Two-Step Hydrothermal Pretreatments for Co-Producing Xylooligosaccharides and Humic-like Acid from Vinegar Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. First-Step Hydrothermal Pretreatment
2.3. Second-Step Hydrothermal Pretreatment
2.4. Endoxylanase Hydrolysis
2.5. Analytical Methods
3. Results and Discussion
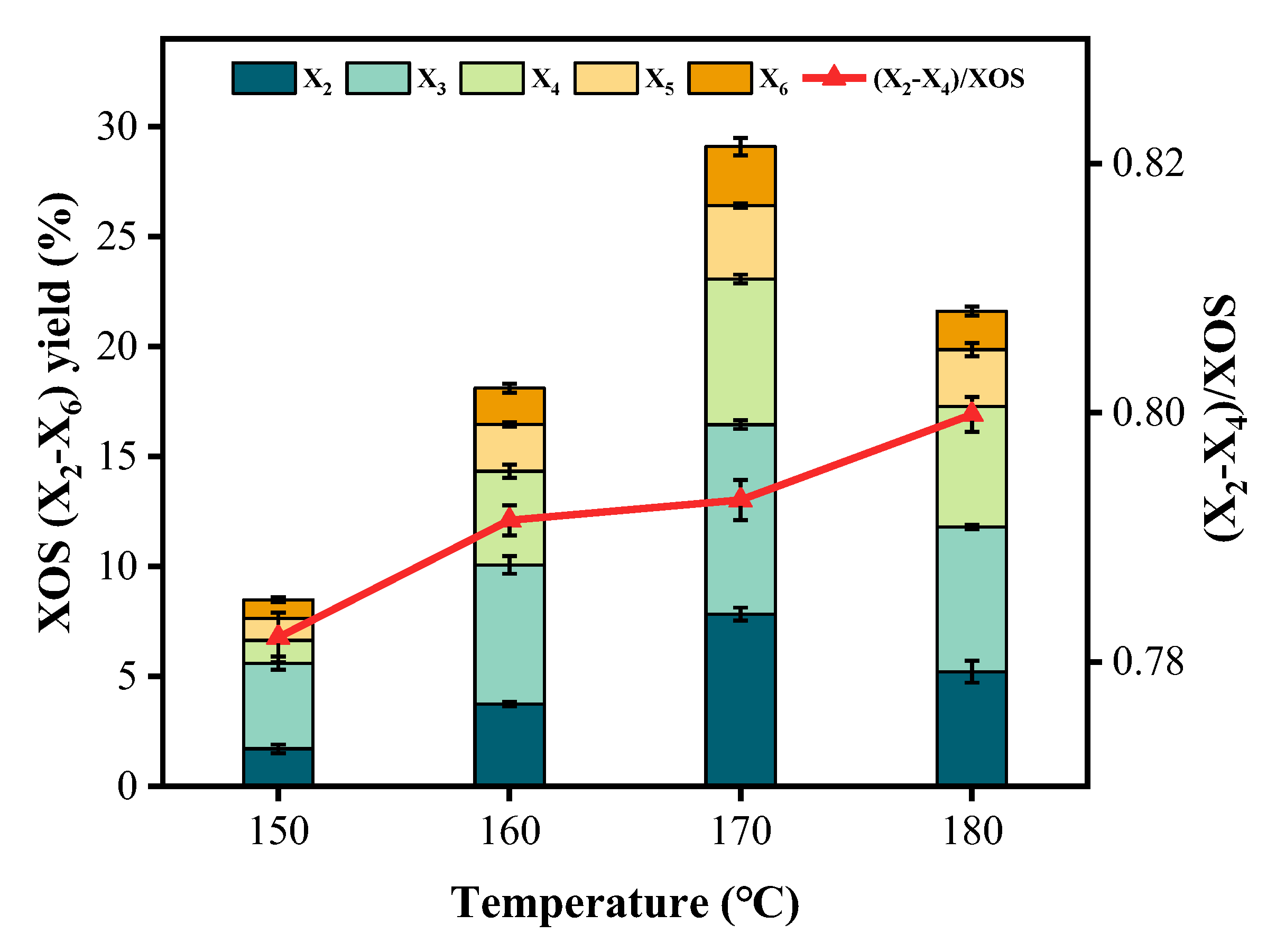
3.1. Xylooligosaccharides Produced from the First-Step Hydrothermal Pretreatment
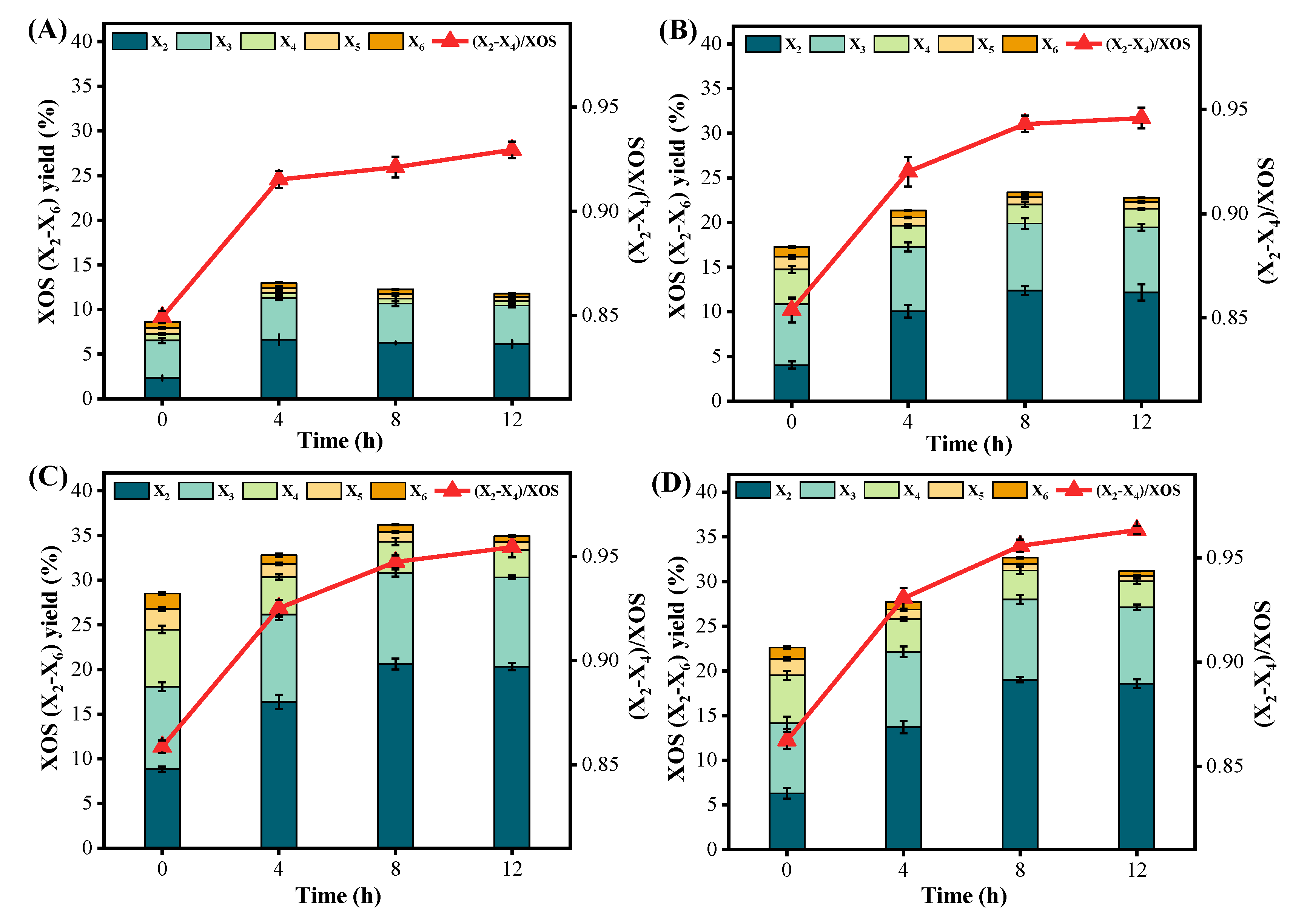
3.2. Endoxylanase-Assisted Hydrolysis of the Xylooligosacchairdes Autohydrolysate
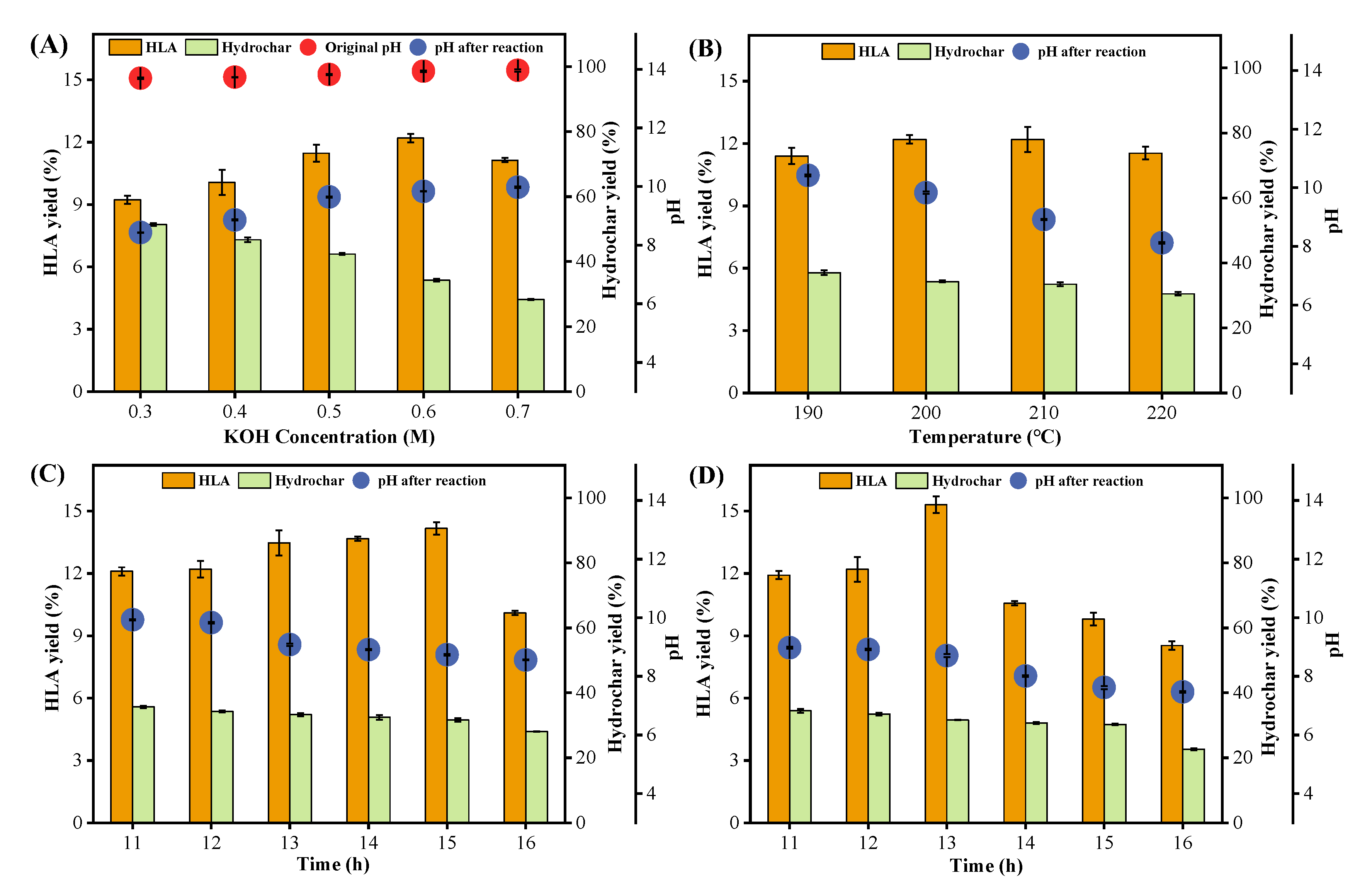
3.3. Humic-like Acid Prepared from the Second-Step Hydrothermal Pretreatment
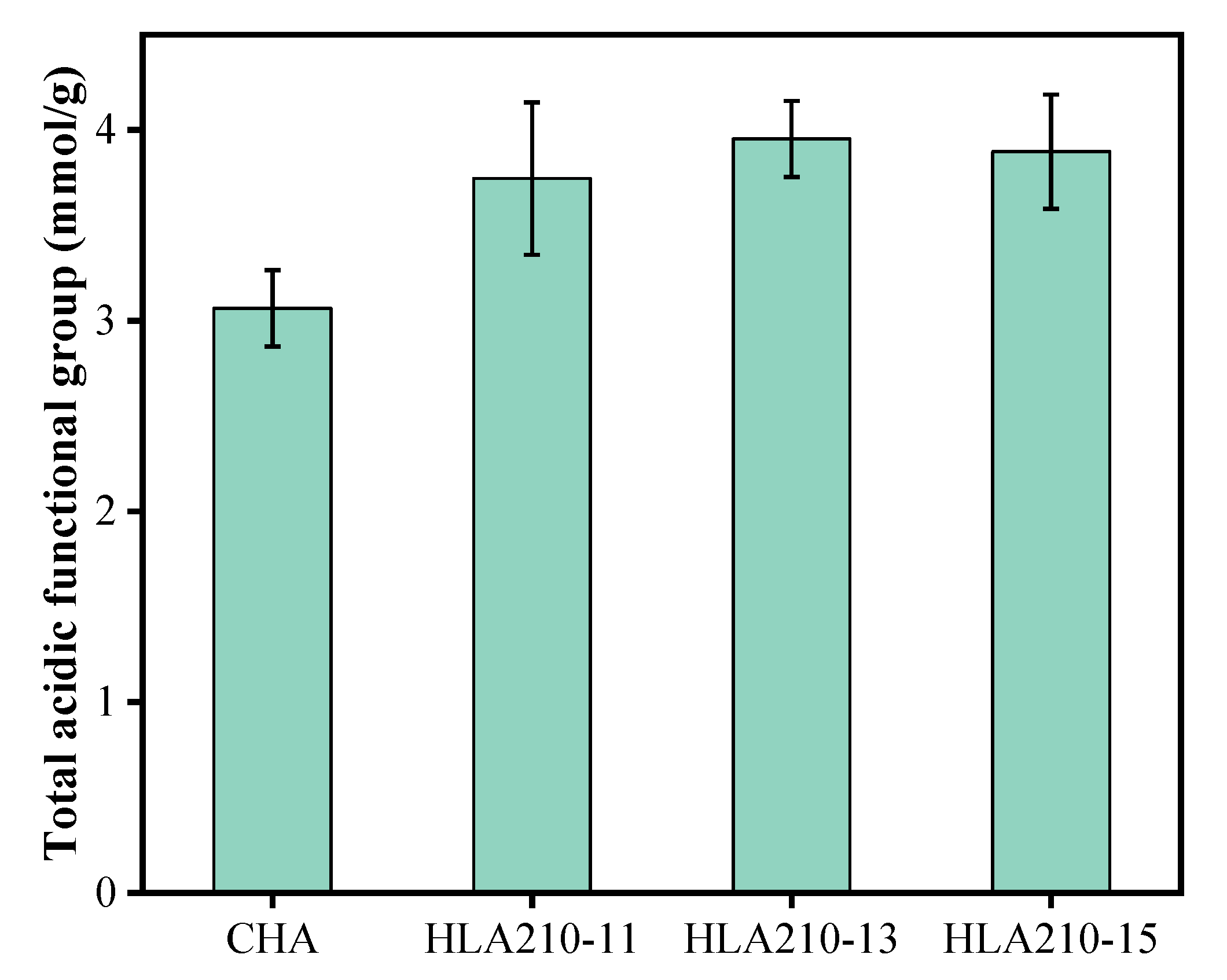
3.4. Characterization of Humic-like Acid
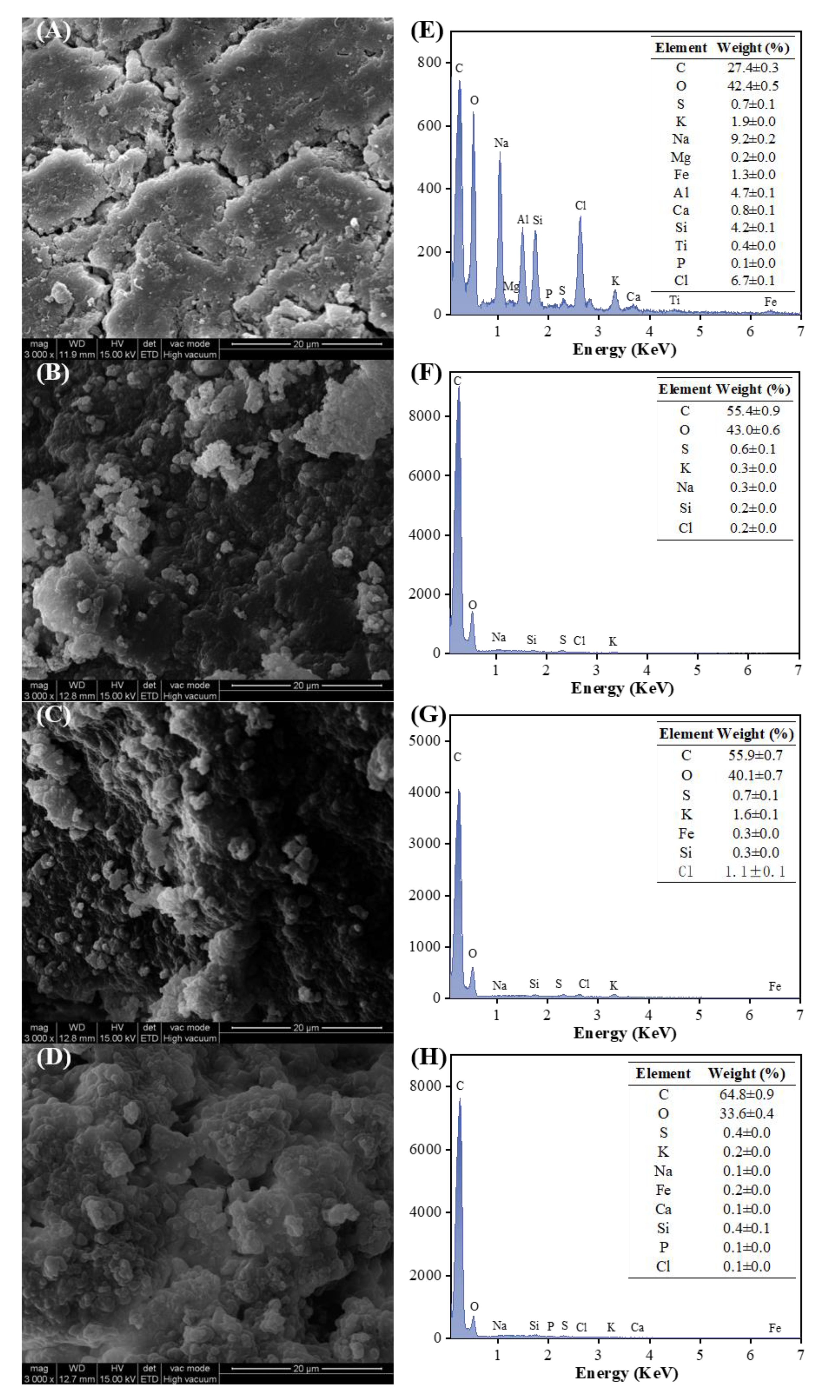
3.5. Characterization of Hydrochar from the Second-Step Hydrothermal Pretreatment
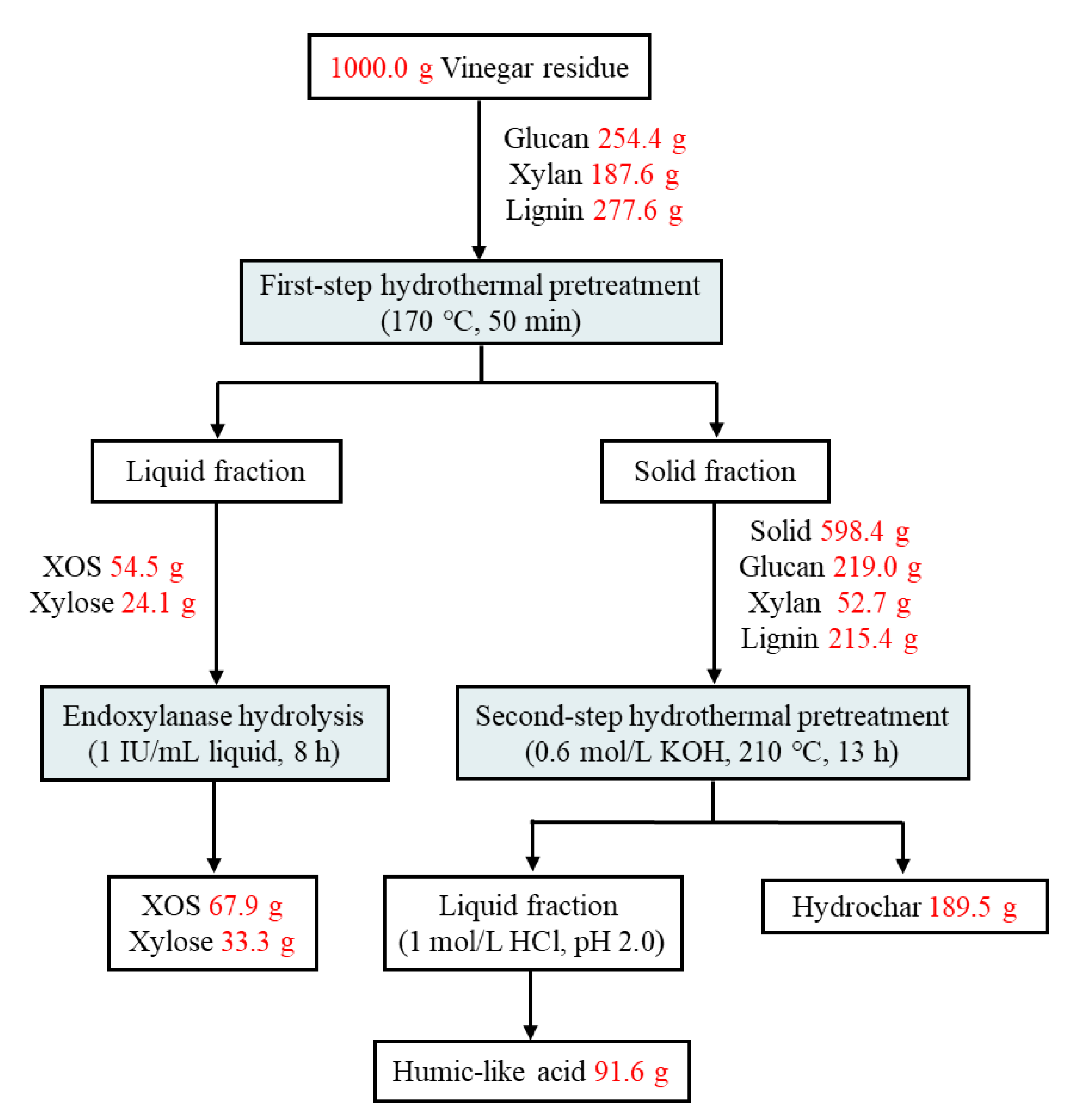
3.6. Mass Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Tang, R.; Yu, Y.; Yu, Z.; Wang, K.; Wang, Y.; Liu, P.; Han, D. Efficient co-production of xylooligosaccharides and glucose from vinegar residue by biphasic phenoxyethanol-maleic acid pretreatment. Fermentation 2023, 9, 61. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, B.; Wang, Q.; Yang, L.; Lu, B.; Li, X.; Yuan, H. Study on the conditions of pretreating vinegar residue with sodium hydroxide for simultaneous saccharification and fermentation to produce alcohol and xylose. Food Sci. Technol. Res. 2020, 26, 381–388. [Google Scholar] [CrossRef]
