Abstract
The purpose of the current work is to produce xylanase from certain agro-industrial wastes in an efficient and effective manner. The culture conditions for three strains of Aspergillus fumigatus are optimized in submerged fermentation (SmF). The most prolific strain (A. fumigatus KSA-2) produces the maximum xylanase at pH 9.0, 30 °C, after 7 days using yeast extract as a nitrogen supply. Aspergillus fumigatus KSA-2 is utilized to produce xylanase at optimum conditions from several agro-industrial wastes. Wheat bran is found to be the most fermentable material, yielding 66.0 U per gram dry substrate (U/gds). The generated xylanase is partly purified using 70% ammonium sulphate, yielding 40 g of dry enzyme powder from 400 g wheat bran. At pH 6.0 and 45 °C, the synthesized xylanase displayed its maximum activity (20.52 ± 1.714 U/mg). In the current study, the effect of ions and inhibitors on xylanase activity is investigated. Both Cu2+ and Mn2+ ions boost the specific activity over the control by 10.2% and 128.0%, respectively. The xylanase enzyme generated has a maximum activity of 4.311 ± 0.36 U/mL/min and the greatest specific activity of 20.53 ± 1.714 U/mg for birchwood xylan, showing a strong affinity for this substrate as opposed to the other xylan and non-xylan substrates.
1. Introduction
The development of a sustainable eco-friendly economy has been made necessary by the rise in fuel prices, global warming, and the need for alternative energy sources. For this purpose, the use of lignocellulosic biomass for the production of value-added components is becoming increasingly important [1]. Cellulose and hemicellulose make up a significant portion of lignocellulosic biomass and may be optimally hydrolyzed to monosaccharides and successfully transformed into a variety of value-added products [2]. Low-cost enzymes are necessary for biotechnological applications to succeed. Therefore, it is not practical to employ pure xylan as an inducer for increased xylanase synthesis. Utilizing lignocellulosic residues, which not only provide for affordable substrates but also have positive environmental effects, is one of the new developments in this field. Use of agro-residues for microbial enzyme synthesis has advanced significantly over the past few decades [3,4,5,6].
Xylanase hydrolytic enzyme releases xylose from lignocellulolytic substrates by rupturing the β-1, 4-glycosidic bond of xylan. Compared to alternative techniques for breaking down xylan, utilizing the enzyme xylanase to convert xylan to xylose has the benefit of not producing any hazardous compounds [7,8,9]. Xylanases are widely distributed in nature and are secreted by a wide range of different species. More xylanases and auxiliary enzymes are released by fungi than by other microorganisms, which are necessary for the breakdown of substituted xylan. The primary hemicellulosic part of xylan, which has a 1,4-glycosidic backbone, is broken down by xylanase, often releasing xylose and various xylooligosaccharides (XOS) as end products [2,7,9].
In recent years, microbial xylanases have shown tremendous biotechnological promise in a number of fields outside of bioenergy production, including food for increasing dough elasticity, animal husbandry for increasing chick weight, clarifying juices for degumming fibers, bread making, pre-bleaching of Kraft pulp, deinking of used newspapers, and winemaking [10]. Filamentous fungi are considered to be good providers of hydrolytic enzymes for the breakdown of holocellulose because they readily adapt to growing on solid substrates by simulating their native environment with lignocelluloses [9]. Fungi have several advantages in producing a diverse array of enzymes, including amylases, cellulases, proteases, lipases, and xylanases [5]. Moreover, fungi can produce enzymes in large quantities, making them ideal for industrial-scale production. They have rapid growth rates, efficient nutrient utilization, and can be cultivated under optimized conditions to maximize enzyme production, resulting in higher yields compared to other organisms. In addition, fungi are cost-effective because they can utilize a variety of inexpensive carbon sources, including agricultural residues, waste materials, and lignocellulosic biomass as substrates for enzyme production. Fungal enzyme production is often considered environmentally friendly and sustainable. Fungi can efficiently degrade organic matter, making use of waste materials and contributing to the circular economy.
One of the most studied fungal genera to be utilized in industrial enzyme production is Aspergillus. The potential of several species of Aspergillus has been recognized. One such species is A. fumigatus. However, it is also known that different strains of fungi differ in their efficiency to produce enzymes, and the most efficient strains need to be found for large-scale industrial purposes.
The aim of this work is to find an efficient strain of A. fumigatus to produce xylanases and optimize their culturing conditions. First, three different strains of A. fumigatus are tested in submerged fermentation (SmF). Second, the most efficient A. fumigatus strain is chosen to study five different agro-industrial wastes as the substrates to produce xylanases. The optimal culturing conditions of solid-state fermentation (SSF), as well as the inhibitors in enzyme production, are tested. The most active xylanase is partially purified and characterized.
2. Materials and Methods
2.1. Fungal Strains and Their Xylanolytic Activity
Utilizing the dilution plate technique [11] on Czapek’s Dox agar (Sigma-Aldrich) [12] at 25 °C, the three strains of Aspergillus fumigatus used in this investigation were isolated from soil samples collected from Rafha, the Northern Border, Saudi Arabia. On sucrose-free Cz agar supplemented with 1% oat spelt xylan, they were tested for their xylanolytic activities. They were chosen for xylanase production since it was found that they were potent xylanase-producing fungi.
