Abstract
This study assessed the potential applicability of Moringa oleifera leaf meal (MO) and fermented Moringa oleifera leaf meal (FMO) as feed supplements for aquatic animals. Five experimental diets, including the basal diet (control), 2.2% and 4.4% MO-supplemented diets (MO2 and MO4), and 2.2% and 4.4% FMO-supplemented diets (FMO2 and FMO4), were prepared for feeding Megalobrama amblycephala juveniles. After the eight-week feeding trial, the growth performance, muscle nutritional composition, plasma and hepatic biochemistry indicators were measured. The results demonstrated that MO and FMO had no detrimental effects on the growth performance of M. amblycephala juveniles. The muscle crude protein, crude lipid, and total free amino acids contents were significantly enhanced in the FMO4 group (p < 0.05). The liver acetyl-CoA carboxylase α mRNA level was significantly increased and the lipoprotein lipase mRNA level was markedly reduced in the FMO4 group (p < 0.05). Therefore, the FMO4 group exhibited a significant increase in plasma low-density lipoprotein cholesterol and triglyceride levels (p < 0.05). Compared to the control group, total superoxide dismutase and catalase activities were significantly increased in the FMO4 group (p < 0.05). The FMO2 and FMO4 groups exhibited an anti-inflammatory response by inhibiting the expression levels of toll-like receptor 4, nuclear factor-kappa B, and tumor protein P53 mRNA (p < 0.05). In conclusion, the 4.4% FMO treatment increased muscle crude protein content, enhanced lipogenesis, and improved the hepatic antioxidant abilities of M. amblycephala juveniles, while 2.2% FMO and 4.4% FMO improved the liver anti-inflammatory capacities.
1. Introduction
In recent years, resource shortages have led to elevated prices of fishmeal, soybean meal, and cottonseed meal in aquatic feed, as well as other protein sources [1]. To alleviate the phenomenon of competition between humans and animals for food, some non-food protein sources obtained widely concerned, including mycoprotein (Clostridium autoethanogenum protein, yeast hydrolysate) [2,3], insect protein (Tenebrio molitor meal, Hermetia illucens meal) [4,5], and plant leaf protein (mulberry leaf meal, Hygrophila spinosa leaf meal) [6,7]. In addition, some processing technologies, such as enzymatic digestion [8] and fermentation [6,9], were also used for improving the quality and utilization efficiency of raw feed ingredients by aquatic animals.
Moringa oleifera is abundant in tropical and subtropical regions. This versatile plant has diverse applications in agriculture, medicine, animal husbandry, and aquaculture due to its rich nutritional value and antioxidant capacity [10]. M. oleifera is used in some developing countries to supply nourishment and guarantee food security. M. oleifera leaf, the main waste material of M. oleifera agriculture, has been reported to contain greater proportions of vitamins C and A, calcium, potassium, iron, and protein compared to other foods such as milk, fruits, and vegetables [11]. M. oleifera leaf contains up to 27.6–35.4% of protein, with as many as 19 kinds of amino acids [12]. In addition to its rich nutritional composition, M. oleifera leaf also contains a wide array of active substances (e.g., kaempferol, isoquercitrin) with hypolipidemic, anti-inflammatory, antioxidant, hepatoprotective, and antibacterial properties [13,14,15].
Several studies have demonstrated that using M. oleifera leaf meal (MO) as a protein source in formulated diets had a positive effect on fish growth and immunity. For example, dietary supplementation with 1.5% MO promoted the development of Nile tilapia (Oreochromis niloticus) and alleviated the damage caused by starvation stress [16]. Furthermore, supplementation with 5% MO improved the systemic immunity (humoral and cellular immunity) of sea bass (Lateolabrax japonicus) [17]. In another study, dietary supplementation with 200 mg/kg of an M. oleifera aqueous extract was found to enhance growth performance and immune organ function in O. niloticus [18]. Other studies reported that the application of MO and M. oleifera leaf extracts enhanced the tissue antioxidant activities of O. niloticus and giant freshwater prawn (Macrobrachium rosenbergii) [13,19,20]. Nevertheless, MO can negatively affect the growth performance of aquatic animals (e.g., Bocourti’s catfish (Pangasius bocourti), African catfish (Clarias gariepinus), and O. niloticus) when supplemented at levels >10% due to its content of anti-nutritional factors such as saponins and tannins, among others [21,22,23,24]. An early study reported that the nutritional quality and nutrient bioavailability of MO could be greatly enhanced through microbial fermentation processes [25]. Zhang et al. [9] reported that fermentation of MO dramatically increased the flavonoid and polysaccharide contents of the fermentation products by 11.18% and 17.39%, respectively. In turn, fermented M. oleifera leaf meal (FMO) improved the non-specific immunity and antioxidant capacity of Gibel carp (Carassius auratus gibelio) [9]. Furthermore, fermentation products promoted the growth performance of aquatic animals by affecting the intestinal environment [26,27]. Replacing 50% and 10% of fishmeal with fermented soy pulp and fermented rice protein, respectively, as protein sources in formulated diets improved the activities of digestive enzymes in C. gariepinus and hybrid grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂) [26,27]. However, very few studies have characterized the effects of MO on lipid metabolism in aquatic animals. MO has a hypolipidemic effect, inhibiting cholesterol synthesis and reducing hepatic lipid vacuolation in mono-sex tilapia (Oreochromis niloticus) [28]. M. oleifera leaf products effectively prevented hypercholesterolemia and lipid deposition in mice by down-regulating plasma low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and total cholesterol (TC) levels [29,30]. The active phenolic and flavonoid substances in MO exhibit anti-lipid peroxidation effects and act as oxygen radical scavengers to protect the meat from oxidation [31]. The antioxidant properties of M. oleifera leaf were also reported to improve goat meat quality (chemical composition, color, and lipid stability) [32].
We previously conducted a pilot study on the liquid fermentation of MO with Bacillus subtilis SIX-15. The fermentation products exhibited a 16.98% reduction in tannin level, an 18.75% increase in 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging, and a 35.75% increase in total phenol content. Blunt snout bream (Megalobrama amblycephala) is a major farmed fish in China. However, this species is highly sensitive to environmental stressors and is vulnerable to disease. Therefore, this research was conducted to study the effects of MO and its fermentation products of FMO on growth performance, feed utilization, and hepatic antioxidant capacity as aquatic animal feed supplements of M. amblycephala juveniles.
2. Materials and Methods
2.1. Ethical Statement
All animal handling procedures were in accordance with the guidelines of the Animal Care Advisory Committee of the Chinese Academy of Fishery Sciences (Authorization No. 20200903001).
