Abstract
Fumaric acid is used in various areas of the chemical industry due to its functional groups. For example, it is used in the polymer industry to produce unsaturated polyester resins, which are nowadays mostly produced from fossil raw materials. With regard to sustainable biotechnological fumaric acid production, the main challenge is to develop a cost-effective and robust fermentation process with industrially relevant final titers, productivities and yields. For biotechnological fumaric acid production, mainly fungi of the genus Rhizopus are used, which require very complex and challenging morphology control. The aim of this work is the development of an effective biotechnological fumaric acid production process with R. arrhizus NRRL 1526. Significant insights into the morphology control of the fungus and optimization of production characteristics were obtained, and a final titer of 86.3 g/L fumaric acid was achieved in a batch cultivation, with a yield of 0.67 g/g and a productivity of 0.60 g/(L∙h). In addition, a fed-batch process was developed, in which the production phase was extended, and a maximum final titer of 195.4 g/L fumaric acid was achieved. According to current knowledge, this value is the highest final concentration of fumaric acid produced using biotechnology.
1. Introduction
Fumaric acid (FA) is a naturally occurring unsaturated dicarboxylic acid with diverse fields of application. Due to its double bond and two carboxylic groups, FA is used as comonomer for polymerization and esterification reactions, resulting in unsaturated polyester resins and alkyd resins. In the pulp industry, FA is applied as acidic tackifier for the production of rosin paper [1,2,3]. Additionally, FA is used as acidulant and nutritional additive in the food and feed sector [1,4,5]. More recently, derivatives of FA like fumaric acid esters have been identified to have potential biomedical applications, such as multiple sclerosis and psoriasis treatment [6,7,8]. All these different fields of application lead to a growing demand for FA, which is currently fulfilled exclusively through the chemical synthesis of petroleum-derived maleic acid anhydride. However, as petroleum prices are rising and the emphasis on low carbon footprint production strategies is increasing, there is a renewed interest in the biotechnological production of FA, which was operational during the 1940s by Pfizer, but was discontinued due to the more economical method of petrochemical-based synthesis [4].
For the microbial production of FA, multiple fungal strains have been identified as natural overproducers, including Rhizopus species (e.g., arrhizus and oryzae) as the most promising productions strains [9,10,11,12]. In fungal metabolism, the substrate glucose, after conversion into pyruvate, is converted into fumaric acid via two different metabolic pathways: (1) in the tricarboxylic acid (TCA) cycle, which is present in all eukaryotic organisms and occurs in the mitochondria and (2) in the reductive TCA cycle, which occurs in the cytosol. The reductive TCA cycle is responsible for the overproduction of fumaric acid in filamentous fungi, and requires CO2 fixation [1,13].
Depending on the metabolic pathway, different theoretical yields are calculated. (1) The formation of fumaric acid via the TCA cycle allows a theoretical yield of a maximum of 1 mol of fumaric acid/mol of glucose consumed, or 0.64 gFA/gglucose. (2) Reductive carboxylation allows a maximum yield of 2 moles of fumaric acid/mole of glucose consumed, or 1.29 gFA/gglucose. In practice, however, a lower yield is expected, since an exclusive course of reductive carboxylation would lead to an energy deficit due to ATP consumption during CO2 fixation. Therefore, in the fermentative production of fumaric acid, the oxidative citric acid cycle is also active to maintain the energy balance [14,15,16].
To reach high product titer, productivity and product yield via submerged fermentations, the control of fungal morphology is one of the most challenging tasks within the bioprocess. In general, the formation of fungal biomass can be distinguished as clumps, disperse filaments or pellets [1,17]. Taking the specific production potential into account, clump and filamentous morphologies show a high tendency to grow on cultivation equipment like bioreactor internals and walls [18,19,20]. This growth behavior causes a poor oxygen supply and leads to the unintentional production of ethanol [13]. In contrast, the formation of small spherical pellets promotes oxygen mass transfer due to a lower overall medium viscosity [13,15,21]. In recent decades, a wide variety of scientific research has been published in the field of morphological control, focusing on the influence of different cultivation parameters. Identified factors affecting the growth behavior and the production performance include physical and chemical parameters like the cultivation system itself, inoculum size, working volume, agitation, aeration, pH and temperature. The second group of factors are medium-related parameters such as nutrient supply, the use of complex medium components and the concentration of metal ions [18]. The concentration of provided nitrogen in particular affects the relationship between additional biomass growth and the accumulation of FA during the production phase [3]. All the previously mentioned parameters influence the final type of morphology and necessitate a targeted optimization of each process stage during the development of a promising strategy for biotechnological FA production.
Comparing the numerous published approaches, Ling and Ng (1989) described in a patent of Du Pont a fermentation procedure with a regulated oxygen concentration of 80% during the production phase [12]. This strategy allowed the production of 135.3 g/L FA with a productivity of 1.77 g/(L∙h) and a yield of 1.04 g/g. To date, this fermentation represents the best biotechnological production of FA with the highest final titer. Furthermore, later published studies stayed remarkably far behind these results [1,13,17,22], making the fermentative procedure of Ling and Ng (1989) [12] a highly interesting template for further investigations [23].
To control the morphology and, therefore, achieve reproducible and comparable cultivations, the effect of the carbon to nitrogen (C/N) ratio was investigated. Based on this batch fermentation, a specific feeding strategy of glucose and ammonium was developed to extend the production phase and reach industrially relevant final titers of FA.
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
Rhizopus arrhizus NRRL 1526 was obtained from the Agricultural Research Service Culture Collection (Peoria, IL, USA). For experimental usage, the strain was stored in the form of spores in 50% glycerol at −80 °C (stock culture). For inoculum preparation, a stock culture was spread on agar plates (medium A) at 32 °C, containing 4 g/L glucose, 10 mL/L glycerol, 6 g/L lactose, 0.6 g/L urea, 0.4 g/L KH2PO4, 1 mL/L corn steep liquid, 1.6 g/L tryptone/peptone, 0.3 g/L MgSO4 × 7 H2O, 0.088 g/L ZnSO4 × 7 H2O, 0.25 g/L FeSO4 × 7 H2O, 0.038 g/L MnSO4 × H2O, 0.00782 g/L CuSO4 × 5 H2O, 40 g/L NaCl, 0.4 g/L KCl and 30 g/L agar-agar. After six days of sporulation, the spores were suspended by adding 0.9 % (w/w) NaCl solution. The resulting spore solution was stored at 4 °C until inoculation. The spore concentration was determined using a counting chamber (Thoma) and a Zeiss microscope (Axioplan, Carl Zeiss AG, Oberkochen, Germany). Chemicals were either purchased from Merck KGaA (Darmstadt, Germany), Carl Roth GmbH and Co. KG (Karlsruhe, Germany) or from Sigma Aldrich (St. Louis, MO, USA) in an appropriate purity for biochemistry.
