Maca (Lepidium meyenii): In Vitro Evaluation of Rumen Fermentation and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Chemical Composition
2.3. In Vitro Fermentation
2.4. In Vitro End-Products
2.5. In Vitro Methane Production
2.6. Measurement of Redox Status and Antioxidant Enzyme Activity Analysis
2.7. Data Analysis
3. Results
3.1. Chemical Composition
3.2. In Vitro Fermentation Characteristics and Methane Production
3.3. In Vitro End-Products
3.4. Oxidative Stress Analysis
4. Discussion
4.1. Chemical Composition
4.2. In Vitro Fermentation
4.3. Methane Production
4.4. Oxidative Stress Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalaf, N.A.; Shakya, A.K.; Al-Othman, A.; El-Agbar, Z.; Farah, H. Antioxidant Activity of Some Common Plants. Turk. J. Biol. 2008, 32, 51–55. [Google Scholar]
- Korkmaz, S. Antioxidants in Maca (Lepidium meyenii) as a Supplement in Nutrition. In Antioxidants in Foods and Its Applications, 1st ed.; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018; pp. 138–154. [Google Scholar]
- Calabrò, S.; Oteri, M.; Vastolo, A.; Cutrignelli, M.I.; Todaro, M.; Chiofalo, B.; Gresta, F. Amaranthus Grain as a New Ingredient in Diets for Dairy Cows: Productive, Qualitative, and in Vitro Fermentation Traits. J. Sci. Food Agric. 2022, 102, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts. Antioxidants 2022, 11, 2366. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Peretti, V.; Ciotola, F.; Albarella, S.; Del Prete, C.; et al. Chemical Analysis of Lepidium meyenii (Maca) and Its Effects on Redox Status and on Reproductive Biology in Stallions. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef]
- Turgud, F.K.; Narinç, D. Influences of Dietary Supplementation with Maca (Lepidium meyenii) on Performance, Parameters of Growth Curve and Carcass Characteristics in Japanese Quail. Animals 2022, 12, 318. [Google Scholar] [CrossRef]
- Greco, A.; Del Prete, C.; De Biase, D.; Palumbo, V.; Albanese, S.; Bruzzese, D.; Carotenuto, D.; Ciani, F.; Tufuri, S.; Meomartino, L.; et al. Effects of Oral Administration of Lepidium meyenii on Morphology of Mice Testis and Motility of Epididymal Sperm Cells After Tetrahydrocannabinol Exposure. Front. Vet. Sci. 2021, 8, 692874. [Google Scholar] [CrossRef]
- Del Prete, C.; Tafuri, S.; Ciani, F.; Pasolini, M.P.; Ciotola, F.; Albarella, S.; Carotenuto, D.; Peretti, V.; Cocchia, N. Influences of Dietary Supplementation with Lepidium meyenii (Maca) on Stallion Sperm Production and on Preservation of Sperm Quality during Storage at 5 °C. Andrology 2018, 6, 351–361. [Google Scholar] [CrossRef]
- Del Prete, C.; Calabria, A.; Longombardi, V.; Palumbo, V.; Merlo, B.; Iacono, E.; Tafuri, S.; Carotenuto, D.; Ciani, F.; Damiano, S.; et al. Effect of Aqueous Extract of Maca Addition to an Extender for Chilled Canine Semen. Animals 2022, 12, 1638. [Google Scholar] [CrossRef]
- da Silva Peres, N.D.; Cabrera, L.P.B.; Medeiros, L.L.M.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Reitz, F.A.C. Medicinal Effects of Peruvian Maca (Lepidium meyenii): A Review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- Sahin, N.; Orhan, C.; Gencoglu, H.; Er, B.; Ozercan, I.H.; Komorowski, J.R.; Sahin, K. Effects of Maca (Lepidium meyenii) on Nutrient Digestibility and Major Nutrient Transporters in Rats Fed a High-Fat Diet. Food Sci. Nutr. 2021, 9, 5765–5773. [Google Scholar] [CrossRef]
- Korkmaz, S.; Eseceli, H.; Korkmaz, I.O.; Bilal, T. Effect of Maca (Lepidium meyenii) powder dietary supplementation on performance, egg quality, yolk cholesterol, serum parameters and antioxidant status of laying hens in the post-peak period. Eur. Poult. Sci. 2016, 80, 147. [Google Scholar] [CrossRef]
