Grape Stalk Valorization: An Efficient Re-Use of Lignocellulosic Biomass through Hydrolysis and Fermentation to Produce Lactic Acid from Lactobacillus rhamnosus IMC501
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomasses and Medium
2.2. Quantification of Cellulose and Hemicellulose in Grape Stalks
2.3. Biomass Pre-Treatment and Hydrolysis
- (1)
- Alkaline hydrolysis: briefly, after size reduction in a benchtop grinder, dry biomass was dissolved in ammonium hydroxide (10% v/v) at 10% (w/v) solid loading and incubated at 70 °C for 22 h. The pH was adjusted to 7 with hydrochloric acid before biomass recovery by centrifugation and washing with water (twice). The supernatant was then discarded, and the pellet was placed in an oven at 70 °C for 24 h [26].
- (2)
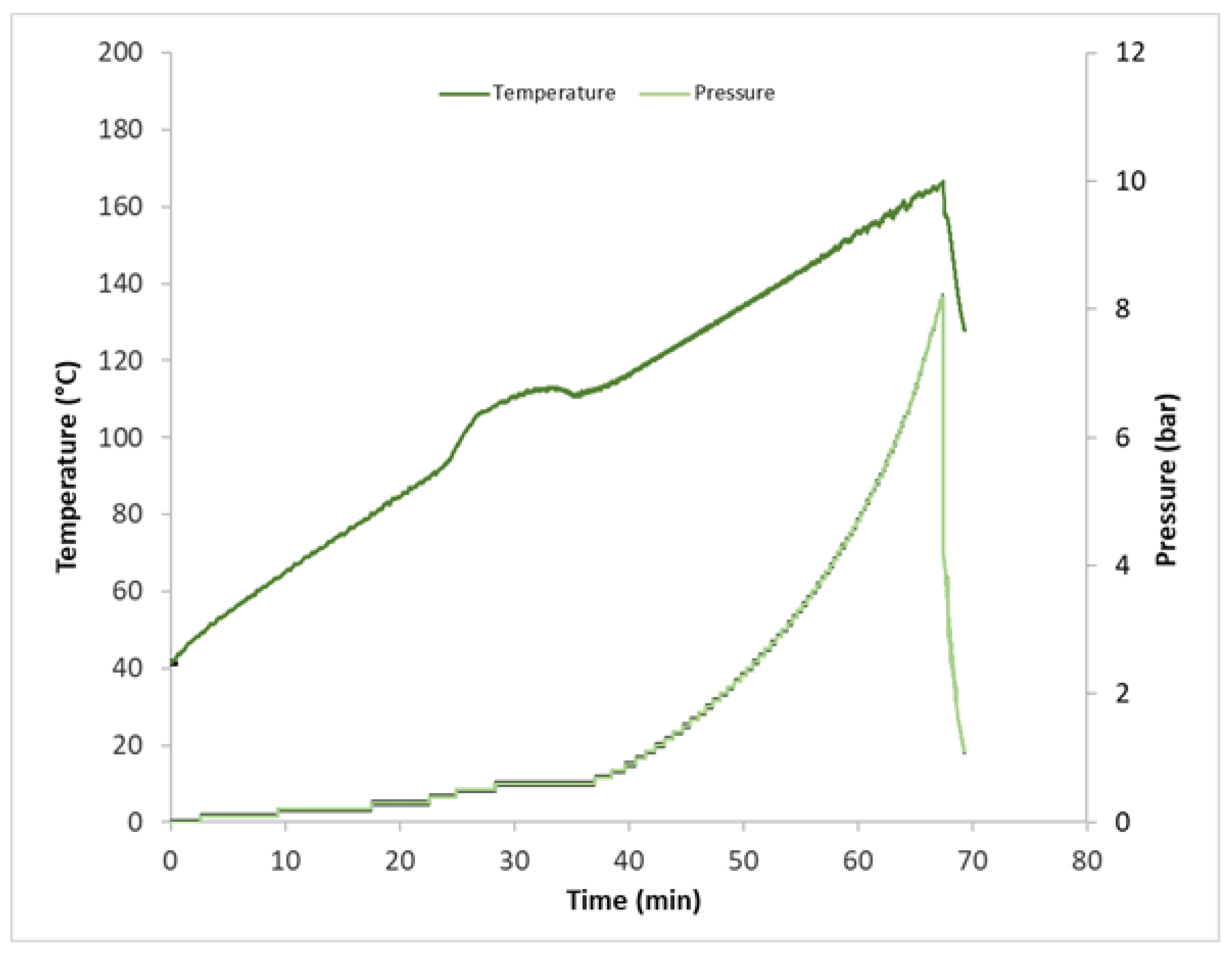
- Steam explosion (SE): the SE pre-treatment of grape stalks was conducted in a 1.5 L stirred-batch vessel made of AISI 316L. The vessel is heated by three electric heating elements of 0.7 kW each. The temperature is guaranteed by a control loop using a type K thermocouple connected to the internal wall of the vessel, a comparator for the set-point temperature, and a voltage controller for tuning the