1. Introduction
Lupinus mutabilis Sweet is an important crop in the Andes. It is known as tarwi in English, tauri in Quechua, and altramuz or chocho in Spanish, and is an important source of protein in the subsistence economy of Andean indigenous communities. Tarwi seeds are characterized by high protein and oleic content that far exceeds that of other legume species, reaching values of 44% dry weight in oils and up to 18% dry weight in proteins. Furthermore, its carbohydrate content is characterized by the virtual absence of starch and the presence of oligosaccharides such as stachyose and raffinose [
1,
2]. Its seeds also contain high content of most essential amino acids (particularly lysine), as well as dietary fiber and healthy fatty acids [
3]. These nutritional characteristics make tarwi a particularly interesting foodstuff, but its cultivation is relegated to the Andean highlands and forms part of the subsistence agriculture of the indigenous communities. This is why
Lupinus mutabilis is considered the lost crop of the Incas.
Tarwi harvest traditionally begins when the stalks are dry and brittle, the leaves turn brown, and the seeds’ moisture has dropped to 14%. At this stage, the seeds have high content of toxic alkaloids (mainly lupanine, sparteine, tetrahydrorombifolin, 4-hydroxylupanine, and 13-hydroxylupanine), as well as protease inhibitors, haemagglutinins, and certain cyanogenic glycosides. These must all be removed before consumption [
4,
5]. The traditional process for removing these toxic components is known as debittering, a method based on cooking and recurrent washing with water. This debittering process involves the use of a considerable amount of water (54 L of water per kilo of seeds) and time (about 6 to 8 days) [
3,
6]. However, it is possible to harvest tarwi seeds at an earlier stage, known as the tender grain stage. At this stage, the seeds have higher moisture content, and their coloration is lime green. Although the objective of harvesting at this stage is to reduce insect damage and improve processing conditions, it is not common practice because harvesting tasks are more onerous and do not offer the possibility of mechanization. However, the physicochemical characteristics of the seeds are slightly better, and they have lower alkaloid content [
4].
Although tarwi crops are concentrated in the Andean areas, there is an important gene pool and genotype variability [
5,
7]. However, many of the cultivated crops have a strong local character, so in Ecuador, the most common genotypes are Guaranguito I-450 and Andino I-451, while in Bolivia, the most common genotypes are Carabuco and Tarwinawi [
2]. Obviously, each cultivar has its own characteristics and differs in terms of its content of both anti-nutritional and functional compounds. In this sense, in the cultivar INIAP-451, around 1 g of tannins are usually found per 100 g, but it is also common to find high amounts of functional compounds. For example, total phenol levels are higher than 1.1 g/100 g in most varieties, and the cultivar Criollo is remarkable, with total phenol content higher than 1.3 g/100 g [
8]. This variability in compound content is also reflected after debittering and agro-industrial transformation processes, which remove a high percentage of anti-nutritional compounds (84% of tannins and about 70% of trypsin inhibitors). Agro-industrial processes, such as fermentation, increase the content of antioxidant compounds and carotenes, for example [
6,
9].
As a consequence of its high nutritional value, tarwi is traditionally used in the production of different food products since its seeds have been properly debittered. In this sense, food products, such as snacks, bread, cakes, biscuits, soups, fresh salads, hamburgers, tarwi stew, and milk substitutes, are commonly consumed by indigenous communities of the Andean highlands [
10,
11]. Additionally, not only are foods manufactured, but beverages are also made using transformed and processed tarwi seeds. These beverages can be obtained via different methods, one of which is fermentation using microorganisms, such as fungi and bacteria. Fermented beverages usually have a low alcohol content but are also high in protein and other bioactive compounds and can, therefore, be a supplement to the diet. Understandably, the composition of functional compounds varies according to the cultivar used. In general, fermented foods, both dairy and non-dairy, are of great global dietary importance, but intolerances and allergies to different compounds and the increase in diets associated with veganism have greatly increased the consumption of non-dairy fermented beverages [
12]. Specifically, functionalized beverages have garnered significant attention for their promising impact on health, such as the potential to reduce cholesterol levels, lower sugar content, provide high fiber content, boost the immune system, and aid in digestion [
13]. Nowadays, consumers are becoming aware of the importance of a healthy diet as a preventive health measure and are faced with a variety of products that provide bioactive components and are known in various ways as functional foods or nutraceuticals. Despite the close relationship between functional food and nutraceuticals, a clear definition and regulation of all these kinds of food is advocated in order to provide clear and precise information to the consumer [
14,
15]. Functional products, probiotics, and nutraceuticals, both dairy and non-dairy, occupy a very significant place in the market due to their health benefits. In fact, in Europe, North America, and Asia alone, fermented dairy products contribute to a market valued at more than 46 billion euros, according to estimations [
13]. Nevertheless, the consumption of dairy beverages faces constraints due to lactose intolerance and milk protein allergies, leading consumers to seek probiotic-rich alternatives, such as fruit, grain, and vegetable juices. This shift has also led manufacturers to create probiotic beverages using a variety of food matrices, taking advantage of the potential of probiotic cultures [
13].