- Ran, G.; Li, D.; Zheng, T.; Liu, X.; Chen, L.; Cao, Q.; Yan, Z. Hydrothermal pretreatment on the anaerobic digestion of washed vinegar residue. Bioresour. Technol. 2018, 248, 265–271. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal pretreatment for the production of oligosaccharides: A review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef] [PubMed]
- Pinales-Márquez, C.D.; Rodríguez-Jasso, R.M.; Araújo, R.G.; Loredo-Treviño, A.; Nabarlatz, D.; Gullón, B.; Ruiz, H.A. Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: A review. Ind. Crop. Prod. 2021, 162, 113274. [Google Scholar] [CrossRef]
- Sonkar, R.M.; Gade, P.S.; Bokade, V.; Mudliar, S.N.; Bhatt, P. Ozone assisted autohydrolysis of wheat bran enhances xylooligosaccharide production with low generation of inhibitor compounds: A comparative study. Bioresour. Technol. 2021, 338, 125559. [Google Scholar] [CrossRef]
- Surek, E.; Buyukkileci, A.O. Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) Shell. Carbohydr. Polym. 2017, 174, 565–571. [Google Scholar] [CrossRef]
- Del Río, P.G.; Gullón, B.; Wu, J.; Saddler, J.; Garrote, G.; Romaní, A. Current breakthroughs in the hardwood biorefineries: Hydrothermal processing for the co-production of xylooligosaccharides and bioethanol. Bioresour. Technol. 2022, 343, 126100. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Y.; Li, X.; Zhu, J.; Zhang, J. Comparison of pH-controlled lactic acid hydrolysis and xylanase hydrolysis for xylo-oligosaccharides production from delignified poplar. Ind. Crop. Prod. 2022, 182, 114902. [Google Scholar] [CrossRef]
