The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Standards
2.2. Yeasts
2.3. Fermentation Conditions
2.4. Determination of Oenological Parameters
2.5. Volatile Compounds Determination
2.5.1. Major Volatile Compounds
2.5.2. Minor Volatile Compounds
2.6. Calculation of Aroma Series
2.7. Statistical Analysis
3. Results
3.1. Fermentation Conditions and Kinetics
3.2. Oenological Parameters
3.3. Volatile Compounds
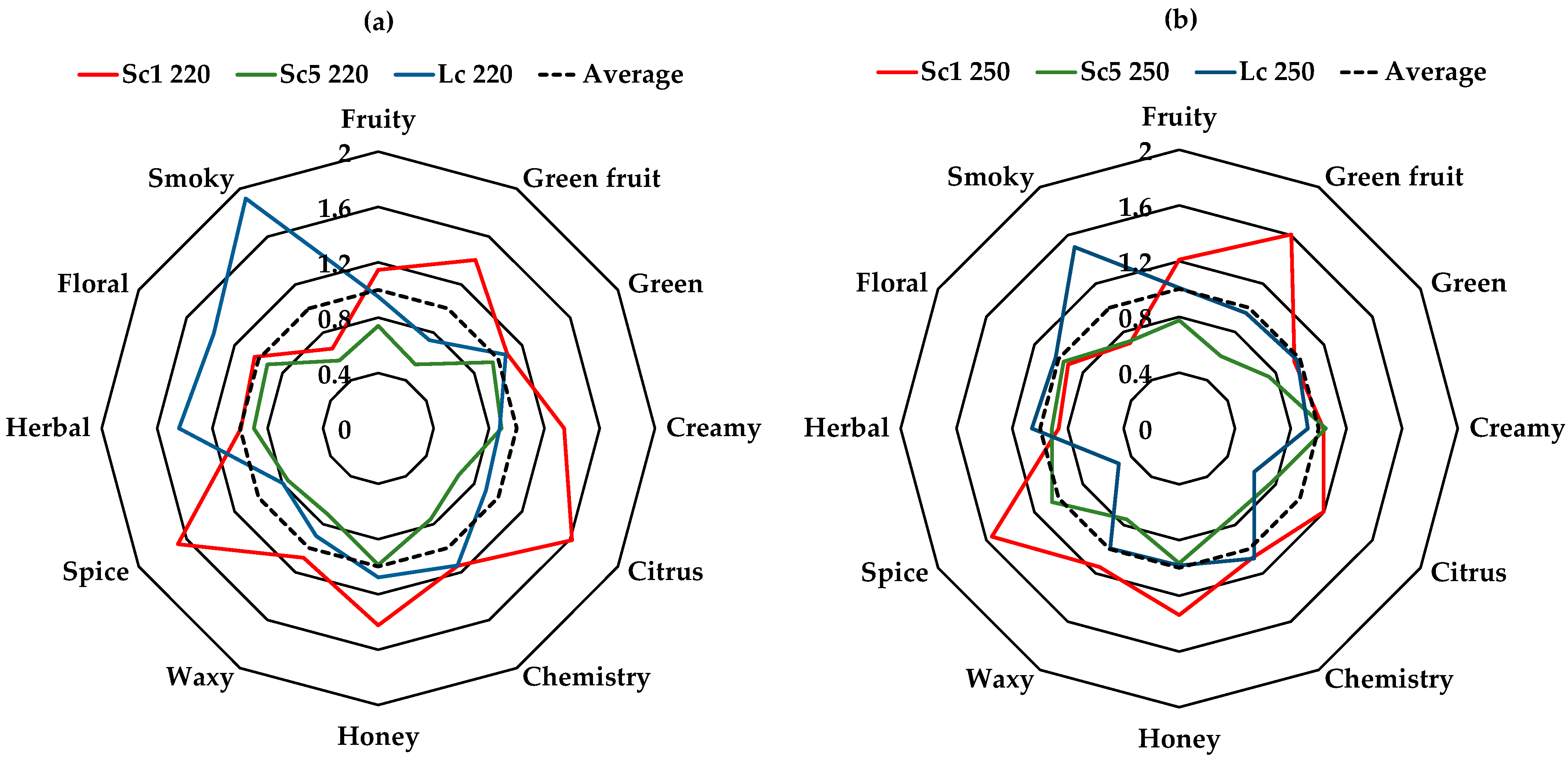
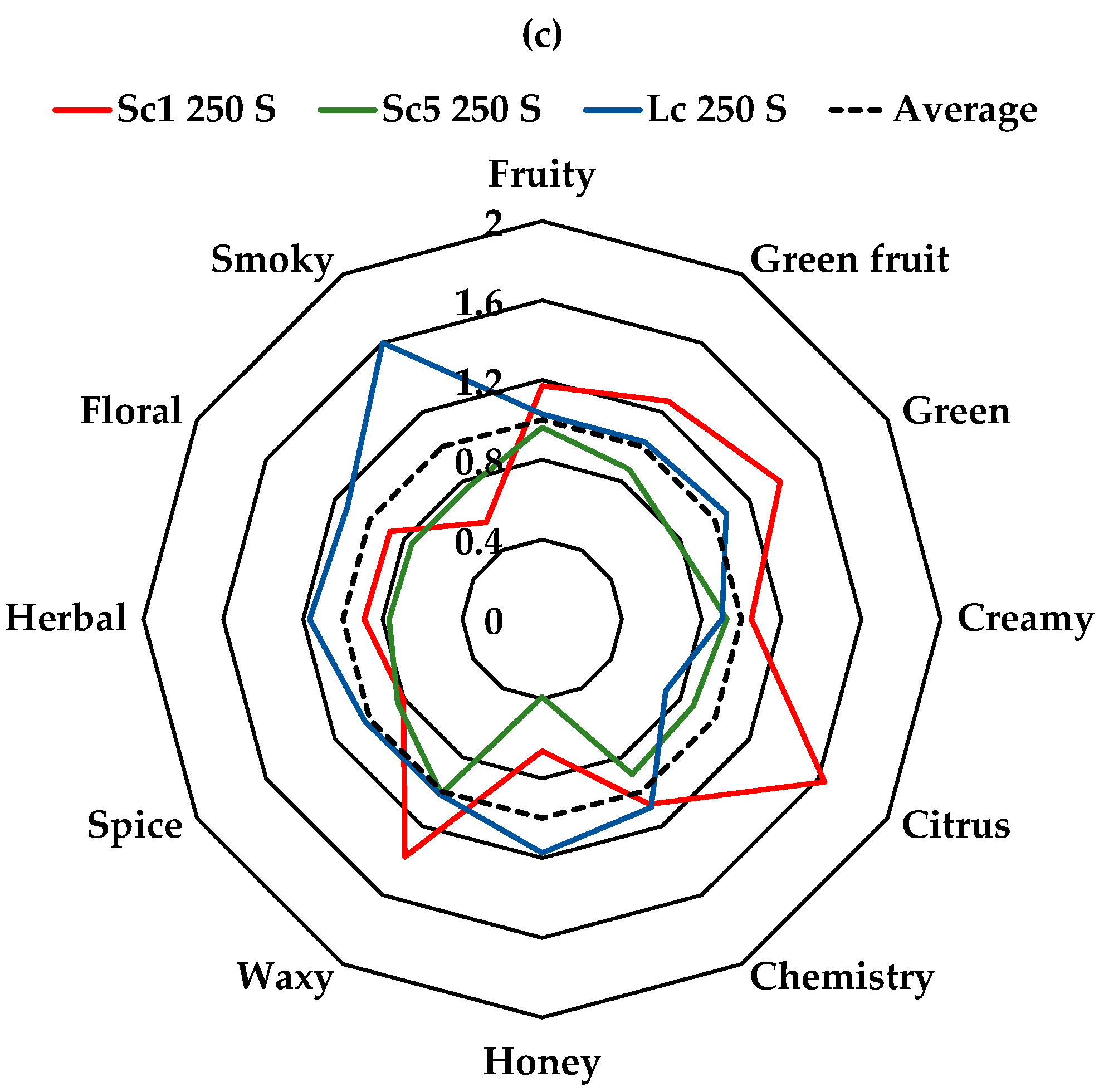
3.4. Aroma Profile
3.5. Multivariate Analysis
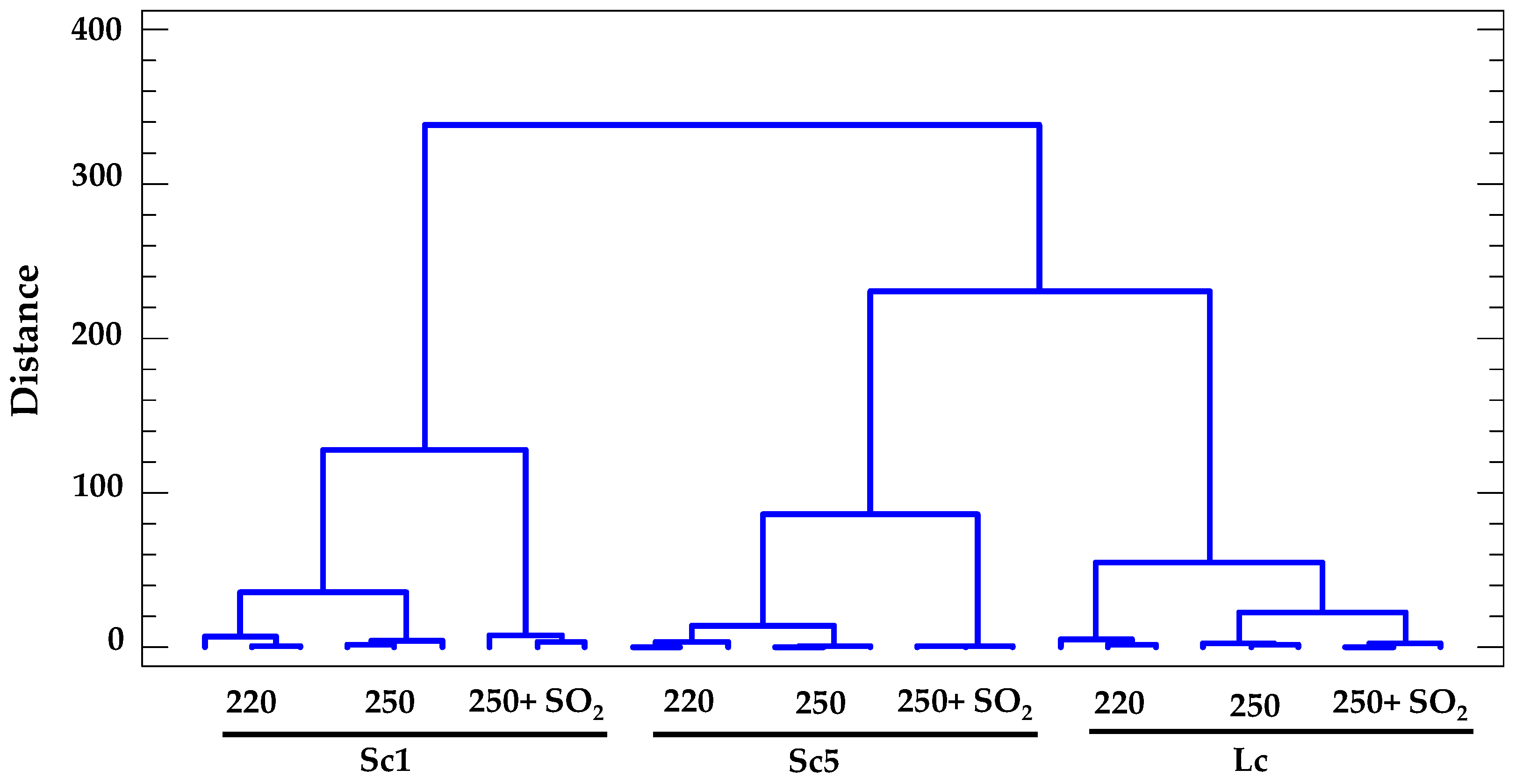
3.6. Cluster Analysis
3.7. Principal Components Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OIV. Available online: https://www.oiv.int/es (accessed on 24 September 2022).