2.2. Molecular Identification of the Aspergillus Isolates
The DNA of the three isolates of Aspergillus was isolated [13], and a PCR reaction was performed using SolGent EF-Taq [14]. The universal primers ITS1 and ITS4 were used for ITS region amplification [15]. Using the DNASTAR computer package (version 5.05), contiguous sequences of the Aspergillus isolates included in this study were produced. An outgroup sequence for Aspergillus versicolor ATCC 9577, 3 sequences for Aspergillus spp. (KSA-1, KSA-2, and KSA-3), and 18 sequences from the genus Aspergillus: section Fumigati retrieved from GenBank made up the 22 sequences in the total ITS dataset included for phylogenetic analysis. In this investigation, all sequences were aligned together using MAFFT (version 6.861b) with the default options [16]. Alignment gaps and parsimony uninformative characters were optimized using BMGE [17]. MEGA X (version 10.2.6) was used to conduct maximum-likelihood (ML) and maximum-parsimony (MP) phylogenetic analyses [18], and the robustness of the most parsimonious trees was evaluated by 1000 replications [19]. Utilizing Modeltest 3.7’s Akaike Information Criterion (AIC), the optimum nucleotide substitution model for ML analysis was identified [20]. The resulting tree was edited and saved in TIF format [21].
2.3. Optimization of Xylanase Production in Submerged Fermentation (SmF)
To maximize the output of xylanases, the respective pH, temperature, nitrogen supply, and fermentation duration of the three stains were varied under one factor at a time (OFAT) conditions. The experiments were carried out in 250 mL Erlenmeyer flasks with 50 mL of sucrose-free Czapek’s broth as the fermentation medium. A total of 1% oat spelt xylan was added as the sole carbon source. Aspergillus fumigatus strains that were 7 days old were used to produce spore suspension (2.0%; v/v), which was used to individually inoculate the flasks. The flasks were then incubated for 1 to 7 days under various operating conditions, including pH (3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0), nitrogen source (peptone, yeast extract, sodium nitrate, sodium nitrite, and ammonium chloride; each at 0.2%), temperature (25, 30, 35, 40, and 45 °C), and incubation duration (1–10) days. For pH adjustment, citrate buffer (pH 3.0–6.0), phosphate buffer (pH 7.0–8.0), and glycine/NaOH buffer (pH 9.0–11.0) were the buffers employed. Three different experiments were conducted.
2.4. Xylanase Production by A. fumigatus KSA-2 from Lignocellulosic Biomass in Solid-State Fermentation (SSF)
The five agro-industrial residues that were selected for enzyme processing in SSF were bean straw (BS), corn cobs (CC), rice husk (RH), wheat bran (WB), and wheat straw (WS). All of these components were acquired from Assiut Governorate’s markets in Egypt. They were cleaned with distilled water, dried in an oven at 50 °C to a constant weight, then ground into 0.2 mm diameter pieces. Separate Erlenmeyer flasks (500 mL) were filled with 10 g of each of the examined agro-industrial residues. Flasks separately received 10 mL of sucrose-free Czapek’s broth amended with 0.1% oat spelt xylan as a precursor of xylanase production. Using distilled water, the biomass was then further saturated to a moisture level of 80%. Each flask was inoculated with 5.0 mL of a spore suspension containing 1 × 108 spores/mL and obtained from 7-day-old cultures of A. fumigatus KSA-2. The pH, nitrogen source, temperature, and fermentation time were adjusted to pH 9.0, yeast extract, 30 °C, and 7 days, respectively.
2.5. Xylanase Assay and Protein Determination
The xylanase assay was performed in accordance with Al-Kolaibe et al. [5]. Xylanase activity was determined by mixing 0.5 mL filtered crude enzyme with 0.5 mL of 1% oat spelt xylan (prepared in 50 mM Na-citrate buffer, pH 5.0). The reaction mixture was incubated at 50 °C for 15 min and the process was stopped by applying 2 mL of 3,5-dinitrosalicylic acid (DNS) and boiling in a water bath for 10 min [22]. After cooling, the color absorbance was measured at 540 nm using UV-Visible spectrophotometer (T80+, Manchester, UK). The amount of reducing sugar liberated was quantified using the standard curve of xylose. One unit of xylanase enzyme is defined as the amount of enzyme that liberates 1 µmol of the xylose equivalent per mL under the standard assay conditions. Total protein content was measured using the method suggested by Lowry et al. [23]. All experiments were conducted in triplicate.
2.6. Procedures for the Extraction and Purification of Xylanase from A. fumigatus KSA-2
The separately removed contents from each flask were filtered using two layers of cheesecloth in 100 mL of 100 mM glycine/NaOH buffer (pH 9.0) [6]. As mentioned above, the enzyme assay and protein content determination were conducted on the cell-free supernatants, which were collected using centrifugation (10,000 rpm for 10 min at 4 °C). Ammonium sulphate precipitation and dialysis was carried out, and a cell-free supernatant was obtained after the incubation period by centrifuging at 10,000 rpm for 10 min. Using ammonium sulphate to achieve a 70% saturated solution, the total protein was extracted at 4 °C. The precipitated protein was separated and lyophilized using a freeze dryer.
2.7. Production of Xylanase from Wheat Bran by A. fumigatus KSA-2 in SSF
The optimum parameters (pH 9.0, yeast extract, at 30 °C for 7 days) for the potent strain A. fumigatus KSA-2 were employed for xylanase production in SSF from wheat bran. The experiment was conducted in 1.8 L-capacity Fernbach flasks, each containing 100 g of wheat bran. The substrate was separately moistened with 100 mL sucrose-free Cz broth supplemented with 0.1% oat spelt xylan. Moisture content was adjusted to 80% by adding buffer solution (pH 9.0) to each flask. After autoclaving, the flasks were individually inoculated with a 10 mL spore suspension containing 1 × 108 spore/mL which was obtained from a 7-day-old culture. After the fermentation time, the enzyme was extracted, and xylanase activity was determined as mentioned above.
