Abstract
This study explores whether guanidinoacetic acid (GAA) addition can regulate nutrient degradability, rumen fermentation characteristics, and gas composition in two sheep-fattening diets. A 2 × 8 factorial in vitro culture was examined to determine the effects of GAA addition at the following levels of 0%, 0.03%, 0.05%, 0.07%, 0.09%, 0.11%, 0.13%, and 0.15% of two total mixed rations (T1 diet: early fattening stage diet; T2 diet: late fattening stage diet). After 72 h in vitro incubation of two diets with mixed rumen liquid obtained from six rumen-cannulated lambs, the T2 diet exhibited higher dry matter (DM) digestibility, higher cumulative gas production at 72 h (GP72), higher asymptotic gas production(A), and longer the time at which half of A is reached (C). However, it exhibited a lower acetic acid and a lower ratio of acetate to propionate than the diet of T1. A quadratic increase occurred in neutral detergent fiber (NDF) and acid detergent fiber (ADF) digestibility, with a maximum point occurring at the 0.09% GAA group. The gas production kinetic result indicated that increasing the level of GAA addition resulted mainly in an increase of GP72 and A, with the maximum point occurring at 0.09% for the T1 diet and 0.07–0.09% for the T2 diet. Moreover, the levels of GAA addition did not affect pH, the proportion of any of the volatile acid, or gas composition, but when the levels of GAA addition were increased, the microbial crude protein (MCP), ammonia nitrogen (NH3-N), and total volatile fatty acid (TVFA) content exhibited a quadratic relationship. The highest MCP contents were seen in the 0.07%, 0.09%, and 0.11% groups, while NH3-N and TVFA were in the 0.07% group. In summary, the appropriate level of GAA addition in early and late fattening stage diets ranged from 0.07% to 0.11%.
1. Introduction
Guanidinoacetic acid (GAA), an amino acid derivative from L-arginine and glycine, is the precursor of creatine and can be methylated to creatine by guanidinoacetate N-methyltransferase (GAMT) [1,2,3]. In normal conditions, creatine and phosphocreatine (PCr) are in constant equilibrium [4]. Creatine and adenosine triphosphate (ATP) generate adenosine diphosphate (ADP) and PCr under the action of creatine kinase (CK), accompanied by the storage of energy [5]. Thereby, creatine is a rich energy source in high energy-demand tissues [6]. The metabolic end products of creatine and PCr are creatinine, which is excreted from the body through urine [7]. The reaction is depicted in Figure 1.
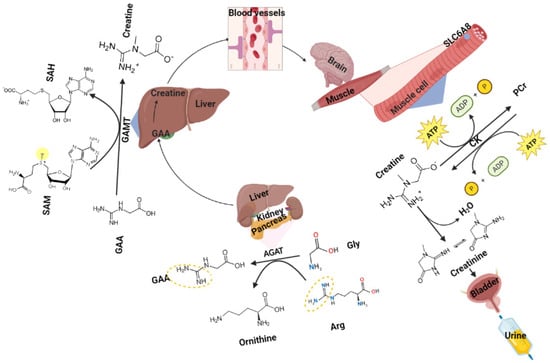
Figure 1.
Guanidinoacetic acid metabolism (Arg, arginine; AGAT, L−arginine: glycine amidinotransferase; CK, creatine kinase; GAMT, guanidinoacetate N−dimethyltransferase; SAH, S−adenosylhomocysteine; SAM, S−adenosylmethionine).