2.2. Pre-Culture Conditions
For pre-culture, 500 mL shaking flasks (unbaffled) with 50 g/L CaCO3 (precipitated, ≥99%, VWR) were sterilized (20 min at 121 °C) then 100 mL of sterile fermentation medium B was added. The composition of medium B was 130 g/L glucose, 1.2 g/L (NH4)2SO4, 0.3 g/L KH2PO4, 0.4 g/L MgSO4 × 7 H2O, 0.044 g/L ZnSO4 × 7 H2O and 0.0075 g/L FeCl3 × 6 H2O. All components of medium B were prepared separately in stock solutions and heat-sterilized; the iron solution was sterile-filtered. For inoculation, spore suspension was added to ensure an initial concentration of 1 × 105 spores/mL. Pre-cultures were carried out at 34 °C and 200 rpm in a rotary shaking flask incubator for 24 h.
2.3. Batch Fermentations
Batch cultivations were studied in 500 mL shaking flasks (unbaffled), each containing 50 g/L CaCO3 and 90 mL medium B at 34 °C, in a rotary incubator at 200 rpm. Then, 10 mL of the 24 h seed culture was transferred into the fermentation medium for inoculation. The solubility of FA at room temperature is very low, 5–7 g/L [22]. CaCO3 is used as neutralizing agent, so calcium fumarate precipitates due to low solubility [17]. Next, 2 mL well-mixed samples were taken periodically, diluted with a 5% (w/w) HCl solution and heated to 80 °C to remove excessive CaCO3 and resolve precipitated calcium fumarate. After 2 d of fermentation, 20 g/L CaCO3 was added under sterile conditions to maintain a pH value of approximately 6.0. Within all shaking flask cultivations, the weight loss due to evaporation was balanced by the addition of deionized water. All cultivations were performed in duplicate.
2.4. Fed-Batch Fermentations
To prolong the phase of FA production, batch fermentations in shaking flasks were extended by the addition of 40 g/L or 80 g/L glucose (solid) before reaching a glucose concentration of less than 30 g/L. In addition, whenever the cultivation showed decreasing FA productivity, 0.6 g/L or 1.2 g/L (NH4)2SO4 (200 g/L stock solution) was transferred into the broth. Depending on the over production of organic acids, portions of 20 g/L CaCO3 were added periodically for pH control. Within all shaking flask cultivations, the weight loss due to evaporation was balanced by the addition of deionized water. All cultivations were performed in duplicate.
2.5. Analytical Methods
Glucose, FA, other organic acids (malic acid and succinic acid) and ethanol were quantified through high-performance liquid chromatography (HPLC) with an HPX-87H ion-exclusion column (300 × 7.8 mm) (Bio-Rad, Hercules, CA, USA), a refraction index (RI) detector (Shodex RI-101, Shōwa Denkō, Tokyo, Japan) and ultraviolet (UV) detector (LaChrom Elite L-2400, Hitachi, Tokyo, Japan) at 250 nm. The column was tempered at 40 °C and eluted with a 5 mM H2SO4 solution at a flow rate of 0.6 mL/min.
The concentration of the cation ammonium and the anion phosphate were determined through ion chromatography (IC) (Dionex ICS-100 IC, Thermo Fisher Scientific Inc., Sunnyvale, CA, USA). Cations were quantified with an IonPac CS16 column (cation-IC, 5 × 250 mm) and a suppressor CSRS-500 (4 mm) at 88 mA and a temperature of 40 °C, and eluted with 30 mM methlysulfonic acid at a flow rate of 1 mL/min. Anions were quantified with an IonPac AG11-HC (cation-IC, 4 × 250) and a suppressor ASRS-300 (4 mm) at 68 mA and a temperature of 40 °C, and eluted with 25 mM sodium hydroxide at a flow rate of 1 mL/min. For measurement, samples were diluted with ultrapure water, filtered using a nylon syringe filter (pore size 0.22 μm), and a sample volume of 2 mL was transferred to a Dionex Polyvial with a filter cap (Thermo Fisher Scientific Inc., Sunnyvale, CA, USA). External cation and anion multi-element standards (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) were measured for each series of measurements to calibrate the IC system.
The morphology of R. arrhizus NRRL 1526 was documented with a phase-contrast microscope (Axioplan, Carl Zeiss AG, Oberkochen, Germany) and processed with the software Analysis (Analysis 5.0, Soft Imaging System GmbH).
The yield is calculated from the product concentration of fumaric acid relative to the concentration of substrate consumed up to that cultivation time and is described by the product yield coefficient (Equation (1)).
where:
YP/S = cFAt/(cs0 − cSt),
- YP/S—product yield coefficient (g/g);
- cFAt—fumaric acid concentration at time t (g/L);
- cS0—initial concentration of the substrate (g/L);
- cSt—substrate concentration at time t (g/L).
Productivity is calculated from the quotient of the concentration of fumaric acid formed and the cultivation period required for this (Equation (2)). Here, the maximum productivity is defined as the productivity that assumes the highest value between two sampling points.
where:
P = (cFat − cFA0)/Δt,
- P—productivity (g/(L∙h));
- cFAt—fumaric acid concentration at time t (g/L);
- cFA0—initial concentration of fumaric acid (g/L);
- Δt—cultivation period (h).
3. Results and Discussion
Based on the procedure of Ling and Ng (1989) as a benchmark, an effective manufacturing process for the biotechnological production of fumaric acid was investigated in the present study [12].
