Abstract
Carotenoids are fat-soluble bioactive compounds found in plants, animals, and microorganisms, which have a range of health benefits and great technological and industrial importance in the fields of pharmaceuticals, food, and biotechnology. Yeasts of the genus Rhodotorula can biosynthesize large amounts of carotenoids under environmental stress conditions, and this process may become industrially feasible if production costs are minimized using low-cost by-products as nutrient sources. As cassava roots are produced and consumed in large quantities in the Amazon biome, our research aimed to investigate the best cultivation conditions in a benchtop bioreactor for the biotechnological production of carotenoids by R. glutinis using manipueira as a low-cost substrate. Manipueira is wastewater generated during cassava flour production, and it was concentrated at 12 °Brix for this study. In addition, the carotenoid composition, at the best cultivation condition, was analyzed by LC-MS. Higher carotenoid contents (1410 μg/g of dried biomass) than described in the literature, and biomass (10 g), were produced by R. glutinis using concentrated manipueira as a low-cost substrate after cultivation at pH 5, 35 °C and agitation at 150 rpm. At the best cultivation conditions, β-carotene, lutein, and 5,8-epoxy-lutein were the major carotenoids, with the latter two compounds being identified for the first time in the biomass of R. glutinis. Therefore, concentrated manipueira can be seen as a feasible alternative and low-cost substrate to be used for the biotechnological production of high carotenoid contents by R. glutinis.
1. Introduction
Carotenoids are fat-soluble natural pigments that play an important role in the photosynthetic process of plant organisms, and when biosynthesized by microorganisms, they are responsible for the protection against damages caused by exposure to oxygen, light radiation, and increased temperature [1]. Yeasts of the genus Rhodotorula spp. are non-photosynthetic microorganisms widely distributed in nature that are able to biosynthesize carotenoids inside their cells, such as β-carotene, torulene, and torularrodine, under certain stress conditions in the environment in which they occur [2,3,4]. During the cultivation of Rhodorotula in a laboratory, some key parameters such as pH, temperature, agitation, aeration rate, light exposure, and substrate composition were reported to affect the metabolism of yeasts and, consequently, carotenogenesis, as reviewed elsewhere [4]. Therefore, the biotechnological production of carotenoids highly depends on environmental factors, which are able to modulate the yield of biomass produced by the microorganism, as well as the carotenoid profile [5,6].
Carotenoids have an extensive series of conjugated double bonds in their chemical structure, which generates resonance systems of electrons moving throughout the polyene chain. These organic compounds can be classified into two structural groups: carotenes—non-polar compounds containing only C and H in their chemical structures—and xanthophylls, which are oxygenated compounds derived from carotenes [7]. Due to these structural features, and depending on the type of terminal group or the presence of oxygenated substituents in the molecule, carotenoids show high reactivity and absorb light in the visible region of the electromagnetic spectrum [8].
The biotechnological production of carotenoids by fermentation can become industrially viable if the production costs are minimized using side streams of the food and agriculture industry as low-cost sources of nutrients for the growth of microorganisms [4]. Chemical syntheses of carotenoids, such as β-carotene and astaxanthin, are less expensive when compared to some existing biotechnological processes [9,10]. However, comprising healthier trends in food science, natural pigments have been extensively investigated to replace synthetic ones by means of alternative and feasible processes that stimulate sustainable approaches for the rational use of natural resources.
Manipueira is one of the main agro-industrial side streams generated during the cassava roots processing to obtain food products, such as flour and starch, and can be characterized as the yellowish liquid fraction obtained after pressing the ground roots. Importantly, both cassava roots and manipueira have cyanogenic glycoside compounds, such as linamarin, that can generate hydrocyanic acid (HCN) by acid or enzymatic hydrolysis, which is a volatile compound that can offer environmental risks if cassava residues are not properly discarded [11,12]. Concerning the presence of linamarin in derived cassava food products, fermentation, and high temperatures were reported to decrease its contents and inactive enzymatic activity [13]. Even if free HCN is released during the food process, exposure to high temperatures, as in oven drying, cooking, or concentration procedures, may quickly volatilize it to safe consumption ranges [13].