GAA is widely used as a nutritional feed additive in animals such as pigs [8] and chickens [9]. Its positive effects have been demonstrated mainly in terms of growth, digestion of nutrients, slaughter performance, and improved lean meat ratio. However, few studies have used GAA in ruminant animals. Previous trials have shown that post-ruminal GAA (30 or 40 g/d) supplementation increased creatine supply to cattle with a methyl group deficiency [10]. The administration of GAA (7.5 and 15 g/d) may improve lean tissue deposition and cattle growth without methyl deficiency [11]. A recent study demonstrated that protozoa and methanogens decreased linearly with increasing levels of GAA addition [12]. Furthermore, according to Dai et al. [13], methane production positively correlates with total protozoa. The livestock industry has recently undergone rapid development, and with the support of national policies, the intensive lamb-fattening industry has grown in scale. However, the effect of GAA addition on rumen fermentation and gas composition in early- and late-stage sheep-fattening diets is not yet known. Therefore, we hypothesize that a suitable amount of GAA may enhance in vitro fermentation, provide energy for microorganisms, and even change the type of fermentation, affecting the composition of fermentation gas in early- and late-stage sheep-fattening diets.
The technique of in vitro gas production is a simulation that uses rumen fermentation, a process closely linked to gas production, to assess the feeding value of forage [14] and feed additives [15] and to predict methane production [16].
Feeding accounts for 70% of lamb meat’s total production cost, whereas a 1% increase in weight at slaughter reduces the total costs by 0.9% [17]. As a result, improving productivity and efficiency is crucial to increasing the competitiveness of lamb meat. Only a few studies have demonstrated that GAA addition (0.09% basal diet) increases final body weight, promotes muscle mass, and changes the distribution of myofiber size of Dorper (♂) × Small Tailed Han sheep (♀) crossed ram lambs (4 months of age, 24.8 ± 1.3 kg) [18]; Zhang et al. [19] recommend dietary supplementation with 500~1000 mg/kg GAA for three-month-old healthy Kazakh male lambs (27.35 ± 0.58 kg). However, the appropriate amount of GAA addition has not been determined for diets at different fattening stages.
Based on the current study selected, two diets were used in the intensive fattening of lambs: an early-stage fattening diet (the T1 diet) and a late-stage fattening diet (the T2 diet). The objective of this study was to evaluate the effects of different levels of GAA addition on fermentation and gas composition, and this evaluation provides preliminary data on the use of GAA in the fattening of lambs. The study used a China-patented Automated Trace Gas Recording System (AGRS-III, China Agricultural University, Beijing, China) [20] to measure the effects of different GAA levels on fermentation and gas composition, and this provides preliminary data on the use of GAA in the fattening of lambs.
2. Materials and Methods
2.1. Animal Ethics Statement
The Animal Care and Use Committee of China Agricultural University approved the experimental protocol. The present study’s experimental animals, designs, and animal management followed the Guidelines of the Beijing Municipal Council on Animal Care (with protocol CAU20171014-1).
2.2. Guanidinoacetic Acid Product
The GAA used in this study was a white powder with a purity of 94.8%, provided by a company in Hebei Province, Shijiazhuang City, China.
2.3. In Vitro System
The AGRS-III provides automatically in vitro gas production (Beijing, China), and it releases pressure automatically during fermentation and constantly monitors gas pressure within 64 modules. Moreover, the temperature can be adjusted as required. The data from electrical chips installed in the lid of a fermentation module are stored on a computer and may be used to characterize the kinetics of gas generation. Gas-tight bags were attached to the fermentation bottles to collect the gas produced during incubation in a constant temperature incubator at 39 °C. The CH4, CO2, and H2 concentrations in the gas sample were analyzed using gas chromatography (GC522, Wufeng Instruments, Shanghai, China) equipped with a thermal conductivity detector, as described by Yang et al. [21]. The reference gas contained 0.499% H2, 15.1% CH4, 1% O2, and 44.9% CO2, pure N2 was used as carrier gas.
2.4. Feed, Experimental Design, and Rumen Fluid Donor Animals
The two types of feeds used in the fermentation study were collected from a scaled sheep farm in Hebei Province, China. Two total mixed rations for the intensive fattening of lambs were used: the early-stage fattening diet (the T1 diet, in which the ratio of concentrate to roughage was 75:25) and the late-stage fattening diet (the T2 diet, in which the ratio of concentrate to roughage was 80:20). The samples were dried in a 65 °C oven for 48 h, rehydrated for 24 h to produce air-dried samples, ground through a 1 mm sieve, and used for subsequent experiments. Table 1 shows the daily diet formulations and nutritional components of the two feed types.