- Olgun, O.; Gül, E.T.; Tüzün, A.E.; Yıldız, A. Effect of Maca Powder Supplementation to Growing Quail Diets on Performance, Carcass, Serum Constituents and Hormones, and Bone and Ileum Characteristics. Trop. Anim. Health Prod. 2022, 54, 239. [Google Scholar] [CrossRef] [PubMed]
- Gül, E.T.; Olgun, O.; Yildiz, A.; Tüzün, A.E.; Sarmiento-García, A. Use of Maca Powder (Lepidium meyenii) as Feed Additive in Diets of Laying Quails at Different Ages: Its Effect on Performance, Eggshell Quality, Serum, Ileum, and Bone Properties. Vet. Sci. 2022, 9, 418. [Google Scholar] [CrossRef]
- Staerfl, S.M.; Kreuzer, M.; Soliva, C.R. In vitro screening of unconventional feeds and various natural supplements for their ruminal methane mitigation potential when included in a maize-silage based diet. J. Anim. Feed Sci. 2010, 19, 651–664. [Google Scholar] [CrossRef]
- Staerfl, S.M.; Zeitz, J.O.; Kreuzer, M.; Soliva, C.R. Methane conversion rate of bulls fattened on grass or maize silage as compared with the IPCC default values, and the long-term methane mitigation efficiency of adding acacia tannin, garlic, maca and lupine. Agric. Ecosyst. Environ. 2012, 148, 111–120. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Dean, P.J. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Cocchia, N.; Vassetti, A.; Carotenuto, D.; Esposito, L.; Maruccio, L.; Avallone, L.; Ciani, F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2019, 35, 4550–4559. [Google Scholar] [CrossRef]
- Czerska, M.; Mikołajewska, K.; Zieliński, M.; Gromadzińska, J.; Wąsowicz, W. Today’s Oxidative Stress Markers. Med. Pr. 2015, 66, 393–405. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and no starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- CSIRO. Nutrient requirements of domesticated ruminants. In Nutrient Requirements of Domesticated Ruminants; Freer, M., Dove, H., Nolan, J.V., Eds.; CSIRO Pub: Clayton, VIC, Australia, 2007. [Google Scholar]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Raso, G.; Todaro, M. Silage of Prickly Pears (Opuntia spp.) Juice by-Products. Animals 2020, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Musco, N.; Trotta, N.; Cutrignelli, M.I.; Di Francia, A.; Infascelli, F.; Tudisco, R.; Lombardi, P.; Vastolo, A.; Calabrò, S. Chemical Characterisation and in Vitro Gas Production Kinetics of Eight Faba Bean Varieties. Animals 2020, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, A.; Calabro, S.; Primi, R.; Carone, F.; Cutrignelli, M.I.; Tudisco, R.; Piccolo, G.; Ronchi, B.; Danieli, P.P. In vitro fermentation patterns and methane production of sainfoin (Onobrychis viciifolia Scop.) hay with different condensed tannin contents. Grass Forage Sci. 2011, 66, 488–500. [Google Scholar] [CrossRef]
- Mancini, S.; Mariani, F.; Sena, P.; Benincasa, M.; Roncucci, L. Myeloperoxidase expression in human colonic mucosa is related to systemic oxidative balance in healthy subjects. Redox Rep. 2017, 22, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ciani, F.; Maruccio, L.; Cocchia, N.; d’Angelo, D.; Carotenuto, D.; Avallone, L.; Namagerdi, A.A.; Tafuri, S. Antioxidants in Assisted Reproductive Technologies: An Overview on Dog, Cat, and Horse. J. Adv. Vet. 2021, 8, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feedstuff. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Voigt, C.; Mosenthin, R.; Verstegen, M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. J. Anim. Sci. 2001, 73, 313–332. [Google Scholar] [CrossRef]
- Bianchi, A. MACA Lepidium meyenii. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2003, 2, 30–36. Available online: http://www.Redalyc.Org/Articulo.Oa?Id=85620303 (accessed on 30 March 2023).