In most parts of Brazil, manipueira is commonly discarded around cassava processing factories, while this practice is less common in the north (Amazon region) than in other Brazilian regions as manipueira is directed to the production of a fermented broth highly appreciated in traditional dishes named tucupi. Manipueira can be seen as a low-cost substrate with great potential for biotechnological processes due to its chemical and nutritional composition, such as total carbohydrates (36–39 g/L), starch (13%), total proteins (1.7 g/L), organic acids, and other micronutrients [14,15].
Therefore, in our study, we investigated the biotechnological production of carotenoids, in a benchtop bioreactor, by determining the best cultivation conditions of a Rhodotorula yeast (R. glutinis) using concentrated manipueira as a substrate. For this purpose, a central composite rotational design (CCRD) with three independent variables (pH, temperature, and agitation) was carried out. Furthermore, the individual profile of carotenoids accumulated in the biomass of R. glutinis, as a result of the tested cultivation conditions, was determined by high-performance liquid chromatography coupled with a diode array detector and mass spectrometry (HPLC-DAD-MS).
2. Materials and Methods
2.1. Manipueira
Cassava roots (10 kg) were collected in the Icoaraci district (Belém, PA, Brazil—01°17′53″ S and 48°29′23″ W) in tucupi-processing units and transported to the Food Technology Laboratory at the Federal University of Pará (UFPA, Belém, PA, Brazil). Manipueira were manually extracted from cassava roots after peeling (with stainless steel knives) and crushing cassava pulp (in a food processor), followed by pressing the crushed mass and filtration through a qualitative paper to obtain the liquid fraction. The fresh manipueira was concentrated in a rotary evaporator (Marconi, São Paulo, Brazil) coupled with a vacuum pump (Quimis, 60LPM, São Paulo, Brazil) at 100 °C until soluble solids content of 12 °Brix, as determined by refractometry. After the concentration step, manipueira (substrate) was sterilized (121 °C/15 min) and stored at −20 °C until used in the fermentation process.
2.2. Physico–Chemical Characterization of Concentrated Manipueira
The physico–chemical characteristics (pH, total acidity, soluble solids) and the proximate composition (moisture, ashes, total lipids, total proteins (conversion factor 6.25 from total nitrogen), total and reducing sugars) were determined in the concentrated manipueira according to the Association of Official Analytical Chemists [16]. Total carbohydrate contents were calculated by the difference of 100% and the sum of the percentages of moisture, ashes, lipids, and proteins (Equation (1)). All analyses were carried out in triplicate, and the results were expressed as meq NaOH/100 mL (total acidity), °Brix (soluble solids), and g/100 mL (proximate composition).
2.3. Starter Culture Preparation
Yeasts strains of Rhodotorula glutinis (CCT 2186, Fundação André Tosello, Campinas, SP, Brazil) were reactivated on YPD agar (2% glucose, 2% bacteriological peptone, 1% yeast extract, 1.5% bacteriological agar, pH 5.6) for 120 h at 28 °C. Subsequently, one isolated colony was transferred to an Erlenmeyer flask with 100 mL of YPD broth for 24 h at 28 °C. Information on the yeasts used for inoculum, as well as the cassava roots used in this study, was registered in the Brazilian National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen, AF18EDC).
2.4. Carotenoids and Biomass Production
The best conditions for the bioproduction of carotenoids and biomass of R. glutinis in a benchtop bioreactor (Electrolab, FerMac 320, Electrolab Biotech, Tewkesbury, UK) were determined by a central composite rotational design (CCRD) 23, with three independent variables (pH, temperature, and agitation), two levels (−1 and +1), three central and six axial points (−α, +α), totaling 17 tests (Table 1).

Table 1.
Total carotenoids and biomass production resulting from the Central Composite Rotational Design (CCRD) for the variation of pH, temperature, and agitation during the fermentation experiments carried out with Rhodotorula glutinis in concentrated manipueira.
Initially, the starter culture (100 mL) containing R. glutinis yeasts was transferred to the bioreactor containing 900 mL of concentrated and sterilized manipueira. The fermentation process was carried out for 120 h [2] under intense exposure to fluorescent light (2086 ± 35 lux, measured with a digital luximeter, ICEL, LD-500, Manaus, AM, Brazil), varying the cultivation parameters pH (1.64 to 7.0), temperature (27 to 43 °C), agitation (66 to 342 rpm), according to the conditions established in the experimental design (Table 1). After the incubation period, the entire suspensions containing the yeast biomass were centrifuged at 6032× g for 10 min at room temperature, and the cells were washed with sterile distilled water and centrifuged again with the same conditions to remove extracellular debris [16]. The biomass was frozen (−18 °C), freeze-dried (L101 Liotop, São Paulo, SP, Brazil), weighed, and stored under a N2 atmosphere at −20 °C until carotenoid extraction.
