Insights into Proteomics Reveal Mechanisms of Ethanol-Enhanced Bacterial Cellulose Biosynthesis by Komagataeibacter nataicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media and Inoculum Preparation
2.2. Cellulose Fermentation and Viable Cell Number Determination
2.3. Extraction of Intracellular Proteins
2.4. Two-Dimensional Electrophoresis and Data Analysis
2.5. Protein Identification and Database Searching
2.6. RNA Extraction and cDNA Synthesis
2.7. RT-qPCR
3. Results
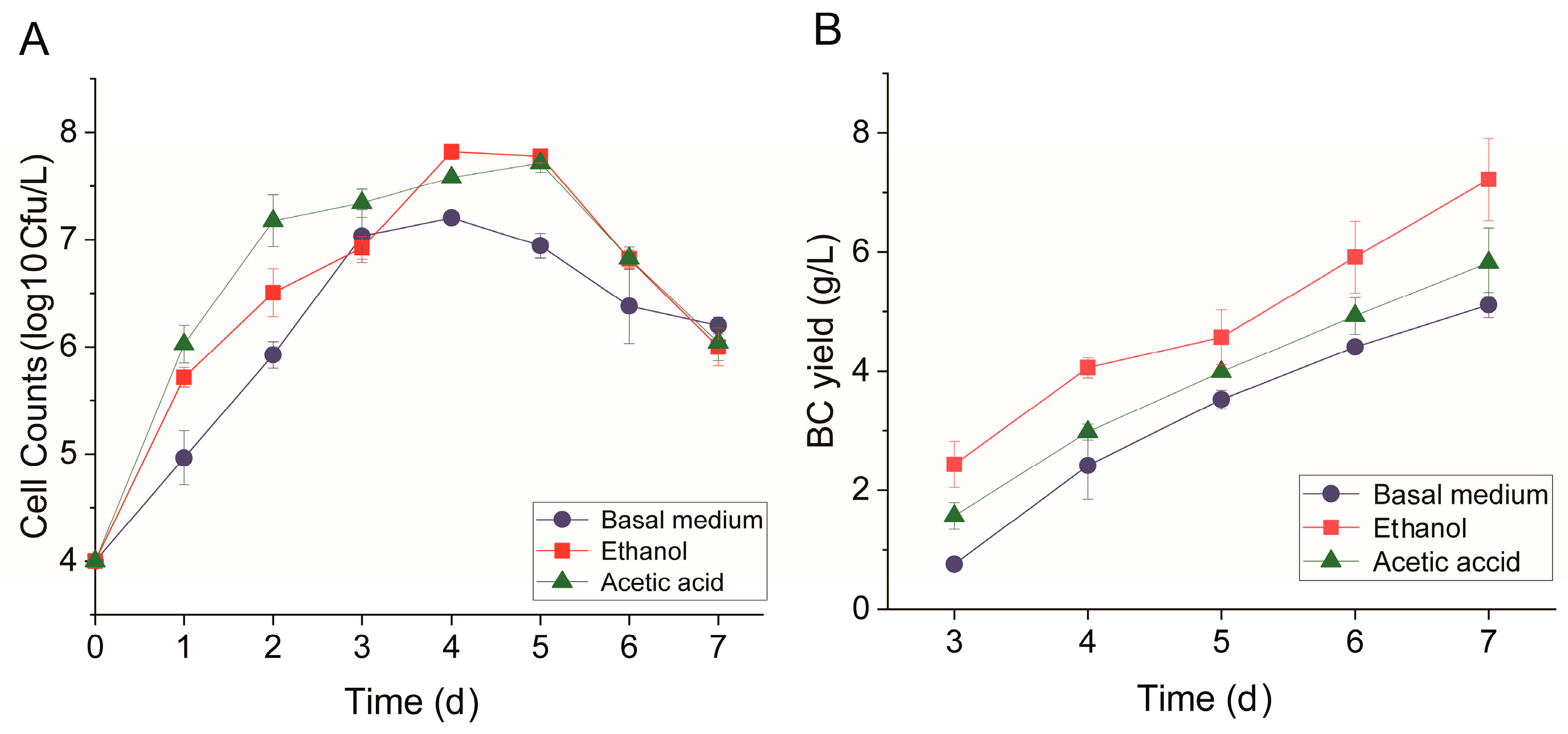
3.1. Effects of Ethanol and Acetic Acid on BC Yield and Structure
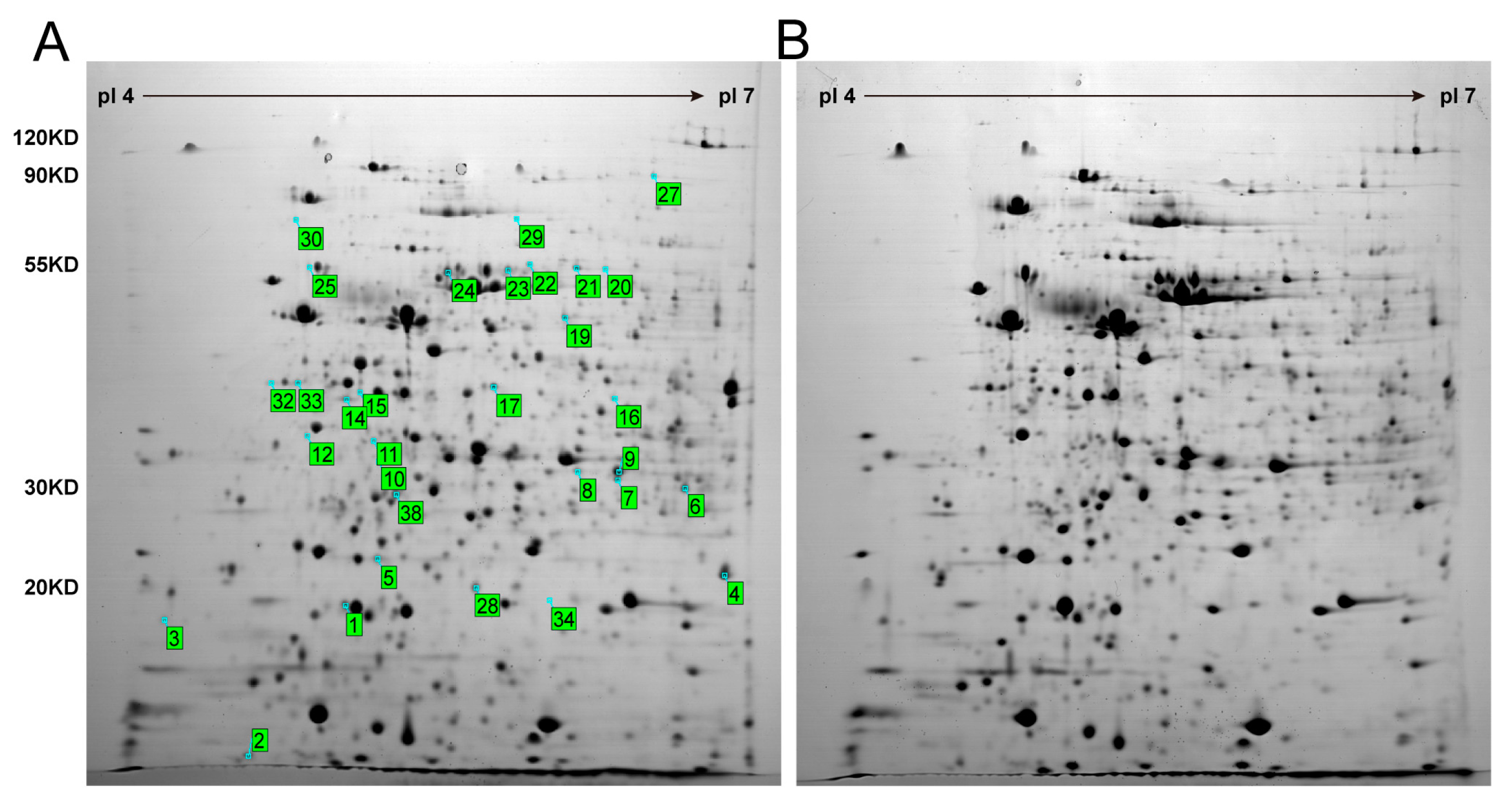
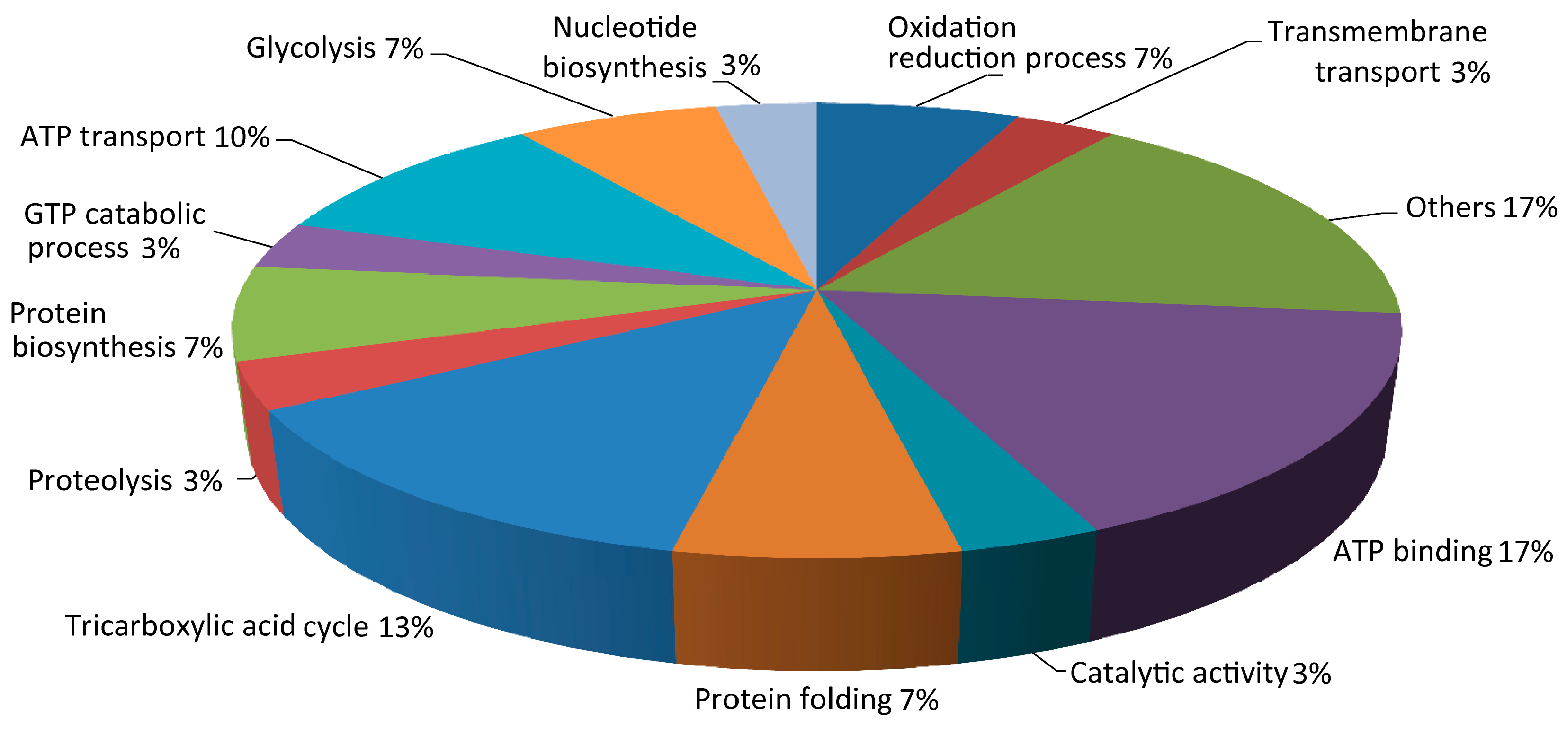
3.2. Proteomics Analysis of K. nataicola during Ethanol-Enhanced Fermentation
3.3. Regulation of Gene Expression in BC Synthesis
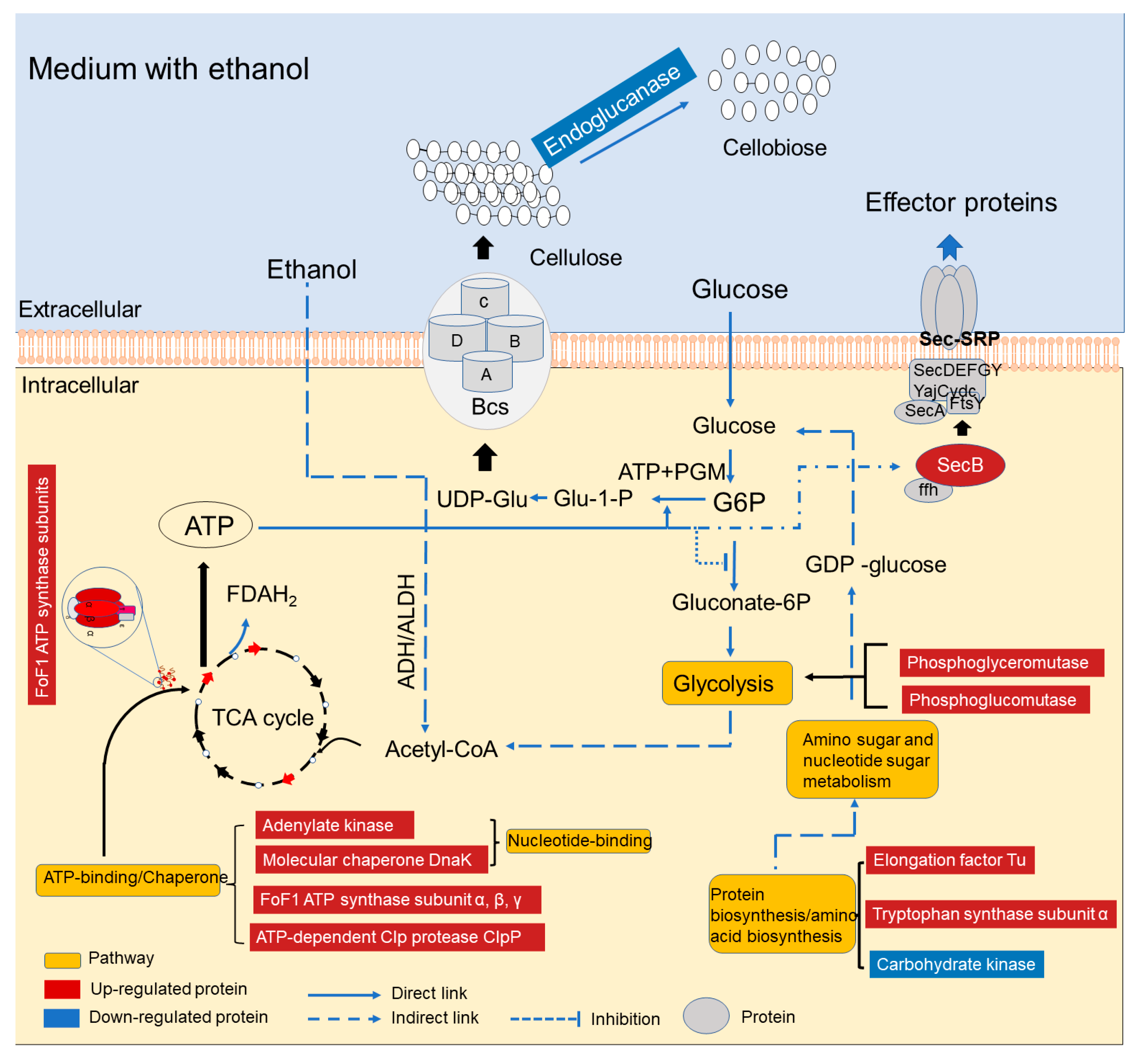
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, N.; Zhou, Z.; Han, Y. Physical and antibacterial properties of bacterial cellulose films supplemented with cell-free supernatant enterocin-producing Enterococcus faecium TJUQ1. Food Microbiol. 2021, 99, 103828. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Guo, C.; Zhang, C.; Liu, S.; Gao, J.; Lin, G.; Yang, H.; Xia, W. Effect of chitosan grafting oxidized bacterial cellulose on dispersion stability and modulability of biodegradable films. Int. J. Biol. Macromol. 2022, 204, 510–519. [Google Scholar] [CrossRef]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Zywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT-Food Sci. Technol. 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, J.; Yuan, X.; Jiang, X.; Wang, Y.; Zhong, C.; Xu, D.; Gu, T.; Wang, F. Bacterial biofilms as platforms engineered for diverse applications. Biotechnol. Adv. 2022, 57, 107932. [Google Scholar] [CrossRef] [PubMed]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Enciso, C.; Torres-Taborda, M.; Zuluaga, R.; Gañán, P.; Rojas, O.J.; Castro, C. Effects of alternative energy sources on bacterial cellulose characteristics produced by Komagataeibacter medellinensis. Int. J. Biol. Macromol. 2018, 117, 735–741. [Google Scholar] [CrossRef]