- Ministry of Agriculture, Fisheries and Food of Spain. Available online: https://www.mapa.gob.es/es (accessed on 30 September 2022).
- Mozell, M.R.; Thach, L. The impact of climate change on the global wine industry: Challenges & solutions. Wine Econ. Policy 2014, 3, 81–89. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Grimplet, J.; Wheatley, M.D.; Ben Jouira, H.; Deluc, L.G.; Cramer, G.R.; Cushman, J.C. Proteomic and selected metabolite analysis of grape berry tissues under well-watered and water-deficit stress conditions. Proteomics 2009, 9, 2503–2528. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Roby, J.P. Préservation des ressources génétiques de la vigne. Nécessité d’une cohabitation entre sélection clonale institutionnelle, sélection massale et sélection clonale privée. Rev. Des. Oenologues 2013, 148, 13–16. [Google Scholar]
- Moreno, J.; Peinado, R. Enological Chemistry, 1st ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Martínez, A.; Aleixandre-Tudó, J.L.; Aleixandre, J.L. Efectos de los fenómenos producidos por el cambio climático sobre la calidad de los vinos. Enoviticultura 2016, 42, 4–16. [Google Scholar]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. JXB 2014, 65, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, H.P. Metabolism of tartaric and malic acids in Vitis–a review. Vitis 1982, 21, 346–358. [Google Scholar]
- Merloni, E.; Camanzi, L.; Mulazzani, L.; Malorgio, G. Adaptive capacity to climate change in the wine industry: A Bayesian Network approach. Wine Econ. Policy 2018, 7, 165–177. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Heuvel, J.E.V. Influence of Grapevine Training Systems on Vine Growth and Fruit Composition: A Review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar] [CrossRef]
- Gatti, M.; Frioni, T.; Garavani, A.; Biagioni, A.; Poni, S. Impact of delayed winter pruning on phenology and ripening kinetics of Pinot Noir grapevines. BIO Web Conf. 2019, 13, 04002. [Google Scholar] [CrossRef]
- De Toda, F.; Sancha, J.; Zheng, W.; Balda, P. Leaf area reduction by trimming, a growing technique to restore the anthocyanins: Sugars ratio decoupled by the warming climate. Vitis 2014, 53, 189–192. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Dos Santos, T.P.; Lopes, C.M.; Rodrigues, M.L.; de Souza, C.R.; da Silva, J.M.R.; Maroco, J.P.; Pereira, J.S.; Chaves, M.M. Effects of deficit irrigation strategies on cluster microclimate for improving fruit composition of Moscatel field-grown grapevines. Sci. Hortic. 2007, 112, 321–330. [Google Scholar] [CrossRef]
- Muñoz-Bernal, E.; Rodríguez, M.E.; Benítez, P.; Fernández-Acero, F.J.; Rebordinos, L.; Cantoral, J.M. Molecular analysis of red wine yeast diversity in the Ribera del Duero D.O. (Spain) area. Arch. Microbiol. 2013, 195, 297–302. [Google Scholar] [CrossRef]
- López de Lerma, N.; García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R.A. Volatile composition of partially fermented wines elaborated from sun dried Pedro Ximénez grapes. Food Chem. 2012, 135, 2445–2452. [Google Scholar] [CrossRef]
- Caridi, A.; Crucitti, P.; Ramondino, D. Winemaking of must at high osmotic strength by thermotolerant yeast. Biotechnol. Lett. 1999, 21, 617–620. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Oenological potential of non-Saccharomyces yeasts to mitigate effects of climate change in winemaking: Impact on aroma and sensory profiles of Treixadura wines. FEMS Yeast Res. 2019, 19, 065. [Google Scholar] [CrossRef]
- Xynas, B.; Barnes, C. Yeast or water: Producing wine with lower alcohol levels in a warming climate: A review. J. Sci. Food Agric. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Del-Real, J.; Contreras-Ruiz, A.; Castiglioni, G.L.; Barrio, E.; Querol, A. The Use of Mixed Populations of Saccharomyces cerevisiae and S. kudriavzevii to Reduce Ethanol Content in Wine: Limited Aeration, Inoculum Proportions, and Sequential Inoculation. Front. Microbiol. 2017, 8, 2087. [Google Scholar] [CrossRef]
- Hidalgo-Togores, J. Operaciones prefermentativas en las vinificaciones. In Procesos Prefermentativos. Calidad de uva y Optimización del uso del SO2; Ayuntamiento de Haro; Dialnet: Haro, Spain, 2010; pp. 23–62. [Google Scholar]
- Abramovič, H.; Košmerl, T.; Poklar Ulrih, N.; Cigić, B. Contribution of SO2 to antioxidant potential of white wine. Food Chem. 2015, 174, 147–153. [Google Scholar] [CrossRef]
- Lallemand Oenology Catalog. Available online: https://www.lallemandwine.com/en/china/products/catalogue (accessed on 15 July 2022).