2.8. Impact of pH, Temperature, Ions, and Inhibitors on the Xylanase Activity
The impact of pH (3.0–11.0) at 30–70 °C on pure xylanase activity was investigated. The reaction mixture contained 0.01 g enzyme powder and 0.01 g oat spelt xylan (each dissolved in 1.0 mL of 50 mM buffer solution). After the reaction time (20 min), the reaction was terminated by introducing 2.0 mL of 3,5-dinitrosalicylic acid (DNS) [22], and the xylanase activity was determined as previously described. In addition, ions such as Na+, K+, Ca+2, Mg+2, Mn+2, Zn+2, Fe+2, Cu+2, Co+2, and Ni+2 were tested by adding them to the reaction mixture at 5 mM/mL concentrations as NaCl, KCl, CaCl2, MgSO4, MnSO4, ZnSO4, FeSO4, CuSO4, CoCl2, and NiSO4. In order to test an enzyme inhibitor, 5 mM/mL ethylenediaminetetraacetic acid (EDTA) and sodium dodecyl sulphate (SDS) were also utilized. Under typical circumstances, the xylanase’s activity without the presence of metal ions, EDTA, or SDS was assessed to determine the residual activity. The experiment was carried out three times.
2.9. Substrate Specificity
Utilizing 1% (w/v) polymeric substrates generated from both xylan (birchwood xylan, oat spelt xylan, corn cob xylan, wheat bran xylan, and corn stalk xylan) [5], and non-xylan sources (carboxy methyl cellulose and microcrystalline cellulose), the substrate specificity of the xylanase was investigated [24]. The reaction mixture contained 0.01 g enzyme powder and 0.01 g oat spelt xylan (each dissolved in 1.0 mL of 50 mM buffer solution, pH 6.0). Activities were measured at 45 °C for 20 min, and the amount of reducing sugars liberated was measured at 540 nm using the DNS method [22].
2.10. Statistical Analysis
Data were subjected to analysis of variance (ANOVA: one-factor with replication) followed by Duncan’s multiple range test [25].
3. Results
3.1. Strain’s Isolation and Identification
Three isolates related to Aspergillus fumigatus group were isolated from soil samples collected from Rafha, the Northern border, Saudi Arabia. The fungal strains shared the identical morphological characteristics of A. fumigatus as fast-growing and greyed-green–colonies, short conidiophores, flask-shaped vesicles, and uniseriate conidial heads (Figure 1).
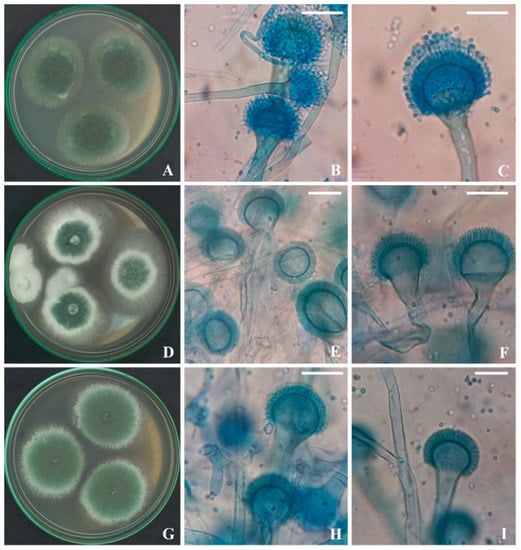
Figure 1.
Seven-day-old colonies on Cz at 25 °C, conidial heads and conidiophores of A. fumigatus KSA-1 (A–C), A. fumigatus KSA-2 (D–F), and A. fumigatus KSA-3 (G–I). (Scale bars = 20 µm).
3.2. Optimization of Xylanase Production Conditions
The three strains produced xylanase in different amounts when the pH was between 3 and 11. At pH 3.0, KSA-1 had its highest xylanase activity (32.53 ± 3.2 U/mL/min). At pH 9.0, KSA-2 had its highest activity (40.3 ± 3.92 U/mL/min), and at pH 7.0, KSA-3 had its highest activity (53.7 ± 4.1 U/mL/min) (Figure 2). Appreciable differences in xylanase activities were observed among different N sources. The most sensitive to N supply was KSA-2. KSA-2 had high activity (100 ± 6.71 U/mL/min) with yeast extract and low activities with Na-nitrate, Na-nitrite, and ammonium chloride (<10 U/mL/min) (Figure 3). The xylanase activity of the strain KSA-3 was relatively stable and independent of its N source. The xylanase activity of KSA-3 varied between ca 60 U/mL/min and 90 U/mL/min depending on the N source. The strain KSA-2 showed the greatest variation due to the culturing temperature. Xylanase activity was the highest (>100 U/mL/min) at 30 °C and ca 20 100 U/mL/min at both 40 °C and 45 °C. The xylanase activity of the KSA-3 strain decreased along with the increased temperature, with 25 °C being the optimum temperature. KSA-1 had its optimum temperature at 35 °C, but the temperature had only a slight effect on its activity (Figure 4). Regarding time, the three strains reached the maximum xylanase activity after 6 to 8 days of fermentation. The three strains KSA-1, KSA-2, and KSA-3 raised their xylanase output to 52.7 ± 6.2 on day 8, 114.6 ± 9.2 on day 7, and 102.4 ± 6.74 U/mL/min on day 6, respectively (Figure 5).
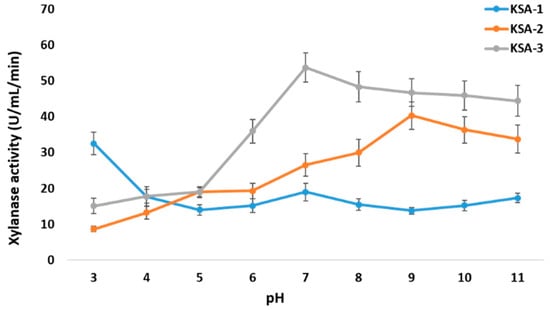
Figure 2.
Effect of medium pH on biosynthesis of xylanases by A. fumigatus strains at 30 °C.