- Ryngajłło, M.; Jacek, P.; Cielecka, I.; Kalinowska, H.; Bielecki, S. Effect of ethanol supplementation on the transcriptional landscape of bionanocellulose producer Komagataeibacter xylinus E25. Appl. Microbiol. Biotechnol. 2019, 103, 6673–6688. [Google Scholar] [CrossRef]
- Wang, S.-S.; Han, Y.-H.; Chen, J.-L.; Zhang, D.-C.; Shi, X.-X.; Ye, Y.-X.; Chen, D.-L.; Li, M. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers 2018, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Abidi, W.; Torres-Sánchez, L.; Siroy, A.; Krasteva, P.V. Weaving of bacterial cellulose by the Bcs secretion systems. FEMS Microbiol. Rev. 2021, 46, fuab051. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, L.; Deng, Y.; Wei, Q. Research progress of the biosynthetic strains and pathways of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab071. [Google Scholar] [CrossRef] [PubMed]
- Naritomi, T.; Kouda, T.; Yano, H.; Yoshinaga, F. Effect of ethanol on bacterial cellulose production from fructose in continuous culture. J. Ferment. Bioeng. 1998, 85, 598–603. [Google Scholar] [CrossRef]
- Yunoki, S.; Osada, Y.; Kono, H.; Takai, M. Role of ethanol in improvement of bacterial cellulose production: Analysis using 13C-labeled carbon sources. Food Sci. Technol. Res. 2007, 10, 307–313. [Google Scholar] [CrossRef]
- Sakurai, K.; Arai, H.; Ishii, M.; Igarashi, Y. Changes in the gene expression profile of Acetobacter aceti during growth on ethanol. J. Biosci. Bioeng. 2012, 113, 343–348. [Google Scholar] [CrossRef]
- Hu, L.; Grim, C.J.; Franco, A.A.; Jarvis, K.G.; Sathyamoorthy, V.; Kothary, M.H.; McCardell, B.A.; Tall, B.D. Analysis of the cellulose synthase operon genes, bcsA, bcsB, and bcsC in Cronobacter species: Prevalence among species and their roles in biofilm formation and cell–cell aggregation. Food Microbiol. 2015, 52, 97–105. [Google Scholar] [CrossRef]