2.2. Experimental Diets
M. oleifera leaves were purchased from Greenway Agriculture Co., Ltd. (Zhongshan, China). MO was obtained by crushing M. oleifera leaves and removing solid impurities at 60 mesh. FMO was prepared using Bacillus subtilis SIX-15 (Table S1 and Figure S1). Firstly, Bacillus subtilis SIX-15 was incubated at 35 °C for 18–20 h in Luria–Bertani broth medium. The fermentation substrate, prepared according to the mass ratio of MO: sterilized water = 1:9. Bacillus subtilis SIX-15, was inoculated into sterilized M. oleifera leaf mixture to 2 × 105 cfu/mL and fermented for 48 h at 35 °C. Thereafter, FMO was prepared after being freeze-dried.
Five iso-nitrogenous and iso-energetic diets were designed in this experiment, including the basal diet (control), MO2 diet (2.2% MO was added to the basal diet in place of 1.1% cottonseed meal), MO4 diet (4.4% MO was added to the basal diet in place of 2.2% cottonseed meal), FMO2 diet (2.2% FMO was added to the basal diet in place of 1.1% cottonseed meal), and FMO4 diet (4.4% FMO was added to the basal diet in place of 2.2% cottonseed meal) (Table 1). All ingredients were sieved to remove any solid impurities and then thoroughly mixed. Afterwards, soybean oil and water were gradually added to produce a sinking pellet (2 mm) using an F-26(II) pelletizer (South China University of Technology, Guangzhou, China). Once air-dried, these diets were placed in airtight bags and kept at −20 °C until further use.

Table 1.
The formulation and nutrient composition of the experimental diets.
2.3. Experimental Fish
The feeding trial was conducted at the Freshwater Fisheries Research Center (FFRC) of the Chinese Academy of Fishery Sciences (120.92 E, 31.43 N). M. amblycephala ‘Huahai No.1’ were obtained from the National M. amblycephala stock farm (Wuhan, China). Healthy and similarly sized M. amblycephala juveniles (initial body weight of 20.27 ± 0.11 g; 300 individuals) were randomly assigned to 15 floating cages (1 m × 1 m × 1 m, 20 fish per floating cage). M. amblycephala juveniles were fed with commercial feed containing 33.0% protein and 7.0% lipid (Tongwei Co., Ltd., Wuxi, China) and were allowed to acclimate to the farming environment for one week. The control, MO2, MO4, FMO2, and FMO4 diets were randomly assigned to three floating cages each. The fish were hand-fed carefully three times daily at 7:30, 11:30, and 17:30 until apparent satiation (based on visual observation) for 56 days. During the feeding trial, the water temperature ranged from 23 °C to 27 °C. The dissolved oxygen, ammonia nitrogen, and pH were ≥6.0 mg/L, 0.029 ± 0.002 mg/L, and 7.2 ± 0.2, respectively.
2.4. Sample Collection
At the end of the feeding trial, all the fish were fasted for 24 h, anesthetized with 100 mg/L of tricaine methanesulfonate (MS-222), and weighed to calculate growth performance in terms of weight gain rate (WGR), specific growth rate (SGR), feed coefficient rate (FCR), protein efficiency ratio (PER), and condition factor (CF).
Four fish were then randomly collected from each cage for sampling. Blood of four fish was obtained using disposable medical syringes from the caudal vein. The supernatant plasma was collected after centrifuging at 4 °C and 4000 r/min for 10 min. The plasma was kept at −20 °C until biochemical parameters were determined. Three fish were randomly selected from each cage and immediately dissected to collect liver tissue and dorsal muscle. Liver samples were divided into two parts, one part stored at −20 °C for antioxidant parameters assay and the other stored at −80 °C for genes relative expressions assay. Dorsal muscle samples from all the groups were stored at −20 °C for muscle composition determination. Dorsal muscle samples from the control, MO4, and FMO4 groups were collected and stored at 4 °C for determining the free amino acid contents.
2.5. Laboratory Analysis
2.5.1. Growth Performance
Weight gain rate (WGR, %) = 100 × (final body weight (g) − initial body weight (g))/initial body weight (g).
Specific growth rate (SGR, %/day) = 100 × [ln (final body weight (g)) − ln (initial body weight (g))]/feeding days.
Feed coefficient rate (FCR) = dry feed intake (g)/wet weight gain (g).
Protein efficiency ratio (PER, %) = 100 × wet weight gain (g)/feed protein intake (g).
Condition factor (CF, g/cm3) = 100 × final body weight (g)/body length (cm)3.
2.5.2. Determination of Feed and Muscle Composition
Feed and muscle nutrient contents were examined according to the AOAC [33] criteria. Briefly, the samples were dried with hot air (105 °C), after which the moisture contents were calculated. Crude protein and crude lipid contents were determined via the Kjeldahl and Soxhlet methods, respectively. Ash contents were measured after burning the samples at 550 °C for 5 h. Gross energy contents were measured with an oxygen bomb calorimeter (IKA C6000, IKA, Staufen, Germany).
The free amino acid contents in muscle were examined based on the method described by Zeng et al. [34]. The samples were analyzed through high-performance liquid chromatography (AG1100, Agilent Technologies Co., Ltd., Santa Clara, CA, USA). Mobile phase A consisted of 0.8% (m/v) sodium acetate and 0.0225% (v/v) triethylamine solution. Mobile phase B was a mixture of 2% (m/v) sodium acetate buffer (pH 7.2), acetonitrile, and methanol at a 1:2:2 (v/v) ratio.
2.5.3. Determination of Plasma Biochemical Parameters
Triglycerides (TG), glucose (GLU), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) were determined on a Mindray BS-400 automated biochemistry analyzer (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) using commercial kits purchased from Zhicheng Bio-Technology Co., Ltd. (Shanghai, China).
2.5.4. Determination of Hepatic Antioxidant Parameters
The liver samples were homogenized in pre-cooled normal saline according to the protocol. The samples were then centrifuged (3500 r/min, 10 min) and the supernatant was collected to measure the liver antioxidant parameters. Catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GPX) activities, and glutathione (GSH), malondialdehyde (MDA) levels were measured by the corresponding commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.5.5. Real-Time PCR (qRT-PCR) Analysis on Genes Relative Expressions
Total RNA was extracted from fish liver using trizol (TaKaRa Biomedical Technology Co., Ltd., Dalian, China). The concentration and purity of the total RNA were quantified using Nanodrop 2000 (Thermo Fisher Scientific Inc., Waltham, MA, USA). The mRNA expression levels of toll-like receptor 4 (tlr4), nuclear factor-kappa B (nf-κb), tumor protein P53 (p53), interleukin 8 (il-8), glucose-6-phosphatase (g6pase), lipoprotein lipase (lpl), pyruvate kinase (pk), peroxisome proliferator activated receptor-β (ppar-β), and acetyl-CoA carboxylase α (acc-α) were determined by qRT-PCR using the TB Green™ Premix Ex Taq™ II (TaKaRa Biomedical Technology Co., Ltd., Dalian, China) on the CFX96 instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). beta-cytoskeletal actin (β-actin) was taken as the reference gene. The sequences of the gene-specific primers were designed by the national center for biotechnology information (NCBI), and the primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) (Table 2). The qRT-PCR procedure was as follows: pre-denaturing at 95 °C for 30 s; 39 cycles of denaturation for 5 s at 95 °C and 30 s at 60 °C (annealing temperature); and extension at 95 °C for 10 s. The relative expressions of the target genes were calculated using the 2−ΔΔCt method.

