From Microalgae to Bioenergy: Recent Advances in Biochemical Conversion Processes
Abstract
1. Introduction
2. Microalgae Biomass and Its Components
3. Biochemical Conversion
3.1. Anaerobic Digestion
3.1.1. Pretreatment Technologies
| Microalgae Species | Pretreatment Strategy and Operating Conditions | Biomethane Yield (mL CH4/g VS) | References | ||
|---|---|---|---|---|---|
| Without Pretreatment | With Pretreatment | % Improvement in Yield | |||
| Physical pretreatment (Mechanical) | |||||
| Scenedesmus sp. | Ultrasound pretreatment at 400 W power for 200 s | 183 ± 25 | 313 ± 15 | 71 | [81] |
| Pinnularia sp. | 152 ± 21 | 250 ± 21 | 65 | ||
| Mixed microalgae and bacteria consortium dominated by green microalgae (Stigeoclonium sp. and Monoraphidium sp.) and diatoms (Nitzschia sp. and Navicula sp. | Ultrasound pretreatment at 70 W power for 0.5 h | 106 ± 2 | 114 ± 2 | 8 | [84] |
| Microwave pretreatment at 900 W power for 180 s | 128 ± 5 | 21 | |||
| Chlorella sorokiniana | Homogenization pretreatment at 200 W power for 0.5 h | 318 ± 1 | 442 ± 29 | 39 | [34] |
| Acutodesmus obliquus | Bead milling pretreatment using 0.35 mm glass beads at 40 g of glass beads/100 g of wet algae for 0.34 h at 8500 rpm | 191 | 289 | 51 | [85] |
| Auxenochlorella protothecoides | Pulsed electric field pretreatment at 40 kV/cm electric field and 1 μs pulse duration (3 Hz) | 425 | 467 | 10 | [86] |
| Physical pretreatment (Thermal) | |||||
| Chlorella sorokiniana | Thermal hydrolysis pretreatment at 60 °C for 0.5 h | 318 ± 1 | 337 ± 12 | 6 | [34] |
| Thermal hydrolysis pretreatment at 70 °C for 0.5 h | 347 ± 39 | 9 | |||
| Thermal hydrolysis pretreatment at 80 °C for 0.5 h | 375 ± 53 | 18 | |||
| Chlorella sp. | Thermal hydrolysis pretreatment at 70 °C for 0.5 h | 155 | 215 | 39 | [87] |
| Thermal hydrolysis pretreatment at 90 °C for 0.5 h | 228 | 47 | |||
| Hydrothermal hydrolysis pretreatment at 121 °C for 0.5 h | 332 | 114 | |||
| Chlorella pyrenoidosa | Solar-driven hydrothermal pretreatment at 723 W/m2 irradiation (~160 °C) for 0.5 h | 222 | 348 | 57 | [89] |
| Chlorella sorokiniana | Steam explosion pretreatment at 4 bars for 0.08 h | 318 ± 1 | 137 ± 5 | −57 | [34] |
| Steam explosion pretreatment at 4 bars for 0.17 h | 128 ± 7 | −60 | |||
| Steam explosion pretreatment at 4 bars for 0.25 h | 230 ± 4 | −28 | |||
| Chemical pretreatment | |||||
| Scenedesmus obliquus | Acidic pretreatment with 0.1% v/v sulfuric acid at 150 °C for 1 h | 131 ± 26 | 253 ± 51 | 93 | [93] |
| Tetraselmis striata M8 | Acidic pretreatment with 2.31 mg/L free nitrous acid at 5.5 pH for 48 h | 161 ± 7 | 250 ± 2 | 55 | [94] |
| Chlorella pyrenoidosa | Alkaline pretreatment with 1.5% (w/v) NaOH at 90 °C for 2 h | 218 | 386 | 77 | [95] |
| Mixed microalgae consortium | Alkaline pretreatment with 0.5 M NaOH at 121 °C for 1 h | 162 | 173 | 7 | [92] |
| Alkaline pretreatment with 2 M NaOH at 121 °C for 1 h | 377 | 133 | |||
| Oxidative pretreatment with 0.5% w/w hydrogen peroxide (11.5 pH) at 50 °C for 1 h | 279 | 72 | |||
| Mixed microalgae consortium of Chlorella sp. and Scenedesmus sp. | Alkaline pretreatment with 4% (w/v) CaO at 55 °C for 24 h | 260 ± 8 | 255 ± 6 | −2 | [96] |
| Alkaline pretreatment with 10% (w/v) CaO at 55 °C for 24 h | 292 ± 11 | 12 | |||
| Alkaline pretreatment with 4% (w/v) CaO at 72 °C for 24 h | 287 ± 4 | 11 | |||
| Alkaline pretreatment with 10% (w/v) CaO at 72 °C for 24 h | 325 ± 12 | 25 | |||
| Nannochloropsis oculata | Organosolv treatment with N-methylmorpholine-N-oxide | 238 ± 6 | 339 ± 4 | 42 | [97] |
| Microcystis sp. | Oxidative pretreatment with 0.1 g peroxymonosulfate/g algae (TSS) | 291 (mL CH4/g COD) | 303 (mL CH4/g COD) | 4 | [99] |
| Mixed microalgae consortium | Oxidative pretreatment with 96 mg of ozone/g algae VS at 23 °C | 260 | 306 | 18 | [100] |
| Oxidative pretreatment with 191 mg of ozone/g algae VS at 23 °C | 334 | 28 | |||
| Oxidative pretreatment with 383 mg of ozone/g algae VS at 23 °C | 433 | 66 | |||
| Ultrasonically pretreated Microcystis sp. | Oxidative pretreatment with 20 g of zero-valent iron/g algae (TS) | 37 (mL CH4/g COD) | 61 (mL CH4/g COD) | 64 | [101] |
| Mixed microalgae consortium | Free ammonia pretreatment with 530 mg NH3-N/L at 22 °C for 24 h (pH 9.5) | 188 | 219 | 17 | [102] |
| Biological pretreatment | |||||
| Porphyridium cruentum | Single enzymatic pretreatment with 0.5 mL/g dry biomass commercial cellulase at 55 °C for 24 h (pH 5–5.5) | 130 | 152 | 17 | [103] |
| Single enzymatic pretreatment with 0.5 mL/g dry biomass commercial protease at 55 °C for 24 h (pH 8–8.5) | 271 | 109 | |||
| Cocktail enzymatic pretreatment with 0.5 mL/g dry biomass commercial viscozyme (carbohydrase mix) at 55 °C for 24 h (pH 4–4.5) | 242 | 86 | |||
| Cocktail enzymatic pretreatment with 0.5 mL/g dry biomass enzyme mix (commercial protease and viscozyme) at 55 °C for 9 h (pH 8–8.5 for first 4.5 h and 4–4.5 for next 4.5 h) | 263 | 102 | |||
| Chlorella vulgaris | Single enzymatic pretreatment with 1% w/v commercial cellulase at 55 °C for 24 h | 120 ± 15 | 183 ± 12 | 53 | [104] |
| Single enzymatic pretreatment with 1% w/v commercial protease at 55 °C for 24 h | 194 ± 1 | 62 | |||
| Single enzymatic pretreatment with 1% w/v commercial amylase at 55 °C for 24 h | 177 ± 14 | 48 | |||
| Cocktail enzymatic pretreatment with 1% w/v enzyme mix (commercial cellulase and protease) at 55 °C for 24 h | 314 ± 11 | 162 | |||
| Cocktail enzymatic pretreatment with 1% w/v enzyme mix (commercial cellulase and amylase) at 55 °C for 24 h | 291 ± 5 | 143 | |||
| Mixed microalgae–bacteria consortium | Single enzyme pretreatment with 100 U/L commercial laccase at 25 °C | 83 ± 1 | 100 ± 7 | 21 | [107] |
| Fungal pretreatment with 100 U/L laccase-rich broth from Trametes versicolor | 144 ± 2 | 74 | |||
| Mixed microalgae consortium | Bacterial pretreatment with bacterial consortium secreting protease, amylase (Bacillus jerish 03 and Bacillus jerish 04) | 0.06 (g CODconverted/g CODadded) | 0.19 (g CODconverted/g CODadded) | 217 | [108] |
| Bacterial pretreatment with cellulase secreting bacteria (Bacillus sp.) | 0.21 (g CODconverted/g CODadded) | 250 | |||
| Bacterial pretreatment with mixed bacteria population (Bacillus jerish 03, Bacillus jerish 04 and Bacillus sp.) | 0.26 (g CODconverted/g CODadded) | 334 | |||
| Mixed microalgae consortium | Cow rumen fluid mixed with anaerobic granular sludge inoculum at 1:4 v/v ratio | 300 | 600 | 100 | [109] |
3.1.2. Co-Digestion
3.2. Biohydrogen Production
3.2.1. Biophotolysis
| Microalgae Species | Biohydrogen Production Strategy and Experimental Conditions | Biohydrogen Production | Biohydrogen Production Duration | References | |
|---|---|---|---|---|---|
| Without Pretreatment | With Pretreatment | ||||
| Oxygen removal by inert gas purging | |||||
| Synechocystis sp. PCC 6803 | Direct photolysis with and without anaerobic conditions | 0.12–0.7 mmol/mg Chla.h | 1–4 mmol/mg Chla.h | 168 h | [134] |
| Parachlorella kessleri EMCCN 3073 | 0.04–0.2 mmol/mg Chla.h | 0.4–1 mmol/mg Chla.h | |||
| Nostoc spongiaeforme | 0.1–0.8 mmol/mg Chla.h | 0.2–2.1 mmol/mg Chla.h | |||
| Nostoc sp. | 0.07–0.2 mmol/mg Chla.h | 0.2–0.9 mmol/mg Chla.h | |||
| Immobilized Tetraspora sp. CU2551 in sodium alginate beads | Two-stage growth (sulfur deprivation in the second stage) with and without anaerobic conditions | 182 nmol/mg DW.h | 1183 nmol/mg DW.h | 108 h (Anaerobic); 1034 h (Aerobic) | [135,136] |
| Oxygen removal by scavenging agents | |||||
| Scenedesmus obliquus 393 | Direct photolysis with and without sodium dithionite addition | ≈0 | 570 μL | 6 h | [137] |
| Chlorococcum minutum | Direct photolysis with and without sodium sulfite addition | NA | 300 μmol | 24 h | [138] |
| Direct photolysis with and without sodium metabisulfite addition | 300 μmol | ||||
| Direct photolysis with and without sodium dithionite addition | 135 μmol | ||||
| Nutrient deprivation | |||||
| Chlamydomonas reinhardtii (CC425) | Two-stage growth with and without sulfur deprivation in the second stage under anaerobic conditions | NA | 61 ± 7 mL/L (17 ± 4 μmol/L.h) | 204 h | [141] |
| Chlamydomonas moewusii (SAG24.91) | NA | 21 ± 3 mL/L (5 ± 0.4 μmol/L.h) | |||
| Coccomyxa chodatii SAG 216–2 | Direct photolysis with and without sulfur deprivation and malate supplementation | <50 mL/L | 200 mL/L | 120 h | [142] |
| Immobilized Tetraspora sp. CU2551 in sodium alginate beads | Two-stage growth with and without sulfur deprivation in the second stage under aerobic conditions | 0.46 mL per 25 mL of medium | 0.55 mL per 25 mL of medium | 48 h | [135] |
| Two-stage growth with and without sulfur and phosphorus deprivation in the second stage under aerobic conditions | 0.61 mL per 25 mL of medium | ||||
| Chlorella sp. IOAC707S | Two-stage growth with and without phosphorus deprivation in the second stage under aerobic conditions | NA | Up to 40 mL/L | 650 h | [144] |