- Jin, K.; Jin, C.; Wu, Y. Synthetic biology-powered microbial co-culture strategy and application of bacterial cellulose-based composite materials. Carbohydr. Polym. 2022, 283, 119171. [Google Scholar] [CrossRef]
- Chien, L.J.; Chen, H.T.; Yang, P.F.; Lee, C.K. Enhancement of cellulose pellicle production by constitutively expressing Vitreoscilla hemoglobin in Acetobacter xylinum. Biotechnol. Progr. 2006, 22, 1598–1603. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Xie, Y.; Jia, S.; Hou, Y.; Zou, Y.; Zhong, C. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2018, 102, 1155–1165. [Google Scholar] [CrossRef]
- Navarrete-Perea, J.; Gygi, S.P.; Paulo, J.A. Growth media selection alters the proteome profiles of three model microorganisms. J. Proteomics 2021, 231, 104006. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Barrao, C.; Saad, M.M.; Ferrete, E.C.; Bravo, D.; Chappuis, M.-L.; Pérez, R.O.; Junier, P.; Perret, X.; Barja, F. Metaproteomics and ultrastructure characterization of Komagataeibacter spp. involved in high-acid spirit vinegar production. Food Microbiol. 2016, 55, 112–122. [Google Scholar] [PubMed]
- Román-Camacho, J.J.; Mauricio, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; García-García, I. Functional metaproteomic analysis of alcohol vinegar microbiota during an acetification process: A quantitative proteomic approach. Food Microbiol. 2021, 98, 103799. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Chi, Y.; Xu, N.; Yao, W.; Sun, B. Effects of alcohols on bacterial cellulose production by Acetobacter xylinum 186. World J. Microbiol. Biotechnol. 2011, 27, 2281–2285. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Hsieh, S.-Y.; Sung, C.-M.; Pai, B.C.-J.; Liu, N.-J.; Chen, C.P.C. Optimizing human bile preparation for Two-Dimensional gel electrophoresis. Biomed. Res. Int. 2016, 2016, 5185317. [Google Scholar] [CrossRef]