Table 1.
Feed ingredients and chemical composition of early and late fattening stages diets.
A 2 × 8 factorial design was applied to in vitro batch cultures with the two feeds (i.e., the T1 and T2 diet) and eight GAA addition levels (0%, 0.03%, 0.05%, 0.07%, 0.09%, 0.11%, 0.13%, and 0.15%) in each diet group, respectively. A 0.5 g sample of one of the feeds (accurate to 0.0001) was weighed into four 120 mL bottles for each additive level. This resulted in a total of 64 bottles, and the treatments were labeled as follows: 0% (control), 0.03%, 0.05%, 0.07%, 0.09%, 0.11%, 0.13%, and 0.15%.
Rumen fluid was collected at the Nankou Pilot Base Chinese Academy of Agricultural Sciences (Changping District, Beijing, China) from six cannulated lambs. The fluid was collected before morning feeding to ensure the tests would isolate the fermentation products resulting from the feed type and the GAA addition [22]. The rumen fluid collection procedure was described in detail by Robles-Jimenez [23]. The rumen liquid was filtered with four layers of cheesecloth and transferred to a thermos prewarmed to 39 °C for in vitro fermentation.
2.5. Experimental Procedures
The buffer solution was prepared according to the procedure developed by Menke [24]. The incubators were 120 mL glass bottles with Hungate’s stoppers and screw caps. The fermentation system included 0.5 g feed substrate, 50 mL prewarmed medium, and 25 mL rumen liquid. All the bottles were flushed with N2 to remove air before attaching the AGRS-III. At the same time, an identical fermentation system was placed in a constant temperature incubator at 39 °C, and all bottles were connected to airbags for gas composition analysis during the 72 h incubation. After 72 h in vitro incubation, all the sample bottle contents were filtered to collect undigested residue in a nylon bag (8 × 12 cm, 42 µm pore size). The nylon bags containing the undegraded residue were cleaned slowly and dried at 65 °C for 48 h, and the undegraded residues were then weighed.
The filtrate was collected from each bottle. A 1 mL subsample was mixed with 0.25 mL of meta-phosphoric acid (250 g/L), which was centrifuged at 20,000× g for 15 min and filtered using a 0.22 μm syringe filter to determine the total volatile fatty acid (TVFA). The TVFA content was analyzed using gas chromatography (HPGC; GC-128; INESA Corporation) with a hydrogen flame detector and a capillary column (FFAP, Zhonghuida Instruments Co., Ltd., Dalian, China; 50 m long, 0.32 mm diameter, 0.50 µm film) as described by Wang et al. [25]. As described by Bremner and Keeney [26], ammonia nitrogen (NH3-N) was measured at 660 nm following a spectrophotometric method. The microbial crude protein (MCP) was measured using Perez’s purine derivative method [27].
2.6. Calculations and Statistical Analyses
The gas production data recorded by the AGRS-III were fitted according to the following exponential model, as described by Groot [28].
where GPt is the cumulative gas production (mL/g DM) at fermentation time t (h), A is the maximum gas production, and B is the shape of the curve.
The average gas production rate (AGPR, mL/h) when 1/2 of the maximum gas production is reached was calculated as follows [29]:
All the statistical analyses for gas production, nutrient disappearance, gas composition, and TVFA content used the MIXED procedure of the Statistical Analysis System Institute (SAS 9.4, Raleigh, NC, USA). The following model was used:
where Yijk is the dependent variable under examination, µ is the overall mean, Fi is the fed-substrate effect where i = 2 for the T1 and T2 diets, Nj is the fixed GAA level effect for j = 8, F × N is the interaction between GAA and the feed-substrate, Rk is the random effect of a repeated batch run (k = 4), and eij is the residual error term. The least-square mean and standard error of the mean were calculated with the least-square mean statement to determine linear and quadratic dosage effects of GAA addition. Significance was declared at p ≤ 0.05 unless otherwise noted.