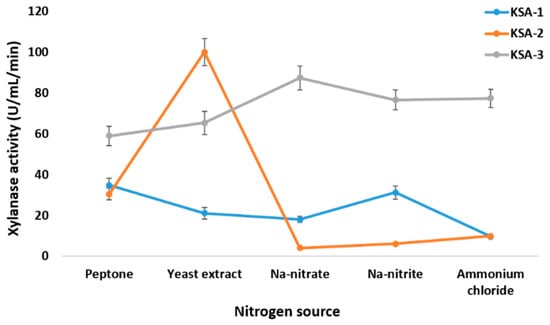
Figure 3.
Effect of nitrogen supply on production of xylanases by A. fumigatus strains at the optimum pH values.
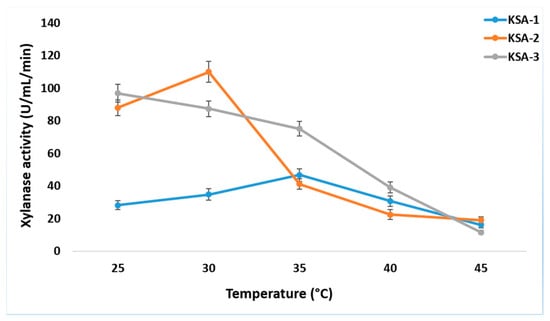
Figure 4.
Effect of culture temperature on production of xylanases by A. fumigatus strains at optimum pH and nitrogen source.
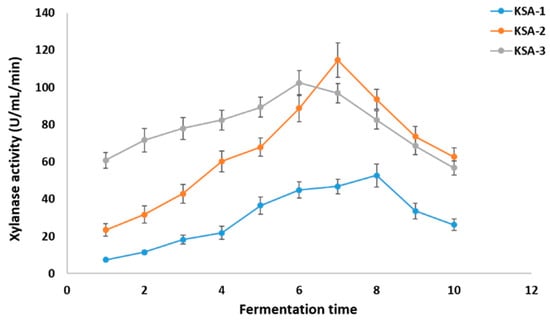
Figure 5.
Effect of fermentation time on the production of xylanases by A. fumigatus strains at optimal pH, temperature, and nitrogen source.
3.3. Molecular Identification of the Aspergillus Strains
Phylogenetic analysis based on ITS sequencing was employed to confirm the identification of the strains. The final ITS dataset contained 22 sequences which produced 640 characters, of which 518 characters could correctly aligned, 90 characters were counted as variable, and 19 as informative. The Tamura 3-parameter using a discrete Gamma distribution (T92 + G) was the perfect model to represent the relationship between taxa. Maximum parsimony method yielded two trees, the most parsimonious of which (Figure 6) had a tree length of 115, highest log likelihood of −1481.80, consistency index of 0.725000, retention index of 0.864198, and a composite index of 0.626543, as shown in Figure 6.
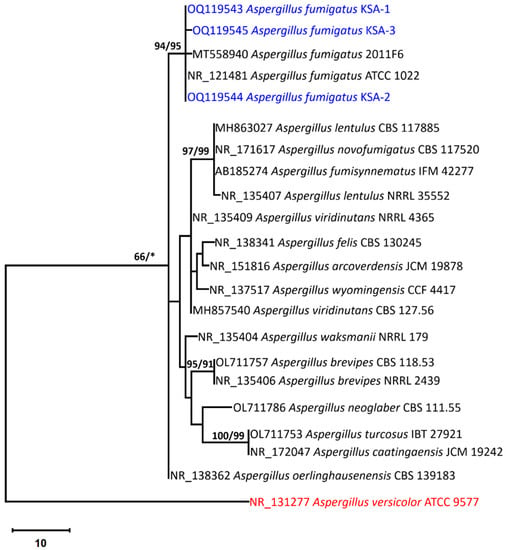
Figure 6.
The first of 1000 equally most parsimonious trees obtained from a heuristic search (1000 replications) of A. fumigatus strains KSA-1, KSA-2, and KSA-3 (in blue color) compared to other closely similar ITS sequences belonging to Aspergillus: Section Fumigati in GenBank. Bootstrap support values for ML/MP ≥ 50% are indicated near the respective nodes. The tree is rooted to Aspergillus versicolor ATCC 9577 as outgroup (in red color).
3.4. Screening Xylanolytic Activity of A. fumigatus KSA-2 on Lignocellulosic Wastes under SSF
Some lignocellulosic wastes were employed as substrates for the environmentally friendly and cost-effective production of xylanase. Although in varying amounts, A. fumigatus KSA-2 was able to ferment all the wastes utilized and create xylanase. The most advantageous substrate was wheat bran, which produced 66.0 ± 8.65 U/gds, while all other substrates produced relatively low amounts. The least productive substrate was maize cobs, producing 13.65 ± 2.1 U/gds (Figure 7).
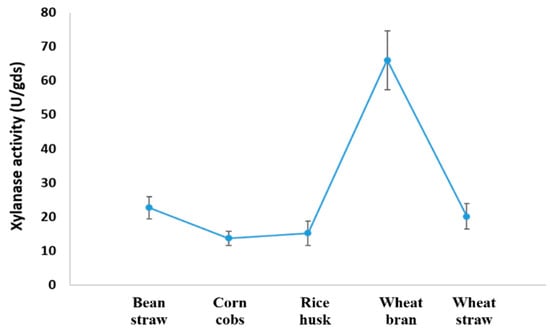
Figure 7.
Xylanase production by A. fumigatus KSA-2 from lignocellulosic residues in SSF.
3.5. Production of Xylanase by A. fumigatus KSA-2 from Wheat Bran in SSF
The optimal conditions (pH 9.0, yeast extract, at 30 °C after 7 days) were employed to produce xylanase from wheat bran in SSF using A. fumigatus KSA-2 as the powerful strain. From 400 g wheat bran, A. fumigatus KSA-2 was able to produce 40 g crude enzyme powder.
