oil Millisecond Pyrolysis and Coke Gasification: A Fundamental Study. Energy Fuels 2016, 30, 6698–6708. [Google Scholar] [CrossRef]
- Akyürek, Z. Synergetic Effects during Co-Pyrolysis of Sheep Manure and Recycled Polyethylene Terephthalate. Polymers 2021, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Madanikashani, S.; Vandewalle, L.A.; De Meester, S.; De Wilde, J.; Van Geem, K.M. Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges. Materials 2022, 15, 4215. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Li, Y.; Zhang, C.; Fang, Y.; Zhao, J. A review on resource utilization of oil sludge based on pyrolysis and gasification. J. Environ. Chem. Eng. 2023, 11, 109692. [Google Scholar] [CrossRef]
- Obileke, K.; Makaka, G.; Nwokolo, N. Recent Advancements in Anaerobic Digestion and Gasification Technology. Appl. Sci. 2023, 13, 5597. [Google Scholar] [CrossRef]
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chem. Ing. Tech. 2018, 90, 127–140. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Teimouri, Z.; Abatzoglou, N.; Dalai, A.K. Kinetics and Selectivity Study of Fischer–Tropsch Synthesis to C5+ Hydrocarbons: A Review. Catalysts 2021, 11, 330. [Google Scholar] [CrossRef]
- Leckner, B. Process aspects in combustion and gasification Waste-to-Energy (WtE) units. Waste Manag. 2015, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.H.; Tao, L. Economic Perspectives of Biogas Production via Anaerobic Digestion. Bioengineering 2020, 7, 74. [Google Scholar] [CrossRef]
- Martínez, E.J.; González, R.; Ellacuriaga, M.; Gómez, X. Valorization of Fourth-Range Wastes: Evaluating Pyrolytic Behavior of Fresh and Digested Wastes. Fermentation 2022, 8, 744. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of Syngas Composition on Anaerobic Fermentation. Reactions 2021, 2, 391–407. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Ravindran, B.; Yang, Y.; Bhatia, S.K. A Mini-Review on Syngas Fermentation to Bio-Alcohols: Current Status and Challenges. Sustainability 2023, 15, 3765. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef]
- Böhme, C. BASF Develops Process for Climate-Friendly Methanol. Available online: https://www.basf.com/global/en/media/news-releases/2019/05/p-19-218.html (accessed on 12 August 2023).
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Shadle, L.J.; Berry, D.A.; Syamlal, M. Coal Conversion Processes, Gasification. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- IGCC PROJECT EXAMPLES. Available online: https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/project-examples (accessed on 12 August 2023).
- Wabash River Generating Station. Available online: https://www.gem.wiki/Wabash_River_Generating_Station#Unit_Retirements (accessed on 12 August 2023).
- Puertollano IGCC Power Station. Available online: https://www.gem.wiki/Puertollano_IGCC_power_station (accessed on 12 August 2023).
- Ward, C.; Goldstein, H.; Maurer, R.; Thimsen, D.; Sheets, B.J.; Hobbs, R.; Isgrigg, F.; Steiger, R.; Revay Madden, D.; Porcu, A.; et al. Making coal relevant for small scale applications: Modular gasification for syngas/engine CHP applications in challenging environments. Fuel 2020, 267, 117303. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.Á.; Sáez, F.; Ballesteros, M.; Moreno, A.D. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. A global approach to obtain biobutanol from corn stover. Renew. Energy 2020, 148, 223–233. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Y.; Xu, Z.; Chen, S.; Shen, G.; Yuan, X.; Deng, Q.; Shen, W.; Yang, S.; Zhang, C.; et al. Efficient Corncob Biorefinery for Ethanol Initiated by a Novel Pretreatment of Densifying Lignocellulosic Biomass with Sulfuric Acid. Fermentation 2022, 8, 661. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Manandhar, A.; Shah, A. Techno-Economic Analysis of the Production of Lactic Acid from Lignocellulosic Biomass. Fermentation 2023, 9, 641. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Mohamed Usman, T.M.; Kavitha, S.; Sachdeva, S.; Thakur, S.; Adish Kumar, S.; Rajesh Banu, J. Lignocellulosic Biorefinery Technologies: A Perception into Recent Advances in Biomass Fractionation, Biorefineries, Economic Hurdles and Market Outlook. Fermentation 2023, 9, 238. [Google Scholar] [CrossRef]
- Schmatz, A.A.; Candido, J.P.; de Angelis, D.d.F.; Brienzo, M. Semi-Simultaneous Saccharification and Fermentation Improved by Lignin and Extractives Removal from Sugarcane Bagasse. Fermentation 2023, 9, 405. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Petersen, I.; Werther, J. Experimental investigation and modeling of gasification of sewage sludge in the circulating fluidized bed. Chem. Eng. Process. Process Intensif. 2005, 44, 717–736. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Song, X.; Zhao, L. Study on the combined sewage sludge pyrolysis and gasification process: Mass and energy balance. Environ. Technol. 2012, 33, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Gökalp, I. Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M.; Szwaja, M. Theoretical and Experimental Analysis on Co-Gasification of Sewage Sludge with Energetic Crops. Energies 2019, 12, 1750. [Google Scholar] [CrossRef]
- Schmid, M.; Hafner, S.; Scheffknecht, G. Experimental Parameter Study on Synthesis Gas Production by Steam-Oxygen Fluidized Bed Gasification of Sewage Sludge. Appl. Sci. 2021, 11, 579. [Google Scholar] [CrossRef]
- Buss, W. Pyrolysis Solves the Issue of Organic Contaminants in Sewage Sludge while Retaining Carbon—Making the Case for Sewage Sludge Treatment via Pyrolysis. ACS Sustain. Chem. Eng. 2021, 9, 10048–10053. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental Investigation into the Effect of Pyrolysis on Chemical Forms of Heavy Metals in Sewage Sludge Biochar (SSB), with Brief Ecological Risk Assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrogen Energy 2010, 35, 11738–11745. [Google Scholar] [CrossRef]
