Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Physicochemical Characterization of Pomegranate By-Products
2.3. Microorganism
2.4. Solid-State Fermentation (SSF)
2.5. Analytical Analysis
2.6. Phenolic Profile
2.7. Statistical Analysis
2.8. Validation of the Model
3. Results
3.1. Physicochemical Characterization of Pomegranate Peel
3.2. Kinetics of TPC Extraction and AA
3.3. Significant Factors for TPC Recovery by SSF
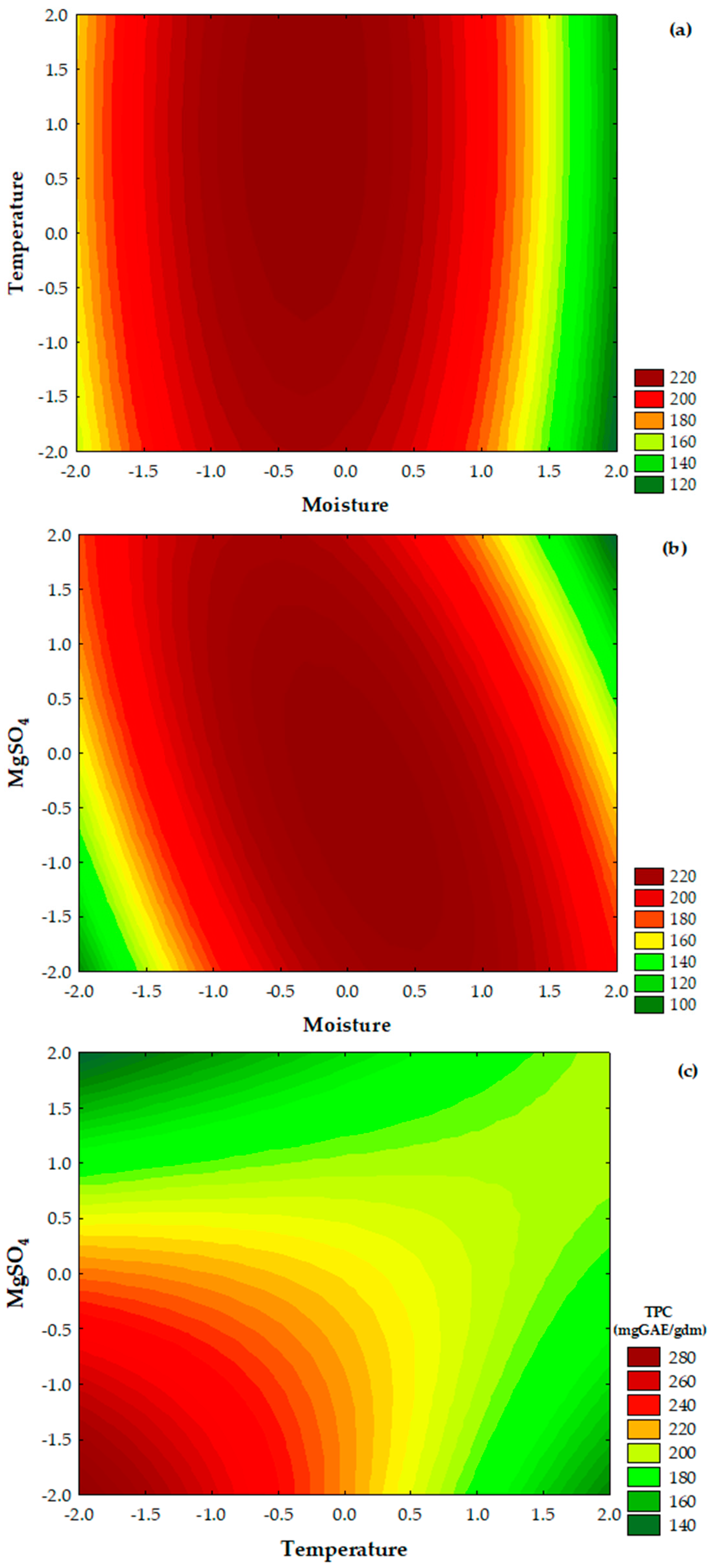
3.4. Optimization of the Culture Conditions for the Release of TPC
3.5. Identification of Phenolic Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J.; Aguilera-Carbó, F.A.; Prado-Barragán, L.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Ellagitannins: Biosynthesis, biodegradation and biological properties. J. Med. Plants Res. 2011, 5, 4696–4703. [Google Scholar]
- Tehranifar, A.; Selahvarzi, Y.; Kharrazi, M.; Bakhsh, V.J. High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind. Crop. Prod. 2011, 34, 1523–1527. [Google Scholar] [CrossRef]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Hamid, A.A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y de Pesca. 2021. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 13 June 2022).