- Wang, S.; Zhu, F. Chemical Composition and Health Effects of Maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Rondán-Sanabria Gerby, G.; Valcarcel-Yamani, B.; Finardi-Filho, F. Effects on Starch and Amylolytic Enzymes during Lepidium meyenii Walpers Root Storage. Food Chem. 2012, 134, 1461–1467. [Google Scholar] [CrossRef]
- Erikssonm, T.; Murphy, M. Ruminal Digestion of Leguminous Forage, Potatoes and Fodder Beets in Batch Culture. I. Fermentation Pattern. Anim. Feed Sci. Technol. 2004, 111, 73–88. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Pearson Education Limited: New York, NY, USA, 2011. [Google Scholar]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Mammi, L.M.E. In Vitro Evaluation of Sugar Digestibility in Molasses. Ital. J. Anim. Sci. 2021, 20, 571–577. [Google Scholar] [CrossRef]
- Vastolo, A.; Matera, R.; Serrapica, F.; Cutrignelli, M.I.; Neglia, G.; Kiatti, D.D.; Calabrò, S. Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow. Fermentation 2022, 8, 351. [Google Scholar] [CrossRef]
- Dong, J.N.; Li, S.Z.; Chen, X.; Qin, G.X.; Wang, T.; Sun, Z.; Wu, D.; Zhao, W.; Demelash, N.; Zhang, X.F.; et al. Effects of Different Combinations of Sugar and Starch Concentrations on Ruminal Fermentation and Bacterial-Community Composition in Vitro. Front. Nutr. 2021, 8, 727714. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.; Titgemeyer, E.; Mamedova, L.; Bradford, B. Dietary molasses increases ruminal pH and enhances ruminal biohydrogenation during milk fat depression. J. Dairy Sci. 2011, 94, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Malhi, M.; Gui, H.; Yao, L.; Aschenbach, J.R.; Gäbel, G.; Shen, Z. Increased Papillae Growth and Enhanced Short-Chain Fatty Acid Absorption in the Rumen of Goats Are Associated with Transient Increases in Cyclin D1 Expression after Ruminal Butyrate Infusion. J. Dairy Sci. 2013, 96, 7603–7616. [Google Scholar] [CrossRef]
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evid. Based Complement. Altern. Med. 2012, 2012, 193496. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules 2022, 27, 8662. [Google Scholar] [CrossRef]
- Meissner, H.O.; Mscisz, A.; Mrozikiewicz, M.; Baraniak, M.; Mielcarek, S.; Kedzia, B.; Piatkowska, E.; Jólkowska, J.; Pisulewski, P. Peruvian Maca (Lepidium peruvianum): (I) Phytochemical and Genetic Differences in Three Maca Phenotypes. Int. J. Biomed. Sci. IJBS 2015, 11, 131–145. [Google Scholar]
- Campos, D.; Chirinos, R.; Barreto, O.; Noratto, G.; Pedreschi, R. Optimized methodology for the simultaneous extraction of glucosinolates, phenolic compounds and antioxidant capacity from maca (Lepidium meyenii). Ind. Crop. Prod. 2013, 49, 747–754. [Google Scholar] [CrossRef]
- Clément, C.; Diaz, D.; Manrique, I.; Avula, B.; Khan, I.A.; Aguirre, D.D.P.; Kunz, C.; Mayer, A.C.; Kreuzer, M. Secondary metabolites in maca as affected by hypocotyl color, cultivation history, and site. Agron. J. 2010, 102, 431–439. [Google Scholar] [CrossRef]
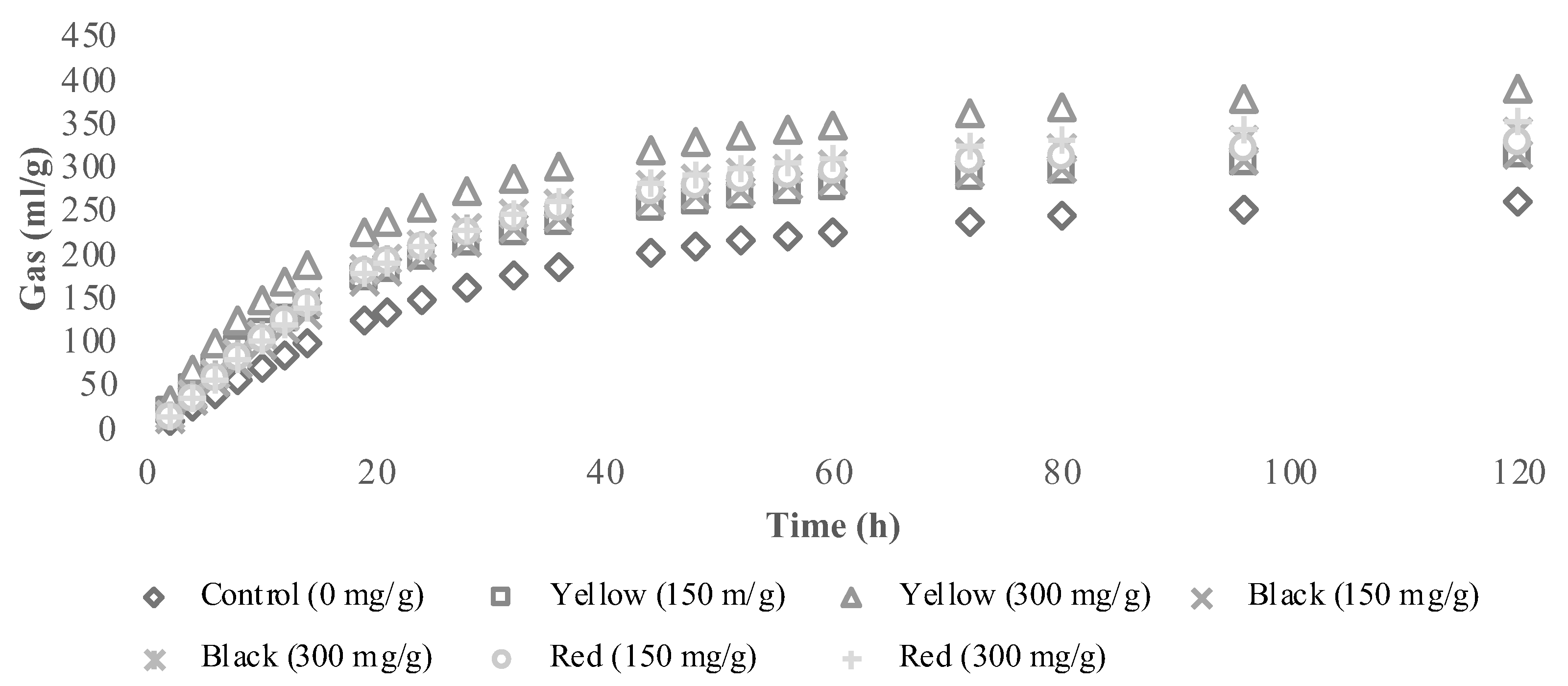
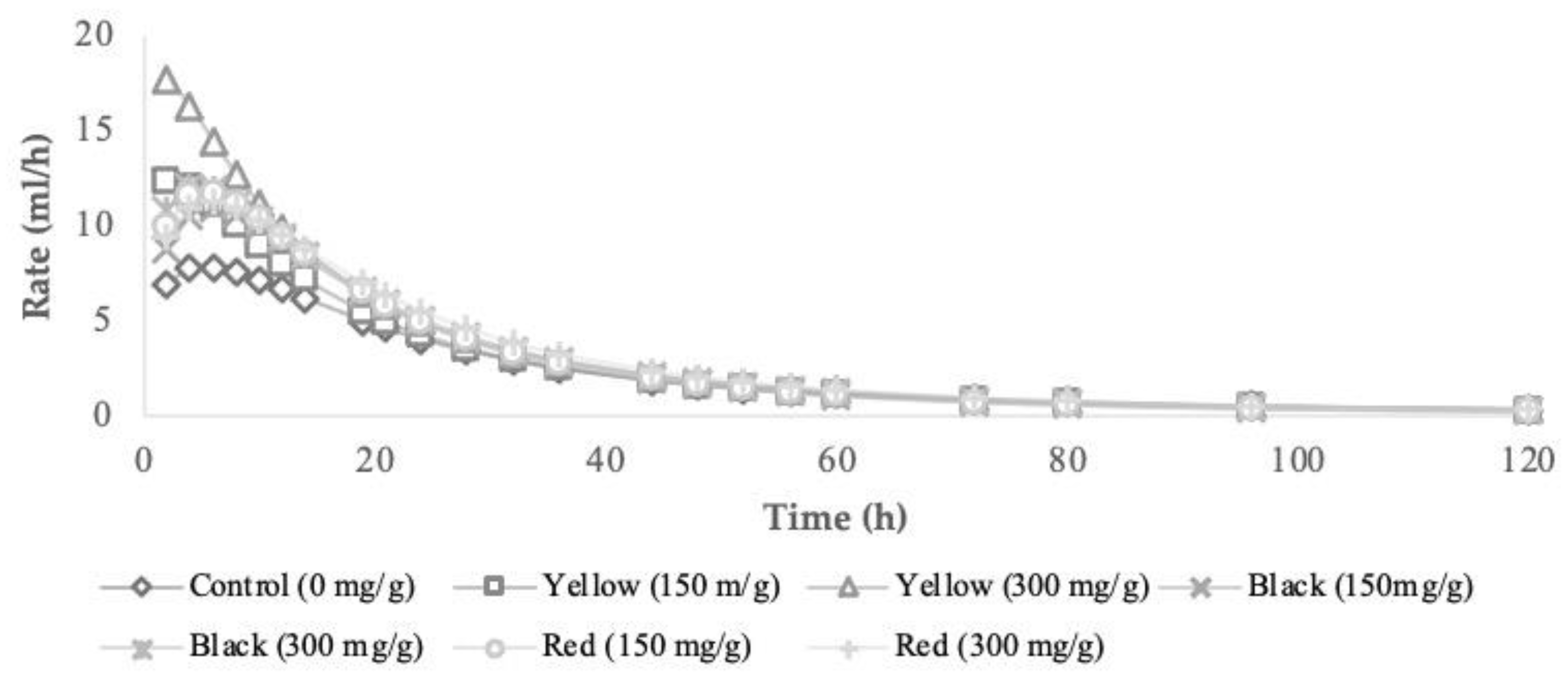
| Ecotype | Ash | CP | NDF | ADF | ADL | EE | NSC |
|---|---|---|---|---|---|---|---|