Table 2.
Primer sequences for qRT-PCR.
2.6. Statistical Analysis
One-way ANOVA was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) after testing normality and homogeneity of variance. A Tukey’s test (p < 0.05) was used to detect differences in means among experimental groups. The data are reported as means ± standard error. A p value < 0.05 was considered statistically significant. All figures were generated using GraphPad Prism 7.0.
3. Results
3.1. Growth Performance
As shown in Table 3, FBW, WGR, SGR, FCR, and PER were not significantly affected by experimental diets (p > 0.05). CF was significantly lower in the FMO4 groups compared to the control group (p < 0.05).

Table 3.
Growth performance of M. amblycephala juveniles fed with the experimental diets.
3.2. Muscle Nutrient Composition
The nutrient concentration of the muscle is summarized in Table 4. The moisture contents of the FMO2 and MO4 groups were prominently higher than that of the MO2 and FMO4 groups (p < 0.05). The MO4 and FMO4 groups increased muscle crude protein contents compared with the MO2 and FMO2 groups (p < 0.05). Moreover, the crude protein content was prominently reduced in the FMO2 group compared to the control group (p < 0.05). A significantly higher content of muscle crude lipid was detected in the FMO4 group versus the MO2 group (p < 0.05). Ash content was not affected by the experimental diets.

Table 4.
Composition of muscles in M. amblycephala juveniles fed with the experimental diets.
The free amino acid profiles in muscle of M. amblycephala juveniles fed with the control, MO4, and FMO4 diets were also analyzed (Table 5). Specifically, the three amino acids with the highest levels in muscle were histidine, glycine, and threonine. Based on their taste properties, the levels of free amino acids exhibited the following order: the total amount of bitter amino acids (TBAA) > the total amount of sweet amino acids (TSAA) > the total amount of umami amino acids (TUAA). Moreover, the MO4 group exhibited significant increases in the total amount of TSAA compared with the FMO4 group (p < 0.05). Specifically, a remarkable improvement in glycine content was observed in the MO4 group compared to the control and the FMO4 groups (p < 0.05). Moreover, the MO4 and FMO4 groups exhibited substantially lower alanine and threonine contents compared to the control group (p < 0.05). The muscle proline content was also significantly enhanced in the FMO4 group compared to the MO4 group (p < 0.05).

Table 5.
Free amino acid composition of muscles in M. amblycephala juveniles fed with the experimental diets.
3.3. Plasma Biochemical Indices
The plasma biochemical indices are illustrated in Figure 1. The FMO2 and FMO4 groups exhibited significantly higher GLU levels compared to the control group (p < 0.05). A further noticeable increase in GLU content was observed in the FMO4 group compared to the MO2 and MO4 groups (p < 0.05). The TG content was significantly increased in the FMO2 group versus the control group (p < 0.05). Moreover, a distinct increment in TG content was observed in the FMO2 and FMO4 groups relative to the MO4 group (p < 0.05). Furthermore, the content of LDL-C was significantly increased in the FMO4 group relative to the MO2 group (p < 0.05).
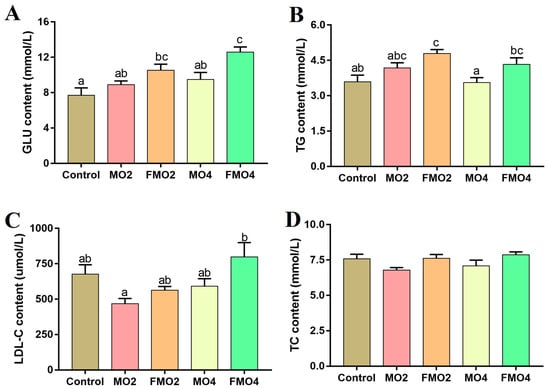
Figure 1.
Effects of dietary MO and FMO on plasma biochemical parameters: GLU: glucose (A); TG: triglycerides (B); LDL-C: low-density lipoprotein cholesterol (C); and TC: total cholesterol (D). Values with different alphabetical superscripts above bars are significantly different (p < 0.05, Tukey’s test).
3.4. Hepatic Antioxidant Parameters
The hepatic antioxidant capacities of M. amblycephala juveniles are illustrated in Figure 2. No significant differences were detected in the levels of MDA and GPX (p > 0.05). CAT activity was significantly increased in the FMO4 group versus the control group (p < 0.05). The MO4, FMO2, and FMO4 groups exhibited a distinct enhancement in T-SOD activities compared to the control group (p < 0.05). Furthermore, the T-SOD activity of the FMO4 group was significantly higher than that of the MO2 group (p < 0.05). The FMO2 group exhibited a notable increase in liver GSH content compared to the MO2 and FMO4 groups (p < 0.05).
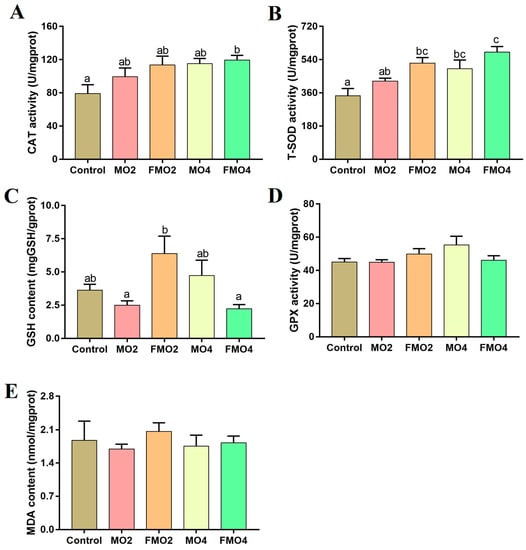
Figure 2.
Effects of dietary MO and FMO on hepatic CAT: catalase (A); T-SOD: total superoxide dismutase (B); GSH: glutathione (C); GPX: glutathione peroxidase (D); and MDA: malondialdehyde (E) levels. Values with different alphabetical superscripts above bars are significantly different (p < 0.05, Tukey’s test).