In the fermentation process, which has been used since ancient times, in addition to the microorganisms that carry out the fermentation, controlled conditions (such as pH, temperature, and the concentration of nutrients in the substrate, among others) are required for the process to be successful [
16]. In the case of tarwi, and Lupinus in general, the technique used to carry out fermentation (anaerobic or aerobic, submerged, or solid-state fermentation) is of significant importance, as the fermentation process reduces the content of anti-nutritional compounds present in lupine grain [
17]. In fact, the fermentation of lupine grain has been explored as a debittering method [
6,
9]. However, it has also been shown that the nutritional quality of the grains is increased after fermentation, as this process improves the bioavailability of elements, such as Fe, Ca, and Zn, as well as the polyphenol content. As a result of the fermentation process, breakdown of the cell walls occurs, which facilitates the synthesis of antioxidant compounds that act as hydrogen donors to free radicals. This improves the antioxidant capacity of the grain [
6,
9,
18]. The end result is that a higher antioxidant capacity is achieved. The aim of this study was to evaluate the influence of genotype on the nutritional content and some organoleptic characteristics of a fermented beverage and tempeh using green seeds from four genotypes of
Lupinus mutabilis. 3. Results
The results concerning the main effects of the germplasm and the dosage of the starter on the proximate analysis, as well as the quality parameters, are shown in
Table 1 and
Table 2 for the fermented beverage and tempeh, respectively. For the fermented beverage, the two independent variables showed statistical differences for all the assessed parameters except for TSC and pH. The influence of the independent variables on tempeh is shown in
Table 2. Effects of the two variables were found for total alkaloids, crude fiber, and protein and calcium content.
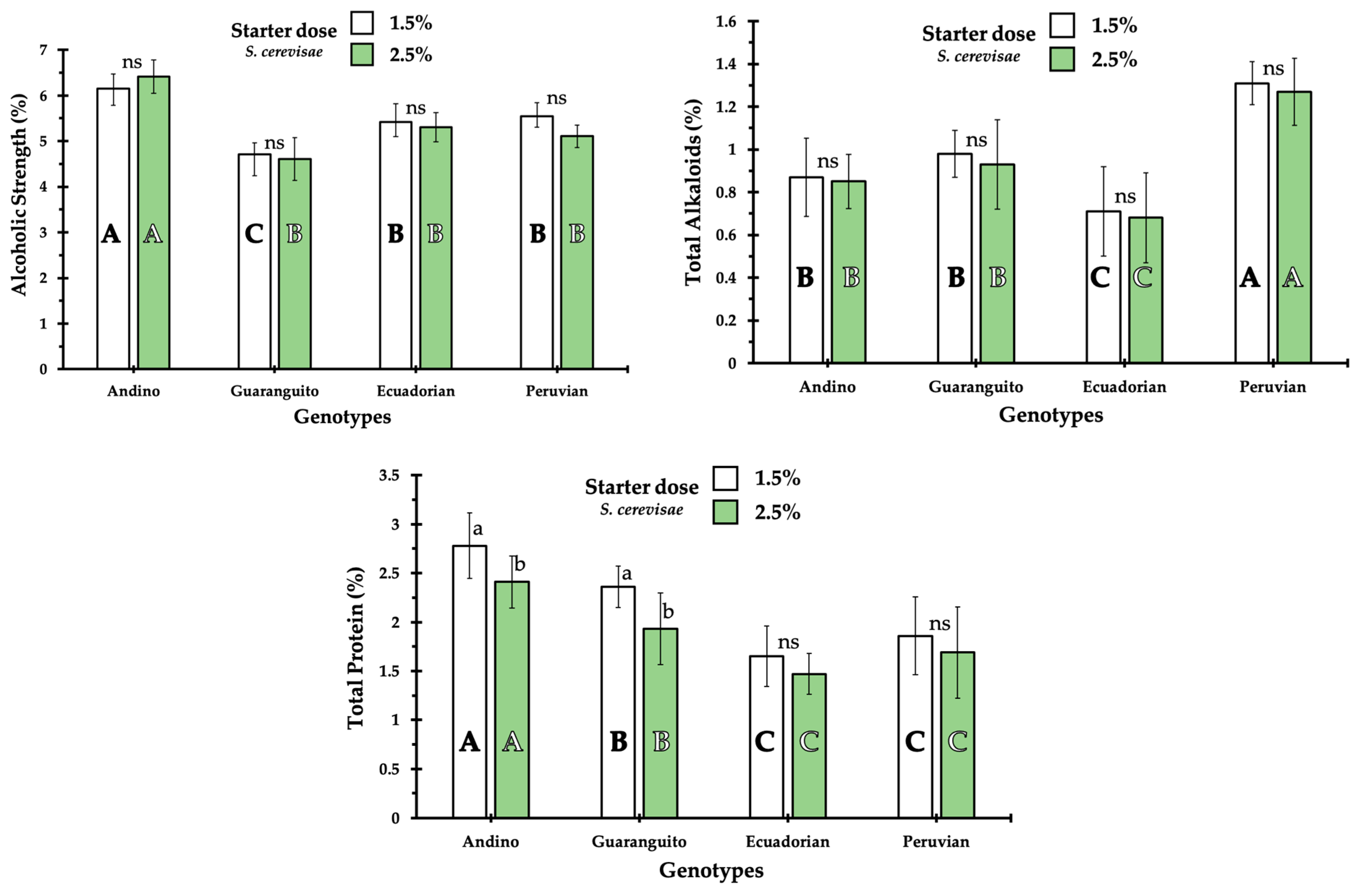