- Ying, W.; Fang, X.; Xu, Y.; Zhang, J. Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenergy 2022, 158, 106377. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Sun, Y.; Zhang, Y.; Liu, Q.; Cao, J.; Huang, G.; Xing, B.; Zhang, C.; Zhang, L.; Cao, Y. Facile and efficient fabrication of bandgap tunable carbon quantum dots derived from anthracite and their photoluminescence properties. Front. Chem. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Bento, L.R.; Melo, C.A.; Ferreira, O.P.; Moreira, A.B.; Mounier, S.; Piccolo, A.; Spaccini, R.; Bisinoti, M.C. Humic extracts of hydrochar and amazonian dark earth: Molecular characteristics and effects on maize seed germination. Sci. Total Environ. 2020, 708, 135000. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Muhmood, A.; Dong, R.; Wu, S. Synthesis of humic-like acid from biomass pretreatment liquor: Quantitative appraisal of electron transferring capacity and metal-binding potential. J. Clean. Prod. 2020, 255, 120243. [Google Scholar] [CrossRef]
- Klavins, M.; Ansone-Bertina, L.; Arbidans, L.; Klavins, L. Biomass waste processing into artificial humic substances. Environ. Clim. Technol. 2021, 25, 631–639. [Google Scholar] [CrossRef]
- Shao, Y.; Bao, M.; Huo, W.; Ye, R.; Liu, Y.; Lu, W. Production of artificial humic acid from biomass residues by a non-catalytic hydrothermal process. J. Clean. Prod. 2022, 335, 130302. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Jiao, N.; Xiao, Y.; Shi, D.; Xu, Y. A green process for producing xylooligosaccharides from poplar: Endoxylanase assisted autohydrolysis, activated carbon separation, and spent liquor for rice growth. Ind. Crop. Prod. 2021, 174, 114187. [Google Scholar] [CrossRef]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA: Madison, WI, USA, 1996. [Google Scholar] [CrossRef]