current into the resistance. A 3-cm-thick insulating layer of glass wool is used to minimize vessel heat dissipation. In the SE process, the biomass was heated with water for several minutes under autogenous pressure and then subjected to a sudden pressure drop. This led to vapor expansion inside the biomass, determining the disruption of the biomass fibers’ structure and thus making cellulose more accessible. For each SE experiment, 1200 mL of deionized water and 200 g of dried biomass were used. Parameters recorded during the steam explosion experiments are displayed in Figure 1, which shows that the water/biomass mixture was heated from 40 °C to 165 °C with a heating rate of about 2 °C/min. At the set temperature, a pressure of 8 bars was recorded. Once the temperature set-point was reached, the ball valve at the bottom of the vessel was opened, and the biomass was exploded/discharged into a collecting tank.
- (3)
- A sequential combination of both. Ammonium hydroxide pre-treatment on steam-exploded grape stalks.
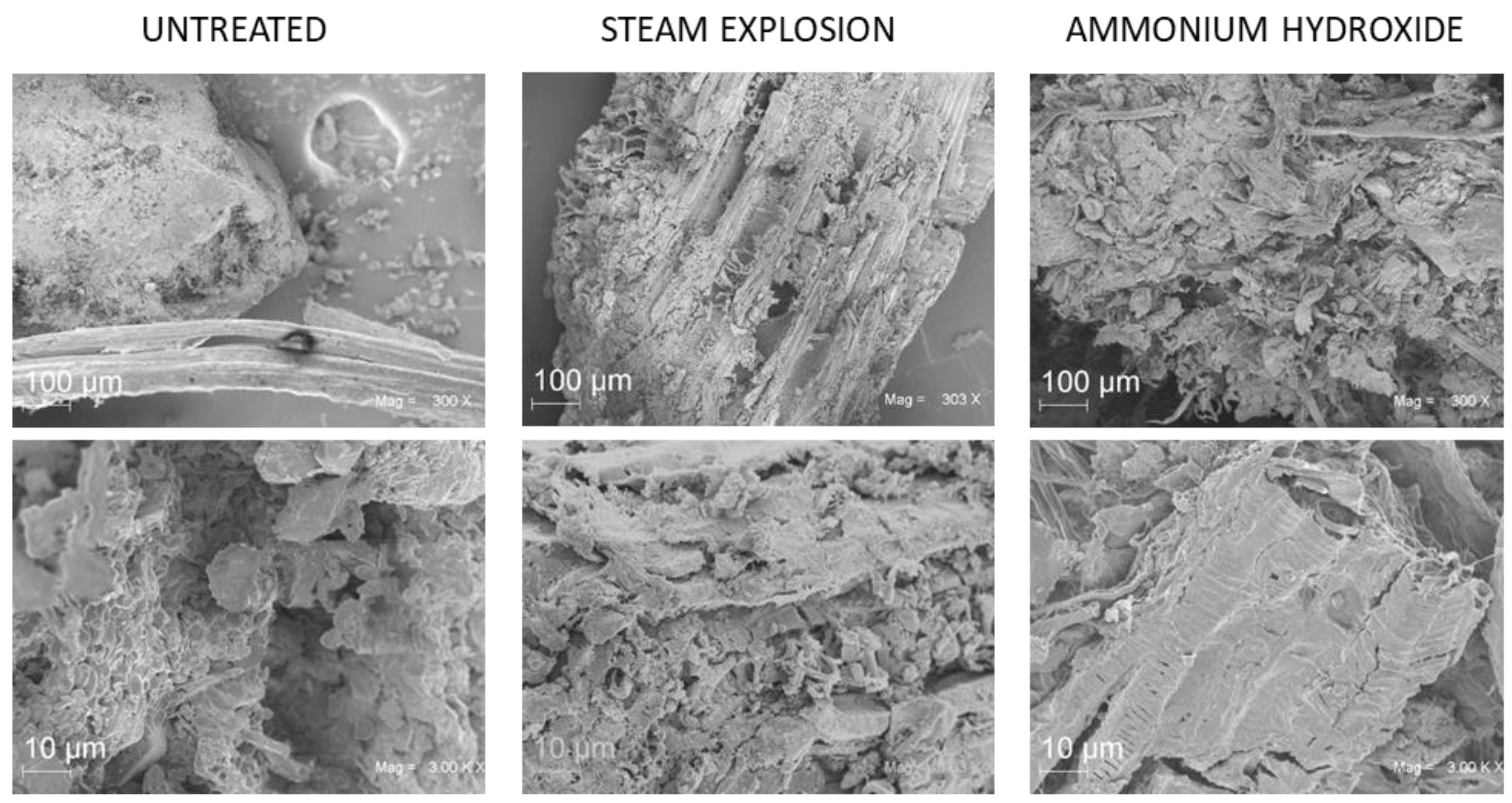
2.4. Morphological Analysis of Grape Stalks by Scanning Electron Microscopy (SEM)
2.5. Small-Scale Bottle Experiments
2.6. Batch Experiments in Controlled Bioreactor Conditions