Cultivation with the process strategy of Ling and Ng (1989) allowed the production of 29.5 g/L fumaric acid within 6 d in shake flasks. Under complete glucose consumption, this corresponds to a yield of 0.26 g/g, with a productivity of 0.21 g/(L∙h). As by-products, malic and succinic acid with, in sum, a maximum of 5.7 g/L after 5 d were detected. The main by-product produced within the first two days of cultivation time was ethanol, formed at a concentration of 18.1 g/L. The accumulation of ethanol here indicates oxygen-limiting conditions within the cultivation system. The biomass of R. arrhizus NRRL 1526 was predominantly present as dense pellets with a diameter of >1 mm in the main culture. This form of compact biomass creates potentially anaerobic conditions inside the pellets that favor ethanol formation while negatively affecting fumaric acid production [24]. Comparable to the findings in the literature, a direct transfer of Ling and Ng’s (1989) cultivation strategy and their published results of 135.3 g/L FA with a productivity of 1.77 g/(L∙h) and a yield of 1.04 g/g was not possible.
Therefore, the influence of different cultivation parameters on morphology, reproducibility, productivity and yield was investigated in the following experiments. Subsequently, a process strategy was developed on the basis of the findings obtained.
3.1. Optimizing Cultivation Parameters
- Influence of process steps
According to Ling and Ng (1989), three individual process steps (spore production, pre-culture and main culture) and glucose as the carbon source were used for FA production [12]. To date, no cultivation conditions have been observed to induce and favor submerged spore production of R. arrhizus NRRL 1526. Therefore, spores were produced via the surface method using agar plates.
Within the overall process, during the pre-culture, the germination of spores and building up biomass take place. Various cultivation parameters, such as the size of shake flasks, the shaking speed, the cultivation temperature or the composition of the medium, have a major influence on the morphology of the pre-culture and, thus, also on the cultivation result of the main culture [17,21,25,26]. The morphology of Rhizopus sp. can be divided into three different growth forms: clumps, pellets and mycelium. The production behavior of FA differs significantly depending on the corresponding growth form of the fungus [1]. Which form of morphology is formed within the cultivation system depends largely on the process parameters described above. However, these influencing factors cannot be considered alone. Rather, the morphology results from a complex interaction of all cultivation parameters. This fact not only limits the comparability of different research studies with each other, but also makes it difficult to identify generally valid regularities in the targeted control of morphology [3,25].
Regarding the potential to accumulate fumaric acid, various publications exist that achieved more effective production of FA with either mycelium or pellet morphology. For example, Rhodes et al. (1962) and Papadaki et al. (2017) realized better FA production using mycelium morphology than pellets [26,27]. Opposing results were obtained in studies by Liao et al. (2007) and Zhou et al. (2011) [21,28]. The contradictory results of these publications thus indicate that a generally valid preference of a certain growth form is not possible. Rather, when developing an effective cultivation system for the production of FA, it makes sense to identify specifically occurring problems and optimization potentials in the context of morphology and, if necessary, to counteract them specifically by controlling the morphology.
In order to perform an optimization of the actual fermentation (main culture) despite this complex interplay of different influencing factors, the main culture was directly inoculated with the spore suspension. By omitting the pre-culture, the influence of process changes on the production of fumaric acid could be directly investigated. The direct spore inoculation of the main culture also allowed an increase in the general reproducibility of cultivations.
- Influence of spore concentration
To investigate the influence of spore concentration, cultivations were inoculated with different amounts of spores (1 × 104, 1 × 105, 1 × 106 spores/mL). Cultivations with concentrations < 1 × 105 spores/mL were found to cause clump morphology and, thus, were not suitable for fumaric acid production. Above a concentration of 1 × 105 spores/mL, R. arrhizus NRRL 1526 grew as loose mycelium, and the production of approximately 50 g/L of fumaric acid was observed after a duration of 7 d, with yields of 0.44 and 0.43 g/g, respectively. A comparison of this result with the literature data shows that the use of higher spore concentrations is quite common in cultivations for the production of fumaric acid. For example, Riscaldati et al. (2000), Zhou et al. (2011) and Das et al. (2015) used a spore concentration of 1 × 106 spores/mL to inoculate the pre-culture [21,29,30]. At 1 × 107 spores/mL, Fu et al. (2010), Ding et al. (2011) and Gu et al. (2014) used the highest spore concentration reported in the literature for inoculating cultivations [31,32,33]. A further increase in spore concentration during cultivation inoculation did not improve fumaric acid production in this work. Thus, a spore concentration of 1 × 105 spores/mL was used for subsequent cultivations with direct spore inoculation.
- Influence of corn steep liquor
According to Ling and Ng (1989), different amounts of corn steep liquor were used for preparing the different culture media (sporulation: 1 mL/L; germination: 0.5 mL/L; production: 0.5 mL/L) for FA production [12]. Corn steep liquor is a complex medium component that can differ significantly in composition depending on the batch and manufacturer. It contains different concentrations of peptides, amino acids, vitamins, nucleotides and various trace elements [34]. Thus, by using two different batches of corn steep liquor and different concentrations in the range of 0.1 to 1 mL/L, corn steep liquor resulted in different morphologies and, thus, a different production of fumaric acid could also be observed. Alternatively, different concentrations of yeast extract (0.1 until 1 g/L) were used in cultivations. However, all cultivations using corn steep liquor or yeast extract yielded lower concentrations of fumaric acid compared to cultivations without the addition of a complex medium component. The morphology obtained exhibited almost exclusively mycelial morphology. In the literature, several publications have already described biomass and fumaric acid production using minimal media [35,36,37]. For example, in Wang et al. (2013), using pre-culture and main culture without complex medium components, cultivation was able to produce 56.5 g/L of fumaric acid, with yields of 0.71 g/g and productivities of 0.67 g/(L∙h) [36].
Therefore, in the adapted cultivation strategy, the addition of complex medium components was completely omitted and, thus, a precisely defined minimal medium was used.
- Influence of tartaric acid
Tartaric acid is a saturated dicarboxylic acid. In the cultivation medium of Ling and Ng (1989), 0.0075 g/L tartaric acid was used [12]. To investigate the principle necessity of tartaric acid for the production of fumaric acid, cultivation with 0.0075 g/L (2S,3R)-tartaric acid and cultivation without the addition of tartaric acid were performed. Cultivation with R. arrhizus NRRL 1526 with and without the addition of tartaric acid demonstrated that a concentration of 0.0084 g/L (2S,3R)-tartaric acid × H2O had no effect on the fermentation process. An identical concentration of fumaric acid of approximately 50.5 g/L, with a yield of 0.41 g/g and productivity of 0.31 g/(L∙h), was obtained in both preparations after 7 d. Thus, the addition of tartaric acid was omitted in subsequent studies.