- Moško, J.; Pohořelý, M.; Skoblia, S.; Beňo, Z.; Jeremiáš, M. Detailed Analysis of Sewage Sludge Pyrolysis Gas: Effect of Pyrolysis Temperature. Energies 2020, 13, 4087. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Analysis of Organic and Inorganic Contaminants in Dried Sewage Sludge and By-Products of Dried Sewage Sludge Gasification. Energies 2014, 7, 462–476. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, H.; Wang, Y.; Huang, H.; Chen, Y. Characteristic of heavy metals in biochar derived from sewage sludge. J. Mater. Cycles Waste Manag. 2016, 18, 725–733. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Chen, H.; Zhai, Y.; Xu, B.; Xiang, B.; Zhu, L.; Qiu, L.; Liu, X.; Li, C.; Zeng, G. Characterization of bio-oil and biochar from high-temperature pyrolysis of sewage sludge. Environ. Technol. 2015, 36, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Werle, S. Impact of feedstock properties and operating conditions on sewage sludge gasification in a fixed bed gasifier. Waste Manag. Res. 2014, 32, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Arjharn, W.; Hinsui, T.; Liplap, P.; Raghavan, G.V. Evaluation of an energy production system from sewage sludge using a pilot-scale downdraft gasifier. Energy Fuels 2013, 27, 229–236. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Brachi, P.; Ruoppolo, G.; Fraia, S.D.; Vanoli, L. Experimental and numerical investigation of biosolid gasification: Equilibrium-based modeling with emphasis on the effects of different pretreatment methods. Ind. Eng. Chem. Res. 2019, 59, 299–307. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis. 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Wu, H.; Hanna, M.A.; Jones, D.D. Fluidized-bed gasification of dairy manure by Box–Behnken design. Waste Manag. Res. 2011, 30, 506–511. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Nam, H.; Maglinao, A.L.; Capareda, S.C.; Rodriguez-Alejandro, D.A. Enriched-air fluidized bed gasification using bench and pilot scale reactors of dairy manure with sand bedding based on response surface methods. Energy 2016, 95, 187–199. [Google Scholar] [CrossRef]
- Pandey, D.S.; Katsaros, G.; Lindfors, C.; Leahy, J.J.; Tassou, S.A. Fast Pyrolysis of Poultry Litter in a Bubbling Fluidised Bed Reactor: Energy and Nutrient Recovery. Sustainability 2019, 11, 2533. [Google Scholar] [CrossRef]
- Ashraf, M.; Ramzan, N.; Azam, M.; Anwar, A.; Khan, R.U.; Durrani, A.K.; Rashid, M.U. Cattle dung conversion to syngas: Solar photovoltaic integrated gasification system. Biomass Convers. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Bergfeldt, B.; Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D. Recovery of Phosphorus and other Nutrients during Pyrolysis of Chicken Manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- Madadian, E.; Orsat, V.; Lefsrud, M. Comparative study of temperature impact on air gasification of various types of biomass in a research-scale down-draft reactor. Energy Fuels 2017, 31, 4045–4053. [Google Scholar] [CrossRef]
- Santana, K.V.R.; Apolônio, F.C.S.O.; Wisniewski, A. Valorization of cattle manure by thermoconversion process in a rotary kiln reactor to produce environmentally friendly products. BioEnergy Res. 2020, 13, 605–617. [Google Scholar] [CrossRef]
- Bläsingä, M.; Oga, I.O.; Müller, M. Co-Gasification of Turkey Manure and Meat Bone with Hard Coal and Lignite: Release of Condensable and Noncondensable Species. Ind. Eng. Chem. Res. 2017, 56, 14396–14400. [Google Scholar] [CrossRef]
- Tian, R.; Li, C.; Xie, S.; You, F.; Cao, Z.; Xu, Z.; Yu, G.; Wang, Y. Preparation of biochar via pyrolysis at laboratory and pilot scales to remove antibiotics and immobilize heavy metals in livestock feces. J. Soils Sediments 2019, 19, 2891–2902. [Google Scholar] [CrossRef]
- Schweitzer, D.; Gredinger, A.; Schmid, M.; Waizmann, G.; Beirow, M.; Spörl, R.; Scheffknecht, G. Steam gasification of wood pellets, sewage sludge and manure: Gasification performance and concentration of impurities. Biomass Bioenerg. 2018, 111, 308–319. [Google Scholar] [CrossRef]
- Vamvuka, D.; Raftogianni, A. Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Appl. Sci. 2021, 11, 12011. [Google Scholar] [CrossRef]
- Chojnacki, J.; Najser, J.; Rokosz, K.; Peer, V.; Kielar, J.; Berner, B. Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System. Energies 2020, 13, 4376. [Google Scholar] [CrossRef]
- Loha, C.; Chatterjee, P.K.; Chattopadhyay, H. Performance of fluidized bed steam gasification of biomass—Modeling and experiment. Energy Convers. Manag. 2011, 52, 1583–1588. [Google Scholar] [CrossRef]
- Meng, F.; Ma, Q.; Wang, H.; Liu, Y.; Wang, D. Effect of gasifying agents on sawdust gasification in a novel pilot scale bubbling fluidized bed system. Fuel 2019, 249, 112–118. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Bijl, A.; Yang, W.; Jönsson, P.G. Effect of H-ZSM-5 and Al-MCM-41 Proportions in Catalyst Mixtures on the Composition of Bio-Oil in Ex-Situ Catalytic Pyrolysis of Lignocellulose Biomass. Catalysts 2020, 10, 868. [Google Scholar] [CrossRef]
- González-Vázquez, M.D.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Comparison of the gasification performance of multiple biomass types in a bubbling fluidized bed. Energy Conver. Manag. 2018, 176, 309–323. [Google Scholar] [CrossRef]
- Osorio, J.; Chejne, F. Bio-Oil Production in Fluidized Bed Reactor at Pilot Plant from Sugarcane Bagasse by Catalytic Fast Pyrolysis. Waste Biomass Valoriz. 2019, 10, 187–195. [Google Scholar] [CrossRef]
- Dahmen, N.; Abeln, J.; Eberhard, M.; Kolb, T.; Leibold, H.; Sauer, J.; Stapf, D.; Zimmerlin, B. The bioliq process for producing synthetic transportation fuels. WIREs Energy Environ. 2017, 6, e236. [Google Scholar] [CrossRef]
- Michler, T.; Wippermann, N.; Toedter, O.; Niethammer, B.; Otto, T.; Arnold, U.; Pitter, S.; Koch, T.; Sauer, J. Gasoline from the bioliq® process: Production, characterization and performance. Fuel Process. Technol. 2020, 206, 106476. [Google Scholar] [CrossRef]
- Michler, T.; Niethammer, B.; Fuchs, C.; Toedter, O.; Arnold, U.; Koch, T.; Sauer, J. Further Development of Gasoline from the bioliq® Process with Focus on Particulate and Hydrocarbon Emissions. Fuels 2023, 4, 205–220. [Google Scholar] [CrossRef]
- Shell: Gasification-Technology. Available online: https://www.shell.com/business-customers/catalysts-technologies/licensed-technologies/refinery-technology/gasification-technology.html (accessed on 15 August 2023).