Figure 1 shows the effects of starter dosage on the main parameters in the proximate analysis of the fermented beverage. In relation to alcoholic strength, it was found that, in all cases, the Andino cultivar produced a beverage with higher alcoholic strength. However, it was found that in all cultivars, the inoculum dose influenced the alcohol content. In general, the alcohol content ranged from 4.61 to 6.45%, but there were differences between the different treatments. For the 1.5% dose, Ecuadorian and Peruvian exhibited a similar alcohol content, with the beverage derived from Guaranguito having a lower alcohol content. However, when the inoculum dose was increased to 2.5%, the beverage from the Andino genotype had the highest alcohol content, with no significant differences found among the remaining three genotypes.
The effects of fermentation on total alkaloid content were clear. This effect was particularly noticeable in the Peruvian cultivar, which showed the highest total alkaloid concentration regardless of the starter dose. Andino and Guaranguito had similar alkaloid contents, both at the 1.5% and 2.5% starter doses, while Ecuadorian was the genotype that contributed the lowest alkaloid content to the beverage. Regarding the protein content, it was the beverage made with the Andino cultivar that exhibited a higher content. There was a certain inoculum dose effect, as a lower protein content was found when the beverage was made with a higher inoculum dose. This effect was observed in the Andino and Guaranguito genotypes. With the Ecuadorian and Peruvian genotypes, a beverage with the lowest protein content was obtained without finding any influence of the inoculum dose.
Regarding the fiber content, the value was logically very low due to the brewing process. Even so, differences were found between the different cultivars, but in general, fiber content showed a similar pattern to that of protein content, i.e., the higher the dose, the lower the fiber content. Similar results were found for fat content, although the values were very low in all cases, and it was found that in beverages from the Andino and Guaranguito cultivars, the fat content was higher. Calcium content in the fermented beverages was always very low, with values found to be between 0.07% and 0.01% (
Supplementary Materials).