3. Results
3.1. In Vitro Degradability
As shown in Table 2, the DM degradability was greater in the T2 diet than in the T1 diet (p < 0.01). The levels of GAA addition did not alter DM degradability in either of the diets (p > 0.05). Moreover, when the level of GAA addition was increased, a quadratic increase (quadratic, p < 0.01) in NDF and ADF degradability was observed. NDF and ADF degradability reached their maximum point when the level of GAA addition was 0.09% in the T1 diet and 0.11% in the T2 diet.

Table 2.
Effect of feeds and GAA addition in vitro disappearance (%, as DM basis).
3.2. Fitted Curves and Kinetic Gas Production
Figure 2 shows the fitted curves for each feed type. As Table 3 shows, a significant interaction between GAA and feed type was observed for GP72 (p < 0.05); the highest GP72 occurred in the T1 diet with 0.09% GAA, whereas the highest GP72 occurred in the T1 diet at 0.07% and 0.09% GAA.
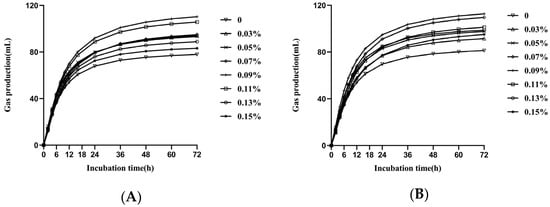
Figure 2.
Effect of GAA addition on gas production kinetics of T1(A) and T2 (B) substrates incubated in batch cultures of mixed rumen microorganisms.

Table 3.
Effect of different addition levels of GAA in culture fluids on the kinetics of gas production of the T1 and T2 diet.
GP72, A, and C were lower in the T1 diet than in the T2 diet, regardless of the level of GAA addition (p < 0.05). Increasing the level of GAA addition resulted in a linear increase in GP72, A, and C (p < 0.01). The AGPR responded quadratically (quadratic, p < 0.01) to an increase in the level of GAA addition, with the maximum point occurring at 0.07% in the T1 diet and 0.09% in the T2 diet.
3.3. Final pH, NH3-N, MCP, and VFA Pattern
As shown in Table 4, the acetic acid ratio and A/P ratio were higher in the T1 diet than in the T2 diet, but the molar proportions of propionic acid and butyric acid were lower in the T1 diet than in the T2 diet (p < 0.05). The MCP, NH3-N, and TVFA content (quadratic, p < 0.01) initially increased but then decreased in response to increasing levels of GAA addition. Neither the pH value nor the isobutyric acid, isovaleric acid, or valeric acid ratios differed in relation to feed type or level of GAA addition (p > 0.05).

Table 4.
Effect of different addition levels of GAA in culture fluids on fermentation characteristics of the T1 and T2 diets.
3.4. Fermentation Gas Composition
There was no significant difference (p > 0.05) in H2, CH4, or CO2 among the GAA treatments (Table 5), but the CO2 was lower for the T1 diet than for the T2 diet (p < 0. 01), although the ratios of H2 and CH4 were unaffected.

Table 5.
Effect of different addition levels of GAA in culture fluids on fermentation gas composition of the T1 and T2 diets.
4. Discussion
4.1. In Vitro Degradability
The chemical composition of the feed plays a crucial role in predicting the degradability of DM and organic matter during in vitro gas production [29]. In the present study, the concentrate-to-roughage ratios of the T1 and T2 diets were 75:25 and 80:20, respectively. The DM degradability of the T1 diet was significantly lower than that of the T2 diet, which can be attributed to the fact that the T1 diet was higher in fiber but lower in starch than the T2 diet (starch is more easily degraded and utilized) [30]. Moreover, NDF degradability and ADF degradability responded quadratically to increases in the level of GAA addition, and NDF and ADF degradability reached their maximum point in the 0.09% and 0.11% groups, which is consistent with previous reports in bulls [31,32]. Further, the improvement in NDF and ADF digestibility was likely related to enhanced enzyme activities and bacterial populations [12]. These findings suggest that GAA addition at an appropriate concentration improves fiber digestibility.