3.6. Effect of pH and Temperature on Xylanase Activity
At 50.0 °C, the effect of pH (3–11) on xylanase activity was assessed. The optimal pH for xylanase activity (15.714 ± 1.9 U/mg of enzyme powder) was 6.0. The enzyme’s activity was decreased at pH 7.0, 8.0, and 9.0 to 72.73%, 63.6%, and 45.44%, respectively (Figure 8). Xylanase activity was the highest (20.52 ± 1.714 U/mg) at 45 °C (Figure 9). Lower and higher temperatures produced remarkably lower activities varying between ca 5 and 15 U/mg.
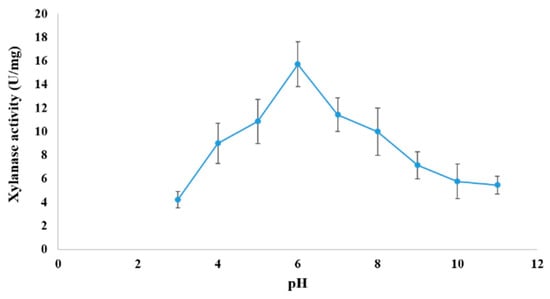
Figure 8.
Optimal pH of xylanase produced by A. fumigatus KSA-2.
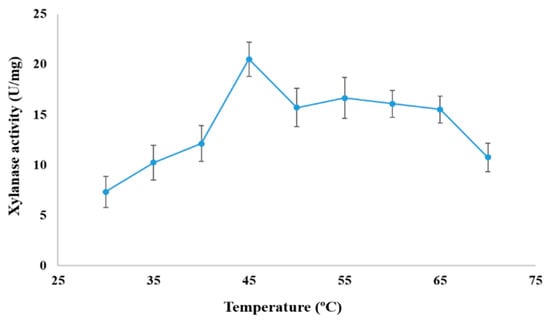
Figure 9.
Optimal temperature of xylanase produced by A. fumigatus KSA-2.
3.7. Substrate Specificity
The enzyme produced resulted in a maximum specific activity of 20.53 ± 1.714 U/mg for birchwood xylan, indicating a high affinity towards birchwood xylan over the remaining xylan and non-xylan substrates (Table 1). The other tested substrate types produced remarkably lower activity.

Table 1.
Substrate specificity of xylanase produced by A. fumigatus KSA-2 from wheat bran in SSF.
3.8. Effect of Ions and Inhibitors on Xylanase Activity
Under the optimal culture conditions of pH 6.0 and 45 °C, the tested ions and inhibitors had different impacts on the produced xylanase. Mn2+ ions had the greatest impact on the specific activity, elevating it to 128.0% (26.3 ± 1.5 U/mg) over the control. The specific activity was somewhat elevated by Cu2+, exhibiting a 10.2% increase (20.95 ± 1.7 U/mg). The specific activity was decreased by the other ions to varying degrees, ranging from 16.0% by Co2+ (17.24 ± 0.9 U/mg) to 63.3% by Na+ (7.52 ± 0.94 U/mg) (Table 2).

Table 2.
Effect of various ions and inhibitors on the activity of xylanase enzyme produced by A. fumigatus KSA-2 (The results are means of three replicates (±SD). Bars carrying different letters are significantly different at p < 0.05).
4. Discussion
Due to the high cost of producing enzymes, a low-cost growth medium is necessary for microbial growth and enzyme synthesis to fulfill industrial demand. Because they may be used in several processes and have the requisite properties to endure challenging circumstances during industrial processes, xylanases are required in massive amounts for applications at the industrial level. As a result, it is necessary to choose microorganisms with the right qualities to create large amounts of xylanases, and then optimize growth to manufacture more enzymes [26]. The production of xylanase is significantly influenced by the nutrient composition of the medium and the culture conditions. Physical and chemical parameters such as pH, temperature, incubation duration, and carbon and nitrogen sources and concentration have been demonstrated to influence xylanase production [27].
The three A. fumigatus strains employed in this investigation had their culture conditions—pH, nitrogen supply, temperature, and incubation period—adjusted for submerged fermentation. With the use of 70% ammonium sulphate, the xylanase produced was partially purified. The produced xylanase showed its greatest activity at pH 6.0 and 45 °C. The specific activity was increased by 10.2% and 128.0%, respectively, above the control, by the Cu2+ and Mn2+ ions. Comparing birchwood xylan to other xylan and non-xylan substrates, the xylanase enzyme that was produced showed the highest specific activity for this substrate.
Utilizing bean straw, corn cobs, rice husk, wheat bran, and wheat straw in solid-state conditions allowed us to use the most productive strain, A. fumigatus KSA-2, at the optimal parameters. Wheat bran, which generated the highest xylanase activity (66.0 ± 8.65 U/gds), was the substrate that A. fumigatus KSA-2 utilized most frequently in solid-state fermentation. Due to wheat bran’s 40.0% xylan content in the cell-wall polysaccharides [28], which broke down into soluble chemicals that the fungus can utilize, wheat bran’s ability to induce the development of xylanase in solid-state fermentation may be ascribed to this.
