The Effect of Guanidinoacetic Acid Addition on In Vitro Rumen Fermentation Characteristics and Gas Production of Early- and Late-Stage Sheep-Fattening Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Guanidinoacetic Acid Product
2.3. In Vitro System
2.4. Feed, Experimental Design, and Rumen Fluid Donor Animals
2.5. Experimental Procedures
2.6. Calculations and Statistical Analyses
3. Results
3.1. In Vitro Degradability
3.2. Fitted Curves and Kinetic Gas Production
3.3. Final pH, NH3-N, MCP, and VFA Pattern
3.4. Fermentation Gas Composition
4. Discussion
4.1. In Vitro Degradability
4.2. Gas-Production Kinetic
4.3. Final pH, NH3-N, MCP, and VFA Pattern
4.4. Fermentation Gas Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poggio, F.; Brofiga, M.; Tedesco, M.; Massobrio, P.; Adriano, E.; Balestrino, M. Lack of Epileptogenic Effects of the Creatine Precursor Guanidinoacetic Acid on Neuronal Cultures In Vitro. Biomolecules 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, H.; Xin, J.; Xu, L.; Li, M.; Yu, H.; Zhang, W.; Ge, Y.; Li, Y.; Qu, M. Effects of Dietary Guanidinoacetic Acid on the Feed Efficiency, Blood Measures, and Meat Quality of Jinjiang Bulls. Front. Vet. Sci. 2021, 8, 684295. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Guanidinoacetic acid as a performance-enhancing agent. Amino Acids. 2016, 48, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Adriano, E.; Garbati, P.; Salis, A.; Damonte, G.; Millo, E.; Balestrino, M. Creatine salts provide neuroprotection even after partial impairment of the creatine transporter. Neuroscience 2017, 340, 299–307. [Google Scholar] [CrossRef]
- Weinsanto, I.; Mouheiche, J.; Laux-Biehlmann, A.; Delalande, F.; Marquette, A.; Chavant, V.; Gabel, F.; Cianferani, S.; Charlet, A.; Parat, M.O.; et al. Morphine Binds Creatine Kinase B and Inhibits Its Activity. Front. Cell. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Wallery, J.J.; Kale, V.P.; Novak, J.; Gibbs, S.; Do, M.T.; McKew, J.C.; Terse, P.S. Evaluation of chronic toxicity of cyclocreatine in beagle dogs after oral gavage administration for up to 23 weeks. Toxicol. Appl. Pharmacol. 2021, 430, 115680. [Google Scholar] [CrossRef] [PubMed]
- Forni Ogna, V.; Ogna, A.; Vuistiner, P.; Pruijm, M.; Ponte, B.; Ackermann, D.; Gabutti, L.; Vakilzadeh, N.; Mohaupt, M.; Martin, P.Y.; et al. New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015, 13, 40. [Google Scholar] [CrossRef]
- Jayaraman, B.; La, K.V.; La, H.; Doan, V.; Carpena, E.M.; Rademacher, M.; Channarayapatna, G. Supplementation of guanidinoacetic acid to pig diets: Effects on performance, carcass characteristics, and meat quality. J. Anim. Sci. 2018, 96, 2332–2341. [Google Scholar] [CrossRef]
- Çenesiz, A.A.; Yavaş, İ.; Çiftci, İ.; Ceylan, N.; Taşkesen, H.O. Guanidinoacetic acid supplementation is favourable to broiler diets even containing poultry by-product meal. Br. Poult. Sci. 2020, 61, 311–319. [Google Scholar] [CrossRef]