- Sun, W.; Xing, B.; Sun, Y.; Du, X.; Lu, M.; Hao, C.; Lu, Z.; Mi, W.; Wu, S.; Wei, H. Proteome analysis of hepatocellular carcinoma by two-dimensional difference gel electrophoresis: Novel protein markers in hepatocellular carcinoma tissues. Mol. Cell. Proteom. 2007, 6, 1798–1808. [Google Scholar] [CrossRef]
- Galisa, P.S.; da Silva, H.A.; Macedo, A.V.; Reis, V.M.; Vidal, M.S.; Baldani, J.I.; Simões-Araújo, J.L. Identification and validation of reference genes to study the gene expression in Gluconacetobacter diazotrophicus grown in different carbon sources using RT-qPCR. J. Microbiol. Methods 2012, 91, 1–7. [Google Scholar] [CrossRef]
- Kawano, S.; Tajima, K.; Kono, H.; Numata, Y.; Yamashita, H.; Satoh, Y.; Munekata, M. Regulation of endoglucanase gene (cmcax) expression in Acetobacter xylinum. J. Biosci. Bioeng. 2008, 106, 88–94. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Gimeno, A.B. Carboxymethyl Bacterial Cellulose from Nata de Coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar] [CrossRef]
- Kornmann, H.; Duboc, P.; Niederberger, P.; Marison, I.; Von Stockar, U. Influence of residual ethanol concentration on the growth of Gluconacetobacter xylinus I 2281. Appl. Microbiol. Biotechnol. 2003, 62, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Kumar, V.; Kumar, V.; Yadav, S.K. Genomic characterization provides genetic evidence for bacterial cellulose synthesis by Acetobacter pasteurianus RSV-4 strain. Int. J. Biol. Macromol. 2020, 156. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Khan, T.; Park, J.K.; Chang, H.N. Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J. Chem. Eng. 2007, 24, 265–271. [Google Scholar] [CrossRef]
- Li, Y.; Tian, C.; Tian, H.; Zhang, J.; He, X.; Ping, W.; Lei, H. Improvement of bacterial cellulose production by manipulating the metabolic pathways in which ethanol and sodium citrate involved. Appl. Microbiol. Biotechnol. 2012, 96, 1479–1487. [Google Scholar] [CrossRef]
