Abstract
Polyunsaturated fatty acids (PUFAs) constitute a significant lipid class with essential nutritional and health benefits for both animal and human health; however, their effect and interaction with the gut microbiota ecosystem are still unclear. Therefore, the present study aims to investigate the effect of fish oil (FO) on ruminal fermentation and bacterial abundance under high- and low-forage diets. Thirty-six ruminal fluid samples were allocated into two experiments. The first was on high-forage diet and included three groups: the control (basal diet with 70% forage and 30% concentrate), group 2 (basal diet + 5 mL/L FO), and group 3 (basal diet + 10 mL/L). The second experiment was on low-forage diet: the control (basal diet with 30% forage and 70% concentrate), group 2 (basal diet + 5 mL/L FO), and group 3 (basal diet + 10 mL/L). The results showed that although FO supplementation did not affect the pH level among different diets, it significantly decreased methane under a high-forage diet. In addition, regarding the fatty acids profile, FO supplementation in high-forage diet significantly decreased fatty acids in both; however, under a low-forage diet, FO groups showed significantly higher fatty acid content than the control. However, FO supplementation increased the abundance of Anaerovibirio, Selenomonas, pseudobutyrivibrio, and butyrivibrio through a high-forage diet. In contrast, the abundance of Prevotella, Rikenellaceae RC9 gut group, and Saccharofermentans was depressed with FO supplementation. Whereas under low-forage diet, FO supplementation increased Ruminobacter, Anaerovibirio, Megasphaera, Pseudobutyrivibrio, Streptococcus, Butyrivibrio, unclassified_lachnospiraceae; it also decreased Prevotella and Rikenellaceae RC9 abundance similar to the high-forage diet. Based on the KEGG pathway results, FO supplementation significantly downregulated genes mainly related to folding, sorting and degradation, environmental adaptation, cell motility, transcription, membrane transport, and signal transduction. The results revealed that FO has a depressing effect on ruminal fermentation and some bacterial population; however, this negative effect can be minimized in high-concentrate diets.
1. Introduction
Over the last decade, oils have been added to ruminant diets to increase the nutritional energy density. However, the quantity and type of dietary lipids significantly impact fatty acids (FA) ruminal metabolism because FA may have an altering impact by inhibiting microorganisms [1]. Polyunsaturated fatty acid (PUFA) is a significant content of fatty acids in fats and oil, especially vegetable oils or oilseeds, marine products, or protected fat sources [2]. PUFA, such as omega-3 FAs, including LNA, EPA, and DHA, contain double bonds in the fatty acyl chain: n-3. PUFA metabolism is complicatedly related to ruminal metabolism depending on H2 metabolism and/or the microbial species engaged in many metabolic pathways indirectly that affect biohydrogenation [2]. Mostly incomplete biohydrogenation produces PUFA in the rumen can influence their concentrations in tissues or milk [3].
Moreover, methane is a greenhouse gas (GHG) with 21 times the global warming potential of CO2, and production from agricultural production represents 10–12% of the total anthropogenic GHG equivalents per year (Gt CO2 Eq/yr) [4]. Previous studies reported the potential of fats and oils to reduce ruminal CH4 production, one of the global environmental side effects of livestock farming, and international agencies recommended their inclusion in ruminant diets as a viable nutritional greenhouse gas (GHG) mitigation strategy [5].
The accumulating knowledge drew attention to the efficiency of fat supplementation using dietary interventions through links between nutrition, metabolism, and the rumen microbiome and their environmental effect [6], especially since lipid supplementation can lead to a shift in the rumen microbial population. However, there is no further investigation into a specific strategy impact on the overall ruminal function and processes.
Specific microbial groups and their interactions are essential in several aspects of ruminant production, including meat and milk quality [7], feed utilization efficiency [8], health and welfare [9], and composition of ruminal microbiota [10]. However, modulation of the fatty acid profile is attributed to various factors, including forage percentage [11] and forage type [10].
Although a previous study by Pirondini [12] showed no adverse effects of fish oil (0.8% dry matter) on milk fat in cattle fed a low-starch diet, fish oil supplementation was responsible for increasing the DHA content in milk by approximately 6.86% (range from 1.35% to 14.4%), and microalgae supplementation increased it by approximately 7.08% (range from 1.09% to 14.0%) [1].
In addition, when marine lipids were added to ruminant diets, several beneficial fatty acids (FA) in milk and meat increased, mostly linked to rumen microbial activity [13]. Marine lipids were found to manipulate the rumen’s microbial community and fermentation processes [14], which could positively change the rumen fatty acid proportions by affecting the activity of rumen microorganisms [10,15].
Nevertheless, marine oil and unsaturated fatty acids have a more substantial antimicrobial effect than saturated ones [16], and different PUFA have been reported to have differential toxicity toward rumen microorganisms [17,18]. However, the microbiological action of ruminal unsaturated FA metabolism remains poorly understood [19,20], especially the crosstalk between unsaturated fatty acids (such as omega-3 acids), diet type, and the rumen microbial community.
Additional research may be needed to identify the relationship between omega-3, the ruminal bacteria, and its effect on fermentation in the rumen. Therefore, more research may be required to determine the relationship between omega-3 and ruminal bacteria and the effect on rumen fermentation. Therefore, the present study was performed using 16sRNA to evaluate the effect of fish oil on in vitro ruminal fermentation and microbial abundance under different diet compositions.