3.5. Gene Expressions Related to Glucose and Lipid Metabolism in the Liver
Figure 3 illustrates the mRNA expression of target genes related to hepatic glucose and lipid metabolism. The hepatic mRNA expressions of g6pase in the MO2, MO4, FMO2, and FMO4 groups were considerably higher than those of the control group (p < 0.05). Furthermore, a noticeable reduction in pk mRNA expression was observed in the FMO2 and FMO4 groups compared to the control and MO2 groups (p < 0.05). The MO4 group exhibited a remarkable reduction in acc-α mRNA expression levels relative to the FMO2 and FMO4 groups (p < 0.05). The lpl mRNA levels of the MO4, FMO2, and FMO4 groups were down-regulated relative to the MO2 group (p < 0.05).
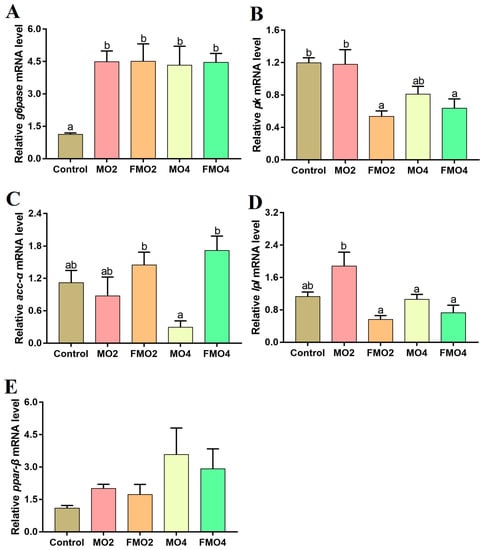
Figure 3.
Effects of dietary MO and FMO on hepatic g6pase: glucose-6-phosphatase (A); pk: pyruvate kinase (B); acc-α: acetyl-CoA carboxylase α (C); lpl: lipoprotein lipase (D); and ppar-β: peroxisome proliferator activated receptor-β (E) expression levels. Values with different alphabetical superscripts above bars are significantly different (p < 0.05, Tukey’s test).
3.6. Gene mRNA Expressions Related to the Inflammatory Response in the Liver
Figure 4 illustrates the mRNA expressions of genes related to inflammatory responses in the liver. The MO2, MO4, FMO2, and FMO4 groups showed a noticeable reduction in tlr4 mRNA expression levels versus the control group (p < 0.05). A noticeable reduction in nf-κb mRNA expression level was observed in the MO4 group compared to the MO2 group (p < 0.05). Furthermore, the nf-κb mRNA expression levels of FMO2 and FMO4 groups were significantly lower than the control and MO2 groups (p < 0.05). The FMO2 group exhibited a remarkable reduction in il-8 mRNA expression level relative to the control and MO2 groups (p < 0.05). Furthermore, the il-8 mRNA expression level was significantly reduced in the MO4 group relative to the MO2 group (p < 0.05). A distinct decrease in the p53 expression level was observed in the FMO2 and FMO4 groups compared to the MO2 group (p < 0.05).
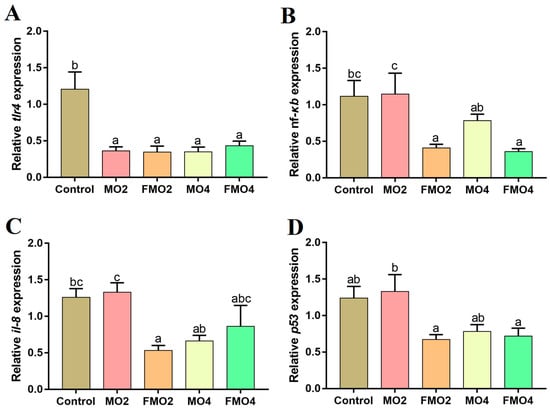
Figure 4.
Effects of dietary MO and FMO on hepatic tlr4: toll-like receptor 4 (A); nf-κb: nuclear factor-kappa B (B); il-8: interleukin 8 (C); and p53 (D) expression levels. Values with different alphabetical superscripts above bars are significantly different (p < 0.05, Tukey’s test).
4. Discussion
MO has been demonstrated to enhance the growth performance of common carp (Cyprinus carpio) [35] and O. niloticus [28], and FMO has been reported to increase the growth performance of C. gibelio juveniles [9]. In this study, supplementation with MO and FMO had no negative effects on the growth performance of M. amblycephala juveniles, which was consistent with previous studies on guppy (Poecilia reticulata) [36] and O. niloticus [37]. These discrepancies may be attributed to differences in fish size and feeding conditions, as well as fish species [28]. Our results indicated that the supplementation of FMO appeared to affect the body shape of M. amblycephala juveniles as reflected in the decreased CF. Previous studies have revealed that supplementation of fermentation products such as protein ingredients reduces CF in largemouth bass (Micropterus salmoides) [38].
Studies have found that replacing 10% soybean meal or fish meal with MO increased the crude protein contents of whole fish such as rohu (Labeo rohita) [39] and O. niloticus [40]. Another study reported that phytase-supplemented MO improved the nutrient profiles (protein and lipid) of Catla catla (Hamilton, 1822) [41]. These results were also reflected in our feeding trial, where the supplementation of 4.4% MO and 4.4% FMO significantly enhanced the muscle crude protein contents of M. amblycephala juveniles. Studies have shown that prolonged consumption of formulated diets containing high levels of plant protein and plant oil results in increased muscle hardness, reduced sweetness, and weakened odor intensity in fish [42]. Threonine, glycine, alanine, proline, and serine are classified as sweet amino acids [43]. Similarly, previous studies reported that supplementing non-fishmeal diets with (2-carboxyethyl) dimethylsulfonium bromide [44] and guanidinoacetic acid [45] significantly increased the contents of sweet amino acids in grass carp (Ctenopharyngodon idella). Moreover, substituting 30% of fish meal with MO had no significant effects on the texture and flavor profile of C. gariepinus meat compared to the control [46]. In the present study, we found that dietary supplementation with 4.4% MO increased the total content of sweet amino acids in muscle, thereby probably improving the meat flavor of M. amblycephala juveniles.