2.7. Quantification of Glucose, Organic Acids, and Potentially Inhibitory Compounds by HPLC
3. Results
3.1. Pre-Treatment and Hydrolysis of Grape Stalks
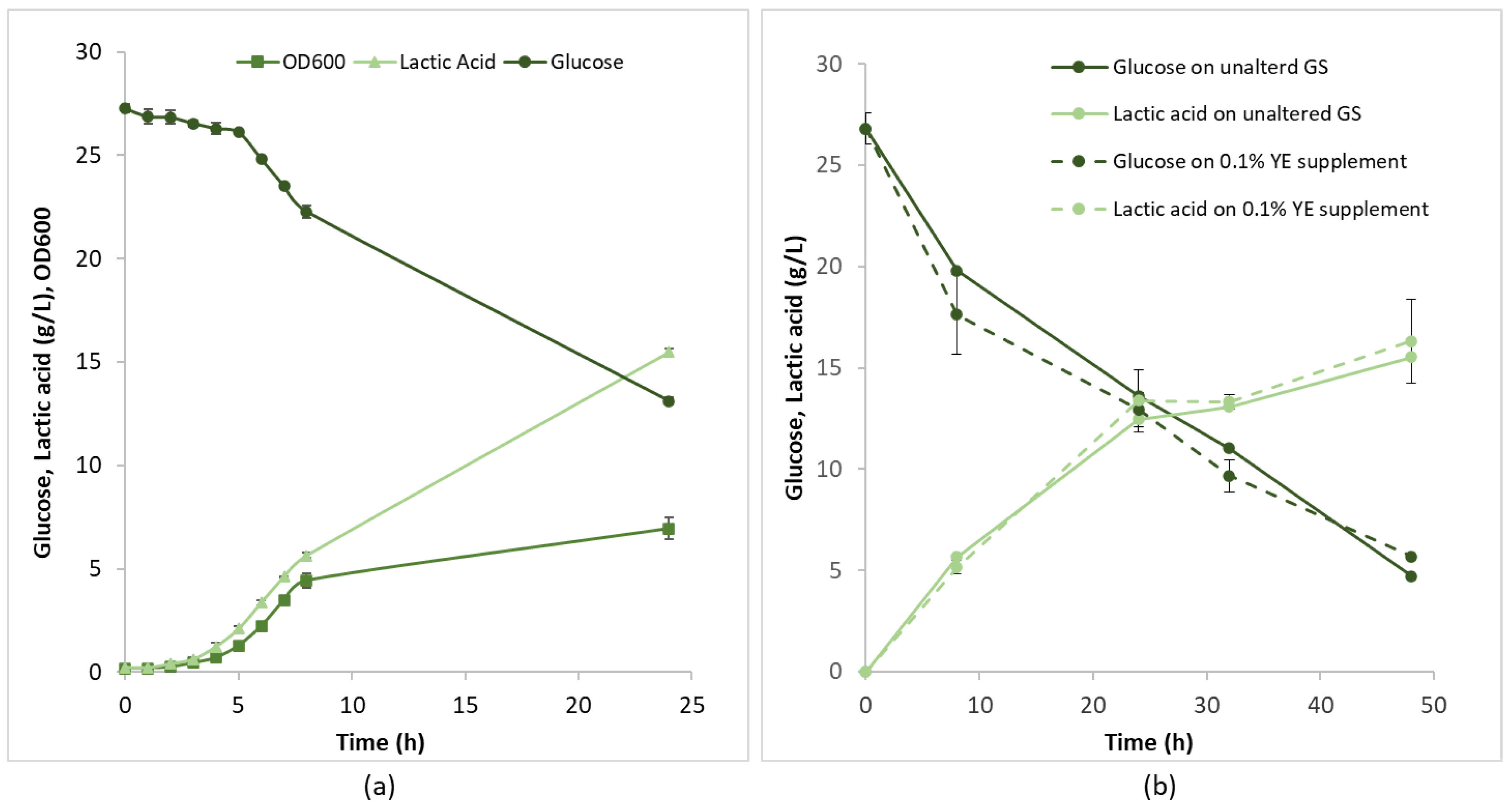
3.2. Growth of L. rhamnosus IMC501 on Grape Stalk Hydrolysate in Bottle and Bioreactor Batch Experiments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culaba, A.B.; Mayol, A.P.; San Juan, J.L.G.; Vinoya, C.L.; Concepcion, R.S.; Bandala, A.A.; Vicerra, R.R.P.; Ubando, A.T.; Chen, W.H.; Chang, J.S. Smart sustainable biorefineries for lignocellulosic biomass. Bioresour. Technol. 2022, 344, 126215–126228. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D. Biomass for energy: A review on supply chain management models. Renew. Sustain. Energy Rev. 2020, 120, 109658–109666. [Google Scholar] [CrossRef]
- Salvachúa, D.; Prieto, A.; López-Abelairas, M.; Lu-Chau, T.; Martínez, A.T.; Martínez, M.J. Fungal pretreatment: An alternative in second—Generation ethanol from wheat straw. Bioresour. Technol. 2011, 102, 7500–7506. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.R.; Padhi, R.K.; Ghosh, G. A review on key pretreatment approaches for lignocellulosic biomass to produce biofuel and value-added products. Int. J. Environ. Sci. Technol. 2022, 20, 6929–6944. [Google Scholar] [CrossRef]
- Chang, J.S.; Show, P.L.; Lee, D.J.; Christakopoulos, P. Recent advances in lignocellulosic biomass refinery. Bioresour. Technol. 2022, 347, 126735–126737. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534–126550. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585–122603. [Google Scholar] [CrossRef]
- Bikash, K.; Pradeep, V. Biomass-based biorefineries: An important architype towards a circular economy. Fuel 2021, 288, 119622–119644. [Google Scholar] [CrossRef]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape stalk valorization for fermentation purposes. Food Chem. 2021, 4, 100067–100078. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342–357. [Google Scholar] [CrossRef]
- Levin, L.; Diorio, L.; Grassi, E.; Forchiassin, F. Grape stalks as substrate for white rot fungi, lignocellulolytic enzyme production and dye decolorization. Rev. Argent. Microbiol. 2012, 44, 105–112. [Google Scholar] [PubMed]
- Statistical Report on World Vitiviniculture. International Organization of Vine and Wine. OIV. 2019. Available online: oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 25 May 2023).