2. Materials and Method
2.1. Sampling and In Vitro Fermentations
Rumen fluid was collected from four cows immediately after slaughtering at a commercial slaughterhouse (Heng Xing Slaughterhouse, Wuhan, China). All cows were fed a total mixed diet consisting mainly of corn silage, excluding disease factors, and rumen fluid was collected through four layers of cheesecloth within 15 min. The animals were slaughtered before morning feeding [21]. After collecting rumen fluid, the bottle was cleared of air at the top, kept at 39 °C, and arrived at the laboratory in 30 min. After that, fresh buffer solutions were prepared per Menke and Steingass [22]. Briefly, rumen fluid and buffer were mixed 1:2 at 39 °C under continuous CO2 flushing. Then, 36 fermentation flasks were used in two conditions in six groups (six flasks/each group). The first experiment used a high-forage, low-concentrate diet (70:30): Control (basal high-forage diet without FO supplementation), group 2:5 mL/L group, and group 3:10 mL/L group. The second experiment included a low-forage, high-concentrate diet (30:70): Control (basal low-forage diet without FO supplementation), T1: 5 mL/L group, and T2: 10 mL/L group. The fish oil was manufactured by Tomson Health Co., Ltd. (Hong Kong, China). Production; No. 19, Xing Han Road, Sanzao Science and Technology Industrial Park, Jinwan District, Zhuhai City, China; approval number G30140593. Each 100 g of fish oil contained 16.1 g eicosapentaenoic acid (EPA) and 10.7 g docosahexaenoic acid (DHA).
The fermentation flasks were then filled with 0.5 g of experimental diets (Table 1) and 50 mL of rumen buffer fluid, injected with CO2, and sealed with a butyl rubber plug and aluminum cap. Finally, the flasks were placed in constant temperature shake incubators at 39 °C.

Table 1.
Experimental Substrate Composition and Analysis.
At the end of fermentation, the fluid pH was measured with a pH meter (FE28—Standard, Mettler Toledo, Greifensee, Switzerland). The samples were separated into 2 mL sterile tubes. SCFA analysis required the collection of isometric samples. The remaining samples were stored at −80 °C, with one isometric sample saved for further microbial composition analysis, and the rest for further analysis.
2.2. Chemical Analysis
For short-chain fatty acids (SCFA) analysis, the supernatant was extracted by 1 mL liquid samples, added to 200 μL of 25% metaphosphoric acid, and centrifuged at 12,000 RPM for 10 min at 4 °C. A gas chromatograph (7890A, Agilent Technologies, Santa Clara, CA, USA), capillary column (30 m × 0.25 mm × 0.25 μm, DB—FFAP, Agilent Technologies, Santa Clara, CA, USA), and a pyrophoric detector were used in this study. The oven temperature was initially set at 80 °C for 5 min, then increased to 190 °C (12.5 °C/min), and held at 190 °C for 3 min. The injector and detector temperatures were set at 250 °C, and the CH4 yield was calculated by the formula of Moss, et al. [23,24] considering a hydrogen recovery of 90% (default):
CH4 (mmol/L) = 0.45 × Acetate (mmol/L) − 0.275 × propionate (mmol/L) + 0.40 × butyrate (mmol/L).
The frozen samples (approximately 200 mg) were thawed according to the manufacturer’s instructions before being subjected to DNA extraction using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). The obtained DNA was subjected to a quality inspection and concentration quantification to ensure that its qualities met the requirements of subsequent analysis. The total microbial DNA was quantified using a UV-Vis spectrophotometer (NanoDrop 2000, New York, NY, USA), and DNA integrity was evaluated by 0.8% agarose gel electrophoresis. The primers used to amplify the V3/V4 regions (338 F: ACTCCTACGGGAGGCAGCA and 806 R: GGACTACHVGGGTWTCTAAT) were synthesized according to conserved regions, and the PCR amplification was conducted in triplicate under the same conditions. The agarose gel electrophoresis (2%) and AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA, USA) were employed to evaluate the PCR amplification product and recycle target fragment, respectively. Based on the first electrophoretic data, fluorescence quantification of PCR-recycled products was carried out on a Microplate Reader (BioTek, Winooski, VT, USA, FLx800). The acquired PCR products were used to build the sequencing library using the TruSeq Nano DNA LT Library Prep Kit from Illumina (San Diego, CA, USA). The final fragment selection and library purification were performed using 2% agarose gel electrophoresis. The sequencing libraries’ quality inspection and fluorescence quantification were performed before sequencing. The qualified libraries were diluted and mixed in the appropriate quantities based on the needed sequencing volume. Then, high-throughput sequencing using a MiSeq sequencing machine was performed on the resulting libraries. The PCR amplification procedure was determined according to previous research [25].
2.3. Sequence Accession Numbers
Sequence data were deposited in the NCBI database with the following accession numbers: OQ538446–OQ539435.
2.4. The 16S rDNA Sequence Data Acquisition and Bioinformatics
A total of 2,880,607 paired-end (PE) reads were generated from 36 samples. Firstly, Trimmomatic v0.33 filtered raw reads; then, the primer sequences were identified and removed by Cutadapt 1.9.1, which finally generated high-quality reads without primer sequences. Based on overlapping sequences, high-quality reads assembled by FLASH v1.2.7 generated clean reads. DADA2 processed de-noise in QIIME2 2020.6 to de-noise and remove chimeric sequences, generating non-chimeric reads. Upon that, 2,874,879 clean reads were obtained after PE reads quality control and assembly, a minimum of 79,385 and an average of 79,858 clean reads for each sample. Raw data were processed for quality control, including removing low-quality reads and length-based filtration to generate high-quality reads (Table 2). As previously described by Li, et al. [25], The rarefaction curve plotted the species counts detected from each group to evaluate whether sequencing data can detect all species from a sample, reflecting species diversity from the side. Simultaneously, each sample rarefaction curve was generated to assess the sequencing depth.

Table 2.
The processed raw-data and estimated data quality based on read length and counts at each stage.
Alpha diversity was used to evaluate the richness and species diversity within a single sample using multiple indexes, including Chao1, ACE, Shannon, and Simpson. Chao1 and ACE count for species richness, i.e., the number of species presented. The top 10 species in each level were selected, and the rest were combined as others for an optimal appearance. Unclassified represents the species that have not been taxonomically annotated. The gut microbial alpha diversity was calculated according to the relative abundance distribution of OTU in each sample. The function prediction of the ruminal microbiota was performed using the phylogenetic investigation of the community by reconstruction of unobserved States 2 (PICRUSt2).
2.5. Statistical Analysis
Statistical analysis of 16sRNA data was performed using R (v3.0.3) and GraphPad Prism (v7.0c). While fermentation parameters data were statistically analyzed using a one-way analysis of variance (ANOVA) using SPSS 22.0, p-values < 0.05 were considered statistically significant.