| Yellow | 1.00 C | 3.60 B | 1.16 B | 0.87 C | ND | 0.30 C | 94.0 A |
| Black | 7.06 A | 13.5 A | 13.6 A | 9.52 A | 1.19 a | 1.50 B | 63.9 C |
| Red | 5.37 B | 12.6 A | 12.5 A | 7.55 B | 1.07 b | 2.23 A | 67.4 B |
| MSE | 0.0003 | 0.05 | 0.28 | 0.02 | 0.002 | 0.0003 | 0.44 |
| 120 h | 24 h | |||||
|---|---|---|---|---|---|---|
| OMD | OMCV | Tmax | Rmax | iCH4 | dCH4 | |
| % | mL/g | h | mL/g | mL/g iOM | mL/g dOM | |
| Variety effect | ||||||
| Yellow | 72.4 ± 0.34 | 339 ± 32.3 | 2.03 ± 0.67 | 15.2 ± 0.28 | 10.8 ± 1.19 | 18.2 ± 5.53 |
| Black | 71.7 ± 0.40 | 328 ± 15.4 | 5.21 ± 0.82 | 11.5 ± 0.68 | 11.3 ± 0.95 | 21.8 ± 2.12 |
| Red | 71.0 ± 0.82 | 337 ± 14.6 | 5.42 ± 1.13 | 11.6 ± 0.41 | 11.5 ± 0.74 | 22.8 ± 1.62 |
| p-Value | 0.022 | 0.149 | <0.001 | <0.001 | 0.331 | 0.008 |
| Dose effect | ||||||
| 0 | 72.6 ± 0.91 | 262 ± 2.42 | 5.29 ± 0.30 | 7.89 ± 0.47 | 13.5 ± 0.69 | 27.8 ± 1.17 |
| 150 | 71.9 ± 0.41 | 318 ± 10.1 | 4.52 ± 0.81 | 11.8 ± 0.71 | 11.9 ± 0.48 | 23.2 ± 1.33 |
| 300 | 71.5 ± 0.98 | 352 ± 13.3 | 3.92 ± 0.93 | 13.7 ± 0.31 | 10.5 ± 0.70 | 18.7 ± 1.25 |
| p-Value | 0.139 | <0.001 | 0.240 | <0.001 | 0.006 | 0.001 |
| Variety × Dose | ||||||
| p-Value | 0.159 | 0.031 | 0.337 | <0.001 | 0.804 | 0.013 |
| pH | VFA | BCFA | Ace | Prop | Iso-But | But | Iso-Val | Val | A/P | |
|---|---|---|---|---|---|---|---|---|---|---|
| mmol/L | % VFA | |||||||||
| Variety effect | ||||||||||
| Yellow | 6.44 ± 0.08 | 86.9 ± 10.6 | 2.17 ± 0.25 | 63.8 ± 4.22 | 19.0 ± 1.14 | 1.02 ± 0.11 | 13.0 ± 0.76 | 1.15 ± 0.15 | 1.94 ± 0.94 | 3.36 ± 0.38 |