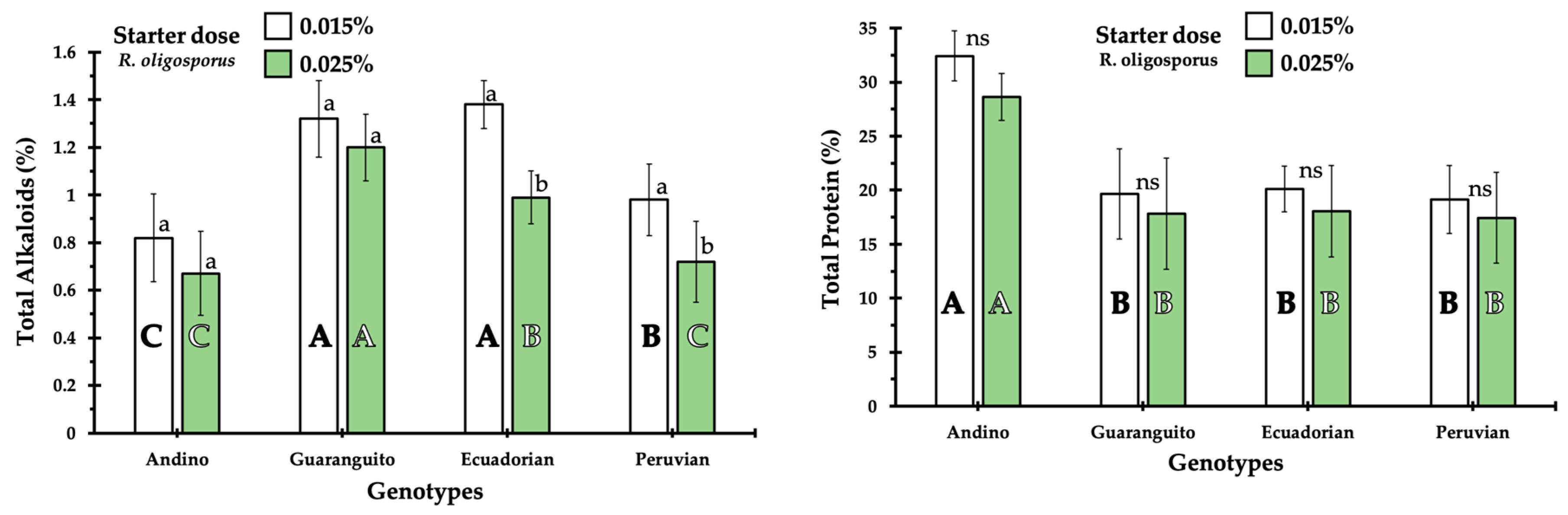
The results of alkaloid and protein content of tempeh are shown in
Figure 2. The alkaloid content of tempeh ranged between 0.49 and 1.38%, with significant differences between the different cultivars. The Guaranguito and Ecuadorian cultivars showed higher alkaloid content than the rest of the cultivars. The effects of fermentation, as a function starter dose, resulted in a reduction in alkaloid content but were noticed only in Ecuadorian and Peruvian genotypes. In this sense, in the Peruvian cultivar, there was an effect of inoculum dose in that a dose of 0.025% produced a lower concentration of alkaloids in the tempeh. In the tempeh, an effect of cultivar on protein content was found, with the Andino cultivar showing higher protein content than the other cultivars. The tempeh samples produced with the Guaranguito, Ecuadorian, and Peruvian cultivars showed similar protein content and were not affected by the starter dose.
With respect to crude fiber, in general, there was no influence of the cultivar on this parameter. This is because, in general, the different tempeh samples had similar fiber content. There was an influence of the starter dose in that the higher the inoculum dose, the less crude fiber was found in the tempeh. The fat content of tempeh was affected by the cultivar used to prepare it, so the use of the Andino and Ecuadorian cultivars generally produced tempeh with higher fat content than when the Guaranguito or Peruvian cultivars were used. In general, the higher the inoculum dose, the lower the fat content. The calcium content fluctuated between 1.68 and 0.08% depending on the cultivar, inoculum dose, and fermentation time used to make the tempeh. The use of Andino in tempeh manufacture always produced tempeh with higher calcium content. (
Supplementary Materials).