- Fakudze, S.; Wei, Y.; Zhou, P.; Han, J.; Chen, J. Synergistic effects of process-generated organic acids during co-hydrothermal carbonization of watermelon peel and high-sulfur coal. J. Environ. Chem. Eng. 2022, 10, 107519. [Google Scholar] [CrossRef]
- Gupta, M.; Bangotra, R.; Sharma, S.; Vaid, S.; Kapoor, N.; Dutt, H.C.; Bajaj, B.K. Bioprocess development for production of xylooligosaccharides prebiotics from sugarcane bagasse with high bioactivity potential. Ind. Crop. Prod. 2022, 178, 114591. [Google Scholar] [CrossRef]
- Yan, B.; Huang, C.; Lai, C.; Ling, Z.; Yong, Q. Production of prebiotic xylooligosaccharides from industrial-derived xylan residue by organic acid treatment. Carbohydr. Polym. 2022, 292, 119641. [Google Scholar] [CrossRef]
- Hao, X.; Xu, F.; Zhang, J. Effect of pretreatments on production of xylooligosaccharides and monosaccharides from corncob by a two-step hydrolysis. Carbohydr. Polym. 2022, 285, 119217. [Google Scholar] [CrossRef] [PubMed]
- Milessi, T.S.; Corradini, F.A.S.; Marçal, J.V.M.; Baldez, T.O.; Kopp, W.; Giordano, R.C.; Giordano, R.L.C. Xylooligosaccharides production chain in sugarcane biorefineries: From the selection of pretreatment conditions to the evaluation of nutritional properties. Ind. Crop. Prod. 2021, 172, 114056. [Google Scholar] [CrossRef]
- Liao, H.; Ying, W.; Li, X.; Zhu, J.; Xu, Y.; Zhang, J. Optimized production of xylooligosaccharides from poplar: A biorefinery strategy with sequential acetic acid/sodium acetate hydrolysis followed by xylanase hydrolysis. Bioresour. Technol. 2022, 347, 126683. [Google Scholar] [CrossRef]