| Chlorella sorokiniana KU204 | Two-stage growth with and without sulfur deprivation in the second stage under anaerobic conditions | 0.7 mL/L | 48 mL/L | 84 h | [143] |
| Two-stage growth with and without sulfur and nitrogen deprivation in the second stage under anaerobic conditions | 98 mL/L | ||||
| Two-stage growth with and without nitrogen deprivation in the second stage under anaerobic conditions | 12 mL/L | ||||
| Two-stage growth with and without phosphorus and nitrogen deprivation in the second stage under anaerobic conditions | 77 mL/L | ||||
| Two-stage growth with and without sulfur, nitrogen, and phosphorus deprivation in the second stage under anaerobic conditions | 125 mL/L | ||||
| Parachlorella kessleri EMCCN 3073 | Two-stage growth with and without nitrogen deprivation in the second stage under anaerobic conditions | 250 μL/L | 300 μL/L | 9 d | [146] |
| Chlorella pyrenoidosa IOAC707S | Two-stage growth with and without nitrogen deprivation in the second stage under anaerobic conditions | 0.003 mL/L | 26.83 mL/L | 92 h | [147] |
| Chlamydomonas Reinhardtii wild type strain 137+ | Two-stage growth with and without sulfur deprivation in the second stage under aerobic conditions | NA | 1.3 mmol/106 cells | 100 h | [148] |
| Three-stage growth with and without magnesium deprivation in the third stage under aerobic conditions | NA | 2.1 mmol/106 cells | 230 h | ||
| Tetraspora sp. CU2551 | Two-stage growth with and without potassium deprivation in the second stage under anaerobic conditions | 3.6 ± 0.1 μmol/mg DW | 9.2 ± 0.1 μmol/mg DW | 32 h | [149] |
| Addition of exogenic substrates | |||||
| Immobilized Chlorella vulgaris in sodium alginate beads | Two-stage growth (under purple light) with and without sulfur deprivation and exogenous organic carbon addition (10 g/L of glucose) in the second stage under anaerobic conditions | NA | 60 mL/L (39 mL/L.d) | 50 h | [151] |
| Immobilized Scenedesmus obliquus in sodium alginate beads | NA | 128 mL/L (205 mL/L.d) | 70 h | ||
| Chlorella pyrenoidosa | Two-stage growth with and without sulfur deprivation and exogenous organic carbon addition (0.7 g/L of glucose) in the second stage under anaerobic conditions | 65.5 mL/L | 121.1 mL/L | 120 h | [150] |
| Chlorella vulgaris | Direct biophotolysis with and without exogenous organic carbon addition (5–10 g/L of glucose) under anaerobic conditions | 0 | 0.75–2 mL/h | 174 h | [153] |
| Chlorella sp. KLSc59 | Two-stage growth with and without exogenous organic carbon addition (glucose) in the second stage under anaerobic conditions | 0 | 128 μmol/mg Chl | 42 h | [154] |
| Two-stage growth with and without exogenous organic carbon addition (acetate) in the second stage under anaerobic conditions | 150 μmol/mg Chl | ||||
| Chlorella sp. | Two-stage growth with and without exogenous organic carbon addition (16 g/L of crude glycerol) in the second stage under anaerobic conditions | 4 mL/L | 10.3 mL/L | 24 h | [155] |
| Tetraselmis subcordiformis | Two-stage growth with and without exogenous organic carbon addition (50% diluted aerobically pretreated dairy wastewater) in the second stage under anaerobic conditions | 54 ± 2 mL/g DW | 69 ± 4 mL/g DW | 120 h | [156] |
| Chlamydomonas reinhardtii UTEX 2243 | Supplementing acetate-rich fermenter effluent to achieve acetate/Cl− ratio of 150 without nutrient deprivation under aerobic conditions | NA | 95 μmol/L | 15 d | [158] |
| Chlorella sorokiniana UTEX 2714 | 80 μmol/L | ||||
| Light intensity manipulation | |||||
| Chlamydomonas reinhardtii | Pulse illumination (strong light pulse for 1 s, followed by dark period for 9 s) vs. continuous illumination under anaerobic conditions | 0 | 3 mmoL/L | 48 h | [159] |
| Chlorella sp. KLSc59 | Illumination with and without light intensity of 7 μmol photons/m2.s | 5 μmol/mg Chl | 63 μmol/mg Chl | 60 h | [154] |
| Illumination with and without light intensity of 14 μmol photons/m2.s | 130 μmol/mg Chl | ||||
| Illumination with and without light intensity of 28 μmol photons/m2.s | 206 μmol/mg Chl | ||||
| Algae–bacteria co-culture | |||||
| Chlamydomonas reinhardtii FACHB-265 | Co-cultured with and without Pseudomonas sp. strain-D in sulfur-deprived media under aerobic conditions | 10 mL/L | 120 mL/L | 15 d | [161] |
| Chlorella vulgaris MACC360 | Co-cultured with and without 5% enriched microbial consortium in pretreated brewery effluent media under aerobic conditions | 52 mL/L.d | 154 mL/L.d | 3 d | [162] |
| Chlamydomonas reinhardtii strain 704 | Co-cultured with and without Escherichia coli K-12 MG1655 with acetic acid under aerobic and low light intensity conditions | NA | 24% higher | 5 d | [163] |
| Co-cultured with and without Pseudomonas stutzeri A1501 with acetic acid under aerobic and low light intensity conditions | 46% higher | ||||
| Co-cultured with and without Pseudomonas putida 12,264 with acetic acid under aerobic and low light 56 intensity conditions | 32% higher | ||||
| Co-cultured with and without unknown bacterial consortium with acetic acid under aerobic and low light intensity conditions | 56% higher | ||||
| Immobilization | |||||
| Tetraspora sp. CU2551 | Two-stage growth with and without immobilized cells in the second stage under aerobic conditions | 0.0025 μmol/mg DW.h | 0.1 μmol/mg DW.h | NA | [135] |
3.2.2. Fermentation
| Microalgae Species | Pretreatment | Fermentation Microorganisms | Operating Conditions | Biohydrogen Yield (mL H2/g VS) | References |
|---|---|---|---|---|---|
| Photofermentation | |||||
| Chlorella sp. | Acid–hydrothermal treatment followed by dark fermentation | Rhodobacter sphaeroides TISTR 1952 | Initial pH: 7 Light: 5000 lux Inoculum size: 20% v/v Temperature: 37 °C | 125 | [171] |
| Arthrospira platensis | Acid–hydrothermal treatment followed by dark fermentation and NaCl-modified zeolite treatment | Rhodopseudomonas palustris | Initial pH: 7 Light: 6000 lux Inoculum size: NA Temperature: 30 °C | 333 | [172] |
| Dark fermentation | |||||
| Chlorella sp. | 1.5% v/v HCl, 180 °C, 0.25 h | Heat-treated (105 °C for 3 h) anaerobic granules (dominated by Clostridium sp.) collected from brewery wastewater treatment plant | Initial pH: 6 F/I: 1 (VS/VS) Temperature: 37 °C | 47 | [171] |
| Arthrospira platensis | 1% v/v H2SO4, 135 °C, 0.25 h | Heat treated (100 °C, 0.5 h) anaerobic digestion sludge dominated by Clostridium sp. | Initial pH: 6 F/I: NA Temperature: 35 °C | 96 | [172] |
| Chlorella sp. | 4% v/v H2SO4, 2.5 h | Heat-treated (105 °C for 3 h) anaerobic granules (dominated by Clostridium sp.) collected from brewery wastewater treatment plant | Initial pH: 6 F/I: 3 (VS/VS) Temperature: 35 °C | 26 | [173] |
| 0.75% v/v H2SO4, 160 °C, 0.5 h | 54 | ||||
| Chlorella sp. | No pretreatment | Heat treated (100 °C, 0.25 h) anaerobic sludge obtained from sewage treatment plant | Initial pH: 6 F/I: NA Temperature: 37 °C | 8 | [175] |
| Chlorella sp. | No pretreatment | Mixed anaerobic bacterial consortia | Initial pH: 7 F/I: NA Temperature: 35 °C | 22 | [176] |
| Deoiled Scenedesmus obliquus UTEX 393 | No pretreatment | Acidogenic mixed consortia (dominated by Clostridium sp.) developed from heat-treated (100 °C for 0.34 h) cow dung | Initial pH: 6.7 Inoculum size: 10% v/v Temperature: 37 °C | 10 | [177] |
| Grinding | 15 | ||||
| Homogenization | 20 | ||||
| Autoclave | 30 | ||||
| Sonication | 36 | ||||
| 1 N NaOH, 121 °C, 0.5 h | 40 | ||||
| 1 N KOH, 121 °C, 0.5 h | 38 | ||||
| 0.5 N H2SO4, 121 °C, 0.5 h | 89 | ||||
| 10% w/v magnetic solid acid, 121 °C, 0.5 h | 53 | ||||
| Scenedesmus obtusiusculus AT-UAM | No pretreatment | Granular sludge obtained from a full-scale up-flow anaerobic sludge blanket reactor fed with tequila vinasses | Initial pH: 7.5 F/I: 12 (VS/VS) Temperature: 37 °C | 29 | [178] |
| 3% HCl, 100 °C, 1.7 h | 48 | ||||
| Algal bloom dominated by Microcystis sp. | No pretreatment | Heat-treated (100 °C, 0.5 h) anaerobic digestion sludge dominated by Clostridium sp. | Initial pH: 6 F/I: 0.5 (VS/VS) Temperature: 35 °C | 0.3 | [179] |
| 2% v/v H2SO4, 135 °C, 0.25 h (steam treatment) | 19 | ||||
| 2% v/v H2SO4, 135 °C, 0.25 h (hydrothermal treatment) | 25 | ||||
| Wastewater-born microalgal biomass | No pretreatment | Heat-treated (90 °C, 0.5 h) anaerobic digestion sludge from sewage treatment plant | Initial pH: 9.5 F/I: 1 (TS) Temperature: 35 °C | 18 | [180] |
| 240–530 mg NH3-N/L, 1 day, pH 9.5 (free ammonia pretreatment) | 20–22 | ||||
3.3. Alcoholic Fermentation
| Dark Fermentation | |||||
| Microalgae Species | Operating Conditions | Starch Content (% of Dry Cell Weight) | % Starch Decomposed | Bioethanol Yield (% of Dry Cell Weight) | References |
| Chlamydomonas reinhardtii UTEX 2247 | Incubation under dark and anaerobic conditions at 25 °C for 46 h Slurry concentration: 15% w/w | 45 | NA | 1 | [182] |
| Chlamydomonas sp. YA-SH-1 | Incubation under dark and anaerobic conditions at 30–35 °C for 44 h Slurry concentration: 15–25% w/w | 30 | NA | 1.3 | [183] |