- Birney, M.; Um, H.-D.; Klein, C. Novel mechanisms of Escherichia coli succinyl-coenzyme A synthetase regulation. J. Bacteriol. 1996, 178, 2883–2889. [Google Scholar] [CrossRef]
- Gardner, P.R. Aconitase: Sensitive target and measure of superoxide. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2002; Volume 349, pp. 9–23. [Google Scholar]
- Sun, L.; Zhou, F.; Shao, Y.; Lv, Z.; Li, C. The iron–sulfur protein subunit of succinate dehydrogenase is critical in driving mitochondrial reactive oxygen species generation in Apostichopus japonicus. Fish Shellfish. Immun. 2020, 102, 350–360. [Google Scholar] [CrossRef]
- Gao, T.; Qian, S.; Shen, S.; Zhang, X.; Liu, J.; Jia, W.; Chen, Z.; Ye, J. Reduction of mitochondrial 3-oxoacyl-ACP synthase (OXSM) by hyperglycemia is associated with deficiency of α-lipoic acid synthetic pathway in kidney of diabetic mice. Biochem. Biophys. Res. Commun. 2019, 512, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.R.; Inglis, K.A.; Urch, J.E.; Müller, S.; Van Aalten, D.M.F.; Fairlamb, A.H. Kinetic, inhibition and structural studies on 3-oxoacyl-ACP reductase from Plasmodium falciparum, a key enzyme in fatty acid biosynthesis. Biochem. J. 2006, 393, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Suzuki, T.; Berney, M.; Yoshida, M.; Cook, G.M. Physiological importance of the ε subunit of bacterial FoF1-ATP synthase. BBA-Bioenergetics 2012, 1817, S25–S26. [Google Scholar] [CrossRef]
- Watanabe, R.; Tabata, K.V.; Iino, R.; Noji, H. Direct observation of the rotation of FoF1-ATP synthase driven by the proton motive force. BBA-Bioenergetics 2012, 1817, S26–S27. [Google Scholar] [CrossRef]
- Terasaki, M.; Suzuki, T.; Hanada, T.; Watanabe, K. Functional compatibility of elongation factors between mammalian mitochondrial and bacterial ribosomes: Characterization of GTPase activity and translation elongation by hybrid ribosomes bearing heterologous L7/12 proteins. J. Mol. Biol. 2004, 336, 331–342. [Google Scholar] [CrossRef]