2.6. Differential Analysis between Groups
Line discriminant analysis (LDA) effect size was applied to find biomarkers with a statistical difference between different groups [25,26].
2.7. 16s Functional Genes Prediction
PICRUSt2 was applied to perform species annotation on feature sequences based on a reference phylogenetic tree. Differential biomarkers with statistical significance were identified between groups by differential analysis. Gene functions, phenotypes, and corresponding abundance of samples were characterized and estimated based on functional prediction [27]. After the standard analysis, STAMP was applied to perform COG analysis [28].
3. Results
3.1. Fermentation and Short Chain Fatty Acid (SCFA) Activity
As shown in Table 3 (Panel a), although pH did not differ between control and fish oil (FO) levels in high-forage conditions, FO supplementation significantly reduced CH4 levels. In addition, the results showed that FO supplementation significantly depressed total SCFA, acetic, and propionic acid; however, the treatments did not affect butyric, isobutyric, valeric, and isovaleric acid.

Table 3.
Fermentation characteristics affected by substrate condition and fish oil (FO) supplementation.
Under low-forage, high-concentrate diet, it was found that fish oil did not change pH or methane levels under different levels. However, the FO groups showed significantly higher fatty acid content except for the acetic and caproic acids, which were not significantly higher in the FO groups Table 3 (Panel b).
3.2. Species Refraction Curve
Venn diagrams (Figure 1) shows that common and unique taxa numbers are represented among different FO levels. Generally, multi-sample refraction curves showed that FO supplementation changed the number of species among different levels and different forage: concentrate ratios.
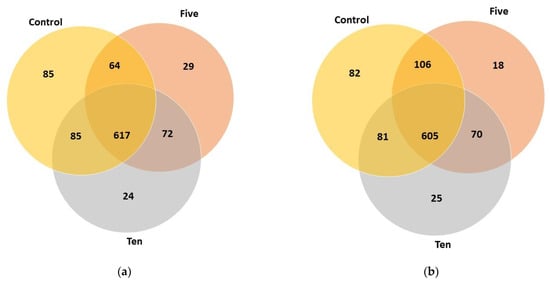
Figure 1.
The Venn diagrams represent common and unique taxa numbers responding to FO supplementation under high-(a) and low-(b) forage ratios.
3.3. Differential Rumen Bacterial Taxa among Different Treatments Differential Alpha Diversity Analysis
Table 4 shows the alpha diversity indexes in the high-forage diet (70% forage:30% concentrate). Although the Simpson index showed no significant difference in species number between FO levels compared to the control group, the Shannon index showed a significant decrease only in the 5 mL group. Whereas, for the richness index, both ACE and Chao index showed a highly significant (p < 0.01) decrease in species number between the control and 5 mL group and only a significant (p < 0.05) decrease between control and 10 mL group as shown in Figure 2 and Figure 3.

Table 4.
Alpha diversity metrics for species richness under high- and low-forage ratios.
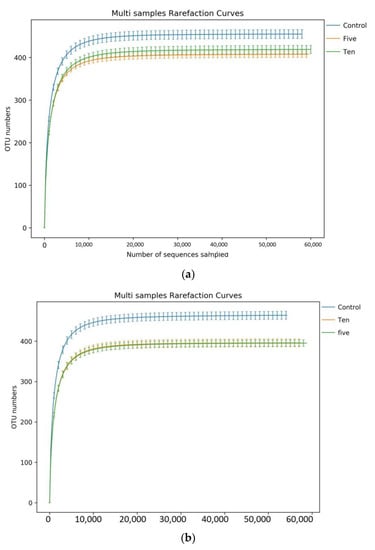
Figure 2.
Rarefaction curve plotting species counts from different FO levels under high-(a) and low-(b) forage ratios.
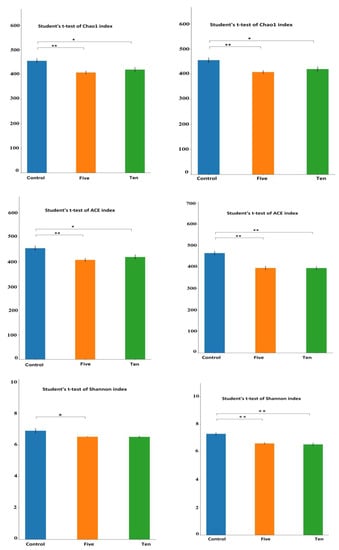
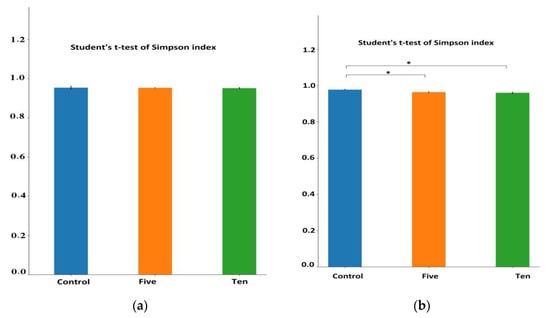
Figure 3.
Differential alpha diversity indexes for different FO levels for richness (ACE and Chao1) and diversity (Shannon and Simpson) under high-(a) and low(b) forage ratios. * means that the ANOVA difference is significant (p < 0.05) while ** means the difference is highly significant (p < 0.01).
For a low-forage diet (30% forage:70% concentrate), richness indexes ACE and Chao showed that both FO levels significantly decreased microbial community. However, the indices for characterizing microbial community diversity, the Shannon index showed that FO groups were significantly lower than the control groups.
These results indicated that the ruminal microorganism’s community richness and diversity were reduced by FO supplementation under different diet compositions.
3.4. Taxonomic Composition and Alteration of Bacterial Community Associated with Different Levels of FO
Overall, under different dietary compositions, FO supplementation could change microbial abundance (Figure 4 and Figure 5).