- Influence of calcium carbonate
To regulate the pH value, 50 g/L calcium carbonate was added to the system in previous cultivations. Due to the optimizations made and the resulting increase in the production of fumaric acid, the calcium carbonate is completely consumed during cultivation. This was recognizable by a drop in the pH value as the production of fumaric acid progressed. To counteract a drop in pH occurring too early on, which negatively influences the formation of fumaric acid, a threefold feed of 10 g/L calcium carbonate was performed after 3, 4 and 5 d. This successfully stabilized the pH at a value of 6, thus realizing an improvement in fumaric acid production. In addition, the calcium carbonate particle size and shape have been shown to be very important parameters for the building of a proper morphology and, therefore, have direct effects on the FA production, productivity and yield [16]. Depending on the particle size of CaCO3, different morphologies of R. arrhizus NRRL 1526 occurred. Using CaCO3 (precipitated, ≥99%, VWR), which has the smallest particle size, the best production of fumaric acid was obtained for loose mycelial morphology [38].
- Influence of ammonium and phosphate
For biomass formation, 1.8 g/L (NH4)2SO4 is used as the nitrogen source in the main culture medium, according to Ling and Ng (1989) [12]. To optimize the amount of growth-relevant ammonium, different initial concentrations of 0.3–3.6 g/L (NH4)2SO4 were used. Using 0.3 g/L (NH4)2SO4, only a low growth of R. arrhizus NRRL 1526 could be detected, which only allowed a very slow production of fumaric acid. In contrast, at concentrations of 3.6 g/L, excessive biomass formation was documented, leading to clump formation. At initial concentrations between 0.6 g/L and 1.8 g/L ammonium sulfate, an increasing production of by-products (e.g., ethanol) could be shown under mycelial morphology. This resulted in a decrease in the yield of fumaric acid with increasing concentrations of ammonium sulfate. With regard to the effective production of fumaric acid, an initial concentration of 1.2 g/L (NH4)2SO4 showed an ideal compromise in terms of productivity and yield. Therefore, for further cultivations, 1.2 g/L (NH4)2SO4 was used in the fermentation medium.
Besides ammonium, phosphate is another component of the main culture medium that significantly influences the biomass growth of R. arrhizus NRRL 1526. Cultivations with 0.05–0.60 g/L KH2PO4 were performed to identify the ideal initial concentration. This series of experiments showed that the best cultivation result in terms of final titer, yield and productivity was achieved with 0.30 g/L KH2PO4. Thus, the concentration of KH2PO4 determined according to Ling and Ng (1989) was not changed.
- Influence of initial glucose concentration
So far, an initial glucose concentration of 130 g/L has been used, according to Ling and Ng (1989) [12]. However, a comparison with the literature shows that the use of lower concentrations such as 80 g/L glucose is quite common [39,40]. In addition to being a carbon and energy source and being used for the production of fumaric acid, glucose is also used for the production of biomass. With regard to an optimal ratio of built-up biomass and the potential to produce fumaric acid, the C/N ratio thus plays a decisive role in cultivation [32,41].
Therefore, cultivations with different initial concentrations of glucose (40–200 g/L) were carried out. In all cultivations, a drop in the pH value was prevented by adding additional calcium carbonate. Furthermore, the medium contained a concentration of 1.20 g/L (NH4)2SO4. The resulting different C/N ratios are shown comparatively in Table 1. In addition, this table contains an overview of the morphology achieved and the corresponding cultivation time until complete conversion of the glucose.

Table 1.
Cultivations with different initial concentrations of glucose (40–200 g/L) using R. arrhizus NRRL 1526 with direct spore inoculation (1 × 105 spores/mL) and optimized medium (100 mL of medium B) in 500 mL shake flasks without baffles at 34 °C and 200 rpm, and 50 g/L CaCO3 with addition of 10 g/L CaCO3 after 3, 4 and 5 d.
In relation to the morphology obtained, the growth of R. arrhizus NRRL 1526 in the form of loose mycelial flakes was observed in a concentration range of 60–200 g/L initial glucose. In contrast, in the cultivation approach with the lowest glucose concentration of 40 g/L, clumps formed, which are unsuitable for the production of fumaric acid. Taking into account the C/N ratio of 63 gC/gN used in this approach, a critical minimum of approximately 60–90 gC/gN can thus be identified, which should not be fallen short of in order to form the preferred mycelial morphology. In the cultivations described in the subsection “Influence of ammonium and phosphate”, the formation of mycelium was observed at a glucose concentration of 130 g/L and 1.80 g/L (NH4)2SO4 (C/N ratio of 136 gC/gN). In contrast, the cultivation batch with 130 g/L glucose and 3.60 g/L (NH4)2SO4 (C/N ratio of 68 gC/gN) showed compact clumps. Thus, based on these results, the influence of the C/N ratio on the growth form could be confirmed and, thus, a targeted control of the morphology in the cultivation system used here could be made possible.
In order to investigate the influence of the initial glucose concentration on the pro-duction of fumaric acid, the results of the individual cultivation approaches were shown in Figure 1 after complete consumption of glucose.
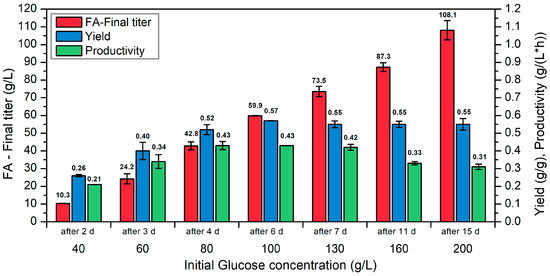
Figure 1.
Cultivations with different initial concentrations of glucose (40–200 g/L) using R. arrhizus NRRL 1526 with direct spore inoculation (1 × 105 spores/mL) and optimized medium (100 mL of medium B) in 500 mL shake flasks without baffles at 34 °C and 200 rpm, and 50 g/L CaCO3 with addition of 10 g/L CaCO3 after 3, 4 and 5 d.