| Chlorococcum littorale | Incubation under dark and anaerobic conditions at 30 °C for 24 h Slurry concentration: 1.4% w/w | 15 | 46 | 1.6 | [184] |
| Photo Fermentation | |||||
| Cyanobacteria | Genes expressed (source of genes) and their expression mechanism | Promotor used | Gene deletion (Effect) | Bioethanol titer (g/L) and days of cultivation (d) | Reference |
| Synechococcus elongatus PCC7942 | pdc (Zymomonas mobilis), Shuttle vector; adhII (Zymomonas mobilis), Shuttle vector | rbcLS | NA | 0.23 in 28 d | [190] |
| Synechocystis sp. PCC 6803 | pdc (Zymomonas mobilis), Homologous recombination; adhII (Zymomonas mobilis), Homologous recombination | psbA2 | NA | 0.46 in 6 d | [191] |
| Synechocystis sp. PCC6803 | pdc (Zymomonas mobilis), Homologous recombination; adh, slr1192 (Endogenous overexpression), Homologous recombination | Prbc | phaA and phaB (Disrupting PHB biosynthesis pathway) | 5.5 in 26 d | [197] |
| Synechocystis sp. PCC6803 | pdc (Zymomonas mobilis), Homologous recombination; adhII (Zymomonas mobilis), Homologous recombination | nblA | glgC (Disrupting glycogen biosynthesis pathway) and phaC + phaE (Disrupting PHB biosynthesis pathway) | 3 in 3 d | [194] |
| Synechocystis sp. PCC6803 | zwf (Endogenous overexpression to enhance NADPH production) Homologous recombination; pdc (Zymomonas mobilis), Homologous recombination; yqhD, NADPH-dependent adh (Escherichia coli), Homologous recombination | Pcpc560 | NA | 0.59 in 14 d | [192] |
| Synechocystis sp. PCC6803 | pdc (Zymomonas mobilis), Shuttle vector; adh, slr1192 (Endogenous), Shuttle vector | PnrsB | NA | 0.45 in 7 d | [196] |
| pdc (Zymomonas mobilis), Shuttle vector; adh, slr1192 (Endogenous), Shuttle vector; rbcSC, slr0009-slr0011-slr0012-FLAG with RuBisCO-encoding genes (Endogenous), Shuttle vector | PnrsB (for pdc and adh) and psbA2 (for rbcSC, 70glpX, tktA, and fbaA) | 0.7 in 7 d | |||
| pdc (Zymomonas mobilis), Shuttle vector; adh, slr1192 (Endogenous), Shuttle vector; 70glpX with FBP/SBPase-encoding genes (Synechococcus PCC 7002), Shuttle vector | 0.75 in 7 d | ||||
| pdc (Zymomonas mobilis), Shuttle vector; adh, slr1192 (Endogenous), Shuttle vector; tktA, sll1070 with TK-encoding genes (Endogenous), Shuttle vector | 0.6 in 7 d | ||||
| pdc (Zymomonas mobilis), Shuttle vector; adh, slr1192 (Endogenous), Shuttle vector; fbaA, sll0018 with FBA-encoding genes (Endogenous), Shuttle vector | 0.75 in 7 d | ||||
| Synechocystis sp. PCC6803 | pdc (Zymomonas mobilis), Shuttle vector; adh, slr1192 (Endogenous), Shuttle vector; fbaA, sll0018 with FBA-encoding genes (Endogenous), Shuttle vector; tktA, sll1070 with TK-encoding genes (Endogenous), Shuttle vector | PnrsB (for pdc and adh) and psbA2 (for tktA and fbaA) | NA | 1.2 in 20 d | [195] |
| Synechocystis sp. PCC6803 (Fe2O3-treated culture) | pdc (Saccharomyces cerevisiae), Shuttle vector; adh (Endogenous), Shuttle vector | psbA1 | NA | 4.9 in 25 d | [198] |
| Synechocystis sp. PCC6803 (MgO-treated culture) | 5.1 in 25 d | ||||
| Microalgae Species | Pretreatment | Fermentation Microorganisms | Fermentation Operating Conditions | Bioethanol Concentration (g/L) and Yield (% of Dry Cell Weight) | References |
|---|---|---|---|---|---|
| Separate hydrolysis and fermentation | |||||
| Carotenoid-free Chromochloris zofingiensis SAG 211-14 | Autoclave (120 °C for 0.34 h) followed by two-stage enzymatic pretreatment with α-Amylase (90 °C, 2 h, 4.5 pH) and glucoamylase (60 °C, 22 h, 6.5 pH) | Saccharomyces cerevisiae CCUG 53310 | Initial pH: 4.8 Inoculum size: NA Temperature: 37 °C Time: | 25 ± 2% | [206] |
| Autoclave (120 °C for 0.34 h) followed by three-stage enzymatic pretreatment with Cellic Ctec2 and Cellic Htec2 (45 °C, 48 h, 5 pH), α-Amylase (90 °C, 2 h, 5 pH) and glucoamylase (60 °C, 22 h, 5 pH) | 62 ± 2% | ||||
| Carotenoid-free Haematococcus pluvialis SAG 192.80 | Autoclave (120 °C for 0.34 h) followed by two-stage enzymatic pretreatment with α-Amylase (90 °C, 2 h, 4.5 pH) and glucoamylase (60 °C, 22 h, 6.5 pH) | Saccharomyces cerevisiae CCUG 53310 | Initial pH: 4.8 Inoculum size: NA Temperature: 37 °C Time: | 35 ± 0.3% | [207] |
| Autoclave (120 °C for 0.34 h) followed by three-stage enzymatic pretreatment with Cellic Ctec2 and Cellic Htec2 (45 °C, 49 h, 5 pH), α-Amylase (90 °C, 2 h, 5 pH) and glucoamylase (60 °C, 22 h, 5 pH) | 88.1 ± 0.5% | ||||
| Chlorella vulgaris | 1 N HCl, 90 °C, 1 h | Saccharomyces cerevisiae | Initial pH: 5 Inoculum size: 3% v/v Temperature: 30 °C Time: | 46% | [205] |
| Chlorella vulgaris FSP-E | 2% H2SO4, 121 °C, 0.34 h | Saccharomyces cerevisiae FAY-1 | Initial pH: NA Inoculum size: NA Temperature: 30 °C | 21% (43 g/L) | [208] |
| Mixed microalgae consortium | 0.5 M H2SO4 and 2.5% (w/v) MgSO4 at 121 °C, 0.67 h | Saccharomyces cerevisiae ATCC 7921 | Initial pH: 6.5 Inoculum size: 3% v/v Temperature: 30 °C | 5 g/L | [200] |
| Three-stage enzymatic pretreatment with β-glucosidase/cellulase (65 °C, 3 h), α-amylase (95 °C, 3 h) and amyloglucosidase (55 °C, 3 h) | 6.4 g/L | ||||
| Arthrospira platensis NIES-39 | 1 g/L lysozyme and 100 mM CaCl2 | Saccharomyces cerevisiae strain BY4741 AASS/GASS | Initial pH: 5.2–5.4 Inoculum size: 5% v/v Temperature: 38–40 °C | 32% | [202] |
| Wastewater-grown microalgae biomass dominated by Microcystis | 0.5 N H2SO4, 120 °C, 4 h | Immobilized Saccharomyces cereviciae ATCC 4126 | Initial pH: 4.5 Inoculum size: 15% v/v Temperature: 30 °C | 19 g/L | [21] |
| Porphyridium cruentum KMMCC-1061 | One-stage enzymatic hydrolysis with pectinase and cellulase (37 °C, 7 h, 4.8 pH) | Saccharomyces cerevisiae KCTC 7906 | Initial pH: 4.5 Inoculum size: 0.1% w/v Temperature: 37 °C | 70–78% (based on initial glucose content) | [204] |
| Simultaneous Saccharification and Fermentation | |||||
| Chlorella vulgaris | Two-stage enzymatic pretreatment with α-Amylase (90 °C, 6 pH) and glucoamylase (80 °C, 5 h, 6 pH) | Saccharomyces cerevisiae | Initial pH: 5 Inoculum size: 3% v/v Temperature: 30 °C | 49% | [205] |
| Porphyridium cruentum KMMCC-1061 | One-stage enzymatic hydrolysis with pectinase and cellulase (37 °C, 10 h, 4.8 pH) | Saccharomyces cerevisiae KCTC 7906 | Initial pH: 4.5 Inoculum size: 0.1% w/v Temperature: 37 °C | 74–80% (based on initial glucose content) | [204] |
| Co-fermentation | |||||
| Dried and milled Chlorella vulgaris biomass powder Neoalgae® (Micro seaweed products B-52501749). | 3% H2SO4, 120 °C, 0.5 h | 75% Saccharomyces cerevisiae Cameo S.p.A. and 25% Pichia stipitis ATCC 58, 785 | Initial pH: 5–6 Inoculum size: 7.5 g/L Temperature: 30 °C | 49 ± 5% | [201] |
| One-stage enzymatic hydrolysis with Viscozyme® L, AMG 300 L, and Pectinex Ultra SP-L (50 °C, 4 h, 5 pH) | 49 ± 0.5% | ||||
| Scenedesmus obliquus SAG 276.7 | 3% H2SO4, 120 °C, 0.5 h | 87 ± 6% | |||
| Ultrasonication followed by One-stage enzymatic hydrolysis with Viscozyme® L, AMG 300 L, and Pectinex Ultra SP-L (50 °C, 8 h, 5 pH) | 41 ± 1.5% | ||||
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilanci, V.; Haouas, I.; Ozgur, O.; Sarkodie, S.A. Energy diversification and economic development in emergent countries: Evidence from fourier function-driven bootstrap panel causality test. Front. Energy Res. 2021, 9, 632712. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2022; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). International Energy Outlook 2021 Narrative; U.S. Department of Energy: Washington, DC, USA, 2021. [Google Scholar]
- Al-Bawwat, A.a.K.; Jurado, F.; Gomaa, M.R.; Cano, A. Availability and the possibility of employing wastes and biomass materials energy in Jordan. Sustainability 2023, 15, 5879. [Google Scholar] [CrossRef]
- Yadav, K.K.; Krishnan, S.; Gupta, N.; Prasad, S.; Amin, M.A.; Cabral-Pinto, M.M.S.; Sharma, G.K.; Marzouki, R.; Jeon, B.-H.; Kumar, S.; et al. Review on evaluation of renewable bioenergy potential for sustainable development: Bright future in energy practice in India. ACS Sustain. Chem. Eng. 2021, 9, 16007–16030. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, W.; Liu, L.; Ma, X. Biomass energy consumption and carbon neutrality in OECD countries: Testing pollution haven hypothesis and environmental Kuznets curve. Front. Environ. Sci. 2022, 10, 1691. [Google Scholar] [CrossRef]
- Duarah, P.; Haldar, D.; Patel, A.K.; Dong, C.-D.; Singhania, R.R.; Purkait, M.K. A review on global perspectives of sustainable development in bioenergy generation. Bioresour. Technol. 2022, 348, 126791. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawwat, A.a.K.; Cano, A.; Gomaa, M.R.; Jurado, F. Availability of biomass and potential of nanotechnologies for bioenergy production in Jordan. Processes 2023, 11, 992. [Google Scholar] [CrossRef]