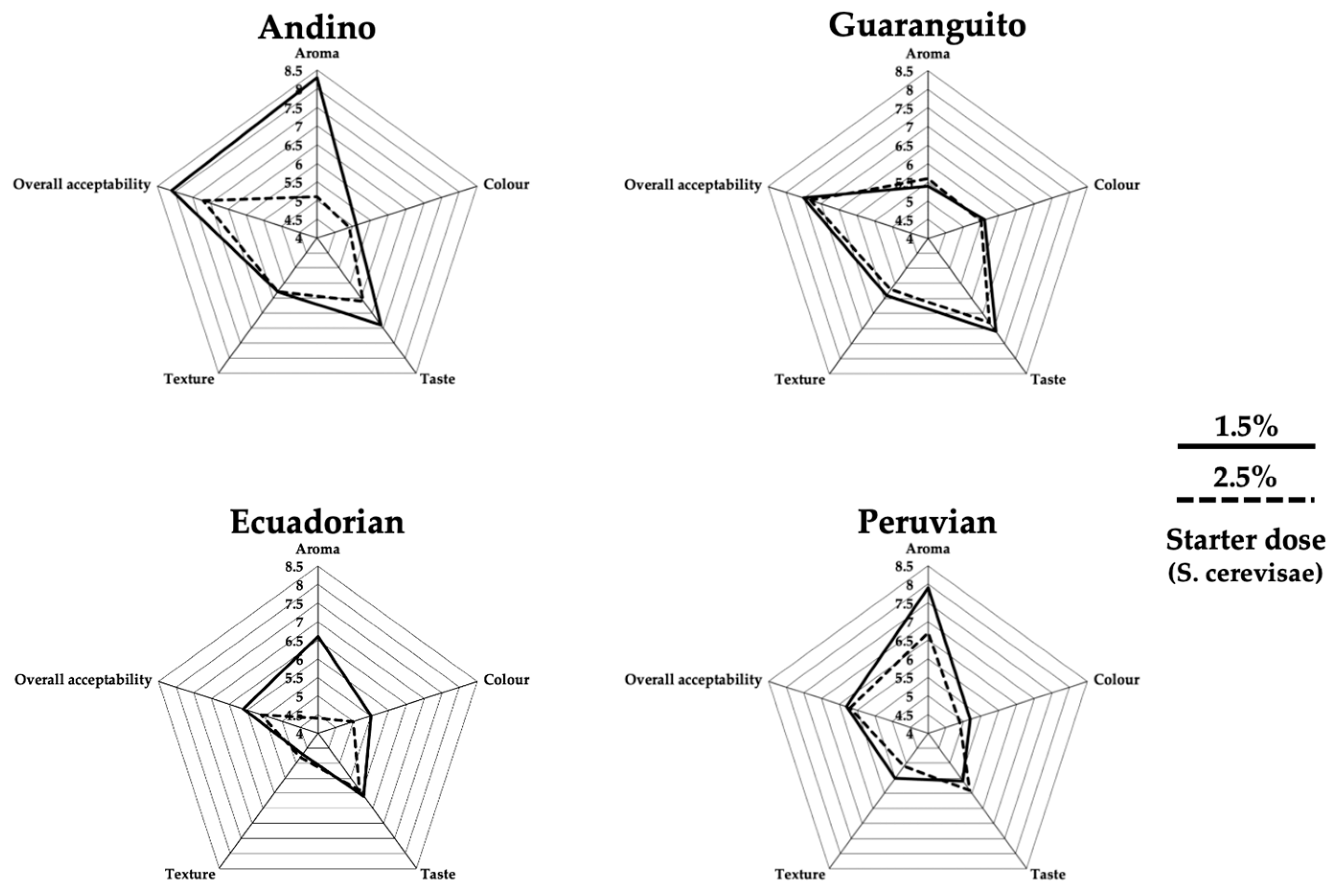
Using the hedonic scale, both the fermented beverage and the tempeh were subjected to a sensory evaluation to assess their acceptability and preference. In the fermented beverage (
Figure 3), the differences were found in aroma, texture, and general acceptability, with the samples formulated with the Andino and Peruvian genotypes standing out, reaching values of around 8 in aroma, making these samples pleasant for the panelists. As for texture, the evaluation, in general, was around 5.5–6 for three genotypes: Andino, Guaranguito, and Peruvian, which showed differences compared to the fourth genotype: Ecuadorian. In terms