- Girodat, D.; Blanchard, S.C.; Wieden, H.J.; Sanbonmatsu, K.Y. Elongation Factor-Tu switch I is a gate for aminoacyl-tRNA selection. J. Mol. Biol. 2020, 432, 3064–3077. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009, 16, 574–581. [Google Scholar] [CrossRef] [PubMed]
- de Cock, H.; Overeem, W.; Tommassen, J. Biogenesis of outer membrane protein PhoE of Escherichia coli: Evidence for multiple SecB-binding sites in the mature portion of the PhoE protein. J. Mol. Biol. 1992, 224, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Bi, R.; Khatri, V.; Oguzlu, H.; Takada, M.; Jiang, J.; Jiang, F.; Bao, J.; Saddler, J.N. Use of endoglucanase and accessory enzymes to facilitate mechanical pulp nanofibrillation. ACS Sustain. Chem. Eng. 2021, 9, 1406–1413. [Google Scholar] [CrossRef]


| Gene Name | Forward Primer | Reverse Primer | PCR Size (bp) |
|---|---|---|---|
| K. nataicola 16S rRNA | 5′ GTGCTACAATGGCGGTGACA 3′ | 5′ TGACGGGCGGTGTGTACAAG 3′ | 137 |
| Phosphoglucomutase (pgm) | 5′ CAGATCCGCATGGACTGTTCT 3′ | 5′ GGTCCGCATCGGTATCATTG 3′ | 100 |
| Phosphoglycerate kinase (pgk) | 5′ CGCGCTGACCTGAATGTTC 3′ | 5′ ATCACCTTCGCACCCTTCTG 3′ | 110 |
| Phosphopyruvate hydratase (eno) | 5′ CCGATCGACATTCAGGAATTC 3′ | 5′ CCCGAGAGGCTTTTTTTAAGC 3′ | 110 |
| Ppa inorganic pyrophosphatase (ppa) | 5′ GACAAGGTGCACCCGTACTATTC 3′ | 5′ GCCCTTCTCCAGGTCCTTGTA 3′ | 111 |
| Protein Name | Spot | NCBI | Relative Change Ratio | p-Value | Protein PI | Protein MW | Protein Score | Total Ion Score |
|---|---|---|---|---|---|---|---|---|
| Accession No. (gi) | Exp./Theor. | Exp./Theor. | ||||||
| Bacterioferritin | 1 | gi|517915025 | −3.56973 | 3.05 × 10−3 | 5.17/5.17 | 23,103.6/23,118.12 | 291 | 259 |
| Short-chain dehydrogenase | 2 | gi|498194358 | 2.04802 | 6.09 × 10−4 | 5.22/5.22 | 30,091.2/30,109.84 | 153 | 170 |
| Preprotein translocase subunit SecB | 3 | gi|503872550 | 5.13468 | 1.97 × 10−2 | 4.43/4.42 | 21,359/21,372.33 | 285 | 246 |
| Keto hydroxyglutarate aldolase | 4 | gi|498193026 | −2.26968 | 4.03 × 10−3 | 5.76/5.75 | 20,515.9/20,528.93 | 210 | 182 |