- Valmet Gasification. Available online: https://www.valmet.com/energyproduction/gasification/ (accessed on 15 August 2023).
- Eqtec Gasification Process. Available online: https://eqtec.com/gasification-projects/ (accessed on 15 August 2023).
- Ducom, G.; Tagutchou, J.-P.; Gautier, M.; Gaignaire, C.; Méhu, J.; Gourdon, R. Olive mill solid waste gasification in a pilot-scale downdraft gasifier with three-stage air supply: Performance, mass-energy balance and fate of inorganic elements. Fuel 2023, 340, 127469. [Google Scholar] [CrossRef]
- Thyssenkrupp-Uhde: Gasification. Available online: https://www.thyssenkrupp-uhde.com/en/products-and-technologies/hydrogen-and-gas-technologies/gasification (accessed on 15 August 2023).
- Sofia, D.; Coca Llano, P.; Giuliano, A.; Iborra Hernández, M.; García Peña, F.; Barletta, D. Co-gasification of coal–petcoke and biomass in the Puertollano IGCC power plant. Chem. Eng. Res. Des. 2014, 92, 1428–1440. [Google Scholar] [CrossRef]
- MHI Gasification Process. Available online: https://www.mhi.com/products/environment/pyrolysis_gasification_melting.html (accessed on 15 August 2023).
- MHI Technology. Available online: http://www.mhi.co.jp/technology/review/pdf/e483/e483037.pdf (accessed on 15 August 2023).
- Enerkem: Carbon Recycling. Available online: https://enerkem.com/process-technology/carbon-recycling/ (accessed on 15 August 2023).
- Bioliq. Available online: https://www.bioliq.de/english/55.php (accessed on 15 August 2023).
- Greentransitionholding: Gasification Technology. Available online: https://www.greentransitionholding.com/group-companies#aitos-gasification-technology (accessed on 15 August 2023).
- Urbas: Wood Gas CHP the Innovation. Available online: https://www.urbas.at/wp-content/uploads/2020/09/p_URBAS_DE_KWK_7.8.web_WF.PDF-en.pdf (accessed on 15 August 2023).
- Syncraft Climate Positive Solutions. Available online: https://en.syncraft.at/ (accessed on 16 August 2023).
- Burkhardt-Gruppe: Heat-and-Power-from-Wood. Available online: https://burkhardt-gruppe.de/en/power-engineering/heat-and-power-from-wood/wood-gas-generator/wood-gasifier-v-3-90/ (accessed on 16 August 2023).
- Repotec: Biomass Steam Gasification. Available online: http://www.repotec.at/index.php/technology.html (accessed on 16 August 2023).
- Pyrox. Available online: http://www.woodgas.eu/pyrox-tecnology (accessed on 16 August 2023).
- Eco20x Energia Rinnovabile. Available online: https://eco20cmd.com/en/eco20x/how-does-eco20x-work/ (accessed on 16 August 2023).
- Espegroup: CGiP50 Biomass Cogenerators. Available online: https://www.espegroup.com//app/uploads/2021/02/Datasheet_CHiP50_ENG.pdf (accessed on 16 August 2023).