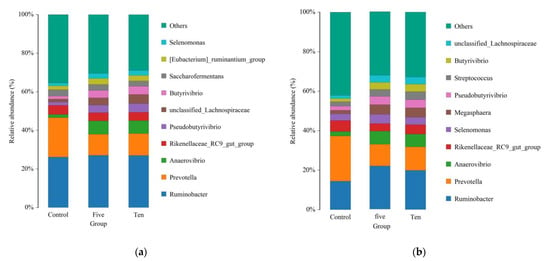
Figure 4.
Relative abundance of genus among different FO levels under high-(a) and low-(b) forage ratios.
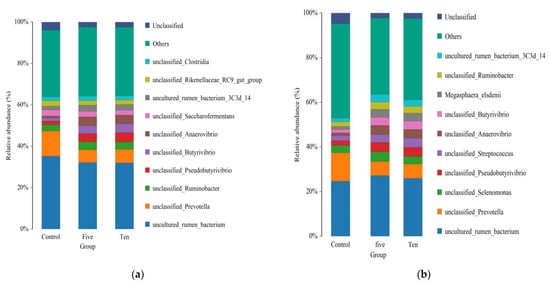
Figure 5.
Relative abundance of species among different FO levels under high-(a) and low-(b) forage ratios.
Under high-forage diet conditions, although at the genus level, the Ruminobacter genus did not change with different FO levels, Anaerovibirio, Selenomonas, pseudobutyrivibrio, and butyrivibrio abundance was increased by FO supplementation. In contrast, the abundance of Prevotella, Rikenellaceae RC9 gut group, and Saccharofermentans was depressed with FO supplementation.
Whereas under low-forage conditions, at the genus level, FO supplementation increased Ruminobacter, anaerovibirio, megasphaera, pseudobutyrivibrio, streptococcus, Butyrivibrio, and unclassified_lachnospiraceae; it also decreased Prevotella and Rikenellaceae RC9 genus abundance similar to high-forage conditions.
3.5. Linear Discriminant Analysis of Effect Size (LDA)
Significant differences in microbial species among the three groups were identified following LEfSe analysis (LDA = 4). Specifically, at a high-forage ratio (70% forage:30% concentrate), the LEfSe analysis revealed that six genera were potential biomarkers to distinguish the control and treatment groups (Figure 7); also, the low-forage diet was distinguished by six genera, except the 10 mL group that was distinguished by one genus. At a high-forage ratio, the control group was characterized by s_unclassified_Prevotella, g_Prevotella, f_prevotellacea, o_Bacteriodales, s_Fibrobacter_succinogenes, g_Fibrobacter, f_Fibrobacteraceae, and o_Fibrobactereales (p < 0.05). However, the 5 mL/L group was marked by s_unclassified_Ananerovibrio, g_Ananerovibrio, f_Selenomonadaceae, g_Megasphera, f_Veillonellaceae, and o_Veillonellaceae_Selenomonadales. On the other hand, the results revealed that FO levels significantly enriched in the 10 mL/L group the abundance of: s_unclassified_Butyrivibrio, g_Butyrivibrio, s_unclassified_Pseudobutyrivibrio, g_Pseudobutyrivibrio, s_Lachnospiraceae_bacterium_CG54, g_unclassified_Lachnospiraceae, f_Lachnospiraceae, and o_Lachnospirales. On the other hand, at low-forage ratio the control group was characterized by s_unclassified_Bacteroidales_bacterium_bact_22, g_Bacteriodales_bacterium_Bact_22, f_F082, s_unclassified_Prevotella, g_Prevotella, f_Prevotellaceae,o_Bacteriodales, s_Fibrobacter_succinogenes, g_Fibrobacter, f_Fibrobacteraceae, and o_Fibrobactereales. The 5 mL/L group was enriched by s_unclassified_Pseudobutyrivibrio, g_Pseudobutyrivibrio, and g_unclassified. These results indicate that different supplemental FO levels could lead to characteristic differences in ruminal bacterial taxa, as Figure 6, Figure 7 and Figure 8 show.
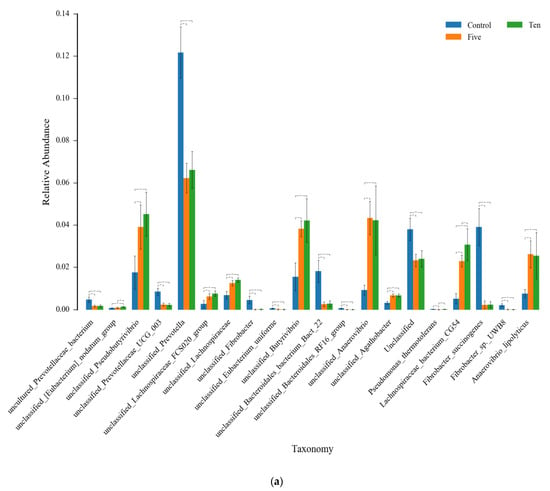
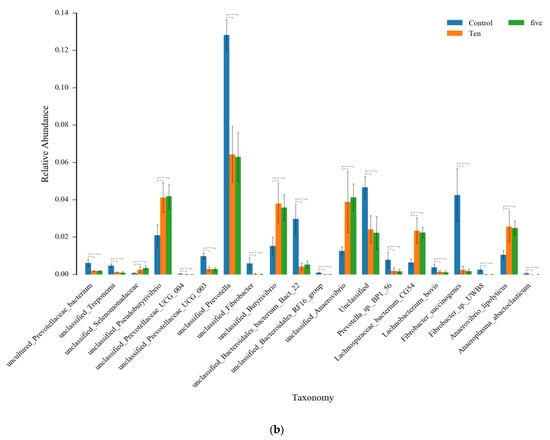
Figure 6.
The ANOVA analysis for the top 20 species in abundance among FO levels under high- (a) and low- (b) forage ratio. * means that the difference is significant (p < 0.05), while ** means the difference is very significant (p < 0.01).