Lipid accumulation is a complex process involving the ingestion, transportation, and catabolism of lipids. The catabolism of triacylglycerols is also a crucial process influencing lipid deposition in particular tissues. Reduced hepatic lipid accumulation and TG catabolism were observed in large yellow croaker (Larimichthys crocea) fed with a low-lipid diet [47]. Intracellular lipid accumulation in muscle and liver reflects a phenomenon of ectopic TG distribution in locations other than adipose tissue, which results from an impairment of the cellular mechanisms that regulate lipid storage and utilization [48]. Studies have found that increased levels of LDL-C, TG, and GLU in the blood promoted lipid deposition in the muscle of golden pompano (Trachinotus ovatus) and pacific abalone (Haliotis discus hannai) [49,50]. In our study, the plasma LDL-C, TG, and GLU levels and muscle lipid content were markedly increased in the group fed with the diet containing 4.4% FMO. We speculated that FMO promoted lipogenesis in the present study. This was consistent with the results of a previous study, in which C. gariepinus fed with a diet containing 20% MO exhibited improvements in muscular lipid deposition [51]. Previous studies have also reported that probiotics may improve the synthesis of vitamin B12, biotin, and fatty acids, among other nutrients [52,53], thereby positively influencing the wellness of organisms. Additionally, we analyzed the expression of target genes related to lipogenesis and glucose metabolism. Our findings indicated that FMO supplementation up-regulated hepatic acc-α mRNA expression and down-regulated lpl mRNA expression. Acc-α is a key enzyme involved in fatty acid biosynthesis and can regulate lipogenesis by participating in the synthesis of long-chain fatty acids and β-oxidation [54]. Moreover, lpl acts as a rate-limiting enzyme within the lipid hydrolysis process and contributes to the absorption of lipids from food and plasma lipoprotein metabolism [55]. Glucose metabolism can preserve the energy homeostasis of vital functions in animals and has a crucial role in modulating glucose and lipid metabolism [56]. A 15 mM dose of glucose was found to induce TG accretion in the plasma of yellow catfish (Pelteobagrus fulvidraco) [57]. Elevated plasma GLU level increases the expression levels of lipogenic genes such as acc-α and fat synthase (fas), which in turn enhances lipid deposition [58,59]. Furthermore, increased plasma GLU level inhibits the transcription of peroxisome proliferator-activated receptors-α (ppar-α) and carnitine palmitoyltransferase 2 (cpt-2), thereby inhibiting fatty acid oxidation [60]. G6pase transforms glucose-6-phosphate to free glucose in the last step of the gluconeogenesis pathway [61]. The process of glycogen utilization can be completed by glycolysis, and pk acts as the rate-limiting enzyme in the last step of glycolysis [62]. In this study, supplementation with FMO increased plasma GLU levels and provided energy to the juvenile fish [63] by enhancing the expression of g6pase mRNA and down-regulating the pk mRNA expression level, thus stimulating hepatic gluconeogenesis and decreasing the glycolytic capacity of the liver. Therefore, we speculated that 4.4% FMO promoted lipid accumulation in M. amblycephala juveniles due to an increase in plasma GLU level, which reduced lpl and increased acc-α mRNA expression levels, thereby promoting lipid accumulation in locations other than adipose tissue.
M. oleifera leaves are rich in vitamin C, vitamin A, and phenolic compounds such as quercetin and flavonoids. Studies have shown that M. oleifera leaf extracts increased antioxidant activity and slowed down oxidative damage in goats, rats, and other mammals [64]. In aquatic animals, dietary administration of MO and its extracts enhanced the tissue antioxidant activities of Nile tilapia and M. rosenbergii [13,19,20]. Moreover, dietary supplementation with FMO increased the plasma SOD, CAT, and GPX activities of Gibel carp [9]. Fermentation changes the type and volume of active ingredients in the substrate, and the resulting small molecule glycosides have stronger antioxidant activities [9]. In this study, dietary supplementation with 4.4% FMO increased the hepatic CAT and T-SOD activities. The primary role of T-SOD is to scavenge intracellular superoxide anions and produce non-toxic oxygen as well as less harmful hydrogen peroxide [13,16]. CAT effectively scavenges intracellular hydrogen peroxide and catalyzes the breakdown of hydrogen peroxide to produce water and oxygen [19,20]. In other words, 4.4% FMO improved the hepatic antioxidant activities of M. amblycephala juveniles. The GSH content of the FMO2 group was significantly higher compared to the MO2 and FMO4 groups. GSH scavenges reactive oxygen species (ROS) to alleviate oxidative stress directly [65]. The present results indicated that FMO improved the hepatic antioxidant capacity of M. amblycephala juveniles, which was stronger in the dietary 4.4% FMO. Non-specific immunity is the first line of defense against invading pathogens. Toll-like receptors (tlrs) serve as the primary sensors that induce innate immune responses in fish by detecting various microbial components [66]. Among these, tlr4 is a pattern-recognition receptor that recognizes pathogenic microbes and is expressed in the cell membrane [67]. Nf-κb serves as the critical downstream activator of the tlr4 pathway, and therefore tlr4 activation is ultimately manifested by nf-κb activation [68]. Recent studies demonstrated that plant polysaccharides inhibit the expressions of tlr2, nf-κb, and their downstream inflammatory genes in tissues of O. niloticus and Jian carp (Cyprinus carpio var. Jian) [69,70]. In this experiment, the diets containing 2.2% and 4.4% FMO down-regulated the mRNA expressions of pro-inflammatory factors of tlr4 and nf-κb, as well as the apoptosis factor p53. Our finding was consistent with those of a previous study in which FMO modulated the inflammatory reaction of Gibel carp and improved their tolerance to pathogenic bacteria by modulating the tlr2 signaling pathway [9]. Moreover, M. oleifera ethanolic extracts decreased the mRNA expressions of pro-inflammatory factors, thereby attenuating the kidney damage induced by tilmicosin [64]. These findings suggested that dietary 2.2% and 4.4% FMO improved the innate immunity of M. amblycephala juveniles by inhibiting the levels of pro-inflammatory factors.
5. Conclusions
Dietary supplementation with 2.2% and 4.4% of M. oleifera leaf meal or fermented M. oleifera leaf meal did not affect the growth performance of M. amblycephala juveniles, while dietary 2.2% and 4.4% fermented M. oleifera leaf meal enhanced anti-inflammatory capacity of the liver. Dietary 4.4% fermented M. oleifera leaf meal supplementation was recommended because it increased the muscle nutrient composition and hepatic antioxidant and anti-inflammatory capacities of the M. amblycephala juveniles. Based on the results of our present study, probiotics fermentation improved the physiological status of M. amblycephala juveniles fed M. oleifera leaf meal. It is necessary to conduct further investigations into the mechanisms by which fermented protein sources enhance nutrient deposition in aquatic animals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9060577/s1, Table S1. The nutrients profile in MO and FMO; Figure S1. Molecular weight distribution of peptides in MO and FMO; * indicates a significant difference (p < 0.05; independent t-test); ** indicates an extremely significant difference (p < 0.01; independent t-test).