The extraction of carotenoids was carried out according to the methodology proposed by Mandelli et al. [17], with adaptations. The freeze-dried biomasses were weighed (50 to 200 mg) and exhaustively extracted by maceration with celite and acetone in a mortar with a pestle until the biomasses became colorless. The carotenoid extract was vacuum-filtered and partitioned with petroleum ether/diethyl ether (1:1, v/v), followed by washing with distilled water. The organic phase was evaporated in a rotary evaporator (Marconi, São Paulo, Brazil) under vacuum (T < 38 °C), the carotenoid extract was solubilized in acetone, and the total carotenoid contents were determined by spectrophotometry at 450 nm. The specific absorption coefficient of β-carotene in acetone (2620) [18] was used, and the results were expressed as μg carotenoids/g of freeze-dried biomass.
2.5. Carotenoid Composition by HPLC-DAD-APCI-MS/MS
The carotenoids were extracted from the freeze-dried biomass of R. glutinis strains, according to Squina and Mercadante [2], with modifications. Briefly, 30 mg of biomass were vortexed for 1 min with 100 mg of glass beads (425–600 μm, Sigma-Aldrich, St. Louis, MI, USA) and 1.5 mL of dimethyl sulfoxide (DMSO, analytical grade, Sigma-Aldrich, St. Louis, MI, USA), followed by the addition of 3 mL of diethyl ether/ethyl acetate (1:1), vortexed again and centrifuged at 3000× g for 5 min at 20 °C. After centrifugation, 4.5 mL of distilled water was added, and the mixture was centrifuged again at the same conditions. The non-polar phase was collected, and the remaining polar phase was re-extracted twice using diethyl ether/ethyl acetate (1:1, v/v). The combined extracts were transferred to petroleum ether and saponified overnight at room temperature using a methanolic solution of 10% KOH. The saponified extracts were washed with deionized water until pH 7.0, the solvent was evaporated to dryness, and the dried extract was stored at −35 °C until analysis.
Carotenoid separation was carried out on a C30 YMC column (5 μm, 250 mm × 4.6 mm) using a Shimadzu HPLC system (Prominence UFLC model, Kyoto, Japan) equipped with a binary pump (LC-20AD), a degasser unit (DGU-20A3R), an automatic injector (SIL-20AHT), and an oven (CTO-20A). The mobile phase was a gradient of methanol and tert-methyl butyl ether from 95:5 to 70:30 in 30 min, followed by 50:50 in 20 min, maintaining this condition for 25 min. The flow rate was 0.9 mL/min, and the column temperature was set as 29 °C [19]. For the tentative identification, the diode array detector (DAD) (SPD-M20A) was connected in series to a mass spectrometer (MS) from Bruker Daltonics (AmaZon speed ETD, Bremen, Germany) with atmospheric pressure chemical ionization (APCI) as the ionization source and an ion trap as a m/z analyzer. The UV-visible spectra were obtained from 250 to 650 nm, and chromatograms were processed at 450 nm. The MS conditions were as follows: APCI positive mode, corona needle discharge current of 4000 nA, source temperature of 450 °C, dry gas (N2) at 350 °C at 5 L/ min, and nebulizer at 60 psi. The carotenoid identification was carried out by a combination of the following information: co-elution with authentic standards, elution order in the C30 column, UV-visible spectra characteristics (maximum absorption wavelength (λmax), spectral-fine structure (%III/II), and cis-peak intensity (%AB/AII)), mass spectra features (protonated molecule ([M+H]+) and MS/MS fragmentation), and comparison with the literature data [7,20].
The quantification of carotenoids was carried out on an Agilent HPLC (model Agilent 1260 Infinity, Santa Clara, CA, USA), equipped with a quaternary pump (G1311C), a Rheodyne injection valve with a 20 μL loop, an oven (G1316A) and a DAD detector (G1328C), following the same procedure described above. The contents of each identified carotenoid were determined by six-point analytical curves with concentrations varying from 3.125 to 100 μg/mL (r2 > 0.99) for (all-E)-β-carotene and (all-E)-lutein, and the results (n = 3) were expressed as μg/g of freeze-dried biomass.