of general acceptance, the beverage from the different genotypes was highly valued, with the Andino and Guaranguito genotypes standing out from the rest. In relation to tempeh (
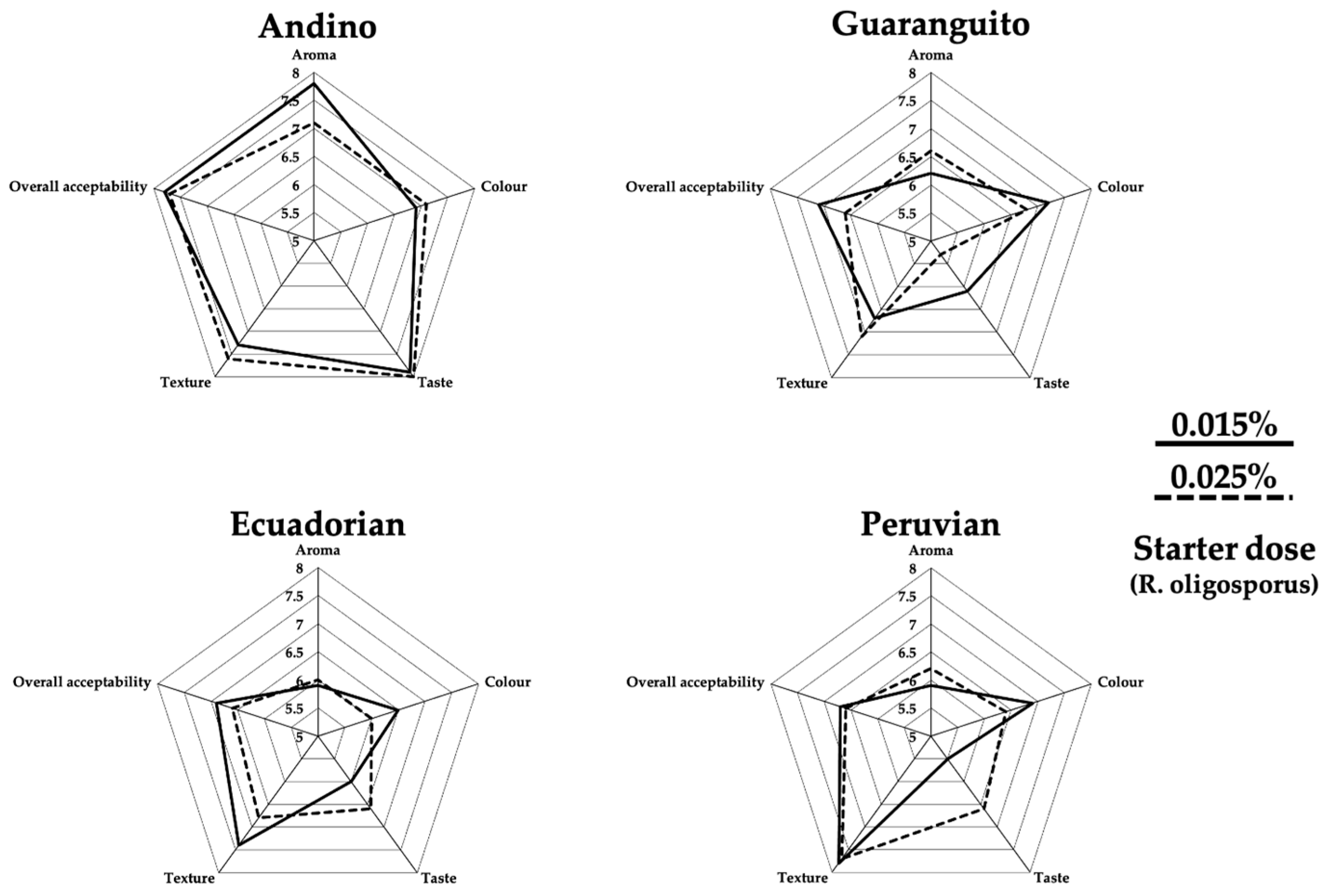
Figure 4), the main differences are in aroma and taste. Although the differences between genotypes used are not excessive, and the values achieved are around 7–8, it should be noted that the Andino cultivar stood out from the rest. In terms of taste, the Andino genotype was also classified with a higher value between 7 and 8 on the hedonic scale. This result shows that the Andino genotype was favored by the consumers in terms of sensory evaluation.