4.2. Gas-Production Kinetic
The process of in vitro feed substrate fermentation produces gas, so the degradability of feed substrates is commonly estimated by measuring gas production [33]. Moreover, gas production is affected by the substrate’s chemical composition and feed type [34]. DM digestibility is typically used to determine how the feed is degraded [25]. In the current study, the change in cumulative gas production at 72 h (GP72), asymptotic gas production (A), and the time at which half of A is reached (C) coincided with the change in DM digestibility. The change of GP72, A, and C suggested that rumen fermentation can be slowed down to some extent by consuming dietary fiber composed of structural carbohydrates (T1) [35]. In addition, GP72 and A increased linearly with increasing levels of GAA addition, providing evidence that GAA is degraded in the rumen to support microbial growth [36].
4.3. Final pH, NH3-N, MCP, and VFA Pattern
The proportion of acetate acid and the A/P ratio in the T1 diet were higher than those in the T2 diet, whereas the proportions of propionate acid and butyrate acid proportion in the T1 diet were lower than those in the T2 diet. Generally, TVFA content is thought to be strongly influenced by the ratio of concentrate to roughage [37], whereas the composition of the proportion of each volatile acid is strongly affected by NDF [38]. Propionic acid is generally thought to be higher in high-concentrate diets, whereas acetic acid is higher in high-roughage diets [39]. Moreover, microbes can synthesize proteins using GAA as a source of N [40]. Therefore, the elevated MCP level may have reflected the growing population of microbes [21]. The increase in TVFA production was in line with the bacteria populations [31], indicating that GAA addition has a positive influence on nutrient degradation and microbial growth [41]. However, the introduction of excessive GAA may have inhibited TVFA production, this may be related to the effect of GAA overdose on rumen homeostasis [42], and it suggests that GAA coating is necessary for ruminants. However, we did not observe a change in the proportion of individual volatile acids, and this finding is inconsistent with the research on cows, which has demonstrated that GAA can alter the fermentation pattern and promote the formation of propionic acid [31]; this may be related to a lower NDF of the basal diet [43].
4.4. Fermentation Gas Composition
In vitro gas production technology is widely used to evaluate the gas composition of microbial fermentation [44]. Liu et al. [12] noted that total protozoa and methanogens respond linearly to decreased levels of GAA addition. Methanogens utilize H2 and CO2 to produce CH4 [45]. It was speculated that GAA had the potential to reduce methane emissions. Moreover, studies have shown that methane production is positively correlated with A/P ratio [46]. In the present study, the level of GAA addition and feed type had little effect on molar CH4 proportions. Except for the significantly lower CO2 ratio in the T1 diet than in the T2 diet, neither the feed type nor the level of GAA addition had a significant effect on the gas composition of rumen fermentation. The insignificant effect on gas composition may be due to the high proportion of concentrate-to-roughage in the basal diet in this study, compared to the 50:50 ratio in dairy cows where an increase in GAA addition resulted in a linear decrease in protozoa and methanogens [31]. There is limited research on using it for energy saving and emission reduction. The result of the current study indicates only that the feed type and GAA addition had no adverse effects on the gas composition of the fermentation system.
In summary, the addition of GAA had a similar effect on the two rapid-growing meat sheep diets, which may have been due to the higher ratio of concentrate to forage in both diets. Thus, the diets exhibited similar nutrient digestibility, gas production, and rumen fermentation characteristics under the conditions of this study.
5. Conclusions
The feed in the late fattening period had a higher DM digestion rate and GP72 than the diet in the early fattening period. Adding GAA can enhance the digestion rate of fiber in the in vitro fermentation system but does not affect the gas composition. Under a high-concentrate feed lotting pattern, the appropriate dosage of GAA, addition in early fattening stage and late fattening stage diets, ranged from 0.07% to 0.11%.