- Bely, M.; Sablayrolles, J.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Sablayrolles, J.M.; Barre, P.; Grenier, P. Design of laboratory automatic system for studying alcoholic fermentations in anisothermal enological conditions. Biotechnol. Tech. 1987, 1, 181–184. [Google Scholar] [CrossRef]
- Compendium of International Methods of Wine and Must Analysis. Organisation Internationale de la Vigne et du Vin. 2023. Available online: https://www.oiv.int/en (accessed on 21 May 2023).
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas Chromatographic Quantification of Major Volatile Compounds and Polyols in Wine by Direct Injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef] [PubMed]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Peinado, R.A. The transformation of must into wine. In Enological Chemistry, 1st ed.; Moreno, J., Peinado, R.A., Eds.; Elsevier: London, UK, 2012; Volume 1, pp. 157–182. [Google Scholar]
- Yang, F.; Heit, C.; Inglis, D.L. Cytosolic Redox Status of Wine Yeast (Saccharomyces Cerevisiae) under Hyperosmotic Stress during Icewine Fermentation. Fermentation 2017, 3, 61. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Stribny, J.; Gamero, A.; Pérez-Torrado, R.; Querol, A. Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int. J. Food Microbiol. 2015, 205, 41–46. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; Wiley: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Morgan, S.C.; Haggerty, J.J.; Johnston, B.; Jiranek, V.; Durall, D.M. Response to Sulfur Dioxide Addition by Two Commercial Saccharomyces cerevisiae Strains. Fermentation 2019, 5, 69. [Google Scholar] [CrossRef]
- De Souza, J.C.; Crupi, P.; Colleta, A.; Antonacci, D.; Toci, A.T. Influence of vinification process over the composition of volatile compounds and sensorial characteristics of greek wines. J. Food Sci. Technol. 2022, 59, 1499–1509. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Lü, H.; Yu, Z.; Li, X. Effect of added sulphur dioxide levels on the fermentation characteristics of strawberry wine. J. Inst. Brew. 2016, 122, 446–451. [Google Scholar] [CrossRef]
- Pretorius, I.S.; Lambrechts, M.G. Yeast and its Importance to Wine Aroma—A Review. SAJEV 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Frivik, S.K.; Ebeler, S.E. Influence of Sulfur Dioxide on the Formation of Aldehydes in White Wine. Am. J. Enol. Vitic. 2003, 54, 31–38. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory Impact, Formation, and Fate of Damascenone in Grapes, Wines, and Other Foods and Beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical Characterization of the Aroma of Five Premium Red Wines. Insights into the Role of Odor Families and the Concept of Fruitiness of Wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Hein, K.; Ebeler, S.E.; Heymann, H. Perception of fruity and vegetative aromas in red wine. J. Sens. Stud. 2009, 24, 441–455. [Google Scholar] [CrossRef]