- Salvati, E.; Brandt, L.R.; Uzun, F.; Zhang, H.; Papadaki, C.; Korsunsky, A.M. Multiscale analysis of bamboo deformation mechanisms following NaOH treatment using X-ray and correlative microscopy. Acta Biomater. 2018, 72, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, Y.; Liu, Q.; Huang, G.; Xing, B.; Zhang, C.; Guo, H.; Pan, J.; Cao, Y. Characterization of coal-based humic acids in relation to their preparation methods. Energ. Source Part A 2020, 1–11. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Cheng, K.; Antonietti, M. A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation. Sci. Total. Environ. 2019, 686, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Antonietti, M. Chemical conversion of sugars to lactic acid by alkaline hydrothermal processes. Chemsuschem 2013, 6, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Z.; Zhang, X.; Min, D.; Wu, Y.; Wen, J.; Yuan, T. Effects of hydrothermal pretreatment on the structural characteristics of organosolv lignin from Triarrhena lutarioriparia. Polymers 2018, 10, 1157. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Liao, Y.; Lv, W.; Ma, L.; Wang, C. Degradation of vanillin during lignin valorization under alkaline oxidation. Top. Curr. Chem. 2018, 376, 29. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Sutradhar, S.; Alam, N.; Christopher, L.P.; Fatehi, P. KOH catalyzed oxidation of kraft lignin to produce green fertilizer. Catal. Today 2022, 404, 49–62. [Google Scholar] [CrossRef]