Author Contributions
Conceptualization, W.-J.L. and H.-J.Y.; methodology, F.Z.; software, S.-T.P.; validation, S.-S.H. and F.-L.X.; formal analysis, L.-K.L. and F.Z.; investigation, W.-J.L.; resources, S.-T.P.; data curation, F.Z.; writing—original draft preparation, W.-J.L.; writing review and editing, W.-J.L. and H.-J.Y.; project administration, H.-J.Y.; funding acquisition, H.-J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (202105510410447) and the National Natural Science Foundation of China (grant No. 31572432).
Institutional Review Board Statement
The Animal Ethics Committee of China Agricultural University approved all procedures with animals. The sampling procedures followed the Guidelines on Ethical Treatment of Experimental Animals (2006) No. 398 set by the Ministry of Science and Technology, China.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to the study are included in the article. Data are available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poggio, F.; Brofiga, M.; Tedesco, M.; Massobrio, P.; Adriano, E.; Balestrino, M. Lack of Epileptogenic Effects of the Creatine Precursor Guanidinoacetic Acid on Neuronal Cultures In Vitro. Biomolecules 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, H.; Xin, J.; Xu, L.; Li, M.; Yu, H.; Zhang, W.; Ge, Y.; Li, Y.; Qu, M. Effects of Dietary Guanidinoacetic Acid on the Feed Efficiency, Blood Measures, and Meat Quality of Jinjiang Bulls. Front. Vet. Sci. 2021, 8, 684295. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Guanidinoacetic acid as a performance-enhancing agent. Amino Acids. 2016, 48, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Adriano, E.; Garbati, P.; Salis, A.; Damonte, G.; Millo, E.; Balestrino, M. Creatine salts provide neuroprotection even after partial impairment of the creatine transporter. Neuroscience 2017, 340, 299–307. [Google Scholar] [CrossRef]
- Weinsanto, I.; Mouheiche, J.; Laux-Biehlmann, A.; Delalande, F.; Marquette, A.; Chavant, V.; Gabel, F.; Cianferani, S.; Charlet, A.; Parat, M.O.; et al. Morphine Binds Creatine Kinase B and Inhibits Its Activity. Front. Cell. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Wallery, J.J.; Kale, V.P.; Novak, J.; Gibbs, S.; Do, M.T.; McKew, J.C.; Terse, P.S. Evaluation of chronic toxicity of cyclocreatine in beagle dogs after oral gavage administration for up to 23 weeks. Toxicol. Appl. Pharmacol. 2021, 430, 115680. [Google Scholar] [CrossRef] [PubMed]
- Forni Ogna, V.; Ogna, A.; Vuistiner, P.; Pruijm, M.; Ponte, B.; Ackermann, D.; Gabutti, L.; Vakilzadeh, N.; Mohaupt, M.; Martin, P.Y.; et al. New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015, 13, 40. [Google Scholar] [CrossRef]
- Jayaraman, B.; La, K.V.; La, H.; Doan, V.; Carpena, E.M.; Rademacher, M.; Channarayapatna, G. Supplementation of guanidinoacetic acid to pig diets: Effects on performance, carcass characteristics, and meat quality. J. Anim. Sci. 2018, 96, 2332–2341. [Google Scholar] [CrossRef]
- Çenesiz, A.A.; Yavaş, İ.; Çiftci, İ.; Ceylan, N.; Taşkesen, H.O. Guanidinoacetic acid supplementation is favourable to broiler diets even containing poultry by-product meal. Br. Poult. Sci. 2020, 61, 311–319. [Google Scholar] [CrossRef]
- Ardalan, M.; Batista, E.D.; Titgemeyer, E.C. Effect of post-ruminal guanidinoacetic acid supplementation on creatine synthesis and plasma homocysteine concentrations in cattle. J. Anim. Sci. 2020, 98, skaa072. [Google Scholar] [CrossRef]