- Allesina, G.; Pedrazzi, S. Barriers to Success: A Technical Review on the Limits and Possible Future Roles of Small Scale Gasifiers. Energies 2021, 14, 6711. [Google Scholar] [CrossRef]
- Patuzzi, F.; Basso, D.; Vakalis, S.; Antolini, D.; Piazzi, S.; Benedetti, V.; Cordioli, E.; Baratieri, M. State-of-the-art of small-scale biomass gasification systems: An extensive and unique monitoring review. Energy 2021, 223, 120039. [Google Scholar] [CrossRef]
- Van De Velde, K.; Kiekens, P. Thermal degradation of flax: The determination of kinetic parameters with thermogravimetric analysis. J. Appl. Polym. Sci. 2002, 83, 2634–2643. [Google Scholar] [CrossRef]
- Abdelouahed, L.; Leveneur, S.; Vernieres-Hassimi, L.; Balland, L.; Taouk, B. Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J. Therm. Anal. Calorim. 2017, 129, 1201–1213. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, S.K. Determination of kinetic parameters of biomass samples using thermogravimetric analysis. Environ. Prog. Sustain. Energy 2014, 33, 256–266. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass. Molecules 2022, 27, 7662. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Fernandez-Lopez, M.; López-González, D.; Valverde, J.L.; Sanchez-Silva, L. Kinetic study of the CO2 gasification of manure samples. J. Therm. Anal. Calorim. 2017, 127, 2499–2509. [Google Scholar] [CrossRef]
- Gil, M.V.; González-Vázquez, M.P.; García, R.; Rubiera, F.; Pevida, C. Assessing the influence of biomass properties on the gasification process using multivariate data analysis. Energy Conver. Manag. 2019, 184, 649–660. [Google Scholar] [CrossRef]
- Rabou, L.P.L.M.; Zwart, R.W.R.; Vreugdenhil, B.J.; Bos, L. Tar in Biomass Producer Gas, the Energy research Centre of The Netherlands (ECN) Experience: An Enduring Challenge. Energy Fuels 2009, 23, 6189–6198. [Google Scholar] [CrossRef]
- Kim, M.; Sohn, G.; Ye, I.; Ryu, C.; Kim, B.; Lee, J. Numerical analysis on the performance of a 300 MW IGCC coal gasifier under various operating conditions. Fuel 2019, 257, 116063. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Wang, C.; Wang, C.; Wu, P.; Che, D. Experimental Study on Coal Gasification in a Full-Scale Two-Stage Entrained-Flow Gasifier. Energies 2020, 13, 4937. [Google Scholar] [CrossRef]
- Ataei, A.; Azimi, A.; Kalhori, S.B.; Abari, M.F.; Radnezhad, H. Performance analysis of a co-gasifier for organic waste in agriculture. Int. J. Recycl. Org. Waste Agric. 2012, 1, 6. [Google Scholar] [CrossRef]
- Fermoso, J.; Arias, B.; Gil, M.V.; Plaza, M.G.; Pevida, C.; Pis, J.J.; Rubiera, F. Co-gasification of different rank coals with biomass and petroleum coke in a high-pressure reactor for H2-rich gas production. Bioresour. Technol. 2010, 101, 3230–3235. [Google Scholar] [CrossRef]
- Martínez González, A.; Silva Lora, E.E.; Escobar Palacio, J.C. Syngas production from oil sludge gasification and its potential use in power generation systems: An energy and exergy analysis. Energy 2019, 169, 1175–1190. [Google Scholar] [CrossRef]
- Ba, Z.; Zhao, J.; Li, C.; Huang, J.; Fang, Y.; Zhang, L.; Kong, L.; Wang, Q. Developing efficient gasification technology for high-sulfur petroleum coke to hydrogen-rich syngas production. Fuel 2020, 267, 117170. [Google Scholar] [CrossRef]
- Chu, Z.; Gong, Z.; Wang, Z.; Zhang, H.; Liu, L.; Wu, J.; Wang, J. Experimental study on gasification of oil sludge with steam and its char characteristic. J. Hazard. Mater. 2021, 416, 125713. [Google Scholar] [CrossRef]
- Ongen, A.; Ozcan, H.K.; Ozbaş, E.E.; Aydin, S.; Yesildag, I. Co-gasification of oily sludge and chicken manure in a laboratory-scale updraft fixed bed gasifier. Clean Technol. Environ. Policy 2022, 24, 2229–2239. [Google Scholar] [CrossRef]
- Pattanayak, S.; Hauchhum, L.; Loha, C.; Sailo, L. Feasibility study of biomass gasification for power generation in Northeast India. Biomass Convers. Biorefin. 2023, 13, 999–1011. [Google Scholar] [CrossRef]
- Porcu, A.; Sollai, S.; Marotto, D.; Mureddu, M.; Ferrara, F.; Pettinau, A. Techno-Economic Analysis of a Small-Scale Biomass-to-Energy BFB Gasification-Based System. Energies 2019, 12, 494. [Google Scholar] [CrossRef]
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzym. Microb. Technol. 2007, 41, 362–367. [Google Scholar] [CrossRef]
- Thi, H.N.; Park, S.; Li, H.; Kim, Y.-K. Medium Compositions for the Improvement of Productivity in Syngas Fermentation with Clostridium autoethanogenum. Biotechnol. Bioprocess Eng. 2020, 25, 493–501. [Google Scholar] [CrossRef]
- Johannes, B.; Volker, M. CO Metabolism in the Acetogen Acetobacterium woodii. Appl. Environ. Microbiol. 2015, 81, 5949–5956. [Google Scholar] [CrossRef]
- Richter, H.; Molitor, B.; Wei, H.; Chen, W.; Aristilde, L.; Angenent, L.T. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy Environ. Sci. 2016, 9, 2392–2399. [Google Scholar] [CrossRef]
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch Syngas Fermentation by Clostridium carboxidivorans for Production of Acids and Alcohols. Processes 2020, 8, 1075. [Google Scholar] [CrossRef]
- Voegele, E. Ineos Bio to Sell Ethanol Business, Including Vero Beach Plant. Available online: https://biomassmagazine.com/articles/13662/ineos-bio-to-sell-ethanol-business-including-vero-beach-plant (accessed on 18 August 2023).
- Dapile, L. Investigation: INEOS Failed Despite $129 Million in Taxpayer Subsidies. Available online: https://eu.tcpalm.com/story/news/2017/01/17/ineos-closes-vero-beach-biofuel-plant/96412616/ (accessed on 18 August 2023).
- Writer, N.E.S. Westinghouse Plasma Operations Delivers Clean Syngas to Coskata Ethanol Facility. Available online: https://www.nsenergybusiness.com/news/newswestinghouse_plasma_operations_delivers_clean_syngas_to_coskata_ethanol_facility_100106/# (accessed on 22 August 2023).
- Ce.eco: Datasheet Plasma. Available online: https://www.ce.eco/it/products/plasma/datasheet-plasma (accessed on 22 August 2023).
- Sassari, N.R.; Marcheti, M. Presentation of Plasma Waste Gasification Technology. Available online: http://www.ecogv.it/wp-content/uploads/2019/12/Presentazione-Serbia-CNR-1.pdf (accessed on 22 August 2023).