- Sui, W.; Li, S.; Zhou, X.; Dou, Z.; Liu, R.; Wu, T.; Jia, H.; Wang, G.; Zhang, M. Potential hydrothermal-humification of vegetable wastes by steam explosion and structural characteristics of humified fractions. Molecules 2021, 26, 3841. [Google Scholar] [CrossRef]
- Shao, Y.; Bao, M.; Huo, W.; Ye, R.; Ajmal, M.; Lu, W. From biomass to humic acid: Is there an accelerated way to go? Chem. Eng. J. 2023, 452, 139172. [Google Scholar] [CrossRef]
- Cheng, G.; Niu, Z.; Zhang, C.; Zhang, X.; Li, X. Extraction of humic acid from lignite by KOH-hydrothermal method. Appl. Sci. 2019, 9, 1356. [Google Scholar] [CrossRef]
- Liao, H.; Ying, W.; Lian, Z.; Xu, Y.; Zhang, J. One-step sodium bisulfate hydrolysis for efficient production of xylooligosaccharides from poplar. Bioresour. Technol. 2022, 355, 127269. [Google Scholar] [CrossRef]
- Chundawat, S.P.; Venkatesh, B.; Dale, B.E. Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol. Bioeng. 2007, 96, 219–231. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Li, X.; Chen, Q.; Hao, L.; Zhang, J.; Zhu, X.; Jia, B. An acidic-groups detection method and its application to analysis of Chinese humic acid samples. PLoS ONE 2020, 15, e238061. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.; Baloch, H.A.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A. Upgradation of chemical; fuel; thermal, and structural properties of rice husk through microwave-assisted hydrothermal carbonization. Environ. Sci. Pollut. Res. 2018, 25, 17529–17539. [Google Scholar] [CrossRef]
- Matuana, L.M.; Balatinecz, J.J.; Sodhi, R.N.S.; Park, C.B. Surface characterization of esterified cellulosic fibers by XPS and FTIR spectroscopy. Wood Sci. Technol. 2001, 35, 191–201. [Google Scholar] [CrossRef]