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2967. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. From Pomegranate Byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods. 2022, 11, 2596. [Google Scholar] [CrossRef]
- de la Torre, I.; Martin-Dominguez, V.; Acedos, M.G.; Esteban, J.; Santos, V.E.; Ladero, M. Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Appl. Microbiol. Biotechnol. 2019, 103, 5975–5991. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.; Huerta-Ochoa, S.; Aguilar, C.; Prado-Barragán, L. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process. Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Robledo, A.; Aguilera-Carbó, A.; Rodriguez, R.; Martinez, J.L.; Garza, Y.; Aguilar, C.N. Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. J. Ind. Microbiol. Biotechnol. 2008, 35, 507–513. [Google Scholar] [CrossRef]
- Saffarzadeh-Matin, S.; Khosrowshahi, F.M. Phenolic compounds extraction from Iranian pomegranate (Punica granatum) industrial waste applicable to pilot plant scale. Ind. Crop. Prod. 2017, 108, 583–597. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Júnior, M.R.M. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Roasa, J.; De Villa, R.; Mine, Y.; Tsao, R. Phenolics of cereal, pulse and oilseed processing by-products and potential effects of solid-state fermentation on their bioaccessibility, bioavailability and health benefits: A review. Trends Food Sci. Technol. 2021, 116, 954–974. [Google Scholar] [CrossRef]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind. Crop. Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef]
- AOAC International; Latimer, G.W. Official Methods of Analysis of AOAC, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- de la Cruz, R.; Ascacio, J.A.; Buenrostro, J.; Sepúlveda, L.; Rodríguez, R.; Prado-Barragán, A.; Contreras, J.C.; Aguilera, A.; Aguilar, C.N. Optimization of Ellagitannase Production by Aspergillus niger GH1 by Solid-State Fermentation. Prep. Biochem. Biotechnol. 2015, 45, 617–631. [Google Scholar] [CrossRef]
- Norma, P.M.; Virginia, N.M.; Ral, R.H.; Jos, C.E.; Cristbal, N.A. A microassay for quantification of 2,2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- Bhol, S.; Lanka, D.; Bosco, S.J.D. Quality characteristics and antioxidant properties of breads incorporated with pomegranate whole fruit bagasse. J. Food Sci. Technol. 2015, 53, 1717–1721. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- López-Flores, A.R.; Luna-Urban, C.; Buenrostro-Figueroa, J.J.; Hernández-Martínez, R.; Huerta-Ochoa, S.; Escalona-Buendía, H.; Aguilar-González, C.N.; Prado-Barragán, L.A. Effect of pH, temperature and protein and carbohydrates source in protease production by Yarrowia lipolytica in solid culture. Rev. Mex. De Ing. Química 2016, 15, 57–67. [Google Scholar]