- Synata Bio. Available online: https://synatabio.com/technology/ (accessed on 22 August 2023).
- True North Eyes Ethanol Investment, Acquires Synata Bio in India. Available online: https://advancedbiofuelsusa.info/true-north-eyes-ethanol-investment-acquires-synata-bio-in-india/ (accessed on 22 August 2023).
- Steelanol, Fueling a Sustainable Future. Available online: http://www.steelanol.eu/en (accessed on 22 August 2023).
- Holmgren, J.; Zarraga, J.; Summers, Z.; Köpke, M.; Trukenbrod, G.; El-Sarkawy, O.; Patel, N.; Lanzatech. Transforming Carbon. Making Products. Available online: https://ir.lanzatech.com/static-files/21eb4d1f-3e47-4224-9957-03bfb658aeab (accessed on 22 August 2023).
- Shougang LanzaTech Obtains Industry’s First Product Carbon Label Certification. Available online: https://www.shougang.com.cn/en/ehtml/ShougangNews/20230313/1749.html (accessed on 22 August 2023).
- Stoll, I.K.; Boukis, N.; Sauer, J. Syngas Fermentation to Alcohols: Reactor Technology and Application Perspective. Chem. Ing. Tech. 2020, 92, 125–136. [Google Scholar] [CrossRef]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Schiel-Bengelsdorf, B.; Dürre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Wood, H.G. Interaction of ferredoxin with carbon monoxide dehydrogenase from Clostridium thermoaceticum. J. Biochem. Chem. 1992, 267, 897–900. [Google Scholar] [CrossRef]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J.M. Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeister, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Weuster-Botz, D.; Dürre, P. Bacterial anaerobic synthesis gas (syngas) and CO2+ H2 fermentation. In Advances in Applied Microbiology; Sarialani, S., Gadd, G., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 103, pp. 143–221. [Google Scholar] [CrossRef]
- Sahoo, J.; Patil, P.; Verma, A.; Lodh, A.; Khanna, N.; Prasad, R.; Pandit, S.; Fosso-Kankeu, E. Biochemical Aspects of Syngas Fermentation. In Recent Developments in Microbial Technologies; Prasad, R., Kumar, V., Singh, J., Upadhyaya, C.P., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Gencic, S.; Grahame, D.A. Diverse energy-conserving pathways in Clostridium difficile: Growth in the absence of amino acid Stickland acceptors and the role of the Wood-Ljungdahl pathway. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Martin, M.E.; Richter, H.; Saha, S.; Angenent, L.T. Traits of selected Clostridium strains for syngas fermentation to ethanol. Biotechnol. Bioeng. 2016, 113, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Ackerson, M.D.; Clausen, E.C.; Gaddy, J.L. Biological conversion of coal and coal-derived synthesis gas. Fuel 1993, 72, 1673–1678. [Google Scholar] [CrossRef]
- Mohammadi, M.; Younesi, H.; Najafpour, G.; Mohamed, A.R. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 837–843. [Google Scholar] [CrossRef]
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour. Technol. 2014, 151, 69–77. [Google Scholar] [CrossRef]
- Elisiário, M.P.; Van Hecke, W.; De Wever, H.; Noorman, H.; Straathof, A.J.J. Acetic acid, growth rate, and mass transfer govern shifts in CO metabolism of Clostridium autoethanogenum. Appl. Microbiol. Biotechnol. 2023, 107, 5329–5340. [Google Scholar] [CrossRef]
- Calvo, D.C.; Luna, H.J.; Arango, J.A.; Torres, C.I.; Rittmann, B.E. Determining global trends in syngas fermentation research through a bibliometric analysis. J. Environ. Manag. 2022, 307, 114522. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Han, W.; Shao, L.; Lü, F. One-step production of C6–C8 carboxylates by mixed culture solely grown on CO. Biotechnol. Biofuels Bioprod. 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Gavala, H.N.; Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V. Gas Biological Conversions: The Potential of Syngas and Carbon Dioxide as Production Platforms. Waste Biomass Valoriz. 2021, 12, 5303–5328. [Google Scholar] [CrossRef]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef]
- Fernández-Naveira, Á.; Abubackar, H.N.; Veiga, M.C.; Kennes, C. Efficient butanol-ethanol (B-E) production from carbon monoxide fermentation by Clostridium carboxidivorans. Appl. Microbiol. Biotechnol. 2016, 100, 3361–3370. [Google Scholar] [CrossRef]
- Ramió-Pujol, S.; Ganigué, R.; Bañeras, L.; Colprim, J. Incubation at 25 °C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour. Technol. 2015, 192, 296–303. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Sultan, I.N.; Khan, M.W.; Parakulsuksatid, P. Cellulase Addition and Pre-hydrolysis Effect of High Solid Fed-Batch Simultaneous Saccharification and Ethanol Fermentation from a Combined Pretreated Oil Palm Trunk. ACS Omega 2021, 6, 26119–26129. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zahoor; Tan, X.; Zhuang, X.; Miao, C.; Guo, Y.; Chen, X.; Yu, Q.; Yuan, Z. Recycling of Black Liquor for Treating Sugarcane Bagasse at Low Temperature to Attain High Ethanol Production without Washing Step. ACS Sustain. Chem. Eng. 2020, 8, 17016–17021. [Google Scholar] [CrossRef]
- Production of Sustainable, Advanced Bio-ethANOL through an Innovative Gas-Fermentation Process Using Exhaust Gases Emitted in the STEEL Industry. Available online: https://cordis.europa.eu/project/id/656437 (accessed on 18 August 2023).