| ATP-dependent Clp protease ClpP | 5 | gi|498193945 | −1.70585 | 4.69 × 10−5 | 5.46/5.46 | 25,122.7/25,138.50 | 322 | 254 |
| Putative 2-keto-4-pentenoate hydratase-like protein | 6 | gi|295977509 | −4.69407 | 3.11 × 10−2 | 5.78/5.78 | 27,325/27,341.80 | 67 | 59 |
| Succinyl-CoA synthetase subunit alpha | 7 | gi|498195575 | 1.92323 | 4.79 × 10−4 | 5.74/5.74 | 29,491.2/29,509.89 | 160 | 125 |
| Dihydrofolate reductase | 8 | gi|498197255 | 0.718128658 | 4.08 × 10−3/ | 6.08/6.08 | 32,787.5/32,808.01 | 155 & 255 & 242 | 143 & 236 & 222 |
| 9 | 5.33 × 10−3 | |||||||
| Endopeptidase | 10 | gi|494639202 | 2.20294 | 2.73 × 10−3 | 6.01/6.01 | 52,500.3/52,532.33 | 89 | 78 |
| Tryptophan synthase subunit alpha | 11 | gi|517915408 | 2.31726 | 6.41 × 10−4 | 4.97/4.97 | 29,003.1/29,021.18 | 171 | 140 |
| Elongation factor Tu | 12/ | gi|494641674 | 2.20755/ | 1.23 × 10−3/ | 5.21/5.21 | 43,038/43,065.11 | 381 & 335 & 384 | 302 & 242 & 304 |
| 32/ | 2.81953/ | 1.00 × 10−5/ | ||||||
| 33 | 2.00149 | 2.78 × 10−3 | ||||||
| Endoglucanase | 14 | gi|517916528 | −4.50838 | 8.01 × 10−5 | 5.69/5.69 | 35,858.8/35,881.21 | 112 | 96 |
| GTP-binding protein TypA | 15 | gi|498201343 | 4.97347 | 2.17 × 10−4 | 5.34/5.34 | 67,064.9/ | 260 | 196 |
| 67,106.43 | ||||||||
| FoF1-ATP synthase subunit gamma | 16 | gi|498194795 | 2.28583 | 2.54 × 10−3 | 9.32/9.32 | 31,908.6/31,928.59 | 189 | 194 |
| Hypothetical protein | 17 | gi|498198670 | −2.34605 | 5.71 × 10−3 | 5.48/5.48 | 30,753.7/30,772.93 | 91 | 78 |
| 3-oxoacyl-ACP synthase | 19 | gi|503870050 | 1.68533 | 2.24 × 10−3 | 5.67/5.67 | 44,656.5/44,684.51 | 318 | 246 |
| Phosphoglucomutase | 21 | gi|498197944 | 2.26054/ | 4.93 × 10−4 | 5.67/5.67 | 59,569.2/59,606.28 | 268 | 187 |
| Phosphoglyceromutase | 23 | gi|517914319 | 3.33922 | 2.47 × 10−3 | 5.67/5.67 | 55,241/55,275.56 | 287 | 258 |
| FoF1-ATP synthase subunit alpha | 24 | gi|498194792 | 2.18281 | 1.67 × 10−3 | 5.5/5.50 | 55,345.9/55,380.43 | 614 | 457 |