- Ardalan, M.; Miesner, M.D.; Reinhardt, C.D.; Thomson, D.U.; Armendariz, C.K.; Smith, J.S.; Titgemeyer, E.C. Effects of guanidinoacetic acid supplementation on nitrogen retention and methionine flux in cattle. J. Anim. Sci. 2021, 99, skab172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Wang, C.; Guo, G.; Huo, W.; Xia, C.; Chen, L.; Zhang, Y.; Pei, C.; Liu, Q. Effects of guanidinoacetic acid supplementation on lactation performance, nutrient digestion and rumen fermentation in Holstein dairy cows. J. Sci. Food Agric. 2023, 103, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Kalscheur, K.F.; Huhtanen, P.; Faciola, A.P. Effects of ruminal protozoa on methane emissions in ruminants—A meta-analysis. J. Dairy Sci. 2022, 105, 7482–7491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Susenbeth, A.; Südekum, K.H. In vitro gas production measurements to evaluate interactions between untreated and chemically treated rice straws, grass hay, and mulberry leaves. J. Anim. Sci. 2002, 80, 517–524. [Google Scholar] [CrossRef]
- Quinn, M.J.; May, M.L.; Hales, K.E.; DiLorenzo, N.; Leibovich, J.; Smith, D.R.; Galyean, M.L. Effects of ionophores and antibiotics on in vitro hydrogen sulfide production, dry matter disappearance, and total gas production in cultures with a steam-flaked corn-based substrate with or without added sulfur. J. Anim. Sci. 2009, 87, 1705–1713. [Google Scholar] [CrossRef]
- Danielsson, R.; Ramin, M.; Bertilsson, J.; Lund, P.; Huhtanen, P. Evaluation of a gas in vitro system for predicting methane production in vivo. J. Dairy Sci. 2017, 100, 8881–8894. [Google Scholar] [CrossRef] [PubMed]
- Raineri, C.; Stivari, T.S.; Gameiro, A.H. Lamb Production Costs: Analyses of Composition and Elasticities Analysis of Lamb Production Costs. Asian-Australas. J. Anim. Sci. 2015, 28, 1209–1215. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Song, P.; Zhao, J.; Zhang, J.; Zhao, J. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, C.; Pan, J.; Ma, C.; Wang, C.; Li, X.; Cai, W.; Yang, K. Effects of dietary guanidinoacetic acid on growth performance, guanidinoacetic acid absorption and creatine metabolism of lambs. PLoS ONE 2022, 17, e0264864. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, Y.L.; Wang, W.K.; Zhang, Z.W.; Si, X.M.; Cao, Z.J.; Li, S.L.; Yang, H.J. Beneficial effect of Rhodopseudomonas palustris on in vitro rumen digestion and fermentation. Benef. Microbes. 2020, 11, 91–99. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, H.; Meng, X.; Zhang, D.; Cao, B. Effect of melamine on in vitro rumen microbial growth, methane production and fermentation of Chinese wild rye hay and maize meal in binary mixtures. J. Agric. Sci. 2014, 152, 686–696. [Google Scholar] [CrossRef]
- Dhakal, R.; Hansen, H.H.; Milora, N.; Copani, G. The effect of direct-fed microbials on grass or maize silage on in vitro rumen fermentation. Fermentation 2023, 9, 347. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Narváez-López, A.C.; Chay-Canul, A.J.; Sainz-Ramirez, A.; Castelan-Ortega, O.A.; Zhang, N.; Gonzalez-Ronquillo, M.; Vargas-Bello-Pérez, E. Effect of different dietary inclusion levels of whole plant green tomato (Physalis philadelphica) silage on nutrient intake and digestibility, and in vitro rumen fermentation kinetics in sheep. Front. Vet. Sci. 2022, 9, 980619. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.H.; Wang, W.K.; Wu, Q.C.; Zhang, F.; Li, W.J.; Li, S.L.; Wang, W.; Cao, Z.J.; Yang, H.J. The Effect of γ-Aminobutyric Acid Addition on In Vitro Ruminal Fermentation Characteristics and Methane Production of Diets Differing in Forage-to-Concentrate Ratio. Fermentation 2023, 9, 105. [Google Scholar] [CrossRef]