- Tang, K.; Sun, Y.; Zhang, X.; Li, J.; Xu, Y. Chemical and Sensory Characterization of Vidal Icewines Fermented with Different Yeast Strains. Fermentation 2021, 7, 211. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Sanchez, I.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation. Fermentation 2022, 8, 51. [Google Scholar] [CrossRef]
- Chambers, J.; Cleveland, W.; Kleiner, B.; Tukey, B. Graphical Methods for Data Analysis, 1st ed.; Wadsworth & Brooks: Belmont, CA, USA, 1983. [Google Scholar]

| 220 g/L of Initial Sugars | 250 g/L of Initial Sugars | 250 g/L of Initial Sugars and 70 mg/L of SO2 | MANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc1 | Sc5 | Lc | Sc1 | Sc5 | Lc | Sc1 | Sc5 | Lc | Yeast | Sugar | SO2 | |
| pH | 3.17 ± 0.05 | 3.28 ± 0.04 | 3.20 ± 0.04 | 3.17 ± 0.03 | 3.28 ± 0.02 | 3.24 ± 0.02 | 3.22 ± 0.03 | 3.26 ± 0.04 | 3.20 ± 0.03 | ns | ns | ns |
| TA | 6.2 ± 0.2 | 6.3 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.25 ± 0.07 | 6.3 ± 0.1 | 6.3 ± 0.2 | 6.2 ± 0.1 | ns | ns | ns |
| VA | 0.47 ± 0.03 | 0.53 ± 0.02 | 0.30 ± 0.01 | 0.53 ± 0.01 | 0.72 ± 0.02 | 0.43 ± 0.03 | 0.96 ± 0.02 | 1.01 ± 0.02 | 0.43 ± 0.01 | *** | ** | *** |
| Glycerol (g/L) | 6.2 ± 0.2 | 5.1 ± 0.2 | 4.6 ± 0.1 | 6.9 ± 0.3 | 5.6 ± 0.1 | 5.2 ± 0.1 | 7.6 ± 0.3 | 6.4 ± 0.2 | 5.8 ± 0.2 | *** | ** | ** |
| RS | 1.8 ± 0.4 | 0.35 ± 0.07 | 6.3 ± 0.4 | 3.8 ± 0.4 | 1.8 ± 0.4 | 7.5 ± 0.7 | 4.3 ± 0.4 | 1.5 ± 0.7 | 8.0 ± 0.7 | *** | *** | ns |
| Ethanol (% v/v) | 12.8 ± 0.1 | 12.9 ± 0.1 | 12.9 ± 0.1 | 14.6 ± 0.1 | 14.8 ± 0.1 | 13.9 ± 0.1 | 14.6 ± 0.1 | 14.9 ± 0.1 | 13.9 ± 0.1 | *** | *** | ns |
| 220 g/L of Initial Sugars | 250 g/L of Initial Sugars | 250 g/L of Initial Sugars and 70 mg/L of SO2 | MANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc1 | Sc5 | Lc | Sc1 | Sc5 | Lc | Sc1 | Sc5 | Lc | Yeast | Sugar | SO2 | |
| Acetate esters (mg/L) | 13.8 ± 0.9 | 6.5 ± 0.4 | 13.0 ± 0.6 | 12.4 ± 0.4 | 6.2 ± 0.2 | 12.0 ± 0.7 | 16.2 ± 1.0 | 10.6 ± 0.3 | 11.4 ± 0.3 | *** | ns | *** |
| Isoamyl acetate | 112 ± 8 | 46 ± 3 | 189 ± 9 | 158 ± 5 | 35 ± 3 | 176 ± 11 | 81 ± 6 | 62 ± 2 | 189 ± 4 | *** | ns | ns |
| Ethyl acetate (mg/L) | 13.4 ± 0.9 | 6.27 ± 0.38 | 12.5 ± 0.6 | 12.0 ± 0.4 | 5.94 ± 0.19 | 11.6 ± 0.7 | 16.0 ± 1.0 | 10.5 ± 0.3 | 11.0 ± 0.3 | *** | ns | *** |
| 2-Phenylethyl acetate | 314 ± 21 | 218 ± 14 | 237 ± 15 | 295 ± 22 | 213 ± 9 | 217 ± 17 | 146 ± 9 | 86 ± 3 | 259 ± 2 | ** | ns | ** |
| Hexyl acetate | 0 ± 0 | 0 ± 0 | 0.62 ± 0.03 | 0 ± 0 | 0 ± 0 | 0.14 ± 0.01 | 0 ± 0 | 0 ± 0 | 0 ± 0 | *** | * | ns |
| Alcohols (mg/L) | 260 ± 12 | 244 ± 15 | 220 ± 12 | 261 ± 1 | 254 ± 1 | 198 ± 16 | 114 ± 5 | 154 ± 3 | 240 ± 6 | ns | ns | ** |
| Isoamyl alcohols (mg/L) | 176 ± 10 | 174 ± 10 | 183 ± 11 | 181 ± 6 | 176 ± 2 | 174 ± 1 | 88 ± 4 | 125 ± 3 | 204 ± 5 | * | ns | ** |
| 2-Phenylethanol (mg/L) | 80 ± 3 | 67 ± 5 | 32 ± 1 | 77 ± 6 | 75 ± 2 | 19 ± 17 | 23 ± 1 | 25 ± 0 | 32 ± 1 | *** | ns | *** |