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Im, H.; An, T.; Kwon, R.; Park, S.; Kim, Y.-K. Effect of Organic Nitrogen Supplements on Syngas Fermentation Using Clostridium autoethanogenum. Biotechnol. Bioprocess Eng. 2021, 26, 476–482. [Google Scholar] [CrossRef]
- Xu, H.; Liang, C.; Yuan, Z.; Xu, J.; Hua, Q.; Guo, Y. A study of CO/syngas bioconversion by Clostridium autoethanogenum with a flexible gas-cultivation system. Enzym. Microb. Technol. 2017, 101, 24–29. [Google Scholar] [CrossRef]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst. Eng. 2009, 32, 369–380. [Google Scholar] [CrossRef]
- Mann, M.; Munch, G.; Regestein, L.; Rehmann, L. Cultivation Strategies of Clostridium autoethanogenum on Xylose and Carbon Monoxide Combination. ACS Sustain. Chem. Eng. 2020, 8, 2632–2639. [Google Scholar] [CrossRef]
- Dykstra, J.C.; van Oort, J.; Yazdi, A.T.; Vossen, E.; Patinios, C.; van der Oost, J.; Sousa, D.Z.; Kengen, S.W.M. Metabolic engineering of Clostridium autoethanogenum for ethyl acetate production from CO. Microb. Cell Fact. 2022, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels Bioprod. 2016, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Park, S.E.; Lee, H.; Yun, J.Y. Enhancement of bioethanol production in syngas fermentation with Clostridium ljungdahlii using nanoparticles. Bioresour. Technol. 2014, 159, 446–450. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Neumann, A. Evaluation of Media Components and Process Parameters in a Sensitive and Robust Fed-Batch Syngas Fermentation System with Clostridium ljungdahlii. Fermentation 2020, 6, 61. [Google Scholar] [CrossRef]
- Anggraini, I.D.; Keryanti; Kresnowati, M.T.A.P.; Purwadi, R.; Noda, R.; Watanabe, T.; Setiadi, T. Bioethanol Production via Syngas Fermentation of Clostridium Ljungdahlii in a Hollow Fiber Membrane Supported Bioreactor. Int. J. Technol. 2019, 10, 291–319. [Google Scholar] [CrossRef]
- Jack, J.; Lo, J.; Maness, P.-C.; Ren, Z.J. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas. Biomass Bioenergy 2019, 124, 95–101. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Ethanol production by syngas fermentation in a continuous stirred tank bioreactor using Clostridium ljungdahlii. Biofuels 2019, 10, 221–237. [Google Scholar] [CrossRef]
- Sertkaya, S.; Azbar, N.; Abubackar, H.N.; Gundogdu, T.K. Design of Low-Cost Ethanol Production Medium from Syngas: An Optimization of Trace Metals for Clostridium ljungdahlii. Energies 2021, 14, 6981. [Google Scholar] [CrossRef]
- Han, Y.-F.; Xie, B.-T.; Wu, G.; Guo, Y.-Q.; Li, D.-M.; Huang, Z.-Y. Combination of Trace Metal to Improve Solventogenesis of Clostridium carboxidivorans P7 in Syngas Fermentation. Front. Microbiol. 2020, 11, 577266. [Google Scholar] [CrossRef] [PubMed]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on Syngas Fermentation with Clostridium carboxidivorans in Stirred-Tank Reactors with Defined Gas Impurities. Front. Microbiol. 2021, 12, 655390. [Google Scholar] [CrossRef]
- Shen, S.; Wang, G.; Zhang, M.; Tang, Y.; Gu, Y.; Jiang, W.; Wang, Y.; Zhuang, Y. Effect of temperature and surfactant on biomass growth and higher-alcohol production during syngas fermentation by Clostridium carboxidivorans P7. Bioresour. Bioprocess 2020, 7, 56. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.; Wen, Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: Evaluating the mass transfer coefficient and ethanol production performance. Biochem. Eng. J. 2014, 85, 21–29. [Google Scholar] [CrossRef]
- Oh, H.J.; Ko, J.K.; Gong, G.; Lee, S.-M.; Um, Y. Production of Hexanol as the Main Product Through Syngas Fermentation by Clostridium carboxidivorans P7. Front. Bioeng. Biotechnol. 2022, 10, 850370. [Google Scholar] [CrossRef]
- Rückel, A.; Oppelt, A.; Leuter, P.; Johne, P.; Fendt, S. Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum. Fermentation 2022, 8, 465. [Google Scholar] [CrossRef]
- Benevenuti, C.; Botelho, A.; Ribeiro, R.; Branco, M.; Pereira, A.; Vieira, A.C.; Ferreira, T.; Amaral, P. Experimental Design to Improve Cell Growth and Ethanol Production in Syngas Fermentation by Clostridium carboxidivorans. Catalysts 2020, 10, 59. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.-Y.; Ko, J.K.; Lee, S.-M.; Gong, G.; Kim, K.H.; Um, Y. Characterization of a Novel Acetogen Clostridium sp. JS66 for Production of Acids and Alcohols: Focusing on Hexanoic Acid Production from Syngas. Biotechnol. Bioprocess Eng. 2022, 27, 89–98. [Google Scholar] [CrossRef]
- Sun, X.; Thunuguntla, R.; Zhang, H.; Atiyeh, H. Biochar amended microbial conversion of C1 gases to ethanol and butanol: Effects of biochar feedstock type and processing temperature. Bioresour. Technol. 2022, 360, 127573. [Google Scholar] [CrossRef] [PubMed]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Effect of nutrient limitation and two-stage continuous fermentor design on productivities during “Clostridium ragsdalei” syngas fermentation. Bioresour. Technol. 2011, 102, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Zhang, H.; Tanner, R.S.; Huhnke, R.L. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Appl. Energy 2019, 236, 1269–1279. [Google Scholar] [CrossRef]
- Patankar, S.; Dudhane, A.; Paradh, A.D.; Patil, S. Improved bioethanol production using genome-shuffled Clostridium ragsdalei (DSM 15248) strains through syngas fermentation. Biofuels 2021, 12, 81–89. [Google Scholar] [CrossRef]
- Gao, J.; Atiyeh, H.K.; Phillips, J.R.; Wilkins, M.R.; Huhnke, R.L. Development of low cost medium for ethanol production from syngas by Clostridium ragsdalei. Bioresour. Technol. 2013, 147, 508–515. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef]
- Ramachandriya, K.D.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R. Critical Factors Affecting the Integration of Biomass Gasification and Syngas Fermentation Technology. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1719&context=biosysengfacpub (accessed on 18 August 2023).