- Maliha, A.; Abu-Hijleh, B. A review on the current status and post-pandemic prospects of third-generation biofuels. Energy Syst. 2022, 1–32. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent advances and viability in biofuel production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- International Energy Agency. Renewables 2022: Analysis and Forecast to 2027; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Ahmed, S.; Warne, T.; Smith, E.; Goemann, H.; Linse, G.; Greenwood, M.; Kedziora, J.; Sapp, M.; Kraner, D.; Roemer, K.; et al. Systematic review on effects of bioenergy from edible versus inedible feedstocks on food security. NPJ. Sci. Food 2021, 5, 9. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of microalgae in achieving sustainable development goals and circular economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef]
- Elalami, D.; Oukarroum, A.; Barakat, A. Anaerobic digestion and agronomic applications of microalgae for its sustainable valorization. RSC Adv. 2021, 11, 26444–26462. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saharia, M.; Srivstava, R.; Kumar, S.; Sahoo, L. Tailoring microalgae for efficient biofuel production. Front. Mar. Sci. 2018, 5, 382. [Google Scholar] [CrossRef]
- Parakh, S.K.; Praveen, P.; Loh, K.C.; Tong, Y.W. Integrating gravity settler with an algal membrane photobioreactor for in situ biomass concentration and harvesting. Bioresour. Technol. 2020, 315, 123822. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- El-Mekkawi, S.A.; Abdo, S.M.; Samhan, F.A.; Ali, G.H. Optimization of some fermentation conditions for bioethanol production from microalgae using response surface method. Bull. Natl. Res. Cent. 2019, 43, 164. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen production from biomass sources: Metabolic pathways and economic analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Mustapha, S.I.; Mohammed, U.A.; Rawat, I.; Bux, F.; Isa, Y.M. Production of high-quality pyrolytic bio-oils from nutrient-stressed Scenedesmus obliquus microalgae. Fuel 2023, 332, 126299. [Google Scholar] [CrossRef]
- Raheem, A.; Abbasi, S.A.; Mangi, F.H.; Ahmed, S.; He, Q.; Ding, L.; Memon, A.A.; Zhao, M.; Yu, G. Gasification of algal residue for synthesis gas production. Algal. Res. 2021, 58, 102411. [Google Scholar] [CrossRef]
- Shahi, T.; Beheshti, B.; Zenouzi, A.; Almasi, M. Bio-oil production from residual biomass of microalgae after lipid extraction: The case of Dunaliella sp. Biocatal. Agric. Biotechnol. 2020, 23, 101494. [Google Scholar] [CrossRef]
- Gomaa, M.R.; Mustafa, R.J.; Al-Dmour, N. Solar thermochemical conversion of carbonaceous materials into syngas by Co-Gasification. J. Cleaner Prod. 2020, 248, 119185. [Google Scholar] [CrossRef]
- Hossain, S.M.Z. Biochemical conversion of microalgae biomass into biofuel. Chem. Eng. Technol. 2019, 42, 2594–2607. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Ballesteros, M.; González-Fernandez, C. Efficient anaerobic digestion of microalgae biomass: Proteins as a key macromolecule. Molecules 2018, 23, 1098. [Google Scholar] [CrossRef]
- Maity, S.; Mallick, N. Trends and advances in sustainable bioethanol production by marine microalgae: A critical review. J. Cleaner Prod. 2022, 345, 131153. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Chew, K.W.; Selvarajoo, A.; Chen, W.-H.; Chang, J.-S.; Show, P.L. Microalgae: The future supply house of biohydrogen and biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Parakh, S.K.; Praveen, P.; Loh, K.-C.; Tong, Y.W. Wastewater treatment and microbial community dynamics in a sequencing batch reactor operating under photosynthetic aeration. Chemosphere 2019, 215, 893–903. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal. Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Parakh, S.K. Enhancing the Sustainability of Microalgae Production through Novel Photobioreactor Engineering and Harvesting Strategies; National University of Singapore: Singapore, 2019. [Google Scholar]
- Córdova, O.; Passos, F.; Chamy, R. Physical pretreatment methods for improving microalgae anaerobic biodegradability. Appl. Biochem. Biotechnol. 2018, 185, 114–126. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Bai, Y.; Usman, M.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Li, X.; Salama, E.-S.; Zhang, C. Highest accumulated microalgal lipids (polar and non-polar) for biodiesel production with advanced wastewater treatment: Role of lipidomics. Bioresour. Technol. 2020, 298, 122299. [Google Scholar] [CrossRef]
- Mimouni, V.; Couzinet-Mossion, A.; Ulmann, L.; Wielgosz-Collin, G. Chapter 5-Lipids from microalgae. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 109–131. [Google Scholar]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a feedstock for the production of biodiesel: A review. Bioresour. Technol. Rep. 2021, 15, 100771. [Google Scholar] [CrossRef]
- Bligh, M.; Nguyen, N.; Buck-Wiese, H.; Vidal-Melgosa, S.; Hehemann, J.-H. Structures and functions of algal glycans shape their capacity to sequester carbon in the ocean. Curr. Opin. Chem. Biol. 2022, 71, 102204. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2022, 1–28. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Severo Gonçalves, I.; Patrícia Held Azambuja, S.; Silva Costa, S.; Garcia Pereira Silva, P.; Oliveira Santos, L.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Heald, R.; Johnson, A.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 7th ed.; W. W. Norton & Company: New York, NY, USA, 2022. [Google Scholar]
- de Oliveira, M.C.; Bassin, I.D.; Cammarota, M.C. Microalgae and cyanobacteria biomass pretreatment methods: A comparative analysis of chemical and thermochemical pretreatment methods aimed at methane production. Fermentation 2022, 8, 497. [Google Scholar] [CrossRef]
- Shokravi, Z.; Shokravi, H.; Chyuan, O.H.; Lau, W.J.; Koloor, S.S.R.; Petrů, M.; Ismail, A.F. Improving ‘lipid productivity’ in microalgae by bilateral enhancement of biomass and lipid contents: A review. Sustainability 2020, 12, 9083. [Google Scholar] [CrossRef]
- Zaparoli, M.; Ziemniczak, F.G.; Mantovani, L.; Costa, J.A.V.; Colla, L.M. Cellular stress conditions as a strategy to increase carbohydrate productivity in Spirulina platensis. BioEnergy Res. 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, S.; Gayen, K.; Bhowmick, T.K. Effects of carbon, nitrogen, and phosphorus supplements on growth and biochemical composition of Podohedriella sp. (MCC44) isolated from northeast India. Environ. Prog. Sustain. Energy 2020, 39, e13378. [Google Scholar] [CrossRef]
- Bibi, F.; Yasmin, H.; Jamal, A.; Al-Harbi, M.S.; Ahmad, M.; Zafar, M.; Ahmad, B.; Samra, B.N.; Ahmed, A.F.; Ali, M.I. Deciphering role of technical bioprocess parameters for bioethanol production using microalgae. Saudi J. Biol. Sci. 2021, 28, 7595–7606. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.K.; Banerjee, I.; Singh, D.; Sajwan, P.; Chhetri, V. Ecological stress stimulus to improve microalgae biofuel generation: A review. Octa. J. Biosci. 2020, 8, 48–54. [Google Scholar]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Yun, C.-J.; Hwang, K.-O.; Han, S.-S.; Ri, H.-G. The effect of salinity stress on the biofuel production potential of freshwater microalgae Chlorella vulgaris YH703. Biomass Bioenergy 2019, 127, 105277. [Google Scholar] [CrossRef]
- Choudhary, S.; Tripathi, S.; Poluri, K.M. Microalgal-based bioenergy: Strategies, prospects, and sustainability. Energy Fuels 2022, 36, 14584–14612. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef]
- Muñoz, C.F.; Weusthuis, R.A.; D’Adamo, S.; Wijffels, R.H. Effect of single and combined expression of lysophosphatidic acid acyltransferase, glycerol-3-phosphate acyltransferase, and diacylglycerol acyltransferase on lipid accumulation and composition in Neochloris oleoabundans. Front. Plant Sci. 2019, 10, 1573. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: A review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-based biorefineries: Challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Paul, R.; Silkina, A.; Melville, L.; Suhartini, S.; Sulu, M. Optimization of ultrasound pretreatment of microalgal biomass for effective biogas production through anaerobic digestion process. Energies 2023, 16, 553. [Google Scholar] [CrossRef]
- Schmid, B.; Navalho, S.; Schulze, P.S.C.; Van De Walle, S.; Van Royen, G.; Schüler, L.M.; Maia, I.B.; Bastos, C.R.V.; Baune, M.-C.; Januschewski, E.; et al. Drying microalgae using an industrial solar dryer: A biomass quality assessment. Foods 2022, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Workie, E.; Kumar, V.; Bhatnagar, A.; He, Y.; Dai, Y.; Wah Tong, Y.; Peng, Y.; Zhang, J.; Fu, C. Advancing the bioconversion process of food waste into methane: A systematic review. Waste Manag. 2023, 156, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; El Haj Assad, M.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A critical review of biogas production and usage with legislations framework across the globe. Int. J. Environ. Sci. Technol. 2022, 19, 3377–3400. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.N.; Hilonga, A.; Machunda, R.L.; Njau, K.N. A review on strategies to optimize metabolic stages of anaerobic digestion of municipal solid wastes towards enhanced resources recovery. Sustain. Environ. Res. 2019, 29, 36. [Google Scholar] [CrossRef]