2.6. Statistical Analysis
The results were analyzed using the Statistica 7.0 software (StatSoft Inc., Tulsa, OK, USA), using analysis of variance (ANOVA) to estimate the statistical parameters and evaluate the mathematical quadratic model fitting. The statistical significance was 10% (α = 0.1), and the evaluation of regression and lack of fit of the model were determined by Fisher’s test.
3. Results and Discussion
3.1. Physico–Chemical Characterization of the Concentrated Manipueira
In relation to the physical–chemical characterization (Table 2), the concentrated manipueira presented contents within the expected range for cassava (Manihot esculenta Crantz) and cassava by-products. Concentrated manipueira was characterized as a slightly acidic product (pH = 6.24), with pH similar to the values reported for freshly extracted manipueira (6.01) [14] and low lipid content (0.77–0.99 g/100 g) [21]. Regarding reducing sugars, manipueira showed similar values to those mentioned in the literature (3.80–4.06 g/100 g) [22,23]. However, the result found in this study for total sugars is higher when compared to the values obtained by some studies; (5.82 g/100 g) [22] and (3.58 g/100 g) [14].

Table 2.
Physico–chemical characterization of the concentrated manipueira.
Yeast cells have basic nutritional needs, such as the need to assimilate simple sugars, which directly influence cell multiplication and growth. For the genus Rhodotorula, the use of glucose seems to be associated with high yields in the specific production of carotenoids [4,24]. Thus, manipueira can be confirmed as an excellent alternative substrate for fermentation processes since the main available nutrients for fermentation purposes are carbohydrates.
3.2. Best Cultivation Conditions for Carotenoids and Biomass Production
Based on the experiments carried out at the CCRD conditions (Table 1), the biomass of R. glutinis produced after cultivation in a benchtop bioreactor using concentrated manipueira as a substrate varied from 1.93 to 11.06 g, and the total carotenoid contents varied from 106 to 1432 μg/g of freeze-dried biomass. On the one hand, the yeast cultivation condition at pH 5.0, 35 °C, and 150 rpm of agitation (experiment 15) provided the highest yields for both carotenoid contents and biomass production. On the other hand, strikingly lower levels of both biomass (≈6 times) and carotenoid production (≈10 times) were observed when R. glutinis was cultivated at the same temperature and agitation conditions (35 °C and 150 rpm, respectively), but at the most acidic condition (pH = 1.64, experiment 9).
Both the total carotenoid contents and biomass yields exhibited low relative standard deviation values (RSD = 1.8% and 3.3%, respectively) at the central points of the experimental design (Table 1), indicating high reproducibility of the fermentation process carried out in the benchtop bioreactor at the proposed experimental conditions. The highest content of total carotenoids observed in this study (1432 μg/g of freeze-dried biomass) was about eight times higher than those found when manipueira was supplemented with ammonium sulfate [(NH4)2SO4] after five days of cell growth in the dark, at 30 °C and shaken at 200 rpm (170 μg/g) [23]. When compared to other agroindustrial substrates and the same microorganism, the highest total carotenoid contents produced by R. glutinis using concentrated manipueira were about 55% higher than the values found for grape must (915 μg/g) and 60% higher than those observed for soybean flour extract (883 μg/g), both cultivated at 30 °C and shaken at 180 rpm [24].
The high content of carotenoids found in this study can also be explained due to the intense exposure of yeasts to light over the entire cultivation time since the previously mentioned study did not follow the same strategy. Moreover, in a preliminary experiment from our research group, the influence of intense light exposure and high temperature (35 °C) on carotenoid production was compared with the growing condition at 28 °C and limited light incidence, using the same substrate and microorganism. As a preliminary result, the total carotenoid contents produced after the intense fluorescent incidence and at a higher temperature (35 °C) was about 34 times higher than the other condition. The use of white light was reported to increase the activity of enzymes related to carotenoid biosynthesis by the photoinduction theory [25], and an increase of about 80% in the production of carotenoids produced by R. glutinis under the influence of light radiation was already reported [26], and reviewed elsewhere [4].