Author Contributions
Conceptualization, Y.L.; data curation, W.J.; formal analysis, Y.Y.; investigation, L.Q.; methodology, Y.Y. and L.M.; project administration, H.S. and X.G.; resources, Y.Z. and Y.L.; software, L.Q.; supervision, X.G. and L.M.; validation, H.S.; writing—original draft, W.J.; writing—review and editing, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by China Agriculture Research System (CARS-45) and Science and Technology Innovation Team (Grant No. 2020TD59).
Institutional Review Board Statement
All animal handling procedures were in accordance with the guidelines of the Animal Care Advisory Committee of the Chinese Academy of Fishery Sciences (Authorization No. 20200903001).
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the manuscript and tables.
Acknowledgments
We would like to thank the postgraduate students of the Fish Disease and Nutrition Department, the Freshwater Fisheries Research Center (FFRC), and the Chinese Academy of Fishery Sciences (CAFS) for their help throughout the research period.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. FAO fisheries and aquaculture information and statistics services. In Aquaculture Production: Quantities; FAO: Rome, Italy, 2020; pp. 1950–2016. [Google Scholar]
- Dai, J.; Chen, T.; Guo, X.; Dai, Z.; He, Z.; Hu, Y. Evaluation of fish meal replacement by Clostridium autoethanogenum protein in diets for juvenile red swamp crayfish (Procambarus clarkii). Aquaculture 2023, 570, 739379. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Liu, W.B.; Liang, C.; Sun, C.X.; Xue, Y.F.; Wan, Z.D.; Jiang, G.Z. Effects of partial replacement of fish meal by yeast hydrolysate on complement system and stress resistance in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2017, 67, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Z.; Liu, S.; Sun, J.; Ji, H. Influence of dietary soybean meal replacement with yellow mealworm (Tenebrio molitor) on growth performance, antioxidant capacity, skin color, and flesh quality of mirror carp (Cyprinus carpio var. specularis). Aquaculture 2022, 561, 738686. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Fish Shellfish Immunol. 2022, 120, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lin, Y.; Qian, L.; Miao, L.; Liu, B.; Ge, X.; Shen, H. Mulberry leaf meal: A potential feed supplement for juvenile Megalobrama amblycephala “Huahai No. 1”. Fish Shellfish Immunol. 2022, 128, 279–287. [Google Scholar] [CrossRef]
- Maiti, M.K.; Sahu, N.P.; Sardar, P.; Shamna, N.; Deo, A.D.; Gopan, A.; Sahoo, S. Optimum utilization of Hygrophila spinosa leaf meal in the diet of Labeo rohita (Hamilton, 1822) fingerlings. Aquac. Rep. 2019, 15, 100213. [Google Scholar] [CrossRef]
- Poolsawat, L.; Yang, H.; Sun, Y.F.; Li, X.Q.; Liang, G.Y.; Leng, X.J. Effect of replacing fish meal with enzymatic feather meal on growth and feed utilization of tilapia (Oreochromis niloticus× O. aureus). Anim. Feed Sci. Technol. 2021, 274, 114895. [Google Scholar] [CrossRef]
- Zhang, X.H.; Sun, Z.Y.; Cai, J.F.; Wang, J.H.; Wang, G.B.; Zhu, Z.L.; Cao, F.L. Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol. 2020, 102, 430–439. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Daim, M.M.; Shukry, M.; Nowosad, J.; Kucharczyk, D. Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: A review. Aquaculture 2022, 547, 737369. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: A promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. S. Afr. J. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Abd El-Gawad, E.A.; El Asely, A.M.; Soror, E.I.; Abbass, A.A.; Austin, B. Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquac. Int. 2020, 28, 389–402. [Google Scholar] [CrossRef]
- Nepolean, P.; Anitha, J.; Emilin, R.R. Isolation, analysis and identification of phytochemicals of antimicrobial activity of Moringa oleifera Lam. Curr. Biotechnol. 2009, 3, 33–37. [Google Scholar]
- Toppo, R.; Roy, B.K.; Gora, R.H.; Baxla, S.L.; Kumar, P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Vet. World 2015, 8, 537–540. [Google Scholar] [CrossRef]
- Elabd, H.; Soror, E.; El-Asely, A.; El-Gawad, E.A.; Abbass, A. Dietary supplementation of Moringa leaf meal for Nile tilapia Oreochromis niloticus: Effect on growth and stress indices. Egypt. J. Aquat. Res. 2019, 45, 265–271. [Google Scholar] [CrossRef]
- Tageldein, M.A.; Liang, M.; Cristóbal, E.; María, G.B.J.; Ceballos, F.; Ángeles, E.M. Effects of dietary inclusion of Moringa oleifera leaves on growth and some systemic and mucosal immune parameters of seabream. Fish Physiol. Biochem. 2018, 44, 1223–1240. [Google Scholar]
- Emam, M.A.; Shourbela, R.M.; El-Hawarry, W.N.; Abo-Kora, S.Y.; Gad, F.A.M.; Abd El-latif, A.M.; Dawood, M.A. Effects of Moringa oleifera aqueous extract on the growth performance, blood characteristics, and histological features of gills and livers in Nile tilapia. Aquac. Fish. 2021, 126, 326–334. [Google Scholar] [CrossRef]
- Monir, W.; Abdel-Rahman, M.A.; El-Din, H.S.; Mansour, E.S.; Awad, S.M.M. Pomegranate peel and moringa-based diets enhanced biochemical and immune parameters of Nile tilapia against bacterial infection by Aeromonas hydrophila. Microb. Pathog. 2020, 145, 104202. [Google Scholar] [CrossRef] [PubMed]
- Kaleo, I.V.; Gao, Q.; Liu, B.; Sun, C.; Zhou, Q.; Zhang, H.; Shan, F.; Xiong, Z.; Bo, L.; Song, C. Effects of Moringa oleifera leaf extract on growth performance, physiological and immune response, and related immune gene expression of Macrobrachium rosenbergii with Vibrio anguillarum and ammonia stress. Fish Shellfish Immunol. 2019, 89, 603–613. [Google Scholar] [CrossRef]
- Stevens, C.G.; Ugese, F.D.; Otitoju, G.T.; Baiyeri, K.P. Proximate and anti-nutritional composition of leaves and seeds of Moringa oleifera in Nigeria: A comparative study. Agro-Sci. 2016, 14, 9. [Google Scholar] [CrossRef]