With increasing initial glucose concentration, more product is formed, and with 200 g/L glucose, the highest final concentration of 108.1 g/L FA was achieved in this set of experiments (Figure 1). However, this tendency could not be confirmed in terms of yield and productivity. Rather, with regard to these two process parameters, an optimum range of 80–130 g/L initial glucose was identified. Thus, the cultivation approach with 100 g/L glucose showed the highest yield (0.57 g/g). At initial glucose concentrations higher than 100 g/L, however, only a minimally lower yield of 0.55 g/g was detected. With regard to the achieved productivities, the highest productivities were detected in the range of 80–130 g/L glucose, with 0.43 and 0.42 g/(L∙h), respectively. A further increase in the initial glucose concentration leads to lower productivities. This showed that FA concentrations above 100 g/L are possible, but that a fed-batch strategy should be investigated.
3.2. Direct Spore Inoculation Compared to Pre-Culture Inoculation with Optimized Parameters
Based on the previously described optimizations (Section 3.1), the cultivation process shown in Figure 2 was developed.
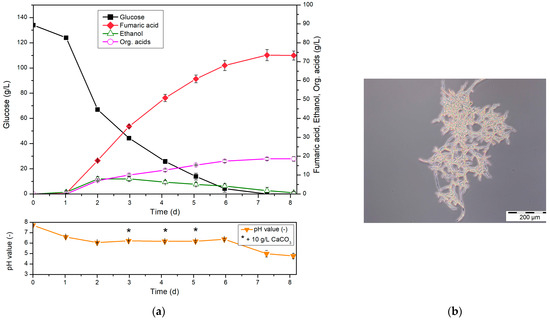
Figure 2.
(a) Cultivation of R. arrhizus NRRL 1526 with direct spore inoculation (1 × 105 spores/mL) and optimized medium (100 mL of medium B) in 500 mL shake flasks without baffles at 34 °C and 200 rpm, and 50 g/L CaCO3 with addition of 10 g/L CaCO3 after 3, 4 and 5 d (left). (b) Morphology of R. arrhizus NRRL 1526 cultured with direct spore inoculation after 3 d (right).
In the optimized cultivation with direct spore inoculation, a lag phase caused by spore germination of 1 day without fumaric acid production was observed. A final titer of 73.5 g/L fumaric acid was achieved after the present concentration of glucose was used up after approximately 7 d. This corresponds to a yield of 0.55 g/g and a total productivity of 0.42 g/(L∙h). A positive aspect is the reduced accumulation of ethanol, which can be attributed to the improved oxygen supply of the biomass.
With regard to the most efficient cultivation process possible for the production of fumaric acid, the cultivation strategy described above with direct spore inoculation still has potential for optimization. For example, due to the initial germination of the spores, no production of fumaric acid takes place within the first 24 h of the main culture. In order to recognize the effects and influences during optimization, the production strategy using spores as an inoculum of the main cultivation has advantages. For an optimized process, on the other hand, the use of a pre-culture has its advantages, e.g., with regard to the key parameters of productivity and yield. Therefore, the use of the previously omitted pre-culture as a process step was reconsidered and further developed. This involves growing biomass (pre-culture) within 1 d, using (10% (vpre-culture/vmain-culture)) as the inoculum. For this purpose, the optimized medium B was used in the pre-culture as well as in the subsequent main culture (Figure 3).
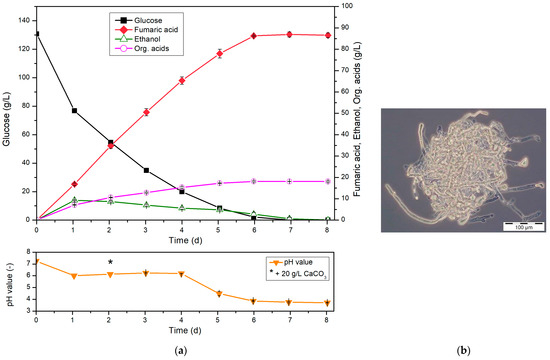
Figure 3.
(a) Cultivation of R. arrhizus NRRL 1526 with 10 % (v/v) pre-culture and optimized medium B in 500 mL shake flasks without baffles at 34 °C and 200 rpm, and 50 g/L CaCO3 with addition of 20 g/L CaCO3 after 2 d (left). (b) Morphology of R. arrhizus NRRL 1526 using pre-culture after 3 d (right).
Due to the outsourced cultivation of the biomass (pre-culture), a direct production of fumaric acid could be detected in this cultivation. With an almost linear increase in fumaric acid, a final titer of 86.3 g/L fumaric acid was reached after 6 d at almost complete conversion of glucose. This corresponds to a yield of 0.67 g/g, with a total productivity of 0.60 g/(L∙h). Thus, compared to cultivation with direct spore inoculation, the lag phase occurring there was successfully transferred to the pre-culture. Since only 10 % (v/v, based on the main culture volume) is required for this, the efficiency of fumaric acid production was successfully improved. In addition, the final titer of fumaric acid as well as the yield and productivity show significantly increased values, which confirm the success of this cultivation strategy. With regard to the yield, it should be noted that a yield higher than 0.64 g/g can no longer be explained exclusively by the oxidative synthesis pathway. Thus, the yield of 0.67 g/g identified here indicates at least partial production of fumaric acid by means of reductive carboxylation [13,16].
Since the production of fumaric acid in this cultivation was completely stopped by the consumption of the supplied amount of glucose, no conclusion can be made about a possible final titer of fumaric acid at this point. However, a comparison of this final titer and especially of the yield with the literature shows that the optimized process conditions and achieved morphology are very well suited for efficient biotechnological production of fumaric acid using R. arrhizus NRRL 1526 (Table 2).

Table 2.
Overview of FA production and yield based on glucose as substrate in batch cultivations using Rhizopus spp.; STR—stirred tank reactor, SF—shaking flask.
3.3. Fed-Batch Cultivation
In order to further increase the final titer of FA, the addition of glucose during the cultivation process was investigated. For this purpose, different process strategies involving an additional dosage of a N-source and CaCO3 were applied, which are described in more detail in the following subsections.
3.3.1. Glucose Feed
The absolute amount of glucose to be converted into FA was increased during cultivation by adding glucose several times. Glucose was added in the form of solids and in an amount leading to an additional 40 g/L in the cultivation medium (Figure 4). The regular addition of calcium carbonate also prevented a drop in pH throughout the cultivation process.