After performing ANOVA with the independent variables, quadratic models were generated by the response surface methodology and showed moderate (R2 = 0.64) and low (R2 = 0.33) determination coefficients for the contents of carotenoid and biomass production, respectively. Moreover, Fisher’s test characterized both the proposed quadratic models as non-significant and non-predictive with inaccuracy to adequately predict the carotenoid production at the studied range, and no response surface was generated to optimize the fermentation process. Perhaps, in future studies, other variables, such as the addition of selected chemical compounds to stimulate carotenogenesis, different types of light, or other options of agroindustrial substrates, may be added in the biotechnological process aiming at the optimization.
Notwithstanding the inaccuracy of the proposed models, contour plots were generated only with the purpose of illustrating the tendencies observed for the experimental design. According to Figure 1A,B, the highest total carotenoid contents and biomass produced by R. glutinis using concentrated manipueira as low-cost substrate tend to be achieved at the same fermentation conditions: pH at 5.0, 35 °C, and agitation at 150 rpm. These conditions are the same ones carried out at the central points of the experimental design, providing 10.8 ± 0.4 g of dry biomass (RSD = 3%) and 1410 ± 26 μg carotenoids/g of dry biomass (RSD = 2%).
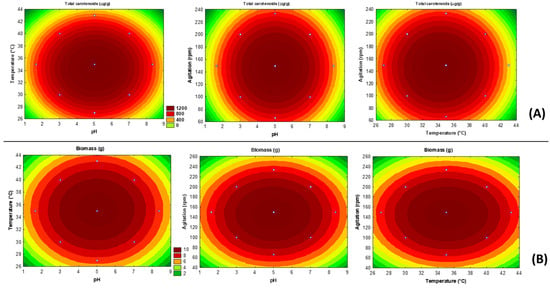
Figure 1.
Contour curves plotted only to illustrate the contents of total carotenoids (A) and biomass accumulation (B) produced by Rhodotorula glutinis in concentrated manipueira.
3.3. Carotenoid Composition Produced by Rhodotorula glutinis
The HPLC-DAD chromatogram (Figure 2) showed the presence of seven major carotenoids, which were identified (Table 3) in the extracts obtained from the biomass of R. glutinis cultivated at the best fermentation conditions proposed in our study. The MS and MS/MS spectra for all the tentatively identified carotenoids can be seen in Supplementary Figure S1.
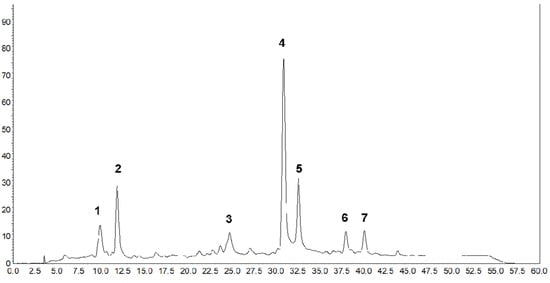
Figure 2.
Chromatographic profile of carotenoids, by HPLC-DAD, produced by Rhodotorula glutinis, produced at pH 5.0, 35 °C, agitation at 150 rpm, and intense exposure to fluorescent light during five days of fermentation. Peak assignments are given in Table 3.

Table 3.
Carotenoid produced by Rhodotorula glutinis using concentrated manipueira as substrate at pH 5.0, temperature at 35 °C, agitation at 150 rpm, and intense exposure to fluorescent light during five days of fermentation.
Peak 1 was tentatively identified as 5,8-epoxy-lutein considering the UV-Vis and MS spectra characteristics. The protonated molecule was not detected due to the in-source fragmentation, and the MS spectrum was characterized by the presence of the parent ion at m/z 567, corresponding to the loss of one hydroxyl group (−18 u). The MS/MS spectrum showed the presence of fragment ions at m/z 549 [M+H-18-18]+ and 531 [M+H-18-18-18]+ resulting from consecutive losses of hydroxyl groups, at m/z 511 corresponding to the consecutive elimination of a hydroxyl group and the ε-ring (−56 u), and at m/z 475, from the consecutive losses of the hydroxyl group (−18 u) and toluene (−92 u). The fragment ion at m/z 221, characteristic of an epoxy substituent in a ring with a hydroxyl group, was also detected.