Both submerged fermentation and solid-state fermentation are capable of producing microbial xylanase. Enzyme synthesis by solid-state fermentation is more cost-effective than enzyme production by submerged fermentation because it requires less capital investment and less water, uses less energy, and produces more enzymes with higher yields and activities [29]. Due to their increased productivity compared to other filamentous fungi and bacteria, filamentous fungi such as species of Aspergillus are particularly significant [30,31]. In accordance with the present investigation, various studies have used submerged as well as solid-state fermentation to produce xylanase using wheat bran. In this respect, A. flavus fermented wheat bran, sugarcane bagasse, wheat bran, and corn cob to generate the maximum xylanase (129.8 and 94.0 U/gds), respectively in submerged fermentation [32]. Penicillium oxalicum produced 38.9 U/gds of xylanase from wheat bran [28]. Several isolates of Aspergillus flavus participated in solid-state fermentation and yielded xylanase at varying concentrations (191–738 U/gds) from wheat bran [33]. Aspergillus oryzae used wheat bran in solid-state fermentation to produce substantially greater levels of xylanase (2830.7 U/gds) [34]. A total of 32.4 IU/gds were generated by A. flavus [35]. Aspergillus niger, A. oryzae, and A. awamori utilized palm kernel cake and palm pressed fiber to yield 18.8, 27.2, and 134.2 U/g xylanase, respectively [36]. A. fumigatus ITBCCL170 was able to ferment the empty fruit bunches to produce 236.30 U/g xylanase [37]. Due to modest methodological variations, it is often challenging to compare the values of enzyme activity between several projects. As a result, care should be used when making comparisons.
In this study, a crude formulation of xylanase was generated by A. fumigatus KSA-2 from wheat bran in solid-state fermentation and precipitated by ammonium sulphate without dialysis. To assess the use of the xylanase precipitation technology, a literature study was conducted. It is typical to utilize ammonium sulphate. As a result, some studies [29,38,39,40,41,42,43,44] mentioned using dialysis to remove salt from the precipitated enzyme solution. Precipitation combined with dialysis results in a slightly greater purification fold than precipitation alone [39,45]. As a result, the procedure without dialysis is preferable since it is less expensive to do and takes less time overall.
The current research revealed that pH 6.0 and 45 °C were the conditions when xylanase activity was at its maximum. For xylanases of species of Aspergillus, the pH range of optimum action has typically been recorded as between pH 3.0 and 6.0 [46]. For example, the optimal pH for xylanase generated by A. oryzae LC1 was found to be 5.0 [47], but A. fumigatus SK1 yielded superior results at a pH of 4.0 [48]. The impact of ions and inhibitors on xylanase activity was examined in the current study. Cu2+ and Mn2+ ions increased the specific activity by 10.2% and 128.0%, respectively, over the control. Enzymes can interact with ionic species present in the reaction medium. Ions can act as co-factors, either enhancing or inhibiting enzymatic activity as a result of the formation of non-active complexes with the enzyme [49]. Similar results have been reported for xylanase produced by Penicillium roquefortii compared to a commercial xylanase. The presence of ionic species in the reaction medium was reported to inhibit enzymatic activity [50]. On the other hand, xylanase produced by solid-state fermentation on yellow mombin residue (Spondias mombin L.) showed 40% higher enzymatic activity in the presence of Mn2+, while the addition of Cu2+ led to a reduction of 50% [51].
Markets provide both pure and raw xylanases. Thermomyces lanuginosus xylanase (Sigma–Aldrich, St. Louis, MO, USA), generated by genetically altered A. oryzae with a specific activity of 2500 U/g, is a good example of pure xylanase and is offered for sale at 199 SGD/10 g [52]. Weifang Yuexiang Chemical sells commercial crude xylanase with specific activity greater than 5500 U/g and sells it for 35 to 85 $US/kg [52]. The enzyme’s specific activity in this study was 20.52 U/mg (=20,520 U/g enzyme), which is higher than that of Weifang Yuexiang Chemical (5500 U/g) and Sigma-Aldrich (2500 U/g) xylanases. As a result, we demonstrate in this work an enzyme that has potential for usage in a number of areas.
5. Conclusions
This study investigated the production of xylanases using A. fumigatus and wheat bran as the substrate. Submerged fermentation (SmF) was used to perfect the culture conditions for three strains of fumigatus. The three strains markedly differed in their efficiency to produce xylanases. Using yeast extract as a nitrogen source, the most productive strain, A. fumigatus KSA-2, produced the greatest amount of xylanase at pH 9.0 and 30 °C after 7 days. Aspergillus fumigatus KSA-2 was used to efficiently manufacture xylanase from various agro-industrial wastes. The highest fermentable substance was wheat bran, which produced 66.0 U/gds. The xylanase produced showed its highest activity (20.52 ± 1.714 U/mg) at pH 6.0 and 45 °C. The impact of ions and inhibitors on xylanase activity was examined in the current study. The xylanase enzyme produced had a maximum specific activity of 20.53 U/mg for birchwood xylan, demonstrating a considerable affinity for this substrate. The specific activity exhibited 10.2% and 128.0% increase, respectively, over the control when Cu2+ and Mn2+ ions were added. The use of wheat bran as a substrate offers several advantages, including its low cost and abundance. The optimized fermentation conditions resulted in enhanced xylanase production, indicating the potential for large-scale industrial applications. The examination of different process parameters, such as pH, temperature, and nutrient supplementation, provides important guidelines for optimizing the production process, allowing for higher yields and improved efficiency. Overall, this study highlights the potential of A. fumigatus as a promising microbial host for xylanase production using wheat bran as a substrate. The insights gained from this research contribute to the development of sustainable and cost-effective strategies for the industrial production of xylanases, with potential applications in various industries including biofuel production, food processing, and animal feed. Future studies could focus on further optimizing the fermentation process, exploring downstream purification techniques, and investigating the application of the produced xylanases in different biotechnological processes. The ability to convert low-cost feedstock into valuable enzymes makes fungal enzyme production attractive.