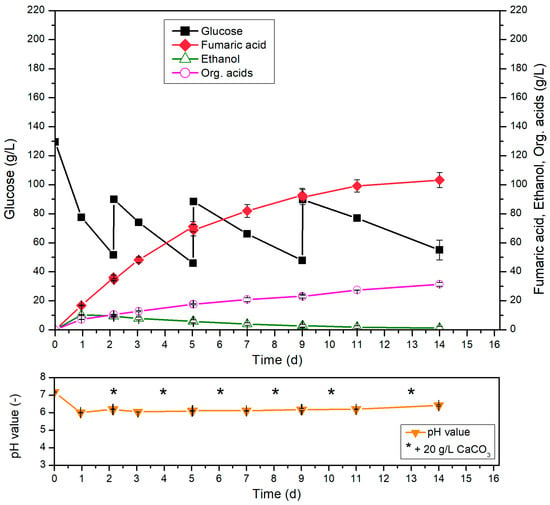
Figure 4.
Fed-batch cultivation of R. arrhizus NRRL 1526 with 10% (v/v) pre-culture and optimized medium B (130 g/L initial glucose) in 500 mL shake flasks without baffles at 34 °C and 200 rpm, and 50 g/L CaCO3 with addition of 40 g/L glucose after 2, 5 and 9 d and addition of 20 g/L CaCO3 after 2, 4, 6, 8, 10 and 13 d.
Under continuous consumption of glucose within 14 d, the total added amount of glucose was not completely metabolized. During the cultivation time, the glucose consumption rate decreased, and a glucose concentration of 55.0 g/L was still detected at the end of cultivation. With respect to the production of fumaric acid, a final titer of 103.2 g/L was achieved after 14 d with the formation of mycelial morphology. This corresponds to a yield of 0.52 g/g, with a productivity of 0.31 g/(L∙h). During the cultivation time, the FA production rate decreased and this prevented effective production of fumaric acid.
Overall, it was demonstrated that glucose can be metabolized even if it is added subsequently during cultivation. However, without further optimization, this process strategy is not suitable for effective FA production, constant glucose consumption rates and constant FA production or FA concentrations higher than 100 g/L.
3.3.2. Glucose and Ammonium Feed
Neither the addition of glucose nor the use of a high initial concentration of glucose provided an effective cultivation system. In particular, the decrease in productivity with advancing cultivation time proved to be problematic. A possible cause for this observation was identified as aging or damage to the biomass formed with increasing cultivation time. In this context, it was hypothesized that the formation of new biomass in an advanced cultivation period could potentially improve productivity. Thus, a process strategy combining the addition of glucose, the usage of a high initial concentration of glucose and the addition of ammonium during cultivation, which, in principle, enables a further growth phase, was investigated (Figure 5).
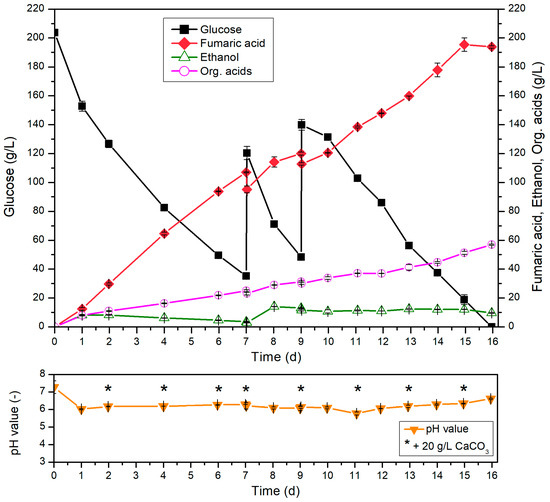
Figure 5.
Fed-batch cultivation of R. arrhizus NRRL 1526 with 10% (v/v) pre-culture and optimized medium B (200 g/L initial glucose) in 500 mL shake flasks without baffles at 34 °C and 200 rpm, and 50 g/L CaCO3 with addition of 80 g/L glucose after 7 and 9 d, addition of 1.2 g/L (NH4)2SO4 after 7 d, addition of 0.6 g/L (NH4)2SO4 after 10, 12 and 14 d and addition of 20 g/L CaCO3 after 2, 4, 6, 7, 9, 11, 13 and 15 d.
During the first 7 days, the usage of a high initial glucose concentration (200 g/L initial glucose) allowed a constant production of 107.0 g/L fumaric acid without any intervention in the cultivation system. At this point, at which approximately 30 g/L glucose remained, glucose and (NH4)2SO4 were added in the form of solids and in amounts leading to an additional 80 g/L glucose and 1.2 g/L (NH4)2SO4 in the cultivation medium. In the following two days, only a small amount of FA was formed under increasing substrate consumption rate. However, ethanol was formed during this period, and its concentration reached a maximum of 14.1 g/L after 8 d. Therefore, the addition of ammonium was omitted during the second addition of 80 g/L glucose (after 9 days). As a result of this adjustment, only low glucose consumption and low FA production were detected between the ninth and tenth day of cultivation. To counteract this trend, a concentration of 0.6 g/L (NH4)2SO4 was added to the cultivation system after 10 d, 12 d and 14 d. Through this process strategy, a complete consumption of the total amount of glucose was documented after 16 d under increased glucose consumption. In relation to the production of FA, a maximum titer of 195.4 g/L could be identified after a cultivation time of 15 d. This corresponds to a yield of 0.54 g/g, with a total productivity of 0.54 g/(L∙h). It was shown that very high productivities are possible at late cultivation times and that effective production of fumaric acid was still possible even at concentrations of >150 g/L fumaric acid. For example, maximum productivities of 0.72 g/(L∙h) (13–14 d) and 0.74 g/(L∙h) (14–15 d) were detected at the end of cultivation.
Related to the obtained morphology of R. arrhizus NRRL 1526, the formation of mycelium was also documented during this cultivation. With increasing cultivation time, the aggregation of individual mycelial flakes into larger agglomerates was observed.
Fumaric acid is currently produced petrochemically. It has a high potential to be produced using biotechnological processes and it has been designated by the US Department of Energy (DOE) as one of the top 12 valuable chemicals to be produced from biomass [43].