- Rajarathnam, S.; Bano, Z.; Steinkraus, K.H. Pleurotus mushrooms. Part III. Biotransformations of natural lignocellulosic wastes: Commercial applications and implications. Crit. Rev. Food Sci. Nutr. 1989, 28, 31–113. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Jekle, M.; Becker, T. Development of fibre-enriched wheat breads: Impact of recovered agroindustrial by-products on physicochemical properties of dough and bread characteristics. Eur. Food Res. Technol. 2017, 243, 1973–1988. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbo, A.; Martínez-Hernández, J.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Euphorbia antisyphilitica residues as a new source of ellagic acid. Chem. Pap. 2010, 64, 528–532. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Ascacio-Valdés, A.; Sepúlveda, L.; De la Cruz, R.; Prado-Barragán, A.; Aguilar-González, M.A.; Rodríguez, R.; Aguilar, C.N. Potential use of different agroindustrial by-products as supports for fungal ellagitannase production under solid-state fermentation. Food Bioprod. Process. 2014, 92, 376–382. [Google Scholar] [CrossRef]
- Martínez-Ávila, G.C.; Aguilera-Carbó, A.F.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal enhancement of the antioxidant properties of grape waste. Ann. Microbiol. 2011, 62, 923–930. [Google Scholar] [CrossRef]
- Coetzee, G.; Joubert, E.; van Zyl, W.H.; Viljoen-Bloom, M. Improved extraction of phytochemicals from rooibos with enzyme treatment. Food Bioprod. Process. 2014, 92, 393–401. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Buenrostro, J.J.; De la Cruz, R.; Sepúlveda, L.; Aguilera, A.F.; Prado, A.; Contreras, J.C.; Rodríguez, R.; Aguilar, C.N. Fungal biodegradation of pomegranate ellagitannins. J. Basic Microbiol. 2013, 54, 28–34. [Google Scholar] [CrossRef]
- da Silveira, J.S.; Durand, N.; Lacour, S.; Belleville, M.-P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- Jamal, P.; Idris, Z.M.; Alam, Z. Effects of physicochemical parameters on the production of phenolic acids from palm oil mill effluent under liquid-state fermentation by Aspergillus niger IBS-103ZA. Food Chem. 2011, 124, 1595–1602. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Aguilera-Carbó, A.; Ascacio-Valdés, J.; Rodríguez-Herrera, R.; Martínez-Hernández, J.; Aguilar, C. Optimization of ellagic acid accumulation by Aspergillus niger GH1 in solid state culture using pomegranate shell powder as a support. Process. Biochem. 2012, 47, 2199–2203. [Google Scholar] [CrossRef]