| Temperature (°C) | Solid Yield (wt %) | Glucan (wt %) | Xylan (wt %) | Arabinan (wt %) | Lignin (wt %) | Removal (wt %) | ||
|---|---|---|---|---|---|---|---|---|
| Glucan | Xylan | Lignin | ||||||
| Raw Material | - | 25.4 ± 0.4 | 18.8 ± 0.5 | 7.9 ± 0.3 | 27.8 ± 0.1 | - | - | - |
| 150 | 78.6 ± 0.5 | 29.9 ± 0.5 | 17.7 ± 0.6 | 1.7 ± 0.1 | 30.9 ± 0.4 | 7.5 ± 0.2 | 26.0 ± 0.1 | 12.5 ± 0.4 |
| 160 | 68.1 ± 0.1 | 34.4 ± 0.1 | 11.9 ± 0.6 | 0.0 ± 0.0 | 35.4 ± 0.8 | 7.9 ± 0.1 | 56.9 ± 0.4 | 13.2 ± 0.4 |
| 170 | 59.8 ± 0.2 | 36.6 ± 0.5 | 8.8 ± 0.2 | 0.0 ± 0.0 | 36.0 ± 0.1 | 13.8 ± 0.2 | 72.0 ± 0.9 | 22.4 ± 0.5 |
| 180 | 49.0 ± 0.4 | 36.8 ± 0.2 | 6.1 ± 0.3 | 0.0 ± 0.0 | 39.8 ± 0.3 | 29.1 ± 0.9 | 84.0 ± 0.7 | 29.8 ± 0.9 |
| Projects | C (wt %) | H (wt %) | O (wt %) | N (wt %) | S (wt %) | O/C | H/C |
|---|---|---|---|---|---|---|---|
| CHA | 41.8 ± 0.4 | 3.4 ± 0.2 | 24.9 ± 0.5 | 1.1 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.1 | 1.0 ± 0.1 |
| HLA 210-11 | 70.7 ± 0.6 | 7.5 ± 0.2 | 18.8 ± 0.6 | 1.5 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 1.3 ± 0.1 |
| HLA 210-13 | 71.20.7 | 7.6 ± 0.4 | 18.6 ± 0.2 | 1.5 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 1.3 ± 0.0 |
| HLA 210-15 | 72.30.5 | 8.0 ± 0.3 | 17.2 ± 0.4 | 1.2 ± 0.1 | 0.0 ± 0.0 | 0.2 ± 0.0 | 1.3 ± 0.1 |
| Projects | a C1 (%) | b C2 (%) | c C3 (%) | O/C |
|---|---|---|---|---|
| CHA | 39.0 ± 0.2 | 43.6 ± 0.6 | 17.4 ± 0.3 | 0.26 ± 0.1 |
| HLA 210-11 | 57.1 ± 0.7 | 35.5 ± 0.6 | 7.4 ± 0.4 | 0.18 ± 0.1 |
| HLA 210-13 | 52.0 ± 0.6 | 38.2 ± 0.4 | 9.8 ± 0.5 | 0.18 ± 0.0 |