- Lim, J.W.; Park, T.; Tong, Y.W.; Yu, Z. Chapter One: The microbiome driving anaerobic digestion and microbial analysis. In Advances in Bioenergy; Li, Y., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 1–61. [Google Scholar]
- Zhang, L.; Yan, M.; Tsui, T.-H.; Lee, J.T.E.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Chapter 16-Functional microbial characteristics in acidogenic fermenters of organic wastes for production of volatile fatty acids. In Biomass, Biofuels, Biochemicals; Pandey, A., Tong, Y.W., Zhang, L., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 367–394. [Google Scholar]
- Detman, A.; Bucha, M.; Treu, L.; Chojnacka, A.; Pleśniak, Ł.; Salamon, A.; Łupikasza, E.; Gromadka, R.; Gawor, J.; Gromadka, A.; et al. Evaluation of acidogenesis products’ effect on biogas production performed with metagenomics and isotopic approaches. Biotechnol. Biofuels 2021, 14, 125. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Nguyen, P.-D.; Tran, N.-S.T.; Nguyen, T.-T.; Dang, B.-T.; Le, M.-T.T.; Bui, X.-T.; Mukai, F.; Kobayashi, H.; Ngo, H.H. Long-term operation of the pilot scale two-stage anaerobic digestion of municipal biowaste in Ho Chi Minh City. Sci. Total Environ. 2021, 766, 142562. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Sharma, P.; Tian, H.; Tsui, T.-H.; Lam, H.T.; Tong, Y.W. Startup performance and microbial communities of a decentralized anaerobic digestion of food waste. Chemosphere 2023, 318, 137937. [Google Scholar] [CrossRef]
- Paulo, L.M.; Castilla-Archilla, J.; Ramiro-Garcia, J.; Escamez-Picón, J.A.; Hughes, D.; Mahony, T.; Murray, M.; Wilmes, P.; O’Flaherty, V. Microbial community redundancy and resilience underpins high-rate anaerobic treatment of dairy-processing wastewater at ambient temperatures. Front. Bioeng. Biotechnol. 2020, 8, 192. [Google Scholar] [CrossRef]
- Heitkamp, K.; Latorre-Pérez, A.; Nefigmann, S.; Gimeno-Valero, H.; Vilanova, C.; Jahmad, E.; Abendroth, C. Monitoring of seven industrial anaerobic digesters supplied with biochar. Biotechnol. Biofuels 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of innovative processing methods on microalgae cell wall: Prospects towards digestibility of protein-rich biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Dunker, S.; Wilhelm, C. Cell wall structure of coccoid green algae as an important trade-off between biotic interference mechanisms and multidimensional cell growth. Front. Microbiol. 2018, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ras, M.; Lardon, L.; Bruno, S.; Bernet, N.; Steyer, J.-P. Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef]
- Tg, I.; Haq, I.; Kalamdhad, A.S. 14-Factors affecting anaerobic digestion for biogas production: A review. In Advanced Organic Waste Management; Hussain, C., Hait, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 223–233. [Google Scholar]
- Shahbaz, M.; Ammar, M.; Korai, R.M.; Ahmad, N.; Ali, A.; Khalid, M.S.; Zou, D.; Li, X. Impact of C/N ratios and organic loading rates of paper, cardboard and tissue wastes in batch and CSTR anaerobic digestion with food waste on their biogas production and digester stability. SN Appl. Sci. 2020, 2, 1436. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Passos, F.; Romero-Güiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Kendir, E.; Ugurlu, A. A comprehensive review on pretreatment of microalgae for biogas production. Int. J. Energy Res. 2018, 42, 3711–3731. [Google Scholar] [CrossRef]
- Agarwalla, A.; Komandur, J.; Mohanty, K. Current trends in the pretreatment of microalgal biomass for efficient and enhanced bioenergy production. Bioresour. Technol. 2023, 369, 128330. [Google Scholar] [CrossRef]
- Rokicka, M.; Zieliński, M.; Dudek, M.; Dębowski, M. Effects of ultrasonic and microwave pretreatment on lipid extraction of microalgae and methane production from the residual extracted biomass. BioEnergy Res. 2021, 14, 752–760. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Świca, I.; Zieliński, M. Ultrasonic disintegration to improve anaerobic digestion of microalgae with hard cell walls-Scenedesmus sp. and Pinnularia sp. Plants 2023, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, A.; Rodriguez, C.; Durrant, A. Sustainable approaches to microalgal pre-treatment techniques for biodiesel production: A review. Sustainability 2022, 14, 9953. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Gruber-Brunhumer, M.R.; Jerney, J.; Zohar, E.; Nussbaumer, M.; Hieger, C.; Bochmann, G.; Schagerl, M.; Obbard, J.P.; Fuchs, W.; Drosg, B. Acutodesmus obliquus as a benchmark strain for evaluating methane production from microalgae: Influence of different storage and pretreatment methods on biogas yield. Algal Res. 2015, 12, 230–238. [Google Scholar] [CrossRef]
- Straessner, R.; Nikolausz, M.; Silve, A.; Nazarova, N.; Wuestner, R.; Papachristou, I.; Akaberi, S.; Leber, K.; Mueller, G.; Frey, W. Holistic exploitation of pulsed electric field (PEF)-treated and lipid extracted microalgae Auxenochlorella protothecoides, utilizing anaerobic digestion (AD). Algal Res. 2023, 69, 102950. [Google Scholar] [CrossRef]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal pretreatment of microalgae for biomethane production: Experimental studies, kinetics and energy analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Arcelus-Arrillaga, P.; Millan, M.; Arancibia-Bulnes, C.A.; Valadés-Pelayo, P.J.; Villafán-Vidales, H.I. Solar integrated hydrothermal processes: A review. Renew. Sustain. Energy Rev. 2021, 139, 110575. [Google Scholar] [CrossRef]
- Xiao, C.; Liao, Q.; Fu, Q.; Huang, Y.; Chen, H.; Zhang, H.; Xia, A.; Zhu, X.; Reungsang, A.; Liu, Z. A solar-driven continuous hydrothermal pretreatment system for biomethane production from microalgae biomass. Appl. Energy 2019, 236, 1011–1018. [Google Scholar] [CrossRef]
- Xiao, C.; Liao, Q.; Fu, Q.; Huang, Y.; Xia, A.; Shen, W.; Chen, H.; Zhu, X. Exergy analyses of biogas production from microalgae biomass via anaerobic digestion. Bioresour. Technol. 2019, 289, 121709. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam explosion pretreatment of lignocellulosic biomass: A mini-review of theoretical and experimental approaches. Front. Chem. 2021, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Martín Juárez, J.; Riol Pastor, E.; Fernández Sevilla, J.M.; Muñoz Torre, R.; García-Encina, P.A.; Bolado Rodríguez, S. Effect of pretreatments on biogas production from microalgae biomass grown in pig manure treatment plants. Bioresour. Technol. 2018, 257, 30–38. [Google Scholar] [CrossRef]
- Marques, A.d.L.; Pinto, F.P.; Araujo, O.Q.d.F.; Cammarota, M.C. Assessment of methods to pretreat microalgal biomass for enhanced biogas production. J. Sustain. Dev. Energy Water Environ. Syst. 2018, 6, 394–404. [Google Scholar] [CrossRef]
- Bai, X.; Lant, P.A.; Jensen, P.D.; Astals, S.; Pratt, S. Enhanced methane production from algal digestion using free nitrous acid pre-treatment. Renew. Energy 2016, 88, 383–390. [Google Scholar] [CrossRef]
- Fu, J.; Yan, B.; Gui, S.; Fu, Y.; Xia, S. Anaerobic co-digestion of thermo-alkaline pretreated microalgae and sewage sludge: Methane potential and microbial community. J. Environ. Sci. 2023, 127, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Carrère, H.; Garfí, M.; Ferrer, I. Enhancement of microalgae anaerobic digestion by thermo-alkaline pretreatment with lime (CaO). Algal Res. 2017, 24, 199–206. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Olkiewicz, M.; Pruvost, J.; Lepine, O.; Legrand, J.; Font, J.; Bengoa, C. A novel pre-treatment for the methane production from microalgae by using N-methylmorpholine-N-oxide (NMMO). Bioresour. Technol. 2016, 201, 370–373. [Google Scholar] [CrossRef]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Song, K.; Li, Z.; Li, L.; Zhao, X.; Deng, M.; Zhou, X.; Xu, Y.; Peng, L.; Li, R.; Wang, Q. Methane production from peroxymonosulfate pretreated algae biomass: Insights into microbial mechanisms, microcystin detoxification and heavy metal partitioning behavior. Sci. Total Environ. 2022, 834, 155500. [Google Scholar] [CrossRef]
- Cardeña, R.; Moreno, G.; Bakonyi, P.; Buitrón, G. Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem. Eng. J. 2017, 307, 948–954. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Song, K.; Gu, Y.; Gao, X. Improving methane production from algal sludge based anaerobic digestion by co-pretreatment with ultrasound and zero-valent iron. J. Cleaner Prod. 2020, 255, 120214. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, S.; Gao, L.; Zhou, X.; Wang, D.; Song, K.; Nghiem, L.D. Free ammonia pretreatment improves anaerobic methane generation from algae. Water Res. 2019, 162, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kendir Çakmak, E.; Ugurlu, A. Enhanced biogas production of red microalgae via enzymatic pretreatment and preliminary economic assessment. Algal Res. 2020, 50, 101979. [Google Scholar] [CrossRef]