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Zaccariello, L.; Mastellone, M.L.; D’Amelia, L.I.; Catauro, M.; Morrone, B. Assessment of Integration between Lactic Acid, Biogas and Hydrochar Production in OFMSW Plants. Energies 2020, 13, 6593–6611. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.C.; Södergård, A. From Lactic Acid to Poly(lactic acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Zhang, Z.; Jackson, J.E.; Miller, D.J. Effect of biogenic fermentation impurities on lactic acid hydrogenation to propylene glycol. Bioresour. Technol. 2008, 99, 5873–5880. [Google Scholar] [CrossRef]
- Alfano, A.; D’ambrosio, S.; D’agostino, A.; Finamore, R.; Schiraldi, C.; Cimini, D. Concentrated buffalo whey as substrate for probiotic cultures and as source of bioactive ingredients: A local circular economy approach towards reuse of wastewaters. Fermentation 2021, 7, 281–290. [Google Scholar] [CrossRef]
- Groff, C.M.; Scaglia, G.; Gaido, M.; Kassuha, D.; Ortiz, O.A.; Noriega, S.E. Kinetic modeling of fungal biomass growth and lactic acid production in Rhizopus oryzae fermentation by using grape stalk as a solid substrate. Biocatal. Agric. Biotechnol. 2022, 39, 102255–102271. [Google Scholar] [CrossRef]
- Abdel-rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Verdenelli, M.C.; Ghelfi, F.; Silvi, S.; Orpianesi, C.; Cecchini, C.; Cresci, A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009, 48, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Salvesi, C.; Silvi, S.; Fiorini, D.; Alessandroni, L.; Sagratini, G.; Palermo, F.A.; De Leone, R.; Egidi, N.; Cifani, C.; Micioni Di Bonaventura, M.V.; et al. Six-Month Synbio® Administration Affects Nutritional and Inflammatory Parameters of Older Adults Included in the PROBIOSENIOR Project. Microorganisms 2023, 11, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Loader, N.J.; Robertson, I.; Barker, A.C.; Switsur, V.R.; Waterhouse, J.S. An improved technique for the batch processing of small wholewood samples to alpha-cellulose. Chem. Geol. 1997, 136, 313–317. [Google Scholar] [CrossRef]
- Battipaglia, G.; Jäggi, M.; Saurer, M.; Siegwolf, R.T.W.; Cotrufo, M.F. Climatic sensitivity of δ18O in the wood and cellulose of tree rings: Results fromamixed stand of Acer pseudoplatanus L. and Fagus sylvatica. Palaeogeogr. Palaeocl. 2008, 261, 193. [Google Scholar] [CrossRef]
- Ventrone, M.; Schiraldi, C.; Squillaci, G.; Morana, A.; Cimini, D. Chestnut Shells as Waste Material for Succinic Acid Production from Actinobacillus succinogenes 130Z. Fermentation 2020, 6, 105–116. [Google Scholar] [CrossRef]