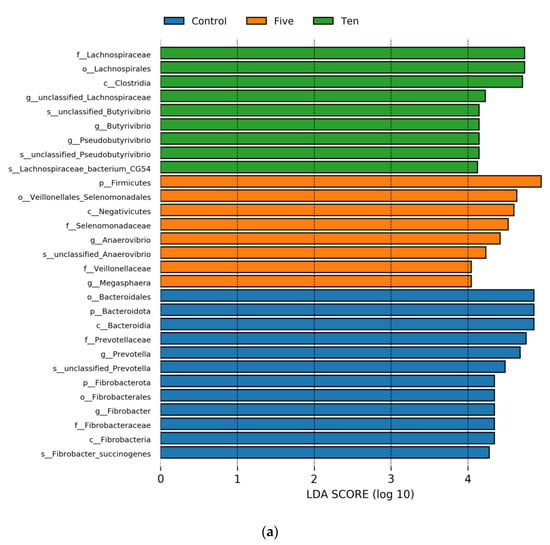
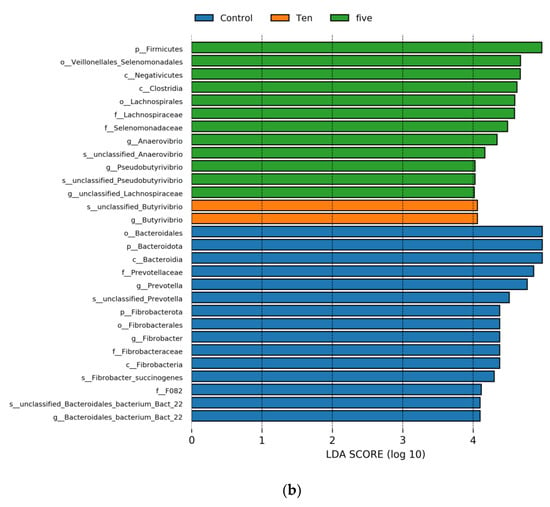
Figure 7.
Shows LDA score exceeding the set value (the default is 4.0). Different colors represent species in different FO groups under high-(a) and low-(b) forage ratios.
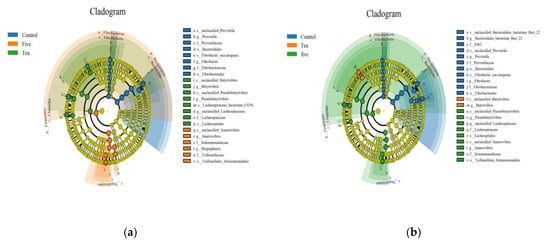
Figure 8.
Cladograms represent the taxonomic level from phylum to species in different FO levels under high-(a) and low-(b) forage ratios. The dots on the circles represents a term on the corresponding taxonomic level. Other colors stand for different groups. Species with specific colors mean this species’ abundance is the highest in the corresponding group, which visualizes each group’s most abundant microbial communities.
3.6. Predictive Functional Profiling Based on the KEGG Pathway Database
Under a high-forage ratio, these genes’ KEGG pathways were significantly (p < 0.05) downregulated by FO supplementation; they were mainly related to membrane transport, cell motility, signal transduction, environmental adaptation, transcription, and xenobiotic biodegradation, and the metabolism was downregulated in 5 mL/L group (Figure 9a).
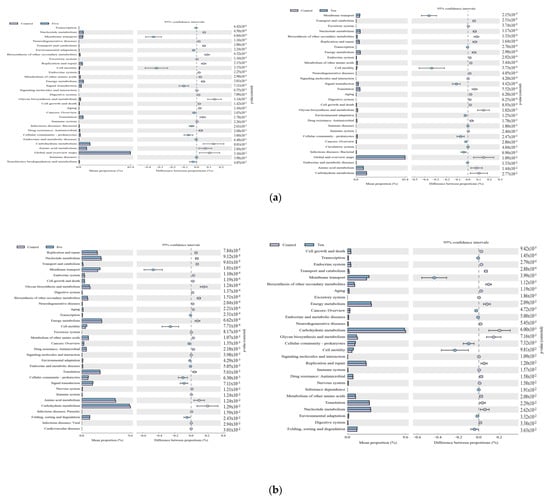
Figure 9.
Comparison of the distribution of the abundance of (KEGG) pathways of the bacterial community in different FO levels under high- (a) and low- (b) forage ratios. Bars on the left indicate the abundance ratio for each function in the two sets of samples; the graph on the right shows the percentage of difference in functional abundance within the 95% confidence interval; and p values are displayed on the far right of each panel. Only the significantly affected KEGG pathways by dietary treatments are shown.
Under low-forage ratios, there were fewer pathways downregulated, and the significant (p < 0.05) functions downregulated by FO supplementation were mainly related to folding, sorting and degradation, environmental adaptation, cell motility, transcription, membrane transport, and signal transduction.
3.7. Predictive Functional Profiling Based on the COGs Database
As shown in Figure 10, the control group under a high-forage ratio (70% forage:30% concentrate) showed significantly higher genes for functions, lipid transport and metabolism, nucleotide transport and metabolism, secondary metabolites biosynthesis transport and metabolism, cell membrane envelop biogenesis, translation ribosomal structure and biogenesis, posttranslational modification, protein turnover, and chaperones. However, under a low-forage ratio (30% forage:70% concentrate), more functions were higher in FO groups; the genes were for defense mechanisms and general function prediction.
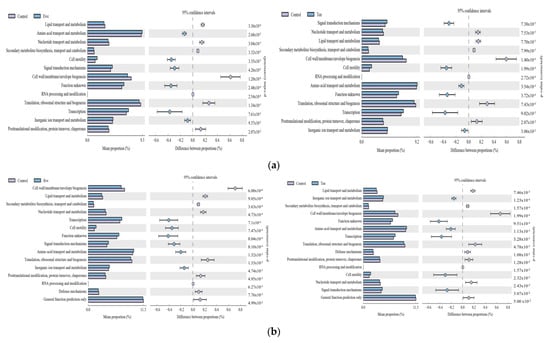
Figure 10.
Comparison of the distribution of the abundance of (COG) pathways of the bacterial community in different FO levels under high-(a) and low-(b) forage ratios. Bars on the left indicate the abundance ratio for each function in the two sets of samples; the graph on the right shows the percentage of difference in functional abundance within the 95% confidence interval; and p values are displayed on the far right of each panel. Only the significantly affected COG pathways by di-etary treatments are shown.