| Hexanol | 1832 ± 91 | 2119 ± 85 | 2801 ± 97 | 1748 ± 32 | 2145 ± 66 | 2527 ± 84 | 1214 ± 90 | 1570 ± 48 | 2831 ± 93 | *** | ns | * |
| 2-Ethyl-1-hexanol | 1014 ± 92 | 1056 ± 29 | 1742 ± 69 | 451 ± 15 | 1133 ± 49 | 774 ± 53 | 1863 ± 114 | 1699 ± 115 | 1622 ± 99 | ns | *** | *** |
| Dodecanol | 45 ± 4 | 30 ± 2 | 20.8 ± 0.9 | 20 ± 1 | 38 ± 2 | 19.6 ± 0.6 | 46 ± 3 | 71 ± 5 | 23 ± 1 | *** | ns | *** |
| Guaiacol | 82 ± 4 | 72 ± 5 | 55 ± 3 | 95 ± 5 | 90 ± 3 | 61 ± 2 | 69 ± 5 | 96 ± 5 | 43 ± 1 | *** | ** | ** |
| 4-Vinylphenol | 24 ± 2 | 14.0 ± 0.7 | 294 ± 8 | 20 ± 2 | 21 ± 1 | 231 ± 10 | 12.0 ± 0.7 | 14.0 ± 0.6 | 276 ± 12 | *** | * | ns |
| 2-Methoxy-4-vinylphenol | 14 ± 1 | 11.0 ± 0.4 | 141 ± 3 | 10.5 ± 0.6 | 15.4 ± 0.9 | 102 ± 8 | 12.6 ± 0.7 | 16 ± 1 | 119 ± 6 | *** | ** | ns |
| Carbonyl compounds | 64 ± 2 | 55 ± 2 | 40 ± 2 | 51 ± 3 | 57 ± 2 | 35.8 ± 0.2 | 77 ± 4 | 43 ± 2 | 48 ± 2 | *** | ns | * |
| Heptanal | 2.0 ± 0.2 | 1.06 ± 0.05 | 1.04 ± 0.09 | 1.15 ± 0.05 | 0.84 ± 0.07 | 0.81 ± 0.06 | 2.4 ± 0.2 | 1.04 ± 0.06 | 1.16 ± 0.06 | *** | *** | *** |
| Octanal | 1.7 ± 0.1 | 1.3 ± 0.1 | 2.4 ± 0.2 | 1.34 ± 0.09 | 1.14 ± 0.09 | 1.91 ± 0.09 | 2.9 ± 0.2 | 1.66 ± 0.09 | 2.0 ± 0.1 | *** | ns | *** |
| Nonanal | 15 ± 1 | 2.4 ± 0.1 | 4.1 ± 0.2 | 12.7 ± 0.9 | 3.1 ± 0.2 | 3.0 ± 0.2 | 14.0 ± 0.7 | 3.5 ± 0.2 | 4.3 ± 0.2 | *** | * | ** |
| Decanal | 2.9 ± 0.1 | 0.84 ± 0.07 | 1.98 ± 0.09 | 0.79 ± 0.06 | 1.9 ± 0.1 | 0.23 ± 0.02 | 1.9 ± 0.2 | 2.4 ± 0.2 | 0.36 ± 0.02 | * | * | ns |
| Benzaldehyde | 30 ± 2 | 31 ± 2 | 23 ± 2 | 17.1 ± 0.8 | 44 ± 1 | 22.5 ± 0.5 | 25 ± 2 | 26 ± 2 | 34 ± 3 | * | ns | ns |
| 6-Methyl-5-hepten-2-one | 12.4 ± 0.7 | 18.3 ± 0.5 | 7.1 ± 0.4 | 18 ± 2 | 6.5 ± 0.3 | 7.5 ± 0.3 | 31 ± 2 | 9.1 ± 0.4 | 5.2 ± 0.3 | *** | ns | ns |
| Ethyl esters | 1435 ± 83 | 934 ± 25 | 1101 ± 26 | 1381 ± 25 | 968 ± 17 | 1070 ± 37 | 1429 ± 36 | 1390 ± 21 | 1067 ± 6 | *** | ns | ** |
| Ethyl propanoate | 142 ± 10 | 74 ± 4 | 108 ± 7 | 127 ± 10 | 108 ± 7 | 71 ± 2 | 86 ± 4 | 115 ± 5 | 71 ± 4 | ** | ns | ns |
| Ethyl butanoate | 404 ± 26 | 426 ± 12 | 464 ± 11 | 357 ± 11 | 396 ± 8 | 447 ± 32 | 317 ± 8 | 386 ± 2 | 425 ± 9 | *** | ** | * |
| Ethyl 3-methylbutanoate | 36 ± 2 | 1.70 ± 0.09 | 4.7 ± 0.3 | 28.9 ± 0.9 | 4.2 ± 0.2 | 20.0 ± 0.6 | 23.7 ± 0.7 | 3.3 ± 0.1 | 25 ± 1 | *** | ns | ns |
| Ethyl 4-hydroxybutanoate | 37 ± 1 | 18.5 ± 0.8 | 22 ± 2 | 47 ± 4 | 25 ± 1 | 2.1 ± 0.1 | 18 ± 1 | 16.1 ± 0.7 | 30 ± 2 | * | ns | ns |
| Ethyl hexanoate | 246 ± 24 | 141 ± 7 | 196 ± 3 | 337 ± 7 | 148 ± 2 | 190 ± 6 | 263 ± 7 | 240 ± 4 | 186 ± 1 | * | ns | ns |
| Ethyl heptanoate | 0.54 ± 0.04 | 1.48 ± 0.09 | 0 ± 0 | 0.68 ± 0.04 | 1.54 ± 0.01 | 0 ± 0 | 0 ± 0 | 0.38 ± 0.03 | 0 ± 0 | *** | ns | *** |
| Ethyl octanoate | 272 ± 13 | 189 ± 6 | 232 ± 6 | 301 ± 11 | 193 ± 1 | 264 ± 10 | 355 ± 19 | 254 ± 5 | 271 ± 2 | *** | ** | *** |