- Zhang, Y.; Caldwell, G.S.; Sallis, P.J. Renewable energy: Evaluation of low energy demand pre-treatments to optimise methane production from microalgae. IET Renew. Power Gener. 2019, 13, 1701–1710. [Google Scholar] [CrossRef]
- Sakhuja, D.; Ghai, H.; Rathour, R.K.; Kumar, P.; Bhatt, A.K.; Bhatia, R.K. Cost-effective production of biocatalysts using inexpensive plant biomass: A review. 3 Biotech 2021, 11, 280. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Passos, F.; Ferrer, I.; Vicent, T.; Blánquez, P. Enzymatic pretreatment of microalgae using fungal broth from Trametes versicolor and commercial laccase for improved biogas production. Algal Res. 2016, 19, 184–188. [Google Scholar] [CrossRef]
- Kavitha, S.; Yukesh Kannah, R.; Rajesh Banu, J.; Kaliappan, S.; Johnson, M. Biological disintegration of microalgae for biomethane recovery-prediction of biodegradability and computation of energy balance. Bioresour. Technol. 2017, 244, 1367–1375. [Google Scholar] [CrossRef]
- Aydin, S.; Yıldırım, E.; Ince, O.; Ince, B. Rumen anaerobic fungi create new opportunities for enhanced methane production from microalgae biomass. Algal Res. 2017, 23, 150–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Caldwell, G.S.; Blythe, P.T.; Zealand, A.M.; Li, S.; Edwards, S.; Xing, J.; Goodman, P.; Whitworth, P.; Sallis, P.J. Co-digestion of microalgae with potato processing waste and glycerol: Effect of glycerol addition on methane production and the microbial community. RSC Adv. 2020, 10, 37391. [Google Scholar] [CrossRef] [PubMed]
- Wágner, D.S.; Radovici, M.; Smets, B.F.; Angelidaki, I.; Valverde-Pérez, B.; Plósz, B.G. Harvesting microalgae using activated sludge can decrease polymer dosing and enhance methane production via co-digestion in a bacterial-microalgal process. Algal Res 2016, 20, 197–204. [Google Scholar] [CrossRef]
- Garoma, T.; Nguyen, D. Anaerobic co-digestion of microalgae Scenedesmus sp. and TWAS for biomethane Production. Water Environ. Res 2016, 88, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Salvadó, H.; Passos, F.; Garfí, M.; Ferrer, I. Strategies to optimize microalgae conversion to biogas: Co-digestion, pretreatment and hydraulic retention time. Molecules 2018, 23, 2096. [Google Scholar] [CrossRef]
- Ferreira, L.O.; Astals, S.; Passos, F. Anaerobic co-digestion of food waste and microalgae in an integrated treatment plant. J. Chem. Technol. Biotechnol. 2022, 97, 1545–1554. [Google Scholar] [CrossRef]
- Panyaping, K.; Khiewwijit, R.; Wongpankamol, P. Enhanced biogas production potential of microalgae and swine wastewater using co-digestion and alkaline pretreatment. Water Sci. Technol. 2018, 78, 92–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Caldwell, G.S.; Zealand, A.M.; Sallis, P.J. Anaerobic co-digestion of microalgae Chlorella vulgaris and potato processing waste: Effect of mixing ratio, waste type and substrate to inoculum ratio. Biochem. Eng. J. 2019, 143, 91–100. [Google Scholar] [CrossRef]
- Carminati, P.; Gusmini, D.; Pizzera, A.; Catenacci, A.; Parati, K.; Ficara, E. Biogas from mono- and co-digestion of microalgal biomass grown on piggery wastewater. Water Sci. Technol. 2018, 78, 103–113. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Garfí, M.; Ferrer, I. Pretreatment and co-digestion of microalgae, sludge and fat oil and grease (FOG) from microalgae-based wastewater treatment plants. Bioresour. Technol. 2020, 298, 122563. [Google Scholar] [CrossRef]
- Vassalle, L.; Passos, F.; Rosa-Machado, A.T.; Moreira, C.; Reis, M.; Pascoal de Freitas, M.; Ferrer, I.; Mota, C.R. The use of solar pre-treatment as a strategy to improve the anaerobic biodegradability of microalgal biomass in co-digestion with sewage. Chemosphere 2022, 286, 131929. [Google Scholar] [CrossRef]
- Mahdy, A.; Fotidis, I.A.; Mancini, E.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Ammonia tolerant inocula provide a good base for anaerobic digestion of microalgae in third generation biogas process. Bioresour. Technol. 2017, 225, 272–278. [Google Scholar] [CrossRef]
- Avila, R.; Carrero, E.; Vicent, T.; Blánquez, P. Integration of enzymatic pretreatment and sludge co-digestion in biogas production from microalgae. Waste Manag. 2021, 124, 254–263. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Alvarado-Morales, M.; Kovalovszki, A.; Corbière, M.; Angelidaki, I. Energy recovery from wastewater microalgae through anaerobic digestion process: Methane potential, continuous reactor operation and modelling aspects. Biochem. Eng. J. 2018, 139, 1–7. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chang, J.-S.; Lee, D.-J. Pretreatment of microalgal biomass for efficient biohydrogen production–Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef]
- Anwar, M.; Lou, S.; Chen, L.; Li, H.; Hu, Z. Recent advancement and strategy on bio-hydrogen production from photosynthetic microalgae. Bioresour. Technol. 2019, 292, 121972. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen production from microalgae for environmental sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Faraloni, C.; Benavides, A.M.S.; Torzillo, G. Recent achievements in microalgal photobiological hydrogen production. Energies 2021, 14, 7170. [Google Scholar] [CrossRef]
- Jiménez-Llanos, J.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Sustainable biohydrogen production by Chlorella sp. microalgae: A review. Int. J. Hydrog. Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Show, K.Y.; Yan, Y.; Zong, C.; Guo, N.; Chang, J.S.; Lee, D.J. State of the art and challenges of biohydrogen from microalgae. Bioresour. Technol. 2019, 289, 121747. [Google Scholar] [CrossRef]
- Gaffron, H.; Rubin, J. Fermentative and photochemical production of hydrogen In algae. J. Gen. Physiol. 1942, 26, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Dong, C.-D.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Biohydrogen production from microalgae—Major bottlenecks and future research perspectives. Biotechnol. J. 2021, 16, 2000124. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, M.L.; Togasaki, R.K.; Seibert, M. Oxygen sensitivity of algal H2- production. Appl. Biochem. Biotechnol. 1997, 63–65, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.; Greenbaum, E. Long-term endurance and selection studies in hydrogen and oxygen photoproduction by Chlamydomonas reinhardtii. Enzym. Microb. Technol. 1985, 7, 169–174. [Google Scholar] [CrossRef]
- Hamed, S.M.; Raut, M.P.; Jaffé, S.R.P.; Wright, P.C. Evaluation of the effect of aerobic–anaerobic conditions on photohydrogen and chlorophyll a production by environmental Egyptian cyanobacterial and green algal species. Int. J. Hydrog. Energy 2017, 42, 6567–6577. [Google Scholar] [CrossRef]
- Maswanna, T.; Lindblad, P.; Maneeruttanarungroj, C. Improved biohydrogen production by immobilized cells of the green alga Tetraspora sp. CU2551 incubated under aerobic condition. J. Appl. Phycol. 2020, 32, 2937–2945. [Google Scholar] [CrossRef]
- Maswanna, T.; Phunpruch, S.; Lindblad, P.; Maneeruttanarungroj, C. Enhanced hydrogen production by optimization of immobilized cells of the green alga Tetraspora sp. CU2551 grown under anaerobic condition. Biomass Bioenergy 2018, 111, 88–95. [Google Scholar] [CrossRef]
- Pow, T.; Krasna, A.I. Photoproduction of hydrogen from water in hydrogenase-containing algae. Arch. Biochem. Biophys. 1979, 194, 413–421. [Google Scholar] [CrossRef]
- Paramesh, K.; Chandrasekhar, T. Improvement of photobiological hydrogen production in Chlorococcum minutum using various oxygen scavengers. Int. J. Hydrog. Energy 2020, 45, 7641–7646. [Google Scholar] [CrossRef]
- Márquez-Reyes, L.A.; Sánchez-Saavedra, M.d.P.; Valdez-Vazquez, I. Improvement of hydrogen production by reduction of the photosynthetic oxygen in microalgae cultures of Chlamydomonas gloeopara and Scenedesmus obliquus. Int. J. Hydrog. Energy 2015, 40, 7291–7300. [Google Scholar] [CrossRef]
- Sung, Y.J.; Yu, B.S.; Yang, H.E.; Kim, D.H.; Lee, J.Y.; Sim, S.J. Microalgae-derived hydrogen production towards low carbon emissions via large-scale outdoor systems. Bioresour. Technol. 2022, 364, 128134. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.R.; Santos, P.V.d.; Giraldi, L.A.; Zaiat, M.; Calijuri, M.d.C. Anaerobic phototrophic processes of hydrogen production by different strains of microalgae Chlamydomonas sp. FEMS Microbiol. Lett. 2018, 365, fny073. [Google Scholar] [CrossRef] [PubMed]