- Ardalan, M.; Batista, E.D.; Titgemeyer, E.C. Effect of post-ruminal guanidinoacetic acid supplementation on creatine synthesis and plasma homocysteine concentrations in cattle. J. Anim. Sci. 2020, 98, skaa072. [Google Scholar] [CrossRef]
- Ardalan, M.; Miesner, M.D.; Reinhardt, C.D.; Thomson, D.U.; Armendariz, C.K.; Smith, J.S.; Titgemeyer, E.C. Effects of guanidinoacetic acid supplementation on nitrogen retention and methionine flux in cattle. J. Anim. Sci. 2021, 99, skab172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Wang, C.; Guo, G.; Huo, W.; Xia, C.; Chen, L.; Zhang, Y.; Pei, C.; Liu, Q. Effects of guanidinoacetic acid supplementation on lactation performance, nutrient digestion and rumen fermentation in Holstein dairy cows. J. Sci. Food Agric. 2023, 103, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Kalscheur, K.F.; Huhtanen, P.; Faciola, A.P. Effects of ruminal protozoa on methane emissions in ruminants—A meta-analysis. J. Dairy Sci. 2022, 105, 7482–7491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Susenbeth, A.; Südekum, K.H. In vitro gas production measurements to evaluate interactions between untreated and chemically treated rice straws, grass hay, and mulberry leaves. J. Anim. Sci. 2002, 80, 517–524. [Google Scholar] [CrossRef]
- Quinn, M.J.; May, M.L.; Hales, K.E.; DiLorenzo, N.; Leibovich, J.; Smith, D.R.; Galyean, M.L. Effects of ionophores and antibiotics on in vitro hydrogen sulfide production, dry matter disappearance, and total gas production in cultures with a steam-flaked corn-based substrate with or without added sulfur. J. Anim. Sci. 2009, 87, 1705–1713. [Google Scholar] [CrossRef]
- Danielsson, R.; Ramin, M.; Bertilsson, J.; Lund, P.; Huhtanen, P. Evaluation of a gas in vitro system for predicting methane production in vivo. J. Dairy Sci. 2017, 100, 8881–8894. [Google Scholar] [CrossRef] [PubMed]
- Raineri, C.; Stivari, T.S.; Gameiro, A.H. Lamb Production Costs: Analyses of Composition and Elasticities Analysis of Lamb Production Costs. Asian-Australas. J. Anim. Sci. 2015, 28, 1209–1215. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Song, P.; Zhao, J.; Zhang, J.; Zhao, J. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, C.; Pan, J.; Ma, C.; Wang, C.; Li, X.; Cai, W.; Yang, K. Effects of dietary guanidinoacetic acid on growth performance, guanidinoacetic acid absorption and creatine metabolism of lambs. PLoS ONE 2022, 17, e0264864. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, Y.L.; Wang, W.K.; Zhang, Z.W.; Si, X.M.; Cao, Z.J.; Li, S.L.; Yang, H.J. Beneficial effect of Rhodopseudomonas palustris on in vitro rumen digestion and fermentation. Benef. Microbes. 2020, 11, 91–99. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, H.; Meng, X.; Zhang, D.; Cao, B. Effect of melamine on in vitro rumen microbial growth, methane production and fermentation of Chinese wild rye hay and maize meal in binary mixtures. J. Agric. Sci. 2014, 152, 686–696. [Google Scholar] [CrossRef]