| FoF1-ATP synthase subunit beta | 25 | gi|517921194 | 3.17124 | 5.57 × 10−3 | 4.92/4.92 | 49,902.8/49,933.85 | 638 | 501 |
| Aconitate hydratase | 27 | gi|517914984 | 2.23475 | 3.57 × 10−4 | 6.09/6.09 | 96,468.8/96,529.47 | 606 | 408 |
| Carbohydrate kinase | 28 | gi|517916350 | −3.89058 | 5.36 × 10−4 | 5.28/5.28 | 18,122.8/18,134.81 | 118 | 97 |
| Molecular chaperone DnaK | 30 | gi|498194452 | 4.11107 | 6.14 × 10−3 | 4.89/4.89 | 67,553/67,594.39 | 297 | 230 |
| Adenylate kinase | 34 | gi|498192553 | 9.06655 | 1.17 × 10−2 | 7.59/7.59 | 24,247.5/24,262.91 | 204 | 184 |
| Succinate dehydrogenase iron-sulfur subunit | 38 | gi|498195041 | 2.31759 | 1.74 × 10−3 | 5.37/5.37 | 29,203.6/29,222.53 | 151 | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, S.; Yang, X.; Xu, W.; Zhang, J.; Li, J.; Chen, H.; Lin, X.; Liu, S.; Li, C. Insights into Proteomics Reveal Mechanisms of Ethanol-Enhanced Bacterial Cellulose Biosynthesis by Komagataeibacter nataicola. Fermentation 2023, 9, 575. https://doi.org/10.3390/fermentation9060575
Fei S, Yang X, Xu W, Zhang J, Li J, Chen H, Lin X, Liu S, Li C. Insights into Proteomics Reveal Mechanisms of Ethanol-Enhanced Bacterial Cellulose Biosynthesis by Komagataeibacter nataicola. Fermentation. 2023; 9(6):575. https://doi.org/10.3390/fermentation9060575
Chicago/Turabian StyleFei, Shuangwen, Xuan Yang, Wentao Xu, Jiachao Zhang, Jun Li, Huamei Chen, Xue Lin, Sixin Liu, and Congfa Li. 2023. "Insights into Proteomics Reveal Mechanisms of Ethanol-Enhanced Bacterial Cellulose Biosynthesis by Komagataeibacter nataicola" Fermentation 9, no. 6: 575. https://doi.org/10.3390/fermentation9060575
APA StyleFei, S., Yang, X., Xu, W., Zhang, J., Li, J., Chen, H., Lin, X., Liu, S., & Li, C. (2023). Insights into Proteomics Reveal Mechanisms of Ethanol-Enhanced Bacterial Cellulose Biosynthesis by Komagataeibacter nataicola. Fermentation, 9(6), 575. https://doi.org/10.3390/fermentation9060575