- Bremner, M.; Keeney, D.R. Distillation Methods for Determination of Ammonium Nitrate and Nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Perez, J.F.J.; Balcells, J.A.; Cebrian; Martin, S.M. Excretion of endogenous and exogenous purine derivatives in sheep: Effect of increased concentrate intake. Br. J. Nutr. 1998, 79, 237–240. [Google Scholar] [CrossRef]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Wang, W.-K.; Wang, Y.-L.; Li, W.-J.; Wu, Q.-C.; Li, S.-L.; Yang, H.-J. Gossypol Exhibited Higher Detrimental Effect on Ruminal Fermentation Characteristics of Low-Forage in Comparison with High-Forage Mixed Feeds. Toxics 2021, 9, 51. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Si, X.; Cao, Z.; Li, S.; Yang, H. Rumen Methanogenesis, Rumen Fermentation, and Microbial Community Response to Nitroethane, 2-Nitroethanol, and 2-Nitro-1-Propanol: An In Vitro Study. Animals 2020, 10, 479. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.Z.; Wang, D.H.; Wu, M.J.; Zheng, C.; Wu, Z.Z.; Wang, C.; Liu, Q.; Zhang, J.; Guo, G.; et al. Effects of guanidinoacetic acid and coated folic acid supplementation on growth performance, nutrient digestion and hepatic gene expression in Angus bulls. Br. J. Nutr. 2021, 126, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, C.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Zhang, Y.L.; Pei, C.X.; et al. Effects of guanidinoacetic acid supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Animal 2020, 14, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7255. [Google Scholar]
- Blu¨mmel, M.; Rskov, E.R. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim. Feed Sci. Technol. 1993, 40, 109–119. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Zhang, Q.; Yang, Y.; Zou, C.; Li, L.; Liang, X.; Wei, S.; Lin, B. Ruminal fermentation and microbial community differently influenced by four typical subtropical forages in vitro. Anim. Nutr. 2018, 4, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.F.; Pearl, K.A.; Titgemeyer, E.C. Relative bioavailability of guanidinoacetic acid delivered ruminally or abomasally to cattle. J. Anim. Sci. 2020, 98, skaa282. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef]
- Friggens, N.C.; Oldham, J.D.; Dewhurst, R.J.; Horgan, G. Proportions of volatile fatty acids in relation to the chemical composition of feeds based on grass silage. J. Dairy Sci. 1998, 81, 1331–1344. [Google Scholar] [CrossRef]
- Iii, R.; Murphy, M.R. An in vitro technique for measuring the production rate of volatile. Small Rumin. Res. 2004, 54, 219–225. [Google Scholar]
- Wright, D.E.; Hungate, R.E. Metabolism of glycine by rumen microorganisms. Appl. Microbiol. 1967, 15, 152–157. [Google Scholar] [CrossRef]
- Andreesen, J.R. Glycine metabolism in anaerobes. Antonie Van Leeuwenhoek 1994, 66, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cui, Z.; Jiang, Y.; Aisikaer, A.; Wu, Q.; Zhang, F.; Wang, W.; Bo, Y.; Yang, H. Dietary Guanidine Acetic Acid Improves Ruminal Antioxidant Capacity and Alters Rumen Fermentation and Microflora in Rapid-Growing Lambs. Antioxidants 2023, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Yin, D.M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef] [PubMed]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J.; Gisi, D.D.; Higginbotham, G.E.; Riordan, T.J. Methane production from commercial dairy rations estimated using an in vitro gas technique. Anim. Feed Sci. Technol. 2005, 123–124, 391–402. [Google Scholar] [CrossRef]
- Mauerhofer, L.M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.S.; Paulik, C.; Rittmann, S.K.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Wagner, A.O.; Reitschuler, C.; Illmer, P. Effect of different acetate:propionate ratios on the methanogenic community during thermophilic anaerobic digestion in batch experiments. Biochem. Eng. J. 2014, 90, 154–161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).