In contrast, supplementing FO under different forage ratios significantly downregulated the genes responsible for signal transduction mechanism, cell motility, amino acid transport and metabolism, transcription, inorganic ion transport, and metabolism.
4. Discussion
Diet interaction with the microbial community, especially bacteria, is a significant factor in modulating the ruminal fermentation pattern, affecting ruminants’ nutrient digestion and metabolism. In the present study, although pH values were not affected by FO supplementation under any forage ratio, the fatty acids profile significantly changed. Under the high-forage ratio, FO supplementation could depress the SCFA yield and acetic and propionate acids among the levels, which also may depress methane production. The present results may be attributed to the depression in the abundance of Saccharofermentans, Prevotella, and Rikenellaceae RC9 gut group at the genus level, whereas Prevotella is central to carbohydrate and hydrogen metabolism. The Prevotella spp. high abundance in the rumen is associated with a healthy microbiome [1] and is one of the significant bacterial members responsible for ruminal fermentation, especially acetic and propionic acid production.
Moreover, the current results showed a link between the drop in TVFA production and the depression in the Prevotella abundance as a response to FO supplementation. These results are supported by Carreno et al. (2019), who reported the detrimental effect of marine lipids and omega-3 fatty acids (EPA, DHA, DPA) on the Prevotella abundance in vitro [29]. In addition, the depression in TVFA was supported by Toral et al. (2017), who discussed the omega-3 fatty acids’ deteriorating effect on ruminal fermentation and fatty acids biohydrogenation [30].
In the present study, under high-forage diet, the significant drop in VFA production after FO supplementing may be explained by the declined ability of ruminal saccharide metabolism, which is a vital VFA-producing step that affect the energy-yielding mechanism in the rumen. In addition, from KEGG pathways results, it was found that genes responsible for carbohydrate metabolism were significantly higher in the control group compared to the FO-supplemented groups, which seems to support the idea that FO supplementation depressed carbohydrate metabolism and, in turn, decreased the bacterial fermentation activity.
A previous study [3] reported a significant link between TVFA production and Saccharofermentans as a central modulator in shaping rumen fermentation activity. The Saccharofermentans belong to the Bacteroidetes phylum, which contains genes responsible for encoding glycosyl hydrolases [31,32]; it also ferments hexoses, polysaccharides, alcohols, sucrose, and aesculin to produce acetate, lactate, and fumarate [33]. These previous findings may explain the effect of FO on depressing the saccharofermentans abundance in the present study and its results in decreasing TVFA production.
Another previous report mentioned that Butyrivibrio fibrisolvens, which produces butyrate via a butyryl CoA transferase, is less sensitive to PUFA than B. hungatei and B. proteoclasticus, which produce butyrate using a butyrate kinase [34]. It may be because B. fibrisolvens cannot metabolize EPA and DHA [17] as opposed to B. proteoclasticus [35].
Regarding the low-forage condition (high-concentrate diet), contrary to the high-forage diet, FO groups showed higher fatty acid production without decreasing pH, similar to Kairenius et al.’s findings [36]. Similarly, in cows fed high-starch, low-fiber diets, reports found that fatty acid formation and FO amount increases were not associated with ruminal pH decrease [37]. In addition, FO groups were characterized by the higher presence of streptococcus and Megasphera genus, especially unclassified streptococcus and Megasphaera elsdenii species, compared to the control (Figure 4 and Figure 5). Megasphaera elsdenii is an important ruminal pH mediator responsible for converting around 60% to 80% of lactate to a volatile fatty acid with less acidity [38], with the ability to prevent ruminal acidosis in elevated dietary step-up regimes [39,40]. The previous results also mentioned the Megasphaera elsdenii buffer’s ability to stabilize pH under high-concentrate low-forage diets avoiding ruminal acidosis [37].
Another explanation for the present study’s result maybe the relation between fibrolytic and non-fibrolytic species [41], which is crucial for ruminal fiber degradation because non-fibrolytic bacteria can activate fibrolytic bacteria through cross-feeding interaction [6]. For example, Prevotella ruminicola has been reported to synergize with fibrolytic bacteria to improve fiber digestion [7]. One of the main synergism pathways is the interspecies hydrogen transfer and removal and/or metabolite exchange [6], which also can explain the pH results. Previous studies reported that feeding lactating cows diets containing FO and omega-3 and omega-6 sources could increase cis-9 and trans-11 CLA concentrations in milk [42,43], but enrichment varies depending on the basal diet composition and the amount and lipid supplement source. Furthermore, dietary supplements of oils containing omega-3 lower the proportions of 12:0, 14:0, and 16:0 fatty acids in milk fat [7]. Another in vivo experiment reported that dietary FO supplementation increases increasingly depress the rumen’s unsaturated fatty acids biohydrogenation, shifting the specific 16- to 22-carbon fatty acids profile at the omasum and inducing vital bacterial population changes capable of biohydrogenation in lactating cows [37].
In the present study, it is assumable that FO supplementation in high-forage conditions may disturb the serial action between the fibrolytic and non-fibrolytic bacteria through the FO toxic action on some ruminal bacterial species, considering that the toxic effect pressure differently from one species to another. It was also found that FO adding during the transition from a high-forage to a high-concentrate diet may result in beneficial impacts on the ruminal fermentation pattern and the fatty acids profile.
This explanation is supported by Maia et al., who concluded that PUFA toxicity in butyrate producers is probably mediated via a metabolic effect involving butyrate production [44]. Beyond butyrate metabolism, other acyl-CoA and ATP pools are affected by exposure to PUFA, suggesting a metabolic interruption [45]. The dietary factor is essential in explaining omega-3 fatty acids’ toxic effect on the microbial community, considering that the toxic effect varies from one species to another beside the fiber to starch ratio may be the central modulator for this deteriorating action.