Funding
The author extends his appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-005-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this manuscript are incorporated only in the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S.; Shukla, P. Bioprospecting of xylanase producing fungal strains: Multilocus phylogenetic analysis and enzyme activity profiling. J. Basic Microbiol. 2022, 62, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Knob, A.; Fortkamp, D.; X Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as alternative for xylanase production by filamentous fungi. BioResources 2014, 9, 5738–5773. [Google Scholar]
- Fasiku, S.A.; Bello, M.A.; Odeniyi, O.A. Production of xylanase by Aspergillus niger Gio and Bacillus sp. (BA) through solid-state fermentation. Access Microbiol. 2022, 5, 000506. [Google Scholar]
- AL-Kolaibe, A.M.; Moharram, A.M.; Al-Bedak, O.A. Worthwhile enzyme production and eco-friendly bioconversion of three agricultural residues by Aspergillus curvatus and Aspergillus gaarensis, promising enzyme-producers isolated from extreme environment. J. Basic Appl. Mycol. (Egypt) 2021, 12, 1–14. [Google Scholar]
- Ismail, M.A.; Moubasher, A.H.; Mohamed, R.A.; Al-Bedak, O.A. Agro–industrial residues as alternative sources for cellulases and xylanases production and purification of xylanase produced by Aspergillus flavus AUMC 10331 isolated from extreme habitat. Curr. Res. Environ. Appl. Mycol. 2018, 8, 313–322. [Google Scholar] [CrossRef]
- Korkmaz, M.N.; Ozdemir, S.C.; Uzel, A. Xylanase production from marine derived Trichoderma pleuroticola 08ÇK001 strain isolated from Mediterranean coastal sediments. J. Basic Microbiol. 2017, 57, 839–851. [Google Scholar] [CrossRef]
- Shrivastava, S. Introduction to glycoside hydrolases: Classification, identification and occurrence. In Industrial Applications of Glycoside Hydrolases; Springer: Singapore, 2020; pp. 3–84. [Google Scholar]
- Dias, L.M.; dos Santos, B.V.; Albuquerque, C.J.B.; Baeta, B.E.L.; Pasquini, D.; Baffi, M.A. Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol. 2018, 124, 708–718. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Glycosyl Hydrolases and biofuel. In Industrial Applications of Glycoside Hydrolases; Springer: Singapore, 2020; pp. 167–190. [Google Scholar]
- Warcup, J. The soil-plate method for isolation of fungi from soil. Nature 1950, 166, 117–118. [Google Scholar] [CrossRef]
- Smith, D.; Onions, A.H. The Preservation and Maintenance of Living Fungi; CAB International: Wallingford, UK, 1994.
- Moubasher, A.H.; Ismail, M.A.; Mohamed, R.A.; Al-Bedak, O.A. Ramophialophora chlamydospora, a new species from an alkaline lake of Wadi-El-Natron, Egypt. Asian J. Mycol. 2019, 2, 110–117. [Google Scholar] [CrossRef]
- Al-Bedak, O.A.; Moubasher, A.H. Aspergillus gaarensis, a new addition to section Circumdati from soil of Lake El-Gaar in Wadi-El-Natron, Egypt. Stud. Fungi 2020, 5, 59–65. [Google Scholar] [CrossRef]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Kumar, S. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Al-Bedak, O.A.; Teama, E.A.; Ali, E.; Said, M.; Shalaby, E.; Moharram, Z.A. Impact of fumigation with phosphine on viability of wheat grains stored for six months at two levels of moisture content, in addition to description of four new records of associated fungi and assessment of their potential for enzymatic production. J. Basic Appl. Mycol. (Egypt) 2020, 11, 77–97. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gaffney, M.; Carberry, S.; Doyle, S.; Murphy, R. Purification and characterisation of a xylanase from Thermomyces lanuginosus and its functional expression by Pichia pastoris. Enzym. Microb. Technol. 2009, 45, 348–354. [Google Scholar] [CrossRef]
- St, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar]
- Uhoraningoga, A.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. The goldilocks approach: A review of employing design of experiments in prokaryotic recombinant protein production. Bioengineering 2018, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef]
- Muthezhilan, R.; Ashok, R.; Jayalakshmi, S. Production and optimization of thermostable alkaline xylanase by Penicillium oxalicum in solid state fermentation. Afr. J. Microbiol. Res. 2007, 1, 20–28. [Google Scholar]
- Ding, C.; Li, M.; Hu, Y. High-activity production of xylanase by Pichia stipitis: Purification, characterization, kinetic evaluation and xylooligosaccharides production. Int. J. Biol. Macromol. 2018, 117, 72–77. [Google Scholar] [CrossRef]
- Ajijolakewu, A.K.; Leh, C.P.; Abdullah, W.N.W.; Lee, C.K. Optimization of production conditions for xylanase production by newly isolated strain Aspergillus niger through solid state fermentation of oil palm empty fruit bunches. Biocatal. Agric. Biotechnol. 2017, 11, 239–247. [Google Scholar] [CrossRef]
- Pithadiya, D.; Nandha, D.; Thakkar, A. Partial purification and optimization of xylanase from Bacillus circulans. Arch. Appl. Sci. Res. 2016, 8, 1–10. [Google Scholar]
- da Silva, P.O.; Guimarães, N.C.A.; Peixoto-Nogueira, S.C.; Betini, J.H.; Marchetti, C.R.; Zanoelo, F.F.; Polizeli, M.L.T.M.; Marques, M.R.; Giannesi, G.C. Production of cellulase-free xylanase by Aspergillus flavus: Effect of polyols on the thermostability and its application on cellulose pulp biobleaching. Afr. J. Biotechnol. 2015, 14, 3368–3373. [Google Scholar]