The (all-E)-lutein (Peak 2) was identified by comparison with the retention time, UV-visible, and MS spectra features of the authentic standard. The positive ion mode spectrum of lutein was characterized by the protonated molecule at m/z 569 and the fragment ions at m/z 551 [M+H-18]+ and 533 [M+H-18-18]+ from the elimination of hydroxyl groups. The intensity of fragment ion m/z 551 was higher than the protonated molecule, corresponding to the loss of the hydroxyl group from the ε-ring. In addition, the MS/MS showed the presence of fragments at m/z 495 and 459 from the consecutive losses of the hydroxyl group and ε-ring [M+H-56-18]+, and hydroxyl group and toluene [M+H-92-18]+. This is the first time that lutein has been reported in the biomass of the R. glutinis species.
Peaks 3, 4, and 5 were assigned to (13Z)-β-carotene, (all-E)-β-carotene, and (9Z)-β-carotene, respectively. All of them exhibited the same protonated molecule at m/z 537 and fragment ion at m/z/ 444 (−92 u, loss of toluene) in the mass spectra. The differentiation was carried out considering the characteristics of UV/Vis spectra: both fine structure (%III/II) and λmax decrease, while cis-peak intensity (%AB/AII) increases as the double bond in Z configuration gets closer to the center of the molecule (from 9Z to 13Z). The (all-E)-β-carotene was also the major carotenoid in R. glutinis biomass found in a previous study [2].
Peaks 6 and 7 were tentatively assigned to (Z)-isomers of β-zeacarotene, according to the retention time, UV-visible spectra, chromatographic behavior, and mass spectra, in comparison to data from the literature [20]. The mass spectrum showed a protonated molecule at m/z 539, and the MS/MS presented the fragment ions at m/z 446 and 401, corresponding to the losses of toluene (92 u) and β-ring (137 u), respectively, and at m/z 309, from the consecutive losses of toluene and the β-ring. The production of β-zeacarotene isomers by R. glutinis was previously reported [2,27,28]. However, as far as we are concerned, this is the first time that the identification of this carotenoid from this yeast species is supported by MS data.
Although E-isomers have greater stabilities than Z-isomers, the presence of Z-isomers of β-carotene and β-zeacarotene was observed, which is related directly to the cultivation method under constant and intense lighting since light is one of the main catalysts in the isomerization reaction of carotenoids [2]. (all-E)-Lutein (242.37 ± 4.11 μg/g of dried biomass) and (all-E)-β-carotene (239.32 μg/g of dried biomass) were the carotenoids with the highest concentration in the cell biomasses of R. glutinis. It is noteworthy that 5,8-epoxy-lutein and (all-E)-lutein were not yet reported in the literature as carotenoids from the biomass of yeasts of the genus Rhodotorula. Some researchers evidenced the influence of the culture medium in the profile of carotenoids produced by microorganisms [29]. El-Banna et al. [30] found that R. glutinis grown in culture media with high concentrations of carbon produced up to 95% of β-carotene in relation to the total composition of carotenoids. β-Zeacarotene is traditionally reported as a metabolic product of R. glutinis yeasts, in addition to citing kinetic evidence that β-carotene can be formed via β-zeacarotene and γ-carotene as an intermediary in the biosynthesis of carotenoids [27,31]. According to Johnson and An [32], the production of the β-zeacarotene by microorganisms is directly related to cellular stress due to exposure to intense light.
Carotenoids in microorganisms have important physiological functions as they contribute to the integrity of cell membranes and also exhibit efficient antioxidant activity to scavenge reactive species generated during normal metabolism, such as singlet oxygen (1O2) and other oxidants [33]. When unfavorable conditions induce stress in R. glutinis, biochemical responses involving an increase in the activity of key enzymes in carotenogenesis take place, resulting in the composition of carotenoids [23].
As already known in the literature [4,34], the composition of carotenoids (contents and profile) produced by R. glutinis is mainly influenced by the substrate composition and cultivation parameters. Thus, this is the reason why investigating the best conditions for the biotechnological production of carotenoids should be determined individually for each yeast strain in combination with different substrates. The proximate composition of manipueira showed low total protein contents (the main source of nitrogen) in relation to carbohydrate contents (the main source of carbon), especially reducing sugars, which are the preferred carbon sources for yeast. This factor may also be responsible for the absence of the most characteristic carotenoids reported for the genus Rhodotorula, such as torulene and torularrodine, as reviewed in detail elsewhere [33].