- Cimini, D.; Argenzio, O.; D’Ambrosio, S.; Lama, L.; Finore, I.; Finamore, R.; Pepe, O.; Faraco, V.; Schiraldi, C. Production of succinic acid from Basfia succiniciproducens up to the pilot scale from Arundo donax hydrolysate. Bioresour. Technol. 2016, 222, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-M.; Lebaka, V.R.; Wee, Y.-J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; Yu, C.W.; Dooley, T.M.; Jenkins, B.M.; Gheynst, J.S.V. Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotech. 2010, 162, 1768–1784. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Abdel-Fattah, Y.R.; Soliman, N.A.; Agblevor, F.A. Comparison of alkaline pulping with steam explosion for glucose production from rice straw. Carbohydr. Polym. 2011, 83, 720–726. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment. Bioresour. Technol. 2010, 101, 6718–6727. [Google Scholar] [CrossRef]
- Sills, D.L.; Gossett, J.M. Assessment of commercial hemicellulases for saccharification of alkaline pretreated perennial biomass. Bioresour. Technol. 2011, 102, 1389–1398. [Google Scholar] [CrossRef]
- Gupta, R.; Khasa, Y.P.; Kuhad, R.C. Evaluation of pretreatment methods in improving the enzymatic saccharification of cellulosic materials. Carbohydr. Polym. 2011, 84, 1103–1109. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244–106267. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Pielhop, T.; Amgarten, J.; von Rohr, P.R.; Studer, M.H. Steam explosion pretreatment of softwood: The effect of the explosive decompression on enzymatic digestibility. Biotechnol. Biofuels 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Alvira, P.; Ballesteros, M.; Negro, M.J. Pretreatment technolo- gies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Spigno, G.; Pizzorno, T.; De Faveri, D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef]
- Jang, S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Potential Use of Gelidium amansii Acid Hydrolysate for Lactic Acid Production by Lactobacillus rhamnosus. Food Technol. Biotechnol. 2013, 51, 131–136. [Google Scholar]
- Ceapa, C.; Davids, M.; Ritari, J.; Lambert, J.; Wels, M.; Douillard, F.P.; Smokvina, T.; de Vos, W.M.; Knol, J.; Kleerebezem, M. The Variable Regions of Lactobacillus rhamnosus Genomes Reveal the Dynamic Evolution of Metabolic and Host-Adaptation Repertoires. Genome Biol. Evol. 2016, 8, 1889–1905. [Google Scholar] [CrossRef] [PubMed]
- Pontes, R.; Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.; Nunes, J. L-lactic Acid Production from Multi-Supply Autohydrolyzed Economically Unexploited Lignocellulosic Biomass. Ind. Crop. Prod. 2021, 170, 113775–113784. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Pejin, J.; Pribic, M.; Kocic-Tanackov, S.; Mladenovic, D.; Djukic-Vukovic, A.; Mojović, L. Brewing and Malting Technology By-Products as Raw Materials in L-(+)-lactic Acid Fermentation. J. Chem. Technol. Biotechnol. 2020, 95, 339–347. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Bioresour. Technol. 2022, 343, 125989–126000. [Google Scholar] [CrossRef]