- Dhakal, R.; Hansen, H.H.; Milora, N.; Copani, G. The effect of direct-fed microbials on grass or maize silage on in vitro rumen fermentation. Fermentation 2023, 9, 347. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Narváez-López, A.C.; Chay-Canul, A.J.; Sainz-Ramirez, A.; Castelan-Ortega, O.A.; Zhang, N.; Gonzalez-Ronquillo, M.; Vargas-Bello-Pérez, E. Effect of different dietary inclusion levels of whole plant green tomato (Physalis philadelphica) silage on nutrient intake and digestibility, and in vitro rumen fermentation kinetics in sheep. Front. Vet. Sci. 2022, 9, 980619. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.H.; Wang, W.K.; Wu, Q.C.; Zhang, F.; Li, W.J.; Li, S.L.; Wang, W.; Cao, Z.J.; Yang, H.J. The Effect of γ-Aminobutyric Acid Addition on In Vitro Ruminal Fermentation Characteristics and Methane Production of Diets Differing in Forage-to-Concentrate Ratio. Fermentation 2023, 9, 105. [Google Scholar] [CrossRef]
- Bremner, M.; Keeney, D.R. Distillation Methods for Determination of Ammonium Nitrate and Nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Perez, J.F.J.; Balcells, J.A.; Cebrian; Martin, S.M. Excretion of endogenous and exogenous purine derivatives in sheep: Effect of increased concentrate intake. Br. J. Nutr. 1998, 79, 237–240. [Google Scholar] [CrossRef]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Wang, W.-K.; Wang, Y.-L.; Li, W.-J.; Wu, Q.-C.; Li, S.-L.; Yang, H.-J. Gossypol Exhibited Higher Detrimental Effect on Ruminal Fermentation Characteristics of Low-Forage in Comparison with High-Forage Mixed Feeds. Toxics 2021, 9, 51. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Si, X.; Cao, Z.; Li, S.; Yang, H. Rumen Methanogenesis, Rumen Fermentation, and Microbial Community Response to Nitroethane, 2-Nitroethanol, and 2-Nitro-1-Propanol: An In Vitro Study. Animals 2020, 10, 479. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.Z.; Wang, D.H.; Wu, M.J.; Zheng, C.; Wu, Z.Z.; Wang, C.; Liu, Q.; Zhang, J.; Guo, G.; et al. Effects of guanidinoacetic acid and coated folic acid supplementation on growth performance, nutrient digestion and hepatic gene expression in Angus bulls. Br. J. Nutr. 2021, 126, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, C.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Zhang, Y.L.; Pei, C.X.; et al. Effects of guanidinoacetic acid supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Animal 2020, 14, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7255. [Google Scholar]
- Blu¨mmel, M.; Rskov, E.R. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim. Feed Sci. Technol. 1993, 40, 109–119. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Zhang, Q.; Yang, Y.; Zou, C.; Li, L.; Liang, X.; Wei, S.; Lin, B. Ruminal fermentation and microbial community differently influenced by four typical subtropical forages in vitro. Anim. Nutr. 2018, 4, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.F.; Pearl, K.A.; Titgemeyer, E.C. Relative bioavailability of guanidinoacetic acid delivered ruminally or abomasally to cattle. J. Anim. Sci. 2020, 98, skaa282. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef]