- Danial, A.W.; Abdel-Basset, R.; Abdel-Kader, H.A.A. Tuning photosynthetic oxygen for hydrogen evolution in synergistically integrated, sulfur deprived consortia of Coccomyxa chodatii and Rhodobium gokarnense at dim and high light. Photosynth. Res. 2023, 155, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Pongpadung, P.; Zhang, L.; Sathasivam, R.; Yokthongwattana, K.; Niran, J.; Jianguo, L. Stimulation of hydrogen photoproduction in Chlorella sorokiniana subjected to simultaneous nitrogen limitation and sulfur- and/or phosphorus-deprivation. J. Pure. Appl. Microbiol. 2018, 12, 1719–1727. [Google Scholar] [CrossRef]
- Batyrova, K.; Gavrisheva, A.; Ivanova, E.; Liu, J.; Tsygankov, A. Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp. Int. J. Mol. Sci. 2015, 16, 2705–2716. [Google Scholar] [CrossRef]
- Jeong, S.; Naidu, G.; Leiknes, T.; Vigneswaran, S. 4.3 Membrane biofouling: Biofouling assessment and reduction strategies in seawater reverse osmosis desalination. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; pp. 48–71. [Google Scholar]
- Hamed, S.M.; Kapoore, R.V.; Raut, M.P.; Vaidyanathan, S.; Wright, P.C. Influence of nutrient status on the biohydrogen and lipid productivity in Parachlorella kessleri: A biorefinery approach. Appl. Microbiol. Biotechnol. 2020, 104, 10293–10305. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Liu, J. Proteomic analysis of hydrogen production in Chlorella pyrenoidosa under nitrogen deprivation. Algal Res. 2021, 53, 102143. [Google Scholar] [CrossRef]
- Volgusheva, A.A.; Jokel, M.; Allahverdiyeva, Y.; Kukarskikh, G.P.; Lukashev, E.P.; Lambreva, M.D.; Krendeleva, T.E.; Antal, T.K. Comparative analyses of H2 photoproduction in magnesium- and sulfur-starved Chlamydomonas reinhardtii cultures. Physiol. Plant. 2017, 161, 124–137. [Google Scholar] [CrossRef]
- Pewnual, T.; Jampapetch, N.; Saladtook, S.; Raksajit, W.; Klinsalee, R.; Maneeruttanarungroj, C. Response of green alga Tetraspora sp. CU2551 under potassium deprivation: A new promising strategy for hydrogen production. J. Appl. Phycol. 2022, 34, 811–819. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Ge, Y.-M.; Sun, J.-Y.; Chen, P.; Addy, M.; Huo, S.-H.; Li, K.; Cheng, P.-F.; Ruan, R. Exogenic glucose as an electron donor for algal hydrogenases to promote hydrogen photoproduction by Chlorella pyrenoidosa. Bioresour. Technol. 2019, 289, 121762. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Canedo-López, Y.; Chávez-Fuentes, P. Biohydrogen production by Chlorella vulgaris and Scenedesmus obliquus immobilized cultivated in artificial wastewater under different light quality. AMB Expr. 2020, 10, 191. [Google Scholar] [CrossRef]
- Lacroux, J.; Seira, J.; Trably, E.; Bernet, N.; Steyer, J.-P.; van Lis, R. Mixotrophic growth of Chlorella sorokiniana on acetate and butyrate: Interplay between substrate, C:N ratio and pH. Front. Microbiol. 2021, 12, 703614. [Google Scholar] [CrossRef] [PubMed]
- Alalayah, W.M.; Al-Zahrani, A.; Edris, G.; Demirbas, A. Kinetics of biological hydrogen production from green microalgae Chlorella vulgaris using glucose as initial substrate. Energy Sources Part A 2017, 39, 1210–1215. [Google Scholar] [CrossRef]
- Sirawattanamongkol, T.; Maswanna, T.; Maneeruttanarungroj, C. A newly isolated green alga Chlorella sp. KLSc59: Potential for biohydrogen production. J. Appl. Phycol. 2020, 32, 2927–2936. [Google Scholar] [CrossRef]
- Sengmee, D.; Cheirsilp, B.; Suksaroge, T.T.; Prasertsan, P. Biophotolysis-based hydrogen and lipid production by oleaginous microalgae using crude glycerol as exogenous carbon source. Int. J. Hydrog. Energy 2017, 42, 1970–1976. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Quattrocelli, P.; Nowicka, A. The cultivation of biohydrogen-producing Tetraselmis subcordiformis microalgae as the third stage of dairy wastewater aerobic treatment system. Sustainability 2022, 14, 12085. [Google Scholar] [CrossRef]
- Khosravitabar, F. Microalgal biohydrogen photoproduction: Scaling up challenges and the ways forward. J. Appl. Phycol. 2020, 32, 277–289. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Lee, M.; Kang, E.H.; Lee, W.H. Renewable algal photo H2 production without S control using acetate enriched fermenter effluents. Int. J. Hydrog. Energy 2021, 46, 1740–1751. [Google Scholar] [CrossRef]
- Kosourov, S.; Jokel, M.; Aro, E.-M.; Allahverdiyeva, Y. A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii. Energy Environ. Sci. 2018, 11, 1431–1436. [Google Scholar] [CrossRef]
- Fakhimi, N.; Gonzalez-Ballester, D.; Fernández, E.; Galván, A.; Dubini, A. Algae-bacteria consortia as a strategy to enhance H2 production. Cells 2020, 9, 1353. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Wu, F.; Luo, J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018, 251, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Boboescu, I.Z.; Pap, B.; Wirth, R.; Kovács, K.L.; Bíró, T.; Futó, Z.; White, R.A.; Maróti, G. Exploitation of algal-bacterial consortia in combined biohydrogen generation and wastewater treatment. Front. Energy Res. 2019, 7, 52. [Google Scholar] [CrossRef]
- Fakhimi, N.; Tavakoli, O. Improving hydrogen production using co-cultivation of bacteria with Chlamydomonas reinhardtii microalga. Mater. Sci. Energy Technol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Liu, P.; Ye, D.M.; Chen, M.; Zhang, J.; Huang, X.H.; Shen, L.L.; Xia, K.K.; Xu, X.J.; Xu, Y.C.; Guo, Y.L.; et al. Scaling-up and proteomic analysis reveals photosynthetic and metabolic insights toward prolonged H(2) photoproduction in Chlamydomonas hpm91 mutant lacking proton gradient regulation 5 (PGR5). Photosynth. Res. 2022, 154, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.S.; Styring, S.; Mamedov, F. Photosystem ratio imbalance promotes direct sustainable H2 production in Chlamydomonas reinhardtii. Green Chem. 2019, 21, 4683–4690. [Google Scholar] [CrossRef]
- Yang, D.-W.; Syn, J.-W.; Hsieh, C.-H.; Huang, C.-C.; Chien, L.-F. Genetically engineered hydrogenases promote biophotocatalysis-mediated H2 production in the green alga Chlorella sp. DT. Int. J. Hydrog. Energy 2019, 44, 2533–2545. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Luo, Z.; Luo, J. Improving hydrogen production of Chlamydomonas reinhardtii by reducing chlorophyll content via atmospheric and room temperature plasma. Bioresour. Technol. 2019, 275, 425–429. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Chen, M.; Zhuang, X.; Wang, C.; Wang, J.; Hu, Z. Improved photobio-H2 production regulated by artificial miRNA targeting psbA in green microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2018, 11, 36. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; He, Y.; Yuan, J. Photo-fermentative hydrogen production performance of a newly isolated Rubrivivax gelatinosus YP03 strain with acid tolerance. Int. J. Hydrog. Energy 2022, 47, 20784–20792. [Google Scholar] [CrossRef]
- Arimbrathodi, S.P.; Javed, M.A.; Hamouda, M.A.; Aly Hassan, A.; Ahmed, M.E. BioH2 production using microalgae: Highlights on recent advancements from a bibliometric analysis. Water 2023, 15, 185. [Google Scholar] [CrossRef]
- Phanduang, O.; Lunprom, S.; Salakkam, A.; Liao, Q.; Reungsang, A. Improvement in energy recovery from Chlorella sp. biomass by integrated dark-photo biohydrogen production and dark fermentation-anaerobic digestion processes. Int. J. Hydrog. Energy 2019, 44, 23899–23911. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Lu, H.; Yue, L.; Zhou, J.; Cen, K. Three-stage gaseous biofuel production combining dark hydrogen, photo hydrogen, and methane fermentation using wet Arthrospira platensis cultivated under high CO2 and sodium stress. Energy Convers. Manag. 2017, 148, 394–404. [Google Scholar] [CrossRef]
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Improvement of hydrogen production from Chlorella sp. biomass by acid-thermal pretreatment. PeerJ 2019, 7, e6637. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Liao, Q.; Fu, Q.; Liu, Z. Enhanced biohydrogen and biomethane production from Chlorella sp. with hydrothermal treatment. Energy Convers. Manag. 2020, 205, 112373. [Google Scholar] [CrossRef]
- Usmanbaha, N.; Jariyaboon, R.; Reungsang, A.; Kongjan, P.; Chu, C.-Y. Optimization of batch dark fermentation of Chlorella sp. using mixed-cultures for simultaneous hydrogen and butyric acid production. Energies 2019, 12, 2529. [Google Scholar] [CrossRef]
- Singh, H.; Rout, S.; Das, D. Dark fermentative biohydrogen production using pretreated Scenedesmus obliquus biomass under an integrated paradigm of biorefinery. Int. J. Hydrog. Energy 2022, 47, 102–116. [Google Scholar] [CrossRef]
- Rincón-Pérez, J.; Razo-Flores, E.; Morales, M.; Alatriste-Mondragón, F.; Celis, L.B. Improving the biodegradability of Scenedesmus obtusiusculus by thermochemical pretreatment to produce hydrogen and methane. BioEnergy Res. 2020, 13, 477–486. [Google Scholar] [CrossRef]