- Puycha, K.; Yuangsoi, B.; Charoenwattanasak, S.; Wongmaneeprateep, S.; Niamphithak, P.; Wiriyapattanasub, P. Effect of moringa (Moringa oleifera) leaf supplementation on growth performance and feed utilization of Bocourti’s catfish (Pangasius bocourti). Agric. Nat. Resour. 2017, 51, 286–291. [Google Scholar] [CrossRef]
- Hlophe, S.N.; Moyo, N. A comparative study on the use of Pennisetum clandestinum and Moringa oleifera as protein sources in the diet of the herbivorous Tilapia rendalli. Aquac. Int. 2014, 22, 1245–1262. [Google Scholar] [CrossRef]
- Richter, N.; Siddhuraju, P.; Becker, K. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture 2003, 217, 599–611. [Google Scholar] [CrossRef]
- Urlings, H.A.P.; Bijker, P.G.H.; Van Logtestijn, J.G. Fermentation of raw poultry byproducts for animal nutrition. J. Anim. Sci. 1993, 71, 2420–2426. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Dawood, M.A.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Mat, K.; Ismail, T.A.; et al. Effect of fish meal substitution with fermented soy pulp on growth performance, digestive enzyme, amino acid profile, and immune-related gene expression of African catfish (Clarias gariepinus). Aquaculture 2022, 546, 737418. [Google Scholar] [CrossRef]
- He, Y.F.; Guo, X.W.; Tan, B.P.; Dong, X.H.; Yang, Q.H.; Liu, H.Y.; Zhang, S.; Chi, S. Replacing fish meal with fermented rice protein in diets for hybrid groupers (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂): Effects on growth, digestive and absorption capacities, inflammatory-related gene expression, and intestinal microbiota. Aquac. Rep. 2021, 19, 100603. [Google Scholar] [CrossRef]
- El-Kassas, S.; Abdo, S.E.; Abosheashaa, W.; Mohamed, R.; El-Naggar, K. Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquac. Rep. 2020, 18, 100422. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.S. Screening Moringa species focused on development of locally available sustainable nutritional supplements. Nutr. Res. Pract. 2019, 13, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Al-Dujaily, A.N.G.; Al-murshidi, M.H.H. Effect of Methanolic Leaf Extract of Moringa oleifera on some Biochemical Markers in obesity induced rats. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2016, 7, 2222–2232. [Google Scholar]
- Amad, A.A.; Zentek, J. Moringa (M. oleifera) leaf meal in diets for broilers and laying hens: A review. J. Agric. Sci. 2022, 14, 12. [Google Scholar] [CrossRef]
- Qwele, K.; Hugo, A.; Oyedemi, S.O.; Moyo, B.; Masika, P.J.; Muchenje, V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 2013, 93, 455–462. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemistry, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2003. [Google Scholar]
- Zeng, X.F.; Xia, W.S.; Jiang, Q.X.; Yang, F. Effect of autochthonous starter cultures on microbiological and physico-chemical characteristics of Suan yu, a traditional Chinese low salt fermented fish. Food Control 2013, 33, 344–351. [Google Scholar] [CrossRef]
- Khalil, F.; Korni, F.M.M. Evaluation of Moringa oleifera leaves and their aqueous extract in improving growth, immunity and mitigating effect of stress on common carp (Cyprinus carpio) fingerlings. Aquat. Sci. Eng. 2017, 32, 170–177. [Google Scholar] [CrossRef]
- Bisht, M.; Kumar, A.; Shah, T.K. Effect of Moringa oleifera leaf powder on skin mucosal immune responses and growth performance of guppy, Poecilia reticulata (Peter, 1860). Aquac. Res. 2020, 51, 4984–4990. [Google Scholar] [CrossRef]
- Bbole, I.; Mumba, C.; Mupenda, N.; Kefi, A.S. Analysis of growth performance and haematological parameters of Oreochromis niloticus fed on a varying diet of Moringa oleifera Lam. leaf meal as an additive protein source. Int. J. Aquac. Fish Sci. 2016, 8, 105–111. [Google Scholar]
- He, M.; Yu, Y.; Li, X.; Poolsawat, L.; Yang, P.; Bian, Y.; Leng, X. An evaluation of replacing fish meal with fermented soybean meal in the diets of largemouth bass (Micropterus salmoides): Growth, nutrition utilization and intestinal histology. Aquac. Res. 2020, 51, 4302–4314. [Google Scholar] [CrossRef]
- Arsalan, M.Z.H.; Hussain, S.M.; Asrar, M.; Anwar, H.; Rehan, M.M.H.; Shahzad, M.M.; Riaz, D.; Ahmad, N.; Wahab, N. Effects of Moringa oleifera leaf meal (MOLM) based diets on carcass composition and hematology of Labeo rohita fingerlings. J. Biodivers. Environ. Sci. 2016, 9, 214–223. [Google Scholar]
- Karina, S.; Akbar, M.; Supriatna, A.; Muchlisin, Z.A. Replacement of soybean meal with moringa oleifera leaf meal in the formulated diets of tilapia (Oreochromis niloticus) fingerlings. Aquac. Aquar. Conserv. Legis. 2015, 8, 790–805. [Google Scholar]
- Shahzad, M.M.; Hussain, S.M.; Jabeen, F.; Hussain, A.I.; Arsalan, M.Z.H.; Ahmad, N. Carcass composition and hematological study of Catla catla fingerlings fed on phytase supplemented Moringa oleifera leaf meal (MOLM) based diet. J. Biodivers. Environ. Sci. 2016, 9, 57–68. [Google Scholar]
- Francesco, M.; Parisi, G.; Médale, F.; Lupi, P.; Kaushik, S.J.; Poli, B.M. Effect of long-term feeding with a plant protein mixture-based diet on growth and body/fillet quality traits of large rainbow trout (Oncorhynchus mykiss). Aquaculture 2004, 236, 413–429. [Google Scholar] [CrossRef]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Liu, X.W.; Lin, F.; Jiang, W.D.; Wu, P.; Jiang, J.; Yang, D.M.; Tang, L.; Kuang, S.Y.; Shi, H.Q.; Zhou, X.Q.; et al. (2-Carboxyethyl) dimethylsulfonium Bromide (Br-DMPT) improves muscle flesh quality and antioxidant status of on-growing grass carp (Ctenopharyngodon idella) fed non-fish meal diets. Aquaculture 2020, 521, 735065. [Google Scholar] [CrossRef]
- Yang, L.L.; Lin, F.; Wu, P.; Jiang, W.D.; Liu, Y.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Guanidinoacetic acid supplementation totally based on vegetable meal diet improved the growth performance, muscle flavor components and sensory characteristics of on-growing grass carp (Ctenopharygodon idella). Aquaculture 2021, 531, 735841. [Google Scholar] [CrossRef]