Fiber digestion is a leading player in changing the ruminal fermentation pattern, which is affected mainly by the dynamic relationship between microbial species, especially fibrolytic and non-fibrolytic bacteria. However, oil supplementation’s effect on microbial abundance among different species must not be studied in a snapshot manner; it must be more dynamic regarding the species fluctuation under different diets.
Recently, interest has been revived because ruminal fermentation’s implications link to subsequent biohydrogenation of PUFA. Further in vivo studies using functional metagenomics approaches are needed to provide greater insight into the diet-induced changes in bacterial biohydrogenation within the rumen. However, such further investigations must consider the dynamics of the microbial community and its interaction with the host feeding strategy.
5. Conclusions
Fish oil is an essential source of PUFA fatty acids, which is significant for many physiological cellular functions; however, the gastrointestinal interaction with the ruminal microbiota community is critical for understanding the different effects of these fatty acids. The dietary factor is essential in explaining omega-3 fatty acids’ toxic effect on the microbial community, considering that the toxic effect varies among different species. This study may revive the potential implications of ruminal microbial alteration during different dietary compositions through PUFA and its effect on crucial fatty acid biohydrogenation. This knowledge can be applied in developing efficient strategies to enhance the valuable FA content of ruminant products such as meat and milk.
Author Contributions
M.A. and Z.A. designed and conducted the experiment. M.A.,W.W. and Z.A. experimented and collected the data. W.W., A.E.A., A.A., Z.A., H.L. and G.H. analyzed the data, and M.A., Z.A. and L.Y. wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (No. 2022YFD1301001), and Modern Agro-industry Technology Research System (CARS-36).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Huang, G.; Zhang, Y.; Xu, Q.; Zheng, N.; Zhao, S.; Liu, K.; Qu, X.; Yu, J.; Wang, J. DHA content in milk and biohydrogenation pathway in rumen: A review. PeerJ 2020, 8, e10230. [Google Scholar] [CrossRef]
- Lourenço, M.; Ramos-Morales, E.; Wallace, R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef]
- Loor, J.; Hoover, W.; Miller-Webster, T.; Herbein, J.; Polan, C. Biohydrogenation of unsaturated fatty acids in continuous culture fermenters during digestion of orchardgrass or red clover with three levels of ground corn supplementation. J. Anim. Sci. 2003, 81, 1611–1627. [Google Scholar] [CrossRef]
- Smith, P.; Clark, H.; Dong, H.; Elsiddig, E.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; Masera, O.; Mbow, C. Agriculture, forestry and other land use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Lee, S.S.; Lee, S.S. Recent insight and future techniques to enhance rumen fermentation in dairy goats. Asian-Australas. J. Anim. Sci. 2019, 32, 1321. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Myer, P.R.; Smith, T.P.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen microbiome from steers differing in feed efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef]
- Nagaraja, T.; Titgemeyer, E. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef]
- Boerman, J.; Lock, A. Effect of unsaturated fatty acids and triglycerides from soybeans on milk fat synthesis and biohydrogenation intermediates in dairy cattle. J. Dairy Sci. 2014, 97, 7031–7042. [Google Scholar] [CrossRef]
- Shingfield, K.; Ahvenjärvi, S.; Toivonen, V.; Ärölä, A.; Nurmela, K.; Huhtanen, P.; Griinari, J.M. Effect of dietary fish oil on biohydrogenation of fatty acids and milk fatty acid content in cows. Anim. Sci. 2003, 77, 165–179. [Google Scholar] [CrossRef]
- Pirondini, M.; Colombini, S.; Mele, M.; Malagutti, L.; Rapetti, L.; Galassi, G.; Crovetto, G. Effect of dietary starch concentration and fish oil supplementation on milk yield and composition, diet digestibility, and methane emissions in lactating dairy cows. J. Dairy Sci. 2015, 98, 357–372. [Google Scholar] [CrossRef]
- Donovan, D.C.; Schingoethe, D.J.; Baer, R.J.; Ryali, J.; Hippen, A.R.; Franklin, S.T. Influence of Dietary Fish Oil on Conjugated Linoleic Acid and Other Fatty Acids in Milk Fat from Lactating Dairy Cows1. J. Dairy Sci. 2000, 83, 2620–2628. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; Snelling, T.J.; López-Ferreras, L.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Effect of sunflower and marine oils on ruminal microbiota, in vitro fermentation and digesta fatty acid profile. Front. Microbiol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- AbuGhazaleh, A.; Ishlak, A. Effects of incremental amounts of fish oil on trans fatty acids and b utyrivibrio bacteria in continuous culture fermenters. J. Anim. Physiol. Anim. Nutr. 2014, 98, 271–278. [Google Scholar] [CrossRef]
- Harfoot, C.; Hazlewood, G.; Hobson, P.; Stewart, C.J.e.H.P.; Stewart CS, C.; Hall, L. The Rumen Microbial Ecosystem; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; pp. 382–426. [Google Scholar]
- Maia, M.R.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Yuan, Z.; Wu, Y.; Wang, J.; Liu, J.; Zhu, W.J. Effect of octadeca carbon fatty acids on microbial fermentation, methanogenesis and microbial flora in vitro. Anim. Feed Sci. Technol. 2008, 146, 259–269. [Google Scholar] [CrossRef]
- Huws, S.A.; Kim, E.J.; Lee, M.R.; Scott, M.B.; Tweed, J.K.; Pinloche, E.; Wallace, R.J.; Scollan, N.D. As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ. Microbiol. 2011, 13, 1500–1512. [Google Scholar] [CrossRef]