| HLA 210-15 | 51.4 ± 0.6 | 42.0 ± 0.5 | 6.6 ± 0.2 | 0.16 ± 0.1 |
| Item | Properties | Vinegar Residue | Hydrochar 210-13 |
|---|---|---|---|
| Higher heating value | HHV (MJ/kg) | 18.6 ± 0.8 | 16.1 ± 0.4 |
| Elemental analysis | Carbon (wt %) | 45.1 ± 0.4 | 41.2 ± 0.6 |
| Oxygen (wt %) | 46.3 ± 0.5 | 52.3 ± 0.7 | |
| Hydrogen (wt %) | 6.6 ± 0.1 | 6.2 ± 0.3 | |
| Nitrogen (wt %) | 1.7 ± 0.1 | 0.3 ± 0.1 | |
| Chemical composition | Glucan (wt %) | 25.4 ± 0.4 | 43.1 ± 0.2 |
| Xylan (wt %) | 18.8 ± 0.5 | 7.4 ± 0.5 | |
| Lignin (wt %) | 27.8 ± 0.1 | 14.5 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, N.; Zhu, Y.; Li, H.; Yu, Y.; Xu, Y.; Zhu, J. Two-Step Hydrothermal Pretreatments for Co-Producing Xylooligosaccharides and Humic-like Acid from Vinegar Residue. Fermentation 2023, 9, 589. https://doi.org/10.3390/fermentation9070589
Jiao N, Zhu Y, Li H, Yu Y, Xu Y, Zhu J. Two-Step Hydrothermal Pretreatments for Co-Producing Xylooligosaccharides and Humic-like Acid from Vinegar Residue. Fermentation. 2023; 9(7):589. https://doi.org/10.3390/fermentation9070589
Chicago/Turabian StyleJiao, Ningxin, Yuanyuan Zhu, Haoran Li, Yongjian Yu, Yong Xu, and Junjun Zhu. 2023. "Two-Step Hydrothermal Pretreatments for Co-Producing Xylooligosaccharides and Humic-like Acid from Vinegar Residue" Fermentation 9, no. 7: 589. https://doi.org/10.3390/fermentation9070589
APA StyleJiao, N., Zhu, Y., Li, H., Yu, Y., Xu, Y., & Zhu, J. (2023). Two-Step Hydrothermal Pretreatments for Co-Producing Xylooligosaccharides and Humic-like Acid from Vinegar Residue. Fermentation, 9(7), 589. https://doi.org/10.3390/fermentation9070589