- Lopez-Trujillo, J.; Medina-Morales, M.A.; Sanchez-Flores, A.; Arevalo, C.; Ascacio-Valdes, J.A.; Mellado, M.; Aguilar, C.N.; Aguilera-Carbo, A.F. Solid bioprocess of tarbush (Flourensia cernua) leaves for β-glucosidase production by Aspergillus niger: Initial approach to fiber–glycoside interaction for enzyme induction. 3 Biotech 2017, 7, 271. [Google Scholar] [CrossRef]
- Beniwal, V.; Rajesh, Goel, G.; Kumar, A.; Chhokar, V. Production of tannase through solid state fermentation using Indian Rosewood (Dalbergia Sissoo) sawdust—A timber industry waste. Ann. Microbiol. 2012, 63, 583–590. [Google Scholar] [CrossRef]
- Vašák, M.; Schnabl, J. Sodium and Potassium Ions in Proteins and Enzyme Catalysis. In The Alkali Metal Ions: Their Role for Life, 1st ed.; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 259–290. [Google Scholar]
- Pande, G.; Akoh, C.C. Antioxidant Capacity and Lipid Characterization of Six Georgia-Grown Pomegranate Cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef]
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef]
- Amyrgialaki, E.; Makris, D.P.; Mauromoustakos, A.; Kefalas, P. Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind. Crop. Prod. 2014, 59, 216–222. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the Major Phenolic Compounds in Pomegranate Juices by HPLC–DAD–ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Gumienna, M.; Szwengiel, A.; Górna, B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur. Food Res. Technol. 2015, 242, 631–640. [Google Scholar] [CrossRef]
- Holic, R.; Xu, Y.; Caldo, K.M.P.; Singer, S.D.; Field, C.J.; Weselake, R.J.; Chen, G. Bioactivity and biotechnological production of punicic acid. Appl. Microbiol. Biotechnol. 2018, 102, 3537–3549. [Google Scholar] [CrossRef]

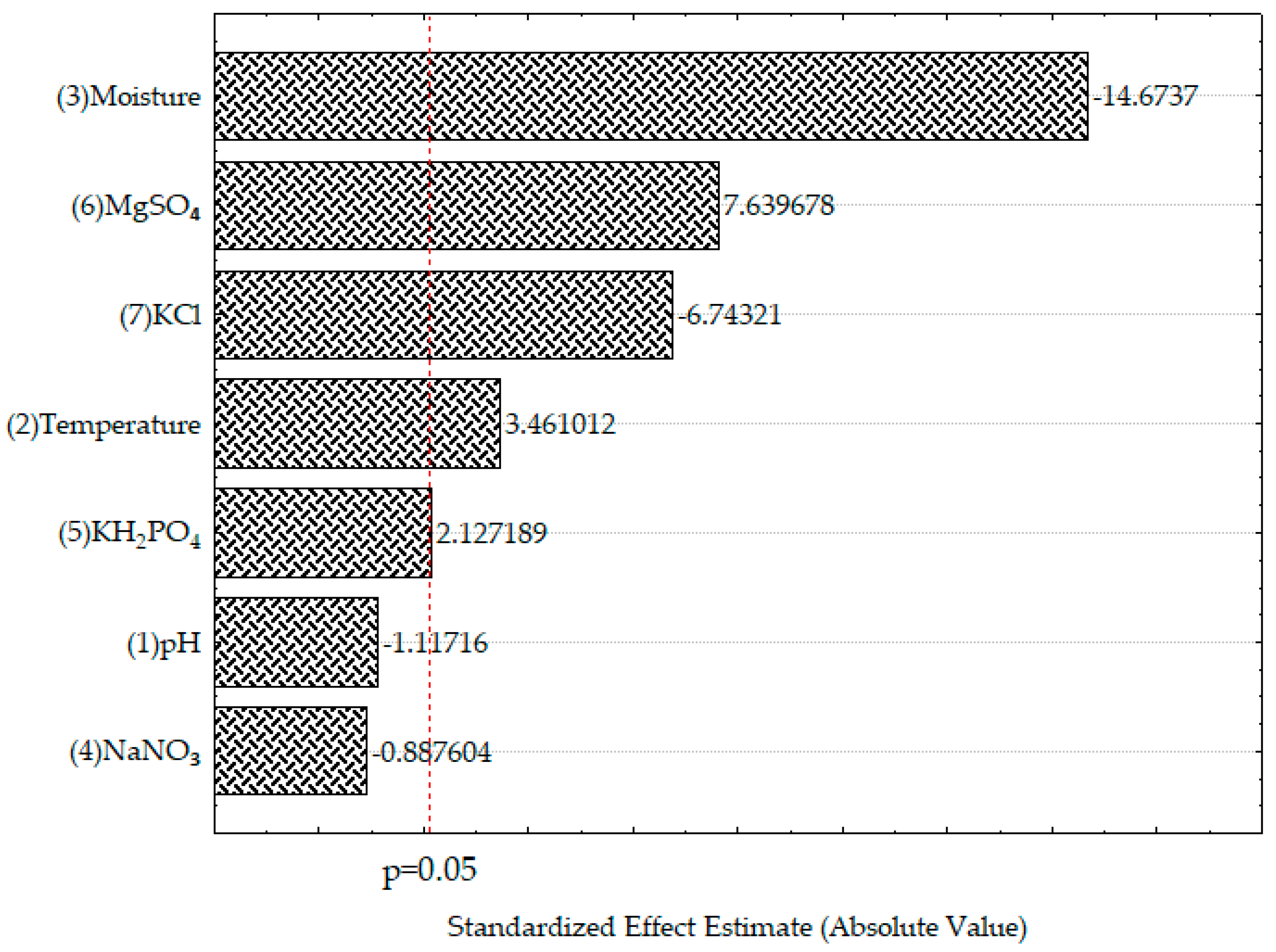

| Treatment | A | B | C | D | E | F | G | TPC (mg/g) * | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 189.93 ± 4.40 a | |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | 171.76 ± 0.66 bc | |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | 1 | 169.90 ± 5.14 bc | |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 175.11 ± 5.55 b | |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | 1 | 127.67 ± 2.64 e | |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | 144.92 ± 8.96 d | |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | 165.07 ± 4.21 c | |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 151.85 ± 3.04 d | |
| Code | Factor | Levels | |||||||
| −1 | +1 | ||||||||
| A | pH | 5 | 6 | ||||||
| B | Temperature (°C) | 30 | 40 | ||||||