- Nair, S.G.; Shashidhar, S. Fungal xylanase production under solid state and submerged fermentation conditions. Afr. J. Microbiol. Res. 2008, 2, 82–86. [Google Scholar]
- Pirota, R.D.P.B.; Tonelotto, M.; Delabona, P.D.S.; Fonseca, R.F.; Paixão, D.A.A.; Baleeiro, F.C.F.; Neto, V.B.; Farinas, C.S. Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under controlled operation conditions. Ind. Crops Prod. 2013, 45, 465–471. [Google Scholar] [CrossRef]
- Mostafa, F.A.; El Aty, A.; Wehaidy, H.R. Improved Xylanase production by mixing low cost wastes and novel co-culture of three marine-derived fungi in solid state fermentation. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 336–349. [Google Scholar]
- Oliveira, A.C.; Amorim, G.M.; Azevêdo, J.A.G.; Godoy, M.G.; Freire, D.M.G. Solid-state fermentation of co-products from palm oil processing: Production of lipase and xylanase and effects on chemical composition. Biocatal. Biotransform. 2018, 36, 381–388. [Google Scholar] [CrossRef]
- Meilany, D.; Anugeraheni, D.; Aziz, A.; Kresnowati, M.T.A.P.; Setiadi, T. The Effects of Operational Conditions in Scaling Up of Xylanase Enzyme Production for Xylitol Production. Reaktor 2020, 20, 32–37. [Google Scholar] [CrossRef]
- Chapla, D.; Patel, H.; Singh, A.; Madamwar, D.; Shah, A. Production, purification and properties of a cellulase-free thermostable endoxylanase from newly isolated Paenibacillus sp. ASCD2. Ann. Microbiol. 2012, 62, 825–834. [Google Scholar] [CrossRef]
- Dhivahar, J.; Khusro, A.; Paray, B.A.; Rehman, M.U.; Agastian, P. Production and partial purification of extracellular xylanase from Pseudomonas nitroreducens using frugivorous bat (Pteropus giganteus) faeces as ideal substrate and its role in poultry feed digestion. J. King Saud Univ.-Sci. 2020, 32, 2474–2479. [Google Scholar] [CrossRef]
- Seemakram, W.; Boonrung, S.; Aimi, T.; Ekprasert, J.; Lumyong, S.; Boonlue, S. Purification, characterization and partial amino acid sequences of thermo-alkali-stable and mercury ion-tolerant xylanase from Thermomyces dupontii KKU–CLD–E2–3. Sci. Rep. 2020, 10, 21663. [Google Scholar] [CrossRef]
- Walia, A.; Mehta, P.; Chauhan, A.; Kulshrestha, S.; Shirkot, C.K. Purification and characterization of cellulase-free low molecular weight endo β-1, 4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J. Microbiol. Biotechnol. 2014, 30, 2597–2608. [Google Scholar] [CrossRef]
- Roy, S.; Dutta, T.; Sarkar, T.S.; Ghosh, S. Novel xylanases from Simplicillium obclavatum MTCC 9604: Comparative analysis of production, purification and characterization of enzyme from submerged and solid state fermentation. SpringerPlus 2013, 2, 382. [Google Scholar] [CrossRef]
- Kamble, R.D.; Jadhav, A.R. Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. Int. J. Microbiol. 2012, 2012, 683193. [Google Scholar] [CrossRef]
- Moharram, A.M.; Zohri, A.A.; Hesham, A.E.; Abdel-Raheam, H.E.F.; Maher, M.A.; Al-Bedak, O.A. Production of cold-active pectinases by three novel Cladosporium species isolated from Egypt and application of the most active enzyme. Sci. Rep. 2022, 12, 15599. [Google Scholar] [CrossRef]
- Seemakram, W.; Boonrung, S.; Katekaew, S.; Aimi, T.; Boonlue, S. Purification and characterization of low molecular weight alkaline xylanase from Neosartorya tatenoi KKU-CLB-3-2-4-1. Mycoscience 2016, 57, 326–333. [Google Scholar] [CrossRef]
- Gupta, P.K.; Choudhary, S.; Chandrananthi, C.; Eveline, J.S.; Sushmitha, S.P.; Hiremath, L.; Srivastava, A.K.; Kumar, S.N. Fungal Biodiversity Producing Xylanase Enzymes Involved in Efficient Uses of Xylanolysis. In Mycodegradation of Lignocelluloses; Springer: Cham, Switzerland, 2019; pp. 51–63. [Google Scholar]
- Bhardwaj, N.; Kumar, B.; Agarwal, K.; Chaturvedi, V.; Verma, P. Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in Xylo-oligosaccharides production from lignocellulosic agricultural wastes. Int. J. Biol. Macromol. 2019, 122, 1191–1202. [Google Scholar] [CrossRef]
- Ang, S.K.; Shaza, E.M.; Adibah, Y.; Suraini, A.A.; Madihah, M.S. Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 2013, 48, 1293–1302. [Google Scholar] [CrossRef]
- Ferraz, J.L.d.A.A.; Souza, L.O.; Soares, G.A.; Coutinho, J.P.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Enzymatic saccharification of lignocellulosic residues using cellulolytic enzyme extract produced by Penicillium roqueforti ATCC 10110 cultivated on residue of yellow mombin fruit. Bioresour. Technol. 2018, 248, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.O.; de Brito, A.R.; Bonomo, R.C.F.; Santana, N.B.; Ferraz, J.L.d.A.; Aguiar-Oliveira, E.; Fernandes, A.G.d.A.; Ferreira, M.L.O.; de Oliveira, J.R.; Franco, M. Comparison of the biochemical properties between the xylanases of Thermomyces lanuginosus (Sigma®) and excreted by Penicillium roqueforti ATCC 10110 during the solid state fermentation of sugarcane bagasse. Biocatal. Agric. Biotechnol. 2018, 16, 277–284. [Google Scholar] [CrossRef]
- Ferraz, J.L.d.A.; Souza, L.O.; Fernandes, A.G.d.A.; Oliveira, M.L.F.; de Oliveira, J.R.; Franco, M. Optimization of the solid-state fermentation conditions and characterization of xylanase produced by Penicillium roqueforti ATCC 10110 using yellow mombin residue (Spondias mombin L.). Chem. Eng. Commun. 2020, 207, 31–42. [Google Scholar] [CrossRef]
- Simanjuntak, B.; Julian, H.; Kresnowati, M. Downstream Process of Xylanase Production from Oil Palm Empty Fruit Bunches: A Review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).