- Toral, P.G.; Belenguer, A.; Shingfield, K.J.; Hervás, G.; Toivonen, V.; Frutos, P. Fatty acid composition and bacterial community changes in the rumen fluid of lactating sheep fed sunflower oil plus incremental levels of marine algae. J. Dairy Sci. 2012, 95, 794–806. [Google Scholar] [CrossRef]
- An, Z.; Luo, G.; Abdelrahman, M.; Riaz, U.; Gao, S.; Yao, Z.; Ye, T.; Lv, H.; Zhao, J.; Chen, C.; et al. Effects of capsicum oleoresin supplementation on rumen fermentation and microbial abundance under different temperature and dietary conditions in vitro. Front. Microbiol 2022, 13, 1005818. [Google Scholar] [CrossRef]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. In Annales de Zootechnie; EDP Sciences: Les Ulis, France, 2000; pp. 231–253. [Google Scholar]
- Colombini, S.; Graziosi, A.R.; Parma, P.; Iriti, M.; Vitalini, S.; Sarnataro, C.; Spanghero, M. Evaluation of dietary addition of 2 essential oils from Achillea moschata, or their components (bornyl acetate, camphor, and eucalyptol) on in vitro ruminal fermentation and microbial community composition. Anim. Nutr. 2021, 7, 224–231. [Google Scholar] [CrossRef]
- Li, A.; Liu, B.; Li, F.; He, Y.; Wang, L.; Fakhar, E.A.K.M.; Li, H.; Fu, Y.; Zhu, H.; Wang, Y.; et al. Integrated Bacterial and Fungal Diversity Analysis Reveals the Gut Microbial Alterations in Diarrheic Giraffes. Front. Microbiol. 2021, 12, 712092. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef]
- Carreño, D.; Toral, P.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.; Hervás, G.; McEwan, N.; Newbold, C.; Frutos, P. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Sci. Rep. 2019, 9, 11857. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Carreño, D.; Leskinen, H.; Belenguer, A.; Shingfield, K.J.; Frutos, P. In vitro response to EPA, DPA, and DHA: Comparison of effects on ruminal fermentation and biohydrogenation of 18-carbon fatty acids in cows and ewes. J. Dairy Sci. 2017, 100, 6187–6198. [Google Scholar] [CrossRef]
- Tomazetto, G.; Hahnke, S.; Wibberg, D.; Pühler, A.; Klocke, M.; Schlüter, A. Proteiniphilum saccharofermentans str. M3/6T isolated from a laboratory biogas reactor is versatile in polysaccharide and oligopeptide utilization as deduced from genome-based metabolic reconstructions. Biotechnol. Rep. 2018, 18, e00254. [Google Scholar] [CrossRef]
- Hao, Y.; Gong, Y.; Huang, S.; Ji, S.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Effects of Age, Diet CP, NDF, EE, and Starch on the Rumen Bacteria Community and Function in Dairy Cattle. Microorganisms 2021, 9, 1788. [Google Scholar] [CrossRef]
- Chen, S.; Niu, L.; Zhang, Y. Saccharofermentans acetigenes gen. nov., sp. nov., an anaerobic bacterium isolated from sludge treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 2010, 60, 2735–2738. [Google Scholar] [CrossRef]
- Paillard, D.; McKain, N.; Chaudhary, L.C.; Walker, N.D.; Pizette, F.; Koppova, I.; McEwan, N.R.; Kopečný, J.; Vercoe, P.E.; Louis, P.; et al. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie Van Leeuwenhoek 2007, 91, 417–422. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Escobar, M.; Wallace, R.J.; Fievez, V.; Vlaeminck, B. Biohydrogenation of 22:6n-3 by Butyrivibrio proteoclasticus P18. BMC Microbiol. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Kairenius, P.; Leskinen, H.; Toivonen, V.; Muetzel, S.; Ahvenjärvi, S.; Vanhatalo, A.; Huhtanen, P.; Wallace, R.; Shingfield, K.J. Effect of dietary fish oil supplements alone or in combination with sunflower and linseed oil on ruminal lipid metabolism and bacterial populations in lactating cows. J. Dairy Sci. 2018, 101, 3021–3035. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Kairenius, P.; Ärölä, A.; Paillard, D.; Muetzel, S.; Ahvenjärvi, S.; Vanhatalo, A.; Huhtanen, P.; Toivonen, V.; Griinari, J.M. Dietary fish oil supplements modify ruminal biohydrogenation, alter the flow of fatty acids at the omasum, and induce changes in the ruminal Butyrivibrio population in lactating cows. J. Nutr. 2012, 142, 1437–1448. [Google Scholar] [CrossRef]
- Counotte, G.; Prins, R.; Janssen, R.; Debie, M.J.A. Role of Megasphaera elsdenii in the fermentation of DL-[2-13C] lactate in the rumen of dairy cattle. Appl. Environ. Microbiol. 1981, 42, 649–655. [Google Scholar] [CrossRef]
- Henning, P.; Horn, C.; Steyn, D.; Meissner, H.; Hagg, F.M. The potential of Megasphaera elsdenii isolates to control ruminal acidosis. Anim. Feed. Sci. Technol. 2010, 157, 13–19. [Google Scholar] [CrossRef]
- Muya, M.; Nherera, F.; Miller, K.; Aperce, C.; Moshidi, P.; Erasmus, L.J. Effect of Megasphaera elsdenii NCIMB 41125 dosing on rumen development, volatile fatty acid production and blood β-hydroxybutyrate in neonatal dairy calves. J. Anim. Physiol. Anim. Nutr. 2015, 99, 913–918. [Google Scholar] [CrossRef]
- Hua, D.; Hendriks, W.H.; Xiong, B.; Pellikaan, W.F. Starch and Cellulose Degradation in the Rumen and Applications of Metagenomics on Ruminal Microorganisms. Animals 2022, 12, 3020. [Google Scholar] [CrossRef]
- Whitlock, L.; Schingoethe, D.; Hippen, A.; Kalscheur, K.; Baer, R.; Ramaswamy, N.; Kasperson, K.J. Fish oil and extruded soybeans fed in combination increase conjugated linoleic acids in milk of dairy cows more than when fed separately. J. Dairy Sci. 2002, 85, 234–243. [Google Scholar] [CrossRef]
- Palmquist, D.; Griinari, J. Milk fatty acid composition in response to reciprocal combinations of sunflower and fish oils in the diet. Anim. Feed. Sci. Technol. 2006, 131, 358–369. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef]
- Enjalbert, F.; Combes, S.; Zened, A.; Meynadier, A. Rumen microbiota and dietary fat: A mutual shaping. J. Appl. Microbiol. 2017, 123, 782–797. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).