- Friggens, N.C.; Oldham, J.D.; Dewhurst, R.J.; Horgan, G. Proportions of volatile fatty acids in relation to the chemical composition of feeds based on grass silage. J. Dairy Sci. 1998, 81, 1331–1344. [Google Scholar] [CrossRef]
- Iii, R.; Murphy, M.R. An in vitro technique for measuring the production rate of volatile. Small Rumin. Res. 2004, 54, 219–225. [Google Scholar]
- Wright, D.E.; Hungate, R.E. Metabolism of glycine by rumen microorganisms. Appl. Microbiol. 1967, 15, 152–157. [Google Scholar] [CrossRef]
- Andreesen, J.R. Glycine metabolism in anaerobes. Antonie Van Leeuwenhoek 1994, 66, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cui, Z.; Jiang, Y.; Aisikaer, A.; Wu, Q.; Zhang, F.; Wang, W.; Bo, Y.; Yang, H. Dietary Guanidine Acetic Acid Improves Ruminal Antioxidant Capacity and Alters Rumen Fermentation and Microflora in Rapid-Growing Lambs. Antioxidants 2023, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Yin, D.M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef] [PubMed]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J.; Gisi, D.D.; Higginbotham, G.E.; Riordan, T.J. Methane production from commercial dairy rations estimated using an in vitro gas technique. Anim. Feed Sci. Technol. 2005, 123–124, 391–402. [Google Scholar] [CrossRef]
- Mauerhofer, L.M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.S.; Paulik, C.; Rittmann, S.K.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Wagner, A.O.; Reitschuler, C.; Illmer, P. Effect of different acetate:propionate ratios on the methanogenic community during thermophilic anaerobic digestion in batch experiments. Biochem. Eng. J. 2014, 90, 154–161. [Google Scholar] [CrossRef]

| Items | T1 | T2 |
|---|---|---|
| Ingredients, g/kg dry matter | ||
| Peanut vine hay | 100 | 80 |
| Foxtail millet silage | 150 | 120 |
| Corn meal | 380 | 500 |
| Soybean meal | 150 | 150 |
| Corn DDGS | 180 | 110 |
| 1 Premix | 40 | 40 |
| Nutrients, g/kg dry matter | ||
| Dry matter | 803 | 799 |
| Crude protein | 183 | 170 |
| Organic matter | 859 | 885 |
| Ether extract | 42 | 37 |
| Neutral detergent fibre | 234 | 194 |
| Acid detergent fiber | 119 | 98 |
| Calcium | 8.5 | 8.6 |
| Phosphorus | 3.8 | 3.4 |
| Metabolic energy MJ/kg | 10.62 | 10.37 |
| Items | Feed | GAA | SEM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.03% | 0.05% | 0.07% | 0.09% | 0.11% | 0.13% | 0.15% | F | G | L | Q | F × G | |||
| DM | T1 | 90.0 | 89.9 | 89.0 | 89.4 | 89.6 | 89.7 | 89.8 | 90.0 | 0.58 | ** | ns | ns | ns | ns |
| T2 | 92.7 | 92.2 | 91.8 | 93.1 | 92.6 | 92.7 | 92.2 | 92.3 | |||||||
| NDF | T1 | 56.6 bc | 56.7 bc | 57.0 bc | 56.9 bc | 57.5 a | 57.5 a | 57.1 ab | 56.5 c | 0.24 | ns | ** | ns | ** | ns |
| T2 | 56.7 b | 56.9 b | 56.7 b | 57.1 ab | 57.7 a | 57.7 a | 56.1 ab | 56.5 b | |||||||