- Odedeyi, D.O. Nutritive values of the carcass of African catfish (Clarias gariepinus) fingerlings fed raw and steam-heated Moringa oleifera diets. In Proceedings of the International Conference on Advances in Environment, Agriculture & Medical Sciences (ICAEAM’14), Kuala Lumpur, Malaysia, 16–17 November 2014; pp. 16–27. [Google Scholar]
- Cai, Z.; Mai, K.; Ai, Q. Regulation of hepatic lipid deposition by phospholipid in large yellow croaker. Br. J. Nutr. 2017, 118, 999–1009. [Google Scholar] [CrossRef]
- Lara-Castro, C.; Garvey, W.T. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol. Metab. Clin. N. Am. 2008, 37, 841–856. [Google Scholar] [CrossRef]
- Song, F.; Qin, Y.; He, C.; Geng, H.; Yang, P.; Wang, W.; Chen, Y. Transcriptome analysis reveals the effects of dietary lipid level on growth performance and immune response in golden pompano (Trachinotus ovatus). Aquaculture 2023, 563, 738959. [Google Scholar] [CrossRef]
- Guo, J.; Fu, Y.; Wu, Z.; Yu, X.; Guo, Y.; Liu, J.; Zhang, W.; Mai, K. Effects of dietary carbohydrate levels on growth performance, body composition, glucose/lipid metabolism and insulin signaling pathway in abalone Haliotis discus hannai. Aquaculture 2022, 557, 738284. [Google Scholar] [CrossRef]
- Ncha, O.S.; Michael, P.B.; Nnabuchi, U.O.; Alex, E. Effect of diets with moringa leaf meal on growth, carcass composition and haematology of Clarias gariepinus. Fish Aquat. Sci. 2015, 3, 397–401. [Google Scholar]
- Sugita, H.; Miyajima, C.; Deguchi, Y. The vitamin B12-producing ability of the intestinal microflora of freshwater fish. Aquaculture 1991, 92, 267–276. [Google Scholar] [CrossRef]
- Vine, N.G.; Leukes, W.D.; Kaiser, H. Probiotics in marine larviculture. FEMS Microbiol. Rev. 2006, 30, 404–427. [Google Scholar] [CrossRef]
- Dyck, J.; Berthiaume, L.G.; Thomas, P.D.; Kantor, P.F.; Barr, A.J.; Barr, R.; Singh, D.; Hopkins, T.A.; Voilley, N.; Prentki, M.; et al. Characterization of rat liver malonyl-CoA decarboxylase and the study of its role in regulating fatty acid metabolism. Biochem. J. 2000, 350, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, H.; Maulu, S.; Ge, X.; Xi, B. Dietary phosphorus affects growth, glucolipid metabolism, antioxidant activity and immune status of juvenile blunt snout bream (Megalobrama amblycephala). Anim. Feed Sci. Technol. 2021, 274, 114896. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.B.; Brown, P.B.; Xu, C.; Li, X.F. Utilization of raw and gelatinized starch by blunt snout bream Megalobrama amblycephala as evidenced by the glycolipid metabolism, glucose tolerance and mitochondrial function. Aquaculture 2020, 529, 735603. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, S.; Chen, G.; Xu, Y.; Luo, Z. Dietary glucose increases glucose absorption and lipid deposition via SGLT1/2 signaling and acetylated ChREBP in the intestine and isolated intestinal epithelial cells of yellow catfish. J. Nutr. 2020, 150, 1790–1798. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, K.; Hogstrand, C.; Xu, Y.; Chen, G.; Wei, C.; Luo, Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell. Mol. Life Sci. 2019, 77, 1987–2003. [Google Scholar] [CrossRef]
- Iizuka, K.; Bruick, R.K.; Liang, G.; Horton, J.D.; Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA 2004, 101, 7281–7296. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–128. [Google Scholar] [CrossRef]
- Michael, M.D.; Kulkarni, R.N.; Postic, C. Loss of Insulin Signaling in Hepatocytes Leads to Severe Insulin Resistance and Progressive Hepatic Dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.L.; Gagliardino, J.J.; Francini, F. Liver glucokinase: An overview on the regulatorymechanisms of its activity. IUBMB Life 2011, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liang, H.; Ren, M.; Ge, X.; Ji, K.; Yu, H.; Maulu, S. Effects of dietary lysine levels on growth performance, whole body composition and gene expression related to glycometabolism and lipid metabolism in grass carp, Ctenopharyngodon idellus fry. Aquaculture 2021, 530, 735806. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; Ahmed, A.I.; Awad, A.; Mohammed, W.A.; Metwally, M.M.M.; Almeer, R.; Abdel-Daim, M.M.; Khalil, S.R. Moringa oleifera ethanolic extract attenuates tilmicosin-induced renal damage in male rats via suppression of oxidative stress, inflammatory injury, and intermediate filament proteins mRNA expression. Biomed. Pharmacother. 2021, 133, 110997. [Google Scholar] [CrossRef] [PubMed]
- Mabe, L.T.; Su, S.; Tang, D.; Zhu, W.; Wang, S.; Dong, Z. The effect of dietary bamboo charcoal supplementation on growth and serum biochemical parameters of juvenile common carp (Cyprinus carpio L.). Aquac. Res. 2018, 49, 1142–1152. [Google Scholar] [CrossRef]
- Kongchum, P.; Palti, Y.; Hallerman, E.M.; Hulata, G.; David, L. SNP discovery and development of genetic markers for mapping innate immune response genes in common carp (Cyprinus carpio). Fish Shellfish Immunol. 2010, 29, 356–361. [Google Scholar] [CrossRef]
- Swanson, L.; Katkar, G.D.; Tam, J.; Pranadinata, R.F.; Chareddy, Y.; Coates, J. TLR4 signaling and macrophage inflammatory responses are dampened by GIV/Girdin. Proc. Natl. Acad. Sci. USA 2020, 117, 26895–26906. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Voulgarelis, M. Toll-Like Receptors, Tissue Injury, and Tumourigenesis. Mediat. Inflamm. 2010, 2010, 581837. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Karimi, A.A.; Hafezieh, M.; Dawood, M.A.O.; Abo-Al-Ela, H.G. Pistachio hulls polysaccharide protects Nile tilapia against LPS-induced excessive inflammatory responses and oxidative stress possibly via TLR2 and Nrf2 signaling pathways. Fish Shellfish Immunol. 2021, 27, 254–268. [Google Scholar] [CrossRef]
- Zhe, Y.; Zhao, L.; Zhao, J.L.; Xu, W.X.; Guo, Z.H.; Zhang, A.Z. Dietary Taraxacum mongolicum polysaccharide ameliorates the growth, immune response, and antioxidant status in association with NF-κB, Nrf2 and TOR in Jian carp (Cyprinus carpio var. Jian). Aquaculture 2022, 547, 737522. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).