4. Conclusions
Rhodotorula glutinis was able to use manipueira as a substrate for carotenoid biosynthesis without the need to supplement the culture medium. Our results demonstrated that the cultivation conditions at pH 5.0, 35 °C, and agitation at 150 rpm, under intense light incidence, provided the highest yields of both carotenoids and biomass. Furthermore, the carotenoid profile showed high contents of β-carotene and lutein, evidencing the added biotechnological and industrial value in the use of regional wastes as strategic sources of low-cost substrates to produce carotenoids by yeasts of the genus Rhodotorula spp., which can be an alternative for the food and pharmaceutical industries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9070617/s1, Supplementary Figure S1: MS and MS/MS spectra, by HPLC-DAD-APCI-MS/MS, of the carotenoids tentatively identified in the biomass of Rhodotorula glutinis cultivated with concentrated manipueira as substrate.
Author Contributions
Conceptualization, W.S.I., A.S.L. and R.C.C.; Methodology, F.d.A.M., W.S.I., A.A.O.X., A.Z.M., A.S.L. and R.C.C.; Software, A.S.L.; Validation, F.d.A.M., W.S.I., A.A.O.X., A.Z.M., A.S.L. and R.C.C.; Formal analysis, F.d.A.M., W.S.I., A.A.O.X., A.Z.M. and R.C.C.; Investigation, F.d.A.M., Willen Silva Igreja, A.A.O.X., A.Z.M., A.S.L. and R.C.C.; Resources, A.S.L. and R.C.C.; Data curation, F.d.A.M., W.S.I., A.A.O.X., A.Z.M., A.S.L.and R.C.C.; Writing—original draft, F.d.A.M., W.S.I., A.A.O.X., A.Z.M., A.S.L. and R.C.C.; Writing—review & editing, W.S.I., A.A.O.X., A.Z.M., A.S.L. and R.C.C.; Visualization, F.d.A.M. and A.A.O.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil—Project 403121/2016-7), the São Paulo Research Foundation (FAPESP, Brazil—multiuser equipment Grant 2018/23752-1), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil—Master’s scholarship—code 001, Process 00214342204).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Acknowledgments
Federal University of Pará (UFPA, Brazil) through PROPESP/UFPA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zoz, L.; Carvalho, J.C.; Soccol, V.T.; Casagrande, T.C.; Cardoso, L. Torularhodin and torulene: Bioproduction, properties and prospective applications in food and cosmetics—A review. Braz. Arch. Biol. Technol. 2015, 58, 278–288. [Google Scholar] [CrossRef]
- Squina, F.M.; Mercadante, A.Z. Análise, por CLAE, de carotenóides de cinco linhagens de Rhodotorula. Rev. Bras. Cienc. Farm. 2003, 39, 309–318. [Google Scholar] [CrossRef]
- Valduga, E.; Tatsch, P.O.; Tiggemann, L.; Treichel, H.; Toniazzo, G.; Zeni, J.; Di Luccio, M.; Fúrigo Júnior, A. Carotenoids production: Microorganisms as source of natural dyes. Quim. Nova 2009, 32, 2429–2436. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.A.; Lopes, A.S.; Chisté, R.C. B’iotechnological Production of Carotenoids Using Low Cost-Substrates Is Influenced by Cultivation Parameters: A Review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Almanza, A.; Montanez, J.C.; Aguilar-González, M.A.; Martínez-Ávila, C.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci. 2014, 5, 64–72. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Britton, G.; Liaanen-Jensen, S.; Pfander, H. Carotenoids; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar] [CrossRef]
- Mercadante, A.Z. Carotenoids in Foods: Sources and Stability during Processing and Storage; Socaciu, C., Ed.; CRC Press: New York, NY, USA, 2007; pp. 213–235. [Google Scholar]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Arthur, E.K.; Azeko, S.T. Surface hardening of ferrous materials with cassava (Manihot spp.) waste: A review. Sci. Afr. 2020, 9, e00483. [Google Scholar] [CrossRef]
- Chaiareekitwat, S.; Latif, S.; Mahayothee, B.; Khuwijitjaru, P.; Nagle, M.; Amawan, S.; Müller, J. Protein composition, chlorophyll, carotenoids, and cyanide content of cassava leaves (Manihot esculenta Crantz) as influenced by cultivar, plant age, and leaf position. Food Chem. 2022, 372, 131173. [Google Scholar] [CrossRef] [PubMed]