4. Discussion
Fermenting legumes or legume-like seeds to create processed foods is a common practice in various regions of the world. Although tempeh is one of the most well-known foods produced in this way, there are numerous variations depending on factors, such as the type of legume, the fermentation technique, and the geographical region. This study focused on the production of both fermented beverages and tempeh using grains from L. mutabilis, a traditional crop among indigenous communities in the Ecuadorian Andean mountains. The production process for this legume differs significantly from that of other grains, with the first step being the removal of anti-nutritional compounds through debittering, given the high content of these compounds in L. mutabilis. The fermentation of legumes or legume-like seeds to produce processed foods is common in different parts of the world. Although one of the best-known foods prepared in this manner is tempeh, there are many variations depending on the geographical region, the type of legume, and the fermentation technique. Both the fermented beverage and the tempeh produced in this work were made from L. mutabilis grains, a traditional crop of the indigenous communities of the Ecuadorian Andean mountains. However, in this study, green lupine grain has been used, which is a novel proposal since traditionally, any food based on lupine grain is made with dry grain. This provides some advantages, such as earlier harvesting and lower alkaloid content.
The production process of this legume entails important differences compared to other grains. The first of these is debittering, as
L. mutabilis is characterized by its high content of anti-nutritional compounds that have to be removed before consumption [
9]. In our experiment, we used tender kernels harvested shortly before they reached the dry seed stage. Under these conditions, the alkaloid content is lower, and therefore, the debittering process is slightly less laborious. The fermented beverage obtained has similar nutritional properties to beverages obtained using other legumes, and logically, it will fundamentally depend on the legume and the fermenting agent used. In our case,
Saccaromices cereviceae enabled the production of a beverage with low alcohol content. Although it is not common to use this yeast to make an alcoholic beverage with tarwi, different works have been published in which tarwi grains are mixed with other compounds, such as amaranth grains [
12]. Other studies use tarwi as an ingredient to enhance the qualities of a fermented beverage. Márquez-Villacorta et al. [
21] designed and optimized a drink based on a mixture of fresh milk, oats, and a tarwi beverage. These authors found that the addition of a tarwi beverage to their formulation resulted in an increase in protein content. Regarding the protein supply that manufactured
Lupinus mutabilis products can offer, it should be noted that vegetable drinks, such as soya milk, or drinks made from Lupinus have slightly higher protein content than cows’ milk [
21,
22], hence their use as an ingredient capable of providing a higher protein content. In this sense, the fermented beverage that we used, obtained using green tarwi beans, had higher protein content than the chickpea-based beverage designed by Wang et al. [
23]. This chickpea drink had a protein content between 1.21 and 2.09%, which is significantly lower than that found in our drink when using the Andino cultivar. Moreover, we can indicate that of all our treatments, fermentation for 92 h using the Ecuadorian cultivar, with a starter dose of 2.5%, presented lower protein content than the chickpea drink proposed by Wang et al. [
23]. A similar argument can be made for the fiber content. The use of Lupinus to make a fermented beverage using brewer’s yeast provides higher fiber content than when other legumes are used. However, this drink is disadvantaged by its alcohol content, which, although low, still renders it an alcoholic beverage, therefore, its consumption must be limited. However, as Pulingundla et al. [
24] point out in their work on new innovations in beers, the probiotic content that can be found in fermented beverages is of interest, and these authors favor
Saccaromices boulardii because of its probiotic contribution and the lower alcohol content of the beverages it produces.
S. boulardii is genetically close to
S. cerevisiae but has different metabolic and physiological characteristics. We used S. cerevisiae in our beverage mainly because it is the most widely available and traditional yeast in indigenous communities. The few studies that have been carried out on
S. boulardii in the fermentation of tarwi grains have focused on debittering [
14] and not on its use as a fermentation starter for beverages. Considering the probiotic properties of
S boulardii and the production of beverages with lower alcoholic content, we believe that in future research, we should try to use this yeast, or a mixture of