| Ethyl decanoate | 223 ± 12 | 69.6 ± 0.6 | 51 ± 1 | 131 ± 10 | 70 ± 2 | 62 ± 3 | 220 ± 9 | 288 ± 5 | 46.4 ± 0.9 | *** | ns | ** |
| Ethyl dodecanoate | 68 ± 2 | 6.2 ± 0.2 | 4.6 ± 0.2 | 44 ± 3 | 13.6 ± 0.9 | 5.43 ± 0.06 | 125 ± 7 | 71 ± 3 | 3.16 ± 0.03 | *** | ns | *** |
| Ethyl hexadecanoate | 4.88 ± 0.09 | 7.6 ± 0.4 | 19.1 ± 0.5 | 7.1 ± 0.4 | 9.0 ± 0.4 | 8.0 ± 0.6 | 21 ± 2 | 16 ± 1 | 8.5 ± 0.5 | ns | ns | ** |
| Lactones (mg/L) | 57 ± 1 | 34 ± 1 | 31 ± 0 | 41 ± 1 | 40 ± 0 | 37 ± 2 | 36 ± 1 | 34 ± 1 | 32 ± 1 | *** | ns | * |
| γ-Crotonolactone (mg/L) | 33 ± 2 | 19 ± 1 | 21 ± 1 | 26 ± 1 | 27 ± 1 | 16 ± 1 | 27 ± 1 | 25 ± 1 | 17 ± 1 | *** | ns | ns |
| γ-Butyrolactone (mg/L) | 24 ± 1 | 15 ± 0 | 9 ± 1 | 15 ± 1 | 13 ± 1 | 21 ± 2 | 9 ± 0 | 8 ± 0 | 15 ± 1 | ns | ns | ** |
| γ-Nonalactone | 9.3 ± 0.7 | 9.3 ± 0.6 | 11.4 ± 0.6 | 9.2 ± 0.9 | 10.8 ± 0.6 | 7.6 ± 0.3 | 14.7 ± 0.6 | 11.1 ± 0.5 | 11 ± 1 | * | ns | ns |
| Valerolactone | 51 ± 4 | 23 ± 2 | 24 ± 2 | 47 ± 3 | 32 ± 2 | 15.2 ± 0.8 | 24 ± 2 | 25 ± 1 | 31 ± 2 | *** | ns | ns |
| Nor-isoprenoids | 344 ± 14 | 295 ± 14 | 546 ± 34 | 279 ± 14 | 300 ± 13 | 363 ± 11 | 293 ± 25 | 227 ± 16 | 418 ± 10 | *** | *** | ns |
| β-Damascenone | 330 ± 13 | 286 ± 14 | 542 ± 34 | 269 ± 15 | 292 ± 13 | 356 ± 10 | 283 ± 25 | 221 ± 16 | 410 ± 10 | *** | *** | ns |
| β-Ionone | 1.47 ± 0.01 | 1.47 ± 0.03 | 1.50 ± 0.01 | 1.47 ± 0.01 | 1.47 ± 0.01 | 1.50 ± 0.01 | 1.46 ± 0.03 | 1.47 ± 0.01 | 1.48 ± 0.02 | *** | ns | ns |
| Vitispirane | 12.7 ± 0.8 | 7.7 ± 0.5 | 3.2 ± 0.1 | 8.6 ± 0.4 | 6.6 ± 0.5 | 5.5 ± 0.5 | 8.8 ± 0.5 | 4.7 ± 0.4 | 6.5 ± 0.4 | *** | ns | ns |
| Terpenoids | 104 ± 3 | 88 ± 4 | 118 ± 3 | 109 ± 5 | 101 ± 0.2 | 113 ± 3 | 113 ± 4 | 114 ± 5 | 120 ± 2 | *** | ns | * |
| Linalol | 1.7 ± 0.2 | 1.34 ± 0.08 | 0.60 ± 0.06 | 1.6 ± 0.2 | 2.4 ± 0.2 | 0 ± 0 | 8.1 ± 0.6 | 0.50 ± 0.04 | 0.32 ± 0.03 | ** | ns | ns |
| Limonene | 28 ± 1 | 23 ± 1 | 22.5 ± 0.7 | 22 ± 1 | 20 ± 1 | 24 ± 1 | 19.0 ± 0.7 | 23 ± 1 | 23 ± 1 | ns | * | ns |
| β-Farnesene | 6.0 ± 0.1 | 6.0 ± 0.2 | 11.8 ± 0.3 | 6.2 ± 0.3 | 6.3 ± 0.1 | 11.6 ± 0.1 | 5.8 ± 0.1 | 6.2 ± 0.3 | 11.7 ± 0.2 | *** | ns | ns |
| E-Nerolidol | 17.9 ± 0.6 | 14.2 ± 0.8 | 15.8 ± 0.7 | 20.0 ± 0.9 | 15.7 ± 0.4 | 15.0 ± 0.2 | 21.8 ± 0.9 | 20.8 ± 0.7 | 17.4 ± 0.6 | *** | ns | *** |
| Z-Dihydrofarnesol | 17 ± 1 | 12.7 ± 0.6 | 21 ± 1 | 16.7 ± 0.9 | 14.2 ± 0.4 | 27 ± 1 | 26 ± 2 | 17 ± 1 | 24 ± 1 | *** | * | * |
| Farnesol 3 | 18.9 ± 0.7 | 18 ± 1 | 32.9 ± 0.3 | 26 ± 2 | 30.3 ± 0.8 | 23 ± 1 | 15.4 ± 0.8 | 33 ± 2 | 32.1 ± 0.9 | ** | ns | ns |
| Geranyl acetone | 15 ± 1 | 13.0 ± 0.6 | 12.8 ± 0.2 | 16.2 ± 0.4 | 12.5 ± 0.6 | 12.0 ± 0.3 | 17 ± 1 | 13.6 ± 0.9 | 11.3 ± 0.9 | *** | ns | ns |
| Methyl esters | 4.4 ± 0.2 | 5.6 ± 0.2 | 2.3 ± 0.2 | 5.1 ± 0.1 | 6.7 ± 0.2 | 5.3 ± 0.2 | 5.5 ± 0.3 | 7.5 ± 0.3 | 5.7 ± 0.2 | *** | *** | * |
| E-Methyl dihydrojasmonate | 4.4 ± 0.2 | 5.6 ± 0.2 | 2.3 ± 0.2 | 5.1 ± 0.1 | 6.7 ± 0.2 | 5.3 ± 0.2 | 5.5 ± 0.3 | 7.5 ± 0.3 | 5.7 ± 0.2 | *** | *** | * |