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting gasification with syngas fermentation: Comparison of the performance of lignin and beech wood. Fuel 2021, 290, 120054. [Google Scholar] [CrossRef]
- Sharma, S.D.; Dolan, M.; Park, D.; Morpeth, L.; Ilyushechkin, A.; McLennan, K.; Harris, D.J.; Thambimuthu, K.V. A critical review of syngas cleaning technologies—fundamental limitations and practical problems. Powder Technol. 2008, 180, 115–121. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Zwart, R.W.R.; Van der Drift, A.; Bos, A.; Visser, H.J.M.; Cieplik, M.K.; Könemann, H.W.J. Oil-based gas washing—Flexible tar removal for high-efficient production of clean heat and power as well as sustainable fuels and chemicals. Environ. Prog. Sustain. Energy 2009, 28, 324–335. [Google Scholar] [CrossRef]
- Thunman, H.; Seemann, M.; Berdugo Vilches, T.; Maric, J.; Pallares, D.; Ström, H.; Berndes, G.; Knutsson, P.; Larsson, A.; Breitholtz, C.; et al. Advanced biofuel production via gasification—Lessons learned from 200 man-years of research activity with Chalmers’ research gasifier and the GoBiGas demonstration plant. Energy Sci. Eng. 2018, 6, 6–34. [Google Scholar] [CrossRef]
- Frilund, C.; Tuomi, S.; Kurkela, E.; Simell, P. Small- to medium-scale deep syngas purification: Biomass-to-liquids multi-contaminant removal demonstration. Biomass Bioenergy 2021, 148, 106031. [Google Scholar] [CrossRef]
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-Up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594. [Google Scholar] [CrossRef]
- Hai, I.U.; Sher, F.; Zarren, G.; Liu, H. Experimental investigation of tar arresting techniques and their evaluation for product syngas cleaning from bubbling fluidized bed gasifier. J. Clean. Prod. 2019, 240, 118239. [Google Scholar] [CrossRef]
- Oliveira, L.; Röhrenbach, S.; Holzmüller, V.; Weuster-Botz, D. Continuous sulfide supply enhanced autotrophic production of alcohols with Clostridium ragsdalei. Bioresour. Bioprocess 2022, 9, 15. [Google Scholar] [CrossRef]
- Oliveira, L.; Rückel, A.; Nordgauer, L.; Schlumprecht, P.; Hutter, E.; Weuster-Botz, D. Comparison of Syngas-Fermenting Clostridia in Stirred-Tank Bioreactors and the Effects of Varying Syngas Impurities. Microorganisms 2022, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide. Front. Microbiol. 2018, 9, 368587. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-Side Comparison of Clean and Biomass-Derived, Impurity-Containing Syngas as Substrate for Acetogenic Fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Monir, M.U.; Aziz, A.A.; Khatun, F.; Yousuf, A. Bioethanol production through syngas fermentation in a tar free bioreactor using Clostridium butyricum. Renew. Energy 2020, 157, 1116–1123. [Google Scholar] [CrossRef]
- Pacheco, M.; Pinto, F.; Brunsvik, A.; André, R.; Marques, P.; Mata, R.; Ortigueira, J.; Gírio, F.; Moura, P. Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation. Energies 2023, 16, 1722. [Google Scholar] [CrossRef]
- Monir, M.U.; Abd Aziz, A.; Yousuf, A.; Alam, M.Z. Hydrogen-rich syngas fermentation for bioethanol production using Sacharomyces cerevisiea. Int. J. Hydrogen Energy 2020, 45, 18241–18249. [Google Scholar] [CrossRef]
- Stoll, I.K.; Boukis, N.; Sauer, J. Syngas Fermentation at Elevated Pressure—Experimental Results. Available online: https://www.researchgate.net/profile/I-Stoll/publication/334697178_SYNGAS_FERMENTATION_AT_ELEVATED_PRESSURE_-EXPERIMENTAL_RESULTS/links/5d3ac241299bf1995b4b5088/SYNGAS-FERMENTATION-AT-ELEVATED-PRESSURE-EXPERIMENTAL-RESULTS.pdf (accessed on 18 August 2023).