| C | Moisture (%) | 50 | 60 | ||||||
| D | NaNO3 (g/L) | 3.83 | 7.65 | ||||||
| E | KH2PO4 (g/L) | 1.52 | 3.04 | ||||||
| F | MgSO4 (g/L) | 1.52 | 3.04 | ||||||
| G | KCl (g/L) | 1.52 | 3.04 | ||||||
| Treatment | X1 | X2 | X3 | TPC (mgGAE/gdm) * | ||
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 236.84 ± 3.89 bc | ||
| 2 | −1 | −1 | 1 | 223.01 ± 4.73 c | ||
| 3 | −1 | 1 | −1 | 174.52 ± 5.41 f | ||
| 4 | −1 | 1 | 1 | 250.78 ± 4.67 a | ||
| 5 | 1 | −1 | −1 | 222.57 ± 8.38 c | ||
| 6 | 1 | −1 | 1 | 203.02 ± 4.84 d | ||
| 7 | 1 | 1 | −1 | 206.66 ± 3.84 d | ||
| 8 | 1 | 1 | 1 | 201.14 ± 8.97 de | ||
| 9 | −1.68 | 0 | 0 | 148.18 ± 4.54 g | ||
| 10 | −1.68 | 0 | 0 | 175.82 ± 6.45 f | ||
| 11 | 0 | −1.68 | 0 | 243.17 ± 2.83 ab | ||
| 12 | 0 | 1.68 | 0 | 184.67 ± 2.72 f | ||
| 13 | 0 | 0 | −1.68 | 226.49 ± 1.72 c | ||
| 14 | 0 | 0 | 1.68 | 186.48 ± 6.94 ef | ||
| 15 | 0 | 0 | 0 | 233.22 ± 4.46 bc | ||
| 16 | 0 | 0 | 0 | 235.39 ± 2.49 bc | ||
| Code | Factor | Levels | ||||
| −1.68 | −1 | 0 | 1 | +1.68 | ||
| X1 | Moisture (%) | 42 | 45 | 50 | 55 | 58 |
| X2 | Temperature (°C) | 31 | 35 | 40 | 45 | 48 |
| X3 | MgSO4 (g/L) | 0.48 | 1.52 | 3.04 | 4.56 | 5.59 |
| Component (%) | Value * |
|---|---|
| Moisture | 11.86 ± 0.05 |
| Fat | 2.64 ± 0.08 |
| Fiber | 8.81 ± 0.07 |
| Protein | 8.66 ± 0.01 |
| Ash | 4.51 ± 0.01 |
| Carbohydrates | 75.38 ± 0.18 |
| C/N | 41.51 |
| WAI ** | 4.38 ± 0.48 |
| CHP *** | 10.13 ± 2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Arely Prado-Barragán, L. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation 2023, 9, 530. https://doi.org/10.3390/fermentation9060530
Buenrostro-Figueroa JJ, Nevárez-Moorillón GV, Chávez-González ML, Sepúlveda L, Ascacio-Valdés JA, Aguilar CN, Pedroza-Islas R, Huerta-Ochoa S, Arely Prado-Barragán L. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation. 2023; 9(6):530. https://doi.org/10.3390/fermentation9060530
Chicago/Turabian StyleBuenrostro-Figueroa, José Juan, Guadalupe Virginia Nevárez-Moorillón, Mónica Lizeth Chávez-González, Leonardo Sepúlveda, Juan Alberto Ascacio-Valdés, Cristóbal Noé Aguilar, Ruth Pedroza-Islas, Sergio Huerta-Ochoa, and Lilia Arely Prado-Barragán. 2023. "Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation" Fermentation 9, no. 6: 530. https://doi.org/10.3390/fermentation9060530
APA StyleBuenrostro-Figueroa, J. J., Nevárez-Moorillón, G. V., Chávez-González, M. L., Sepúlveda, L., Ascacio-Valdés, J. A., Aguilar, C. N., Pedroza-Islas, R., Huerta-Ochoa, S., & Arely Prado-Barragán, L. (2023). Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation, 9(6), 530. https://doi.org/10.3390/fermentation9060530