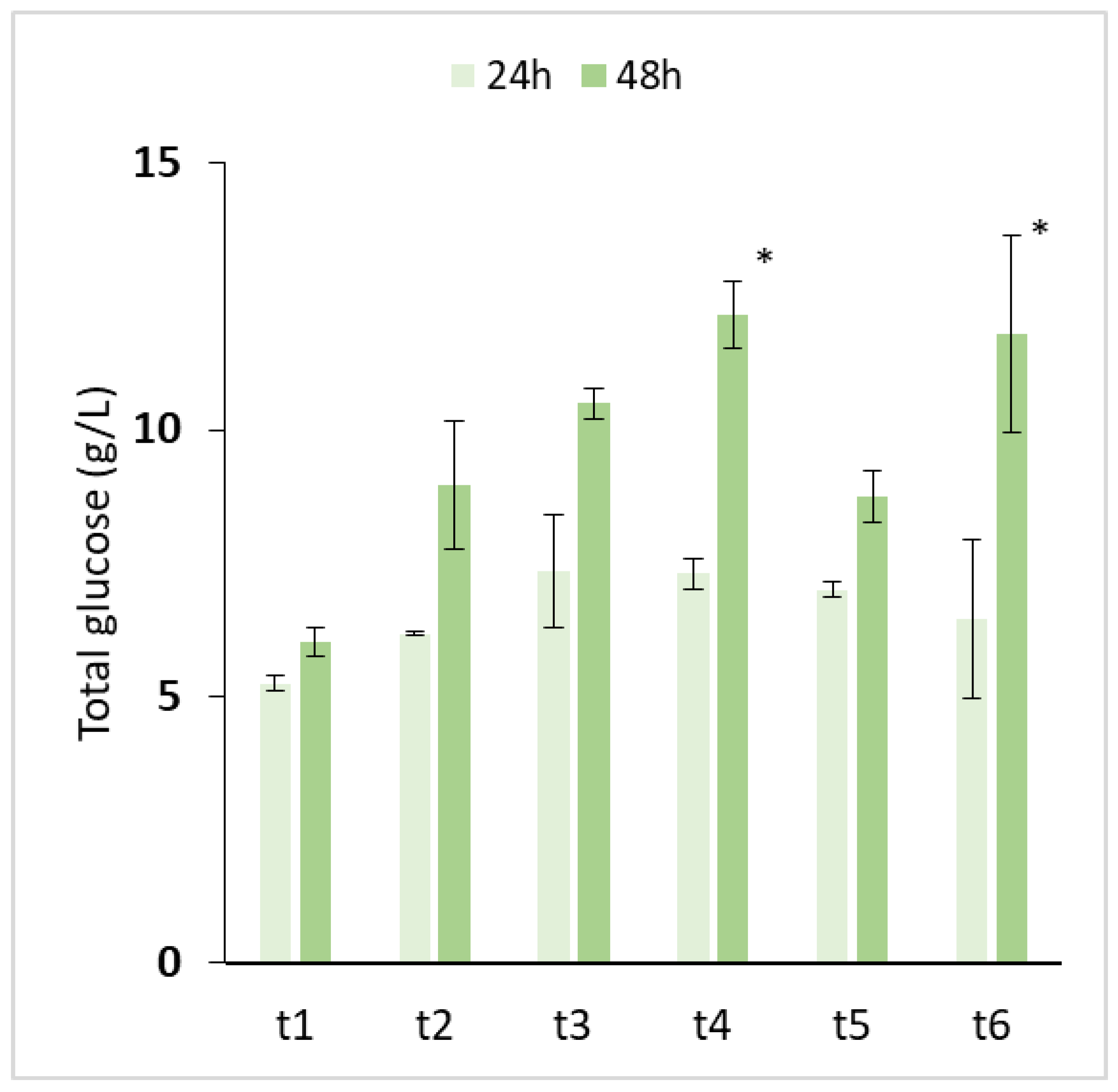

| Test | Conditions | Bioconversion Yield (%) |
|---|---|---|
| t1 | Biomass 10% (w/v) Enzyme 2.7% w/w (genz/gcellulose) | 18.7 |
| t2 | Biomass 10% (w/v) Enzyme 2.7 + 2.7% w/w (genz/gcellulose) | 27.8 |
| t3 | Biomass 10% (w/v) Enzyme 5.5% w/w (genz/gcellulose) | 32.8 |
| t4 | Biomass 10% (w/v) Enzyme 5.5 + 5.5% w/w (genz/gcellulose) | 38.1 |
| t5 | Biomass 15% (w/v) Enzyme 3.6% w/w (genz/gcellulose) | 16.4 |
| t6 | Biomass 15% (w/v) Enzyme 3.6 + 3.6% w/w (genz/gcellulose) | 22.9 |
| Inhibitor | Steam-Exploded GS before Strain Inoculation (mg/L) | Steam-Exploded GS after 48 h of Growth (mg/L) |
|---|---|---|
| 4-Hydroxybenzoic acid | 12.6 ± 1.3 | 11.5 ± 0.1 |
| Furfural | 16.1 ± 0.5 | 17.7 ± 0.6 |
| Vanillin | 6.6 ± 0.25 | 6.7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’ambrosio, S.; Zaccariello, L.; Sadiq, S.; D’Albore, M.; Battipaglia, G.; D’Agostino, M.; Battaglia, D.; Schiraldi, C.; Cimini, D. Grape Stalk Valorization: An Efficient Re-Use of Lignocellulosic Biomass through Hydrolysis and Fermentation to Produce Lactic Acid from Lactobacillus rhamnosus IMC501. Fermentation 2023, 9, 616. https://doi.org/10.3390/fermentation9070616
D’ambrosio S, Zaccariello L, Sadiq S, D’Albore M, Battipaglia G, D’Agostino M, Battaglia D, Schiraldi C, Cimini D. Grape Stalk Valorization: An Efficient Re-Use of Lignocellulosic Biomass through Hydrolysis and Fermentation to Produce Lactic Acid from Lactobacillus rhamnosus IMC501. Fermentation. 2023; 9(7):616. https://doi.org/10.3390/fermentation9070616
Chicago/Turabian StyleD’ambrosio, Sergio, Lucio Zaccariello, Saba Sadiq, Marcella D’Albore, Giovanna Battipaglia, Maria D’Agostino, Daniele Battaglia, Chiara Schiraldi, and Donatella Cimini. 2023. "Grape Stalk Valorization: An Efficient Re-Use of Lignocellulosic Biomass through Hydrolysis and Fermentation to Produce Lactic Acid from Lactobacillus rhamnosus IMC501" Fermentation 9, no. 7: 616. https://doi.org/10.3390/fermentation9070616
APA StyleD’ambrosio, S., Zaccariello, L., Sadiq, S., D’Albore, M., Battipaglia, G., D’Agostino, M., Battaglia, D., Schiraldi, C., & Cimini, D. (2023). Grape Stalk Valorization: An Efficient Re-Use of Lignocellulosic Biomass through Hydrolysis and Fermentation to Produce Lactic Acid from Lactobacillus rhamnosus IMC501. Fermentation, 9(7), 616. https://doi.org/10.3390/fermentation9070616