S. cerviceae and
S. boulardi, to brew a beverage fermented with tarwi grains.
The most common processed product obtained from
Lupinus mutabilis is tempeh, after fermentation with different strains of
Rhizopus oligosporus. The product obtained is rich in protein and is a good source of fiber. As the raw material used in the production of tempeh has high alkaloid content, it must be ensured that in the processing of tempeh, its alkaloid content is safe for human consumption. It is for this reason that Lupinus seeds have to be debittered. However, there are differences in tempeh manufacturing processes. Carvajal-Arenas et al. [
1] point out that the processes of debittering and fermentation can be carried out simultaneously as long as the tarwi beans are soaked and cooked. This results in a reduction in the alkaloid content because contact between the alkaloids and the enzymes produced by the fungus is facilitated. In our case, we started from previously debittered seeds so that the alkaloid content was further reduced after fermentation with the fungus. The importance of tempeh lies mainly in the fact that it is a good source of protein. In this respect, the use of one genotype over another may lead to differences in protein content in the diet. It should not be forgotten that the use of
Lupinus mutabilis is generally restricted to indigenous communities in the Andean highlands [
25]. These communities have a way of life in which they practice self-sufficient agriculture [
26], and in which the tarwi crop is fully integrated. Therefore, different projects are underway to promote alternative uses of tarwi, with one of the most promising being the production of tempeh because it can easily be integrated, with few modifications, into the culinary processes and habits of these communities. This is because the required infrastructure is not particularly expensive and there is no need for special technology. In fact, the traditional culinary use of tarwi is based on several aspects, i.e., tarwi flour is mixed with wheat flour to prepare bread, which is beneficial, because it provides the bread with an extra supply of lysine. Moreover, tarwi is used as a main ingredient in many soups, salads, and stews, and is regularly combined with quinoa or amaranth [
27]. Tempeh could therefore be a promising alternative because, as indicated by Villacrés et al. [
28], fried tempeh has a characteristic and pleasant flavor with a meaty taste. Moreover, its texture is improved by the fermentation process due to the hydrolysis and degradation of different polymers by the fungus, making it palatable to children and the elderly [
28].
A concern regarding both fermented beverages and tempeh is their alkaloid content, although, with debittering and subsequent fermentation, the alkaloid content is greatly reduced. The Ecuadorian Institute for Standardisation [
29] has established that the maximum total alkaloid content in debittered tarwi beans is 0.07%, which is much lower than the content of our products. However, it should be taken into account that according to Aguilera and Trier [
30], a dose of 500 mg per day is considered safe, and according to Carvajal et al. [
1], an adult can consume a quantity of around 750 g of debittered lupin per day. In addition to these data, the tarwi seeds used in our experiment were in the green state, during which they have lower alkaloid content [
4]. We, therefore, consider the use of seeds at this stage of development to be a viable option for obtaining foodstuffs with lower alkaloid content.
The fermented beverage was generally well accepted by the panelists, but there were differences in acceptance based on genotype, with Andino and Guaranguito being particularly favored. The aroma of the beverage was influenced by the inoculum dose and fermentation time, with higher doses and longer fermentation times resulting in lower scores from the panelists. The fermented and slightly pungent odor was disliked by the panelists, and this characteristic increased as the fermentation parameters were increased. This was considered the most negative characteristic of the beverage. While the Andean consumer, particularly the Quechua, is accustomed to the traditional fermented corn drink, chicha, which has a pungent, sour taste and strong fermented smell [
31,
32], a comparison is inevitable, and the fermented beverage presented in this study scored lower in aspects, such as color, texture, and aroma. In contrast, tempeh made from
Lupinus mutabilis was generally well-accepted in terms of taste, texture, and overall acceptability. Tempeh is not a traditional food in Andean communities, however, both the traditional Asian tempeh and the one made in this study have been made with legumes and with similar inoculums. In general, according to Jiménez-Martínez et al. [
27], foods made with
Lupinus tend to have a good sensory acceptability, which may even be superior to the acceptability of soy-based foods [
1,
27]. This is reflected in our sensory analysis results, where tempeh made from
Lupinus scored generally well in terms of taste, texture, and overall acceptability. The preference of the panelists for the Andino cultivar may be due to its familiarity, as it is one of the most common cultivars. There is potential to improve the sensory properties of the alternatives presented in this study by adding different flavorings, colorings, and other additives, as well as gently roasting the tarwii beans, which has been shown to greatly improve the acceptability of
Lupinus products [
33]. Consumer acceptance of the sensory characteristics of these two formulations is crucial, as they may have different tastes and textures than other similar soybean foods. It is important to note that our alternatives have been produced with green grain, whereas the available literature on similar products made with tarwii is based on dry grain. This may be a factor to take into account in two ways. One is the lower content of anti-nutritional compounds, and the other is its influence on the palatability of the processed products.