This requires the development of a fermentation process that allows specific morphology control of the production strain used and, at the same time, enables effective production of fumaric acid. Biotechnologically produced fumaric acid, in a multi-feedstock biorefinery, could contribute to the development of the circular economy and the bioeconomy [44,45].
The results of this work successfully demonstrated that effective biotechnological fumaric acid production with a high final titer of FA is possible using the provided process strategy. The combined addition of glucose and ammonium provides a promising possibility to build up new biomass during cultivation and, thus, to produce FA constantly over long cultivation periods. As shown, this resulted in very high final titers of 195.4 g/L FA. To our knowledge, this is the highest biotechnologically produced concentration of FA (Table 2). Furthermore, the only slight decrease in yield and productivity compared to the reference cultivation (without the addition of glucose and ammonium, Figure 3) illustrates the high potential of this cultivation approach.
4. Conclusions
In this work, different fermentation strategies were developed on the basis of the substrate glucose and with the production strain Rhizopus arrhizus NRRL 1526, and the respective production behavior was characterized. The patented cultivation procedure of Ling and Ng (1989) served as a template. Based on this, an initial cultivation strategy could be developed on a shake flask-scale, consisting of direct spore inoculation of the main culture. By omitting a pre-culture, a significantly simplified process could be investigated, which allowed systematic medium and process optimization. Significant insights regarding morphology control could be gained. For example, the use of calcium carbonate with a smaller particle size, which was used to regulate pH, allowed the formation of individual mycelial flakes, instead of the unsuitable clump morphology or growth in the form of large and compact pellets. In addition, by varying the C/N ratio used, medium optimization identified a critical minimum of about 90 gC/gN, which should not be fallen below to avoid lumps. Deviating from the starting point, the optimized fermentation medium did not contain any complex medium constituents such as corn steep liquor or yeast extract, the use of tartaric acid was also omitted and a reduced concentration of growth-relevant ammonium sulfate was used. Based on these optimizing cultivation parameters, a different cultivation strategy could be developed on a shake flask-scale.
In another cultivation strategy, the germination of the spores and, thus, the first cultivation of biomass were carried out in a pre-culture. In contrast to cultivation with direct spore inoculation, the occurrence of a lag phase could thus be successfully outsourced to the pre-culture. This had a positive effect on the overall balance of the production process and resulted in a final titer of 86.3 g/L fumaric acid, with a yield of 0.67 g/g and an overall productivity of 0.60 g/(L∙h).
Furthermore, the maximum possible final titer of fumaric acid was determined on the shake flask-scale. For this purpose, the cultivation strategy was modified and extended by adding additional glucose and ammonium during the main culture. Depending on the absolute amount of substrate used, the production phase could be successfully extended to a duration of 15 d. This allowed a final concentration of fumaric acid to be determined. As a result, a final titer of fumaric acid of 195.4 g/L could be achieved. These values are by far the highest final titers of fumaric acid achieved through fermentation that have been published, according to the best of our knowledge.
Author Contributions
Conceptualization, L.E., A.K. and U.P.; methodology, L.E.; investigation, L.E.; data curation, L.E.; writing—original draft preparation, A.K.; writing—review and editing, L.E., A.K. and U.P.; supervision, A.K. and U.P.; project administration, U.P. and A.K.; funding acquisition, A.K. and U.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the German Federal Ministry of Food and Agriculture, following a decision of the German Bundestag, via the Agency of Renewable Resources (Grant No. 22029515).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting this paper results are included in this document.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Roa Engel, C.A.; Straathof, A.J.J.; Zijlmans, T.W.; Gulik, W.M.; van der Wielen, L.A.M. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Tsao, G.T.; Cao, N.J.; Du, J.; Gong, C.S. Production of multifunctional organic acids from renewable resources. In Recent Progress in Bioconversion of Lignocellulosics; Springer: Berlin/Heidelberg, Germany, 1999; pp. 243–280. [Google Scholar]
- Das, R.K.; Brar, S.K.; Verma, M. Chapter 8—Fumaric acid: Production and application aspects. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–157. [Google Scholar]
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic acids: Old metabolites, new themes. J. Chem. Technol. Biotechnol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- McGinn, S.; Beauchemin, K.; Coates, T.; Colombatto, D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Weißert, R. Multiple Sklerose-Risiken und Nutzen der neuen antiinflammatorischen Substanzen. J. für Neurol. Neurochir. und Psychiatr. 2014, 16, 95–101. [Google Scholar]
- Smith, D. Fumaric acid esters for psoriasis: A systematic review. Ir. J. Med. Sci. 2017, 186, 161–177. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Recent advances in the biomedical applications of fumaric acid and its ester derivatives: The multifaceted alternative therapeutics. Pharmacol. Rep. 2016, 68, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S.A. Process for the Production of Fumaric Acid. US Patent 2,326,986 (to Merck & Co., Inc. and Pfizer & Co., Inc.), 1943. [Google Scholar]
- Lubowitz, H.R.; La, R.E.G. Fumaric Acid Fermentation Process. US Patent 2,861,922 (to National Distillers and Chemical Corporation), 1958. [Google Scholar]
- Goldberg, I.; Stieglitz, B. Fermentation Process for Production of Carboxylic Acids. US Patent 4,564,594 (to E. I. Du Pont de Nemours and Company, Wilmington, Del.), 1986. [Google Scholar]
- Ling, L.B.; Ng, T.K. Fermentation Process for Carboxylic Acids. US Patent 4,877,731 (to E. I. Du Pont de Nemours and Company, Wilmington, Del.), 1989. [Google Scholar]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef]