| Black | 6.45 ± 0.03 | 89.4 ± 9.08 | 2.05 ± 0.25 | 69.1 ± 1.54 | 17.7 ± 0.83 | 0.97 ± 0.12 | 9.23 ± 0.45 | 1.08 ± 0.13 | 1.65 ± 0.54 | 3.86 ± 0.26 |
| Red | 6.42 ± 0.09 | 92.7 ± 7.66 | 2.39 ± 0.25 | 67.4 ± 0.72 | 19.0 ± 0.18 | 1.10 ± 0.09 | 9.12 ± 0.44 | 1.29 ± 0.17 | 2.11 ± 0.71 | 3.54 ± 0.07 |
| p-Value | 0.612 | 0.029 | 0.018 | <0.001 | 0.093 | 0.074 | <0.001 | 0.027 | <0.001 | 0.005 |
| Dose effect | ||||||||||
| 0 | 6.60 ± 0.04 | 80.8 ± 1.32 | 3.42 ± 0.12 | 66.1 ± 0.76 | 19.0 ± 0.40 | 1.51 ± 0.37 | 9.62 ± 0.11 | 1.90 ± 0.08 | 1.89 ± 0.13 | 3.48 ± 0.11 |
| 150 | 6.49 ± 0.02 | 81.9 ± 4.31 | 2.29 ± 0.34 | 68.2 ± 1.77 | 18.3 ± 0.92 | 1.05 ± 0.14 | 9.46 ± 0.49 | 1.23 ± 0.20 | 1.71 ± 0.41 | 3.74 ± 0.28 |
| 300 | 6.39 ± 0.05 | 97.4 ± 1.71 | 2.12 ± 0.17 | 65.3 ± 3.99 | 19.0 ± 0.84 | 1.00 ± 0.08 | 11.4 ± 0.73 | 1.11 ± 0.10 | 2.09 ± 0.10 | 3.44 ± 0.31 |
| p-Value | 0.002 | 0.077 | <0.001 | <0.001 | 0.121 | 0.246 | <0.001 | 0.048 | <0.001 | 0.010 |
| Variety × Dose | ||||||||||
| p-Value | 0.307 | 0.009 | 0.353 | <0.001 | 0.257 | 0.013 | <0.001 | 0.015 | <0.001 | 0.027 |
| 24 h | 120 h | |||||
|---|---|---|---|---|---|---|
| OXY | T-SOD | LP Cholox | OXY | T-SOD | LP Cholox | |
| mmol HClO/mL | U/mL | mEq/L | mmol HClO/mL | U/mL | mEq/L | |
| Variety effect | ||||||
| Yellow | 43.3 ± 16.1 | 20.4 ± 1.05 | 1045 ± 58.9 | 58.7 ± 17.5 | 23.3 ± 1.32 | 1656 ± 154 |
| Black | 32.1 ± 15.3 | 16.9 ± 1.35 | 772 ± 123 | 62.1 ± 20.0 | 22.8 ± 1.19 | 1580 ± 125 |
| Red | 57.7 ± 15.2 | 14.7 ± 1.84 | 904 ± 222 | 60.7 ± 21.7 | 20.5 ± 0.87 | 1266 ± 114 |
| p-Value | 0.182 | 0.004 | 0.001 | 0.997 | 0.002 | <0.001 |
| Dose effect | ||||||