| ADF | T1 | 47.1 c | 47.9 b | 47.8 b | 47.6 bc | 48.7 a | 48.7 a | 47.9 b | 47.7 b | 0.25 | ns | ** | ns | ** | ns |
| T2 | 47.2 c | 47.9 bc | 47.9 bc | 47.3 bc | 49.0 a | 48.9 a | 48.1 b | 47.7 bc | |||||||
| Items | Feed | GAA | SEM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.03% | 0.05% | 0.07% | 0.09% | 0.11% | 0.13% | 0.15% | F | G | L | Q | F × G | |||
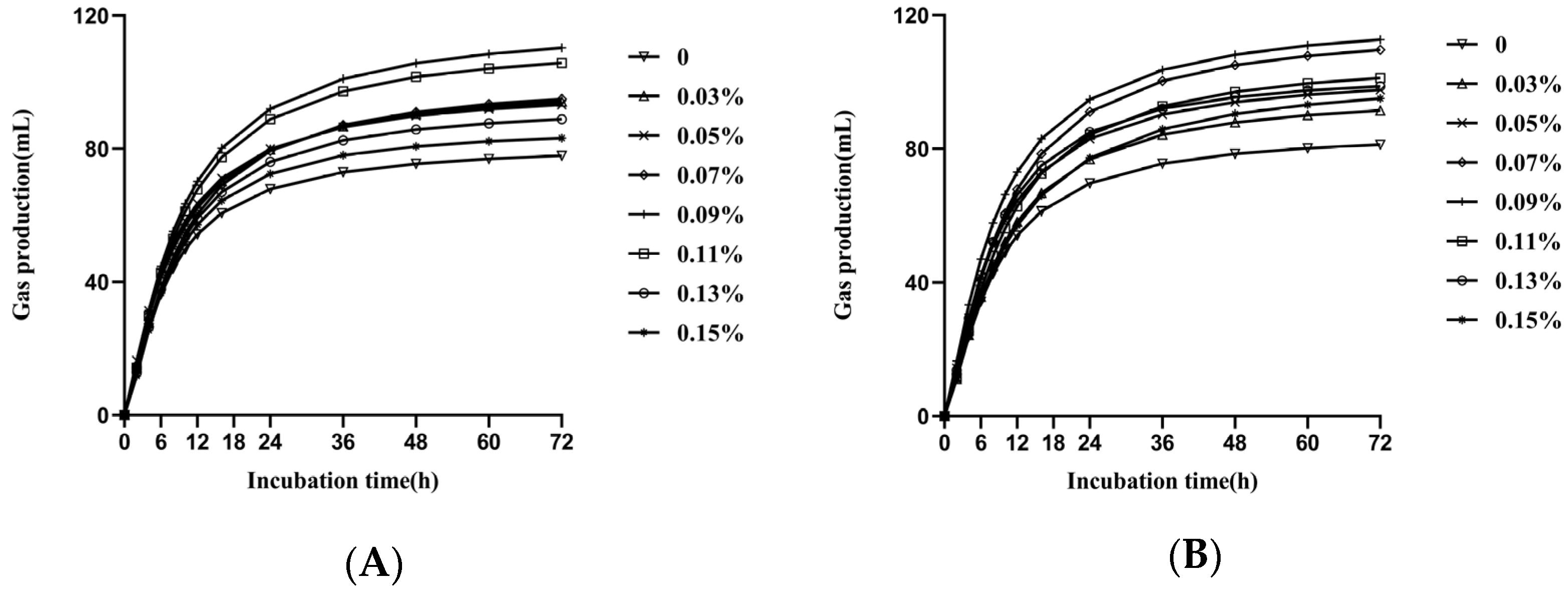
| GP72, mL/g DM | T1 | 76.41 d | 90.61 c | 91.60 c | 94.42 b | 107.85 a | 102.55 b | 87.85 c | 74.46 d | 2.34 | ** | ** | ** | ns | * |
| T2 | 79.66 d | 88.57 c | 95.55 b | 107.49 a | 109.33 a | 98.83 b | 93.81 bc | 82.86 d | |||||||
| A, mL/g DM | T1 | 81.66 d | 100.56 bc | 99.01 bc | 101.36 bc | 118.04 a | 112.44 ab | 93.53 cd | 86.68 cd | 3.80 | * | ** | ** | ns | ns |
| T2 | 85.42 d | 96.62 cd | 103.37 c | 116.5 ab | 120.18 a | 107.41 bc | 103.54 c | 102.75 c | |||||||
| B | T1 | 1.31 | 1.22 | 1.23 | 1.29 | 1.27 | 1.31 | 1.34 | 1.40 | 0.07 | ns | ns | ns | ns | ns |
| T2 | 1.36 | 1.37 | 1.30 | 1.36 | 1.35 | 1.37 | 1.29 | 1.28 | |||||||
| C, h | T1 | 7.11 | 8.08 | 7.47 | 6.94 | 8.87 | 8.73 | 8.01 | 7.49 | 0.67 | * | ns | ns | ns | ns |
| T2 | 8.04 | 8.88 | 8.13 | 9.39 | 8.50 | 9.35 | 7.90 | 10.09 | |||||||
| AGPR, mL/h | T1 | 3.79 | 3.90 | 4.23 | 4.74 | 4.28 | 4.35 | 3.89 | 4.10 | 0.35 | ns | ns | ns | ** | ns |
| T2 | 3.64 b | 3.72 ab | 4.21 ab | 4.24 ab | 4.80 a | 4.01 ab | 4.20 ab | 3.32 b | |||||||
| Items | Feed | GAA | SEM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.03% | 0.05% | 0.07% | 0.09% | 0.11% | 0.13% | 0.15% | F | G | L | Q | F × G | |||
| pH | T1 | 7.17 | 7.14 | 7.16 | 7.13 | 7.13 | 7.19 | 7.19 | 7.16 | 0.03 | ns | ns | ns | ns | ns |
| T2 | 7.17 | 7.15 | 7.13 | 7.21 | 7.14 | 7.21 | 7.17 | 7.13 | |||||||
| MCP, mg/mL | T1 | 0.31 b | 0.33 b | 0.33 b | 0.37 a | 0.38 a | 0.37 a | 0.34 b | 0.33 b | 0.01 | ns | ** | ns | ** | ns |
| T2 | 0.31 c | 0.32 bc | 0.35 ab | 0.36 a | 0.37 a | 0.37 a | 0.34 abc | 0.33 bc | |||||||
| NH3-N, mg/dL | T1 | 22.33 | 23.73 | 24.32 | 25.36 | 23.01 | 22.99 | 22.86 | 22.83 | 0.49 | ns | ** | ** | ** | ns |
| T2 | 23.16 b | 24.41 ab | 24.59 ab | 25.72 a | 23.85 ab | 23.32 b | 23.15 b | 22.83 b | |||||||
| TVFA, mM | T1 | 74.27 cd | 82.36 abc | 83.36 ab | 85.72 a | 84.13 ab | 83.64 ab | 76.57 bcd | 69.58 d | 2.11 | ns | ** | ns | ** | ns |
| T2 | 75.45 c | 80.51 b | 82.00 ab | 84.82 a | 82.19 ab | 80.47 b | 79.80 b | 74.86 b | |||||||
| VFA pattern (%, molar) | |||||||||||||||
| acetic acid | T1 | 79.11 | 79.09 | 79.60 | 79.11 | 79.59 | 79.19 | 79.54 | 79.52 | 0.30 | ** | ns | ns | ns | ns |
| T2 | 78.98 | 78.56 | 78.60 | 78.91 | 78.62 | 78.51 | 78.61 | 78.86 | |||||||
| propionic acid | T1 | 10.99 | 10.93 | 10.81 | 10.98 | 11.06 | 11.05 | 10.93 | 11.38 | 0.18 | * | ns | ns | ns | ns |
| T2 | 10.96 | 11.07 | 11.06 | 11.26 | 11.31 | 11.22 | 11.36 | 11.44 | |||||||
| isobutyric acid | T1 | 0.64 | 0.70 | 0.67 | 0.74 | 0.59 | 0.78 | 0.80 | 0.65 | 0.06 | ns | ns | ns | ns | ns |