- Kenealy, W.; Zaady, E.; du Preez, J.C.; Stieglitz, B.; Goldberg, I. Biochemical aspects of fumaric acid accumulation by Rhizopus arrhizus. Appl. Environ. Microbiol. 1986, 52, 128–133. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, B.; Yang, S.T. Production of citric, itaconic, fumaric and malic acids in filamentous fungal fermentations. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals and Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 375–397. [Google Scholar]
- Martin-Dominguez, V.; Cabrera, P.I.A.; Eidt, L.; Pruesse, U.; Kuenz, A.; Ladero, M.; Santos, V.E. Production of Fumaric Acid by Rhizopus arrhizus NRRL 1526: A Simple Production Medium and the Kinetic Modelling of the Bioprocess. Fermentation 2022, 8, 64. [Google Scholar] [CrossRef]
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.D.B.; Santos, V.E.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef]
- Byrne, G.S.; Ward, O.P. Growth of Rhizopus arrhizus in fermentation media. J. Ind. Microbiol. 1989, 4, 155–161. [Google Scholar] [CrossRef]
- Kosakai, Y.; Soo Park, Y.; Okabe, M. Enhancement of L(+)-lactic acid production using mycelial flocs of Rhizopus oryzae. Biotechnol. Bioeng. 1997, 55, 461–470. [Google Scholar] [CrossRef]
- Ilica, R.A.; Kloetzer, L.; Galaction, A.I.; Caşcaval, D. Fumaric acid: Production and separation. Biotechnol. Lett. 2018, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Du, G.; Hua, Z.; Zhou, J.; Chen, J. Optimization of fumaric acid production by Rhizopus delemar based on the morphology formation. Bioresour. Technol. 2011, 102, 9345–9349. [Google Scholar] [CrossRef]
- Sebastian, J.; Hegde, K.; Kumar, P.; Rouissi, T.; Brar, S.K. Bioproduction of fumaric acid: An insight into microbial strain improvement strategies. Crit. Rev. Biotechnol. 2019, 39, 817–834. [Google Scholar] [CrossRef]
- Eidt, L. Nutzung Nachwachsender Rohstoffe für die Biotechnologische Produktion von Fumarsäure. Ph.D. Thesis, Technical University of Braunschweig, Braunschweig, Germany, 2021. [Google Scholar] [CrossRef]
- Roa Engel, C.A.; van Gulik, W.M.; Marang, L.; van der Wielen, L.A.M.; Straathof, A.J.J. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzyme Microb. Technol. 2011, 48, 39–47. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Papadaki, A.; Androutsopoulos, N.; Patsalou, M.; Koutinas, M.; Kopsahelis, N.; Castro, A.M.d; Papanikolaou, S.; Koutinas, A.A. Biotechnological production of fumaric acid: The effect of morphology of Rhizopus arrhizus NRRL 2582. Fermentation 2017, 3, 33. [Google Scholar] [CrossRef]
- Rhodes, R.A.; Lagoda, A.A.; Misenheimer, T.J.; Smith, M.L.; Anderson, R.F.; Jackson, R.W. Production of fumaric acid in 20-liter fermentors. Appl. Microbiol. 1962, 10, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. A new approach of pellet formation of a filamentous fungus—Rhizopus oryzae. " Bioresour. Technol. 2007, 98, 3415–3423. [Google Scholar] [CrossRef]
- Riscaldati, E.; Moresi, M.; Federici, F.; Petruccioli, M. Direct ammonium fumarate production by Rhizopus arrhizus under phosphorous limitation. Biotechnol. Lett. 2000, 22, 1043–1047. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Valorization of egg shell biowaste and brewery wastewater for the enhanced production of fumaric acid. Waste Biomass Valorization 2015, 6, 535–546. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Li, S.; Chen, Y.; Xu, Q.; Huang, H.; Sheng, X.Y. Enhancement of fumaric acid production by Rhizopus oryzae using a two-stage dissolved oxygen control strategy. Appl. Biochem. Biotechnol. 2010, 162, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, S.; Dou, C.; Yu, Y.; Huang, H. Production of fumaric acid by Rhizopus oryzae: Role of carbon–nitrogen ratio. Appl. Biochem. Biotechnol. 2011, 164, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xu, Q.; Huang, H.; Li, S. Alternative respiration and fumaric acid production of Rhizopus oryzae. Appl. Microbiol. Biotechnol. 2014, 98, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S. Biotechnisch Hergestellte D-Milchsäure—Substitution von Hefeextrakt Durch Agrarische Rohstoffhydrolysate. Ph.D. Thesis, Technical University of Braunschweig, Braunschweig, Germany, 2017. [Google Scholar] [CrossRef]
- Yu, S.; Huang, D.; Wen, J.; Li, S.; Chen, Y.; Jia, X. Metabolic profiling of a Rhizopus oryzae fumaric acid production mutant generated by femtosecond laser irradiation. Bioresour. Technol. 2012, 114, 610–615. [Google Scholar] [CrossRef]
- Wang, G.; Huang, D.; Qi, H.; Wen, J.; Jia, X.; Chen, Y. Rational medium optimization based on comparative metabolic profiling analysis to improve fumaric acid production. Bioresour. Technol. 2013, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Application of calcium carbonate nanoparticles and microwave irradiation in submerged fermentation production and recovery of fumaric acid: A novel approach. RSC Adv. 2016, 6, 25829–25836. [Google Scholar] [CrossRef]
- Eidt, L.; Kuenz, A.; Prüße, U. Biotechnologische Produktion von Fumarsäure: Prozessoptimierung und Kontrolle der Morphologie. Chem. Ing. Tech. 2018, 90, 1272. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, Q.; Li, S.; Huang, H.; Chen, Y. A novel multi-stage preculture strategy of Rhizopus oryzae ME-F12 for fumaric acid production in a stirred-tank reactor. World J. Microbiol. Biotechnol. 2009, 25, 1871–1876. [Google Scholar] [CrossRef]
- Xu, Q.; He, S.; Jiang, L.; Li, S.; Wen, J.; Guan, R.; Huang, H. Extractive fermentation for fumaric acid production by Rhizopus oryzae. Sep. Sci. Technol. 2017, 52, 1512–1520. [Google Scholar] [CrossRef]
- Swart, R.M.; Ronoh, D.K.; Brink, H.; Nicol, W. Continuous Production of Fumaric Acid with Immobilised Rhizopus oryzae: The Role of pH and Urea Addition. Catalysts 2022, 12, 82. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, J.; Tsao, G.T. Comparison of fumaric acid production by Rhizopus oryzae using different neutralizing agents. Bioprocess. Biosyst. Eng. 2002, 25, 179–181. [Google Scholar] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas Energy Efficiency and Renewable Energy; PNLL: Richland, WA, USA, 1992. [Google Scholar]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: A biorefinery perspective. Int. J. Food Sci.Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- Di Lorenzo, R.D.; Serra, I.; Porro, D.; Branduardi, P. State of the Art on the Microbial Production of Industrially Relevant Organic Acids. Catalysts 2022, 12, 234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).