| 220 g/L of Initial Sugars | 250 g/L of Initial Sugars | 250 g/L of Initial Sugars and 70 mg/L of SO2 | MANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc1 | Sc5 | Lc | Sc1 | Sc5 | Lc | Sc1 | Sc5 | Lc | Yeast | Sugar | SO2 | |
| Chemistry | 23 ± 1 | 15.4 ± 0.6 | 23 ± 1 | 21.6 ± 0.4 | 15.1 ± 0.3 | 21.9 ± 0.8 | 22 ± 1 | 18.3 ± 0.4 | 22.2 ± 0.3 | *** | * | * |
| Citrus | 12.4 ± 0.6 | 5.2 ± 0.2 | 6.9 ± 0.3 | 9.2 ± 0.6 | 5.9 ± 0.3 | 4.8 ± 0.2 | 12.5 ± 0.6 | 6.7 ± 0.3 | 5.5 ± 0.2 | *** | ** | ** |
| Creamy | 1.93 ± 0.01 | 1.28 ± 0.04 | 1.26 ± 0.03 | 1.48 ± 0.05 | 1.51 ± 0.02 | 1.32 ± 0.05 | 1.51 ± 0.05 | 1.33 ± 0.05 | 1.30 ± 0.07 | *** | ns | ns |
| Floral | 80 ± 2 | 71 ± 2 | 106 ± 5 | 71 ± 2 | 74 ± 2 | 79 ± 2 | 68 ± 4 | 58 ± 2 | 87 ± 2 | *** | ** | ns |
| Fruity | 124 ± 7 | 80 ± 2 | 103 ± 3 | 131 ± 2 | 84 ± 1 | 109 ± 3 | 127 ± 5 | 104 ± 2 | 113 ± 1 | *** | ns | * |
| Green | 1.47 ± 0.07 | 1.30 ± 0.04 | 1.45 ± 0.03 | 1.30 ± 0.06 | 1.01 ± 0.03 | 1.33 ± 0.04 | 1.9 ± 0.1 | 1.07 ± 0.01 | 1.45 ± 0.02 | *** | * | ** |
| Green fruit | 30 ± 2 | 11.3 ± 0.5 | 15.6 ± 0.3 | 34 ± 1 | 12.6 ± 0.2 | 21 ± 1 | 27 ± 1 | 18.4 ± 0.3 | 21.7 ± 0.2 | *** | * | ns |
| Herbal | 68 ± 2 | 62 ± 2 | 99 ± 5 | 60 ± 2 | 63 ± 2 | 73 ± 2 | 62 ± 4 | 53 ± 2 | 80 ± 2 | *** | *** | ns |
| Honey | 1.25 ± 0.08 | 0.87 ± 0.06 | 0.95 ± 0.06 | 1.18 ± 0.09 | 0.85 ± 0.04 | 0.87 ± 0.07 | 0.58 ± 0.04 | 0.34 ± 0.01 | 1.03 ± 0.01 | ** | ns | ** |
| Smoky | 1.49 ± 0.03 | 1.26 ± 0.06 | 4.28 ± 0.05 | 1.57 ± 0.07 | 1.61 ± 0.06 | 3.4 ± 0.2 | 1.25 ± 0.05 | 1.69 ± 0.08 | 3.6 ± 0.2 | *** | ns | ns |
| Spice | 5.1 ± 0.4 | 2.3 ± 0.2 | 2.4 ± 0.2 | 4.7 ± 0.3 | 3.2 ± 0.2 | 1.52 ± 0.08 | 2.5 ± 0.2 | 2.5 ± 0.1 | 3.1 ± 0.2 | *** | ns | ns |
| Waxy | 58 ± 3 | 39 ± 1 | 48 ± 1 | 62 ± 2 | 41 ± 1 | 53 ± 2 | 74 ± 4 | 54 ± 1 | 55 ± 1 | *** | * | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-García, F.J.; Palacios-Fernández, S.; López de Lerma, N.; García-Martínez, T.; Mauricio, J.C.; Peinado, R.A. The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine. Fermentation 2023, 9, 541. https://doi.org/10.3390/fermentation9060541
Martín-García FJ, Palacios-Fernández S, López de Lerma N, García-Martínez T, Mauricio JC, Peinado RA. The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine. Fermentation. 2023; 9(6):541. https://doi.org/10.3390/fermentation9060541
Chicago/Turabian StyleMartín-García, Francisco José, Sandra Palacios-Fernández, Nieves López de Lerma, Teresa García-Martínez, Juan C. Mauricio, and Rafael A. Peinado. 2023. "The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine" Fermentation 9, no. 6: 541. https://doi.org/10.3390/fermentation9060541
APA StyleMartín-García, F. J., Palacios-Fernández, S., López de Lerma, N., García-Martínez, T., Mauricio, J. C., & Peinado, R. A. (2023). The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine. Fermentation, 9(6), 541. https://doi.org/10.3390/fermentation9060541