- Cereda, M.P.; Vasconcelos, S.P. Varieties and Landraces Cultural Practices and Traditional Uses; Volume 2 in Underground Starchy Crops of South American Origin; Chapter 9—Cassava Cyanogenic Glycosides: Importance, Toxicity, and Dosage Methods; Academic Press: Cambridge, MA, USA, 2023; pp. 179–209. [Google Scholar] [CrossRef]
- Brito, B.N.C.; Chisté, R.C.; Lopes, A.S.; Glória, M.B.A.; Pena, R.S. Influence of spontaneous fermentation of manipueira on bioactive amine and carotenoid profiles during tucupi production. Food Res. Int. 2019, 120, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Maróstica, M.R., Jr.; Pastore, G.M. Biotransformation of citronellol in rose-oxide using cassava wastewater as a medium. Food Sci. Technol. 2006, 26, 690–696. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1997; Volume 2, 850 p. [Google Scholar]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z. Identification and Quantification, by HPLC-DAD-MS/MS, of Carotenoids and Phenolic Compounds from the Amazonian Fruit Caryocar villosum. J. Agric. Food Chem. 2012, 60, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Santana Neto, J.A.; Muniz, E.N.; Santos, G.R.A.; Macedo, F.A.F.; Rangel, J.H.D.A.; Gomes, L.C. Effect of cassava wastewater on physicochemical characteristics and fatty acids composition of meat from feedlot-finished lambs. Acta Sci.—Anim. Sci. 2017, 39, 377. [Google Scholar] [CrossRef]
- Damasceno, S.; Cereda, M.P.; Pastore, G.M.; Oliveira, J.G. Production of volatile compounds by Geotrichum fragrans using cassava wastewater as substrate. Process Biochem. 2003, 39, 411–414. [Google Scholar] [CrossRef]
- Ribeiro, J.E.S.; Sant’Ana, A.M.S.; Martini, M.; Sorce, C.; Andreucci, A.; Melo, D.J.N.; Silva, F.L.H. Rhodotorula glutinis cultivation on cassava wastewater for carotenoids and fatty acids generation. Biocatal. Agric. Biotechnol. 2019, 22, 101419. [Google Scholar] [CrossRef]
- Buzzini, P.; Martini, A. Production of carotenoids by strains of Rhodotorula glutinis cultured in raw materials of agro-industrial origin. Bioresour. Technol. 2000, 71, 41–44. [Google Scholar] [CrossRef]
- Bhosale, P.; Gadre, R. β-Carotene production in sugarcane molasses by a Rhodotorula glutinis mutant. J. Ind. Microbiol. Biotechnol. 2001, 26, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, H.; Nakanishi, T.; Tada, A.; Miki, W.; Komemushi, S. Activation of torularhodin production by Rhodotorula glutinis using weak white light irradiation. J. Biosci. Bioeng. 2001, 92, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Goodwin, T.W. A comparison of synthetic β-zeacarotene and samples obtained from Rhodotorula glutinis and yellow corn. Phytochemistry 1965, 4, 193–195. [Google Scholar] [CrossRef]
- Maldonade, I.R.; Rodriguez-Amaya, D.B.; Scamparini, A.R.P. Carotenoids of yeasts isolated from the Brazilian ecosystem. Food Chem. 2008, 107, 145–150. [Google Scholar] [CrossRef]
- Squina, F.M.; Yamashita, F.; Pereira, J.L.; Mercadante, A.Z. Production of carotenoids by Rhodotorula rubra and R. glutinis in culture medium supplemented with sugar cane juice. Food Biotechnol. 2002, 16, 227–235. [Google Scholar] [CrossRef]
- El-Banna, A.A.; El-Razek, A.M.A.; El-Mahdy, A.R. Some Factors Affecting the Production of Carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr. Sci. 2012, 3, 64–71. [Google Scholar] [CrossRef]
- Simpson, K.L.; Nakayama, T.O.M.; Chichester, C.O. Biosynthesis of yeast carotenoids. J. Bacteriol. 1964, 88, 1688–1694. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.H. Astaxanthin from Microbial Sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Berera, R.; van Stokkum, I.H.M.; Kennis, J.T.M.; van Grondelle, R.; Dekker, J.P. The light-harvesting function of carotenoids in the cyanobacterial stress-inducible IsiA complex. Chem. Phys. 2010, 373, 65–70. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).