| 0 | 63.8 ± 2.58 | 20.5 ± 2.61 | 903 ± 46.7 | 163 ± 18.5 | 21.2 ± 0.35 | 1604 ± 9.90 |
| 150 | 52.0 ± 12.1 | 17.9 ± 2.16 | 1013 ± 118 | 66.6 ± 15.5 | 22.9 ± 2.03 | 1596 ± 216 |
| 300 | 36.8 ± 10.0 | 16.7 ± 3.38 | 801 ± 172 | 52.9 ± 19.0 | 21.6 ± 0.85 | 1406 ± 178 |
| p-Value | 0.174 | 0.232 | <0.001 | 0.717 | 0.014 | 0.004 |
| Variety × Dose | ||||||
| p-Value | 0.115 | 0.401 | 0.209 | 0.997 | 0.085 | 0.589 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vastolo, A.; Calabrò, S.; Carotenuto, D.; Cutrignelli, M.I.; Kiatti, D.d.; Tafuri, S.; Ciani, F. Maca (Lepidium meyenii): In Vitro Evaluation of Rumen Fermentation and Oxidative Stress. Fermentation 2023, 9, 568. https://doi.org/10.3390/fermentation9060568
Vastolo A, Calabrò S, Carotenuto D, Cutrignelli MI, Kiatti Dd, Tafuri S, Ciani F. Maca (Lepidium meyenii): In Vitro Evaluation of Rumen Fermentation and Oxidative Stress. Fermentation. 2023; 9(6):568. https://doi.org/10.3390/fermentation9060568
Chicago/Turabian StyleVastolo, Alessandro, Serena Calabrò, Domenico Carotenuto, Monica Isabella Cutrignelli, Dieu donné Kiatti, Simona Tafuri, and Francesca Ciani. 2023. "Maca (Lepidium meyenii): In Vitro Evaluation of Rumen Fermentation and Oxidative Stress" Fermentation 9, no. 6: 568. https://doi.org/10.3390/fermentation9060568
APA StyleVastolo, A., Calabrò, S., Carotenuto, D., Cutrignelli, M. I., Kiatti, D. d., Tafuri, S., & Ciani, F. (2023). Maca (Lepidium meyenii): In Vitro Evaluation of Rumen Fermentation and Oxidative Stress. Fermentation, 9(6), 568. https://doi.org/10.3390/fermentation9060568