- Sieblist, C.; Hägeholz, O.; Aehle, M.; Jenzsch, M.; Pohlscheidt, M.; Lübbert, A. Insights into large-scale cell-culture reactors: II. Gas-phase mixing and CO2 stripping. Biotechnol. J. 2011, 6, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jia, Y.; Wang, L.; Cao, Y. Drag coefficient fluctuation prediction of a single bubble rising in water. Chem. Eng. J. 2017, 316, 553–562. [Google Scholar] [CrossRef]
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Syngas fermentation to biofuel: Evaluation of carbon monoxide mass transfer coefficient (kLa) in different reactor configurations. Biotechnol. Prog. 2010, 26, 1616–1621. [Google Scholar] [CrossRef]
- Ungerman, A.J.; Heindel, T.J. Carbon Monoxide Mass Transfer for Syngas Fermentation in a Stirred Tank Reactor with Dual Impeller Configurations. Biotechnol. Prog. 2007, 23, 613–620. [Google Scholar] [CrossRef]
- De Tissera, S.; Köpke, M.; Simpson, S.D.; Humphreys, C.; Minton, N.P.; Dürre, P. Syngas Biorefinery and Syngas Utilization. In Biorefineries; Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer: Cham, Switzerland, 2017; Volume 166, pp. 247–280. [Google Scholar] [CrossRef]
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346. [Google Scholar] [CrossRef]
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous Ethanol Production from Synthesis Gas by Clostridium ragsdalei in a Trickle-Bed Reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef]
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble column reactors. Process Biochem. 2005, 40, 2263–2283. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Duduković, M.P. 18 Gas holdup and liquid recirculation in gas-lift reactors. Chem. Eng. Sci. 1980, 35, 135–141. [Google Scholar] [CrossRef]
- Ham, P.; Bun, S.; Painmanakul, P.; Wongwailikhit, K. Effective Analysis of Different Gas Diffusers on Bubble Hydrodynamics in Bubble Column and Airlift Reactors towards Mass Transfer Enhancement. Processes 2021, 9, 1765. [Google Scholar] [CrossRef]
- Elisiário, M.P.; De Wever, H.; Van Hecke, W.; Noorman, H.; Straathof, A.J.J. Membrane bioreactors for syngas permeation and fermentation. Crit. Rev. Biotechnol. 2022, 42, 856–872. [Google Scholar] [CrossRef]
- Sauer, M. Industrial production of acetone and butanol by fermentation—100 years later. FEMS Microbiol. Lett. 2016, 363, fnw134. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H. Recent innovations for reviving the ABE fermentation for production of butanol as a drop-in liquid biofuel. Biofuel Res. J. 2020, 7, 1256–1266. [Google Scholar] [CrossRef]
- Schüler, M.A.; Stegmann, B.A.; Poehlein, A.; Daniel, R.; Dürre, P. Genome sequence analysis of the temperate bacteriophage TBP2 of the solvent producer Clostridium saccharoperbutylacetonicum N1-4 (HMT, ATCC 27021). FEMS Microbiol. Lett. 2020, 367, fnaa103. [Google Scholar] [CrossRef] [PubMed]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N.; Ferreira, T. Clostridium sp. As Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef]
- Gungormusler, M.; Azbar, N.; Keskin, T. Bioethanol production from C1 gases using alternative media by syngas fermentation. Int. J. Glob. Warm. 2022, 28, 42–59. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.W.; Chae, C.G.; Kwon, S.J.; Kim, Y.J.; Lee, J.-H.; Lee, H.S. Domestication of the novel alcohologenic acetogen Clostridium sp. AWRP: From isolation to characterization for syngas fermentation. Biotechnol. Biofuels Bioprod. 2019, 12, 228. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Tsang, D.C.W.; Kwon, E.E.; Wang, C.-H. Towards practical application of gasification: A critical review from syngas and biochar perspectives. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1165–1213. [Google Scholar] [CrossRef]
- Straczewski, G.; Mai, R.; Gerhards, U.; Garbev, K.; Leibold, H. Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems. Catalysts 2021, 11, 1231. [Google Scholar] [CrossRef]
- Hartwell, A.R.; Wilhelm, C.A.; Welles, T.S.; Milcarek, R.J.; Ahn, J. Effects of Synthesis Gas Concentration, Composition, and Operational Time on Tubular Solid Oxide Fuel Cell Performance. Sustainability 2022, 14, 7983. [Google Scholar] [CrossRef]
- Costa, P.; Pinto, F.; André, R.N.; Marques, P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes 2021, 9, 254. [Google Scholar] [CrossRef]
- Ajvad, M.; Shih, H.Y. Combustion Analysis of Syngas Fuels Applied in a Micro Gas Turbine Combustor with a Rotating Casing. In Turbo Expo: Power for Land, Sea, and Air, Proceedings of the Turbomachinery Technical Conference and Exposition, Phoenix, AZ, USA, 17–21 June 2019; American Society of Mechanical Engineers: New York, NY, USA, 2019; Volume 58615, p. V04AT04A047. [Google Scholar] [CrossRef]
- Colantoni, S.; Della Gatta, S.; De Prosperis, R.; Russo, A.; Fantozzi, F.; Desideri, U. Gas Turbines Fired with Biomass Pyrolysis Syngas: Analysis of the Overheating of Hot Gas Path Components. J. Eng. Gas Turbines Power. 2010, 132. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State-of-the-art review and perspectives. Biofuels Bioprod. Biorefin. 2018, 12, 139–158. [Google Scholar] [CrossRef]
- Paniagua, S.; Lebrero, R.; Muñoz, R. Syngas biomethanation: Current state and future perspectives. Bioresour. Technol. 2022, 358, 127436. [Google Scholar] [CrossRef]
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.A.; Vasile, N.S.; Pirri, C.F.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064. [Google Scholar] [CrossRef]
- Westman, S.Y.; Chandolias, K.; Taherzadeh, M.J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2, 8. [Google Scholar] [CrossRef]
- Mauerhofer, L.-M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.-S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Feickert Fenske, C.; Md, Y.; Strübing, D.; Koch, K. Preliminary gas flow experiments identify improved gas flow conditions in a pilot-scale trickle bed reactor for H2 and CO2 biological methanation. Bioresour. Technol. 2023, 371, 128648. [Google Scholar] [CrossRef]
- Janković, T.; Straathof, A.J.J.; Kiss, A.A. Advanced downstream processing of bioethanol from syngas fermentation. Sep. Purif. Technol. 2023, 322, 124320. [Google Scholar] [CrossRef]
- Yao, P.; Xiao, Z.; Chen, C.; Li, W.; Deng, Q. Cell growth behaviors of Clostridium acetobutylicum in a pervaporation membrane bioreactor for butanol fermentation. Biotechnol. Appl. Biochem. 2016, 63, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum. Fermentation 2016, 2, 13. [Google Scholar] [CrossRef]
- Arregoitia-Sarabia, C.; González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. PEBA/PDMS Composite Multilayer Hollow Fiber Membranes for the Selective Separation of Butanol by Pervaporation. Membranes 2022, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Michailos, S.; Parker, D.; Webb, C. Design, sustainability analysis and multiobjective optimisation of ethanol production via syngas fermentation. Waste Biomass Valoriz. 2019, 10, 865–876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).