| T2 | 0.51 | 0.73 | 0.71 | 0.67 | 0.68 | 0.71 | 0.60 | 0.66 | |||||||
| butyric acid | T1 | 6.86 | 6.81 | 6.52 | 6.72 | 6.70 | 6.57 | 6.62 | 6.66 | 0.14 | ** | ns | ns | ns | ns |
| T2 | 7.29 | 7.31 | 7.17 | 7.26 | 7.30 | 7.31 | 7.33 | 7.01 | |||||||
| isovaleric acid | T1 | 1.56 ab | 1.65 a | 1.45 bc | 1.62 ab | 1.52 abc | 1.50 abc | 1.50 abc | 1.34 c | 0.06 | ns | ns | ns | ns | ns |
| T2 | 1.45 b | 1.52 a | 1.54 a | 1.44 ab | 1.45 ab | 1.47 a | 1.39 ab | 1.38 ab | |||||||
| valeric acid | T1 | 0.84 ab | 0.82 ab | 0.80 ab | 1.02 a | 0.70 b | 0.92 ab | 0.81 ab | 0.62 b | 0.09 | ns | ns | ns | ns | ns |
| T2 | 0.82 ab | 0.81 ab | 0.92 a | 0.62 b | 0.79 ab | 0.77 ab | 0.70 ab | 0.65 ab | |||||||
| A/P | T1 | 7.21 | 7.24 | 7.38 | 7.21 | 7.20 | 7.17 | 7.28 | 7.00 | 0.13 | ** | ns | ns | ns | ns |
| T2 | 7.21 | 7.11 | 7.11 | 7.02 | 6.96 | 7.00 | 6.92 | 6.90 | |||||||
| Items | Feed | GAA | SEM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.03% | 0.05% | 0.07% | 0.09% | 0.11% | 0.13% | 0.15% | F | G | L | Q | F × G | |||
| H2, % | T1 | 0.14 | 0.18 | 0.29 | 0.14 | 0.13 | 0.12 | 0.16 | 0.19 | 0.07 | ns | ns | ns | ns | ns |
| T2 | 0.43 | 0.15 | 0.10 | 0.15 | 0.15 | 0.20 | 0.16 | 0.09 | |||||||
| CH4, % | T1 | 10.59 | 11.35 | 11.89 | 11.45 | 11.70 | 11.79 | 11.68 | 11.05 | 0.45 | ns | ns | ns | ns | ns |
| T2 | 11.97 | 11.03 | 10.53 | 11.18 | 10.65 | 11.75 | 11.09 | 10.81 | |||||||
| CO2, % | T1 | 89.28 | 88.47 | 87.82 | 88.41 | 88.17 | 88.09 | 88.16 | 88.76 | 0.49 | ** | ns | ns | ns | ns |
| T2 | 87.59 | 88.81 | 89.37 | 88.67 | 89.20 | 88.04 | 88.76 | 89.09 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-J.; Zhang, F.; Pei, S.-T.; He, S.-S.; Xiong, F.-L.; Lv, L.-K.; Yang, H.-J. The Effect of Guanidinoacetic Acid Addition on In Vitro Rumen Fermentation Characteristics and Gas Production of Early- and Late-Stage Sheep-Fattening Diets. Fermentation 2023, 9, 549. https://doi.org/10.3390/fermentation9060549
Li W-J, Zhang F, Pei S-T, He S-S, Xiong F-L, Lv L-K, Yang H-J. The Effect of Guanidinoacetic Acid Addition on In Vitro Rumen Fermentation Characteristics and Gas Production of Early- and Late-Stage Sheep-Fattening Diets. Fermentation. 2023; 9(6):549. https://doi.org/10.3390/fermentation9060549
Chicago/Turabian StyleLi, Wen-Juan, Fan Zhang, Shi-Teng Pei, Shan-Shan He, Feng-Liang Xiong, Liang-Kang Lv, and Hong-Jian Yang. 2023. "The Effect of Guanidinoacetic Acid Addition on In Vitro Rumen Fermentation Characteristics and Gas Production of Early- and Late-Stage Sheep-Fattening Diets" Fermentation 9, no. 6: 549. https://doi.org/10.3390/fermentation9060549
APA StyleLi, W.-J., Zhang, F., Pei, S.-T., He, S.-S., Xiong, F.-L., Lv, L.-K., & Yang, H.-J. (2023). The Effect of Guanidinoacetic Acid Addition on In Vitro Rumen Fermentation Characteristics and Gas Production of Early- and Late-Stage Sheep-Fattening Diets. Fermentation, 9(6), 549. https://doi.org/10.3390/fermentation9060549