- Cheng, J.; Yue, L.; Ding, L.; Li, Y.-Y.; Ye, Q.; Zhou, J.; Cen, K.; Lin, R. Improving fermentative hydrogen and methane production from an algal bloom through hydrothermal/steam acid pretreatment. Int. J. Hydrog. Energy 2019, 44, 5812–5820. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, Y.; Liu, S.; Wang, D.; Liu, R.; Zhou, X.; Nghiem, L.D.; Zhao, Y. Free ammonia pretreatment to improve bio-hydrogen production from anaerobic dark fermentation of microalgae. ACS Sustain. Chem. Eng. 2019, 7, 1642–1647. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Hirayama, S.; Ueda, R.; Ogushi, Y.; Hirano, A.; Samejima, Y.; Hon-Nami, K.; Kunito, S. Ethanol production from carbon dioxide by fermentative microalgae. In Studies in Surface Science and Catalysis; Inui, T., Anpo, M., Izui, K., Yanagida, S., Yamaguchi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 114, pp. 657–660. [Google Scholar]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Gundolf, R.; Oberleitner, S.; Richter, J. Evaluation of new genetic toolkits and their role for ethanol production in cyanobacteria. Energies 2019, 12, 3515. [Google Scholar] [CrossRef]
- Mund, N.K.; Liu, Y.; Chen, S. Advances in metabolic engineering of cyanobacteria for production of biofuels. Fuel 2022, 322, 124117. [Google Scholar] [CrossRef]
- Kopka, J.; Schmidt, S.; Dethloff, F.; Pade, N.; Berendt, S.; Schottkowski, M.; Martin, N.; Dühring, U.; Kuchmina, E.; Enke, H.; et al. Systems analysis of ethanol production in the genetically engineered cyanobacterium Synechococcus sp. PCC 7002. Biotechnol. Biofuels 2017, 10, 56. [Google Scholar] [CrossRef]
- Chou, H.-H.; Su, H.-Y.; Chow, T.-J.; Lee, T.-M.; Cheng, W.-H.; Chang, J.-S.; Chen, H.-J. Engineering cyanobacteria with enhanced growth in simulated flue gases for high-yield bioethanol production. Biochem. Eng. J. 2021, 165, 107823. [Google Scholar] [CrossRef]
- Deng, M.-D.; Coleman John, R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef]
- Dexter, J.; Fu, P. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2009, 2, 857–864. [Google Scholar] [CrossRef]
- Choi, Y.-N.; Park, J.M. Enhancing biomass and ethanol production by increasing NADPH production in Synechocystis sp. PCC 6803. Bioresour. Technol. 2016, 213, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, T.; Passarinho, P.C.; Galriça, R.; Zenóglio, A.; Armshaw, P.; Pembroke, J.T.; Sheahan, C.; Reis, A.; Gírio, F. Evaluation of the ethanol tolerance for wild and mutant Synechocystis strains by flow cytometry. Biotechnol. Rep. 2018, 17, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Namakoshi, K.; Nakajima, T.; Yoshikawa, K.; Toya, Y.; Shimizu, H. Combinatorial deletions of glgC and phaCE enhance ethanol production in Synechocystis sp. PCC 6803. J. Biotechnol. 2016, 239, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Roussou, S.; Albergati, A.; Liang, F.; Lindblad, P. Engineered cyanobacteria with additional overexpression of selected Calvin-Benson-Bassham enzymes show further increased ethanol production. Metab. Eng. Commun. 2021, 12, e00161. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Englund, E.; Lindberg, P.; Lindblad, P. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio. Metab. Eng. 2018, 46, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Metal oxide mediated extracellular NADPH regeneration improves ethanol production by engineered Synechocystis sp. PCC 6803. Front. Bioeng. Biotechnol. 2019, 7, 148. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. Bioethanol production from acidic and enzymatic hydrolysates of mixed microalgae culture. Fuel 2017, 200, 380–386. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Meneghello, D.; Bertucco, A. A systematic study regarding hydrolysis and ethanol fermentation from microalgal biomass. Biocatal. Agric. Biotechnol. 2018, 14, 172–182. [Google Scholar] [CrossRef]
- Aikawa, S.; Inokuma, K.; Wakai, S.; Sasaki, K.; Ogino, C.; Chang, J.S.; Hasunuma, T.; Kondo, A. Direct and highly productive conversion of cyanobacteria Arthrospira platensis to ethanol with CaCl2 addition. Biotechnol. Biofuels 2018, 11, 50. [Google Scholar] [CrossRef]
- Phwan, C.K.; Ong, H.C.; Chen, W.H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Kim, H.M.; Oh, C.H.; Bae, H.J. Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production. Bioresour. Technol. 2017, 233, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Megawati, M.; Bahlawan, Z.A.S.; Damayanti, A.; Putri, R.D.A.; Triwibowo, B.; Prasetiawan, H. Comparative study on the various hydrolysis and fermentation methods of Chlorella vulgaris biomass for the production of bioethanol. Int. J. Renew. Energy Dev. 2022, 11, 515–522. [Google Scholar] [CrossRef]
- Mirzaei, D.; Jazini, M.; Rahimi, M.; Mahdieh, M.; Karimi, K. Production of astaxanthin, ethanol and methane from Chromochloris zofingiensis microalga in an integrated biorefinery. Algal Res. 2022, 68, 102905. [Google Scholar] [CrossRef]
- Hosseini, A.; Jazini, M.; Mahdieh, M.; Karimi, K. Efficient superantioxidant and biofuel production from microalga Haematococcus pluvialis via a biorefinery approach. Bioresour. Technol. 2020, 306, 123100. [Google Scholar] [CrossRef] [PubMed]
- Condor, B.E.; de Luna, M.D.G.; Chang, Y.-H.; Chen, J.-H.; Leong, Y.K.; Chen, P.-T.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Bioethanol production from microalgae biomass at high-solids loadings. Bioresour. Technol. 2022, 363, 128002. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to produce cost-effective third-generation biofuel from microalgae. Front. Energy Res. 2021, 9, 749968. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal biorefinery concepts’ developments for biofuel and bioproducts: Current perspective and bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef] [PubMed]
- Mahant, B.; Linga, P.; Kumar, R. Hydrogen economy and role of hythane as a bridging solution: A perspective review. Energy Fuels 2021, 35, 15424–15454. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
- Nazarpour, M.; Taghizadeh-Alisaraei, A.; Asghari, A.; Abbaszadeh-Mayvan, A.; Tatari, A. Optimization of biohydrogen production from microalgae by response surface methodology (RSM). Energy 2022, 253, 124059. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Xia, A.; Shah, A.A.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. The role of machine learning to boost the bioenergy and biofuels conversion. Bioresour. Technol. 2022, 343, 126099. [Google Scholar] [CrossRef]
- Ahmad Sobri, M.Z.; Redhwan, A.; Ameen, F.; Lim, J.W.; Liew, C.S.; Mong, G.R.; Daud, H.; Sokkalingam, R.; Ho, C.-D.; Usman, A.; et al. A review unveiling various machine learning algorithms adopted for biohydrogen productions from microalgae. Fermentation 2023, 9, 243. [Google Scholar] [CrossRef]
- Ullah, H.; Haq, Z.U.; Naqvi, S.R.; Khan, M.N.A.; Ahsan, M.; Wang, J. Optimization based comparative study of machine learning methods for the prediction of bio-oil produced from microalgae via pyrolysis. J. Anal. Appl. Pyrolysis 2023, 170, 105879. [Google Scholar] [CrossRef]
- Mutlu, A.Y.; Yucel, O. An artificial intelligence based approach to predicting syngas composition for downdraft biomass gasification. Energy 2018, 165, 895–901. [Google Scholar] [CrossRef]
- Shafizadeh, A.; Shahbeig, H.; Nadian, M.H.; Mobli, H.; Dowlati, M.; Gupta, V.K.; Peng, W.; Lam, S.S.; Tabatabaei, M.; Aghbashlo, M. Machine learning predicts and optimizes hydrothermal liquefaction of biomass. Chem. Eng. J. 2022, 445, 136579. [Google Scholar] [CrossRef]
- Salameh, T.; Sayed, E.T.; Olabi, A.G.; Hdaib, I.I.; Allan, Y.; Alkasrawi, M.; Abdelkareem, M.A. Adaptive network fuzzy inference system and particle swarm optimization of biohydrogen production process. Fermentation 2022, 8, 483. [Google Scholar] [CrossRef]
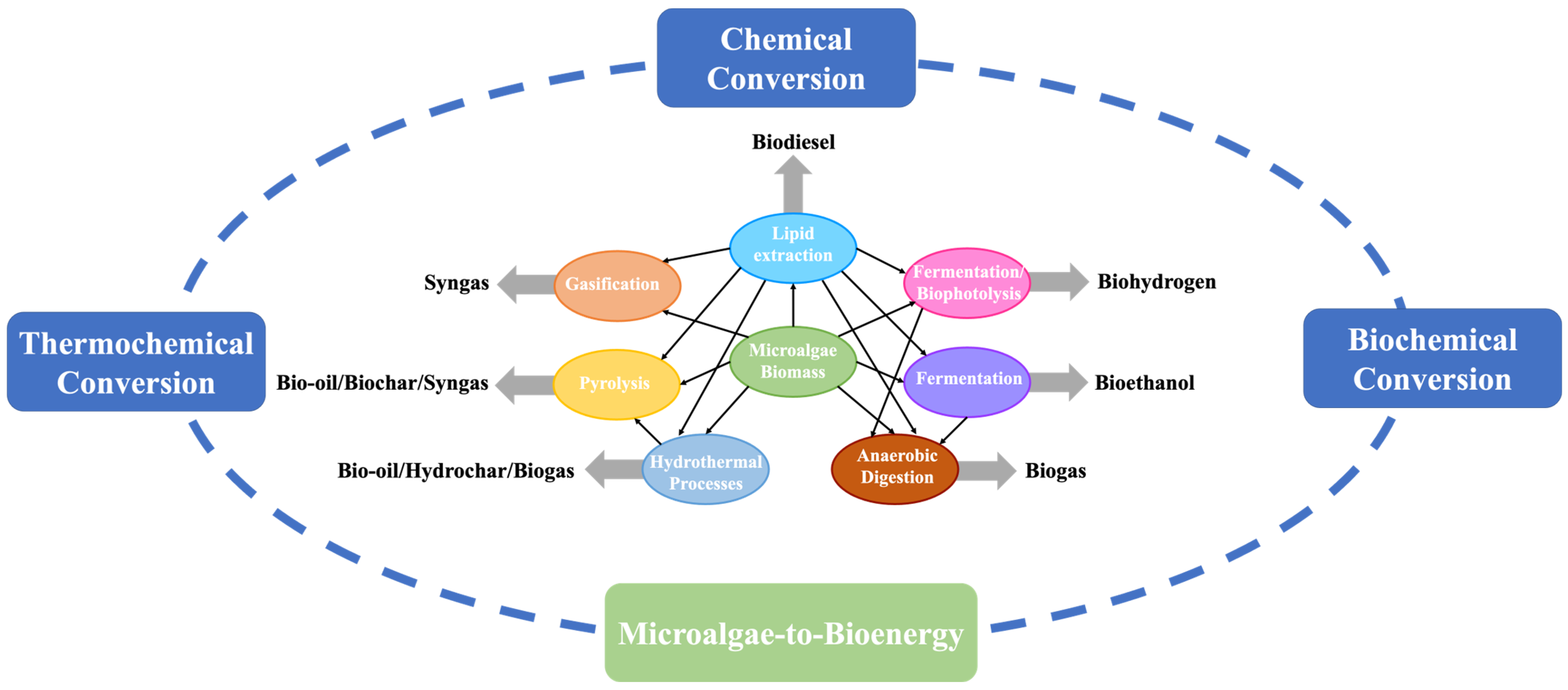
| Microalgae Species | Lipid | Protein | Carbohydrate | References |
|---|---|---|---|---|
| Botryococcus braunii | 25–75 | 1.5 | 4–55 | [16] |
| Chlorella emersonii | 23–63 | 36 | 41 | [19] |
| Chlorella protothecoides | 40–60 | 10–28 | 11–15 | [19] |
| Chlamydomonas reinhardtii | 15–18 | 9.2 | 59.7 | [16] |
| Chlorella sorokiniana | 26.2 | 45.5 | 23.7 | [34] |
| Chlorella vulgaris | 41–58 | 51–58 | 12–17 | [19] |
| Dunaliella salina | 6–25 | 57 | 32 | [16] |
| Euglena gracilis | 4–20 | 39–61 | 14–18 | [16] |
| Isochrysis galbana | 11 | 27 | 34 | [35] |
| Nannochloropsis gaditana | 23.3 | 48.3 | 9.3 | [20] |
| Nannochloropsis granulata | 24–28 | 27–36 | 18–34 | [35] |
| Neochloris oleoabundans | 35–65 | 10–27 | 17–27 | [19] |
| Porphyridium cruentum | 9–14 | 28–39 | 40–57 | [20] |
| Scenedesmus dimorphus | 16–40 | 8–18 | 21–52 | [19] |
| Scenedesmus obliquus | 30–50 | 10–45 | 20–40 | [19] |
| Tetraselmis chuii | 12 | 31–46 | 12 | [35] |
| Approach | Outcomes | References | ||
|---|---|---|---|---|
| Lipid | Carbohydrate | Biomass | ||
| Nutrient stress | ||||
| Nitrogen deprivation/starvation | ↑ | ↑ | ↓ | [19,45] |
| Phosphorus deprivation/starvation | ↑/↓ | -/↓ | ↓ | [19,45] |
| Trace metal availability | ↑/↓ | ↑ | ↑/↓ | [19,46] |
| Carbon availability | ↑ | - | ↑ | [19,47] |
| pH stress | ||||
| Acidic | ↑/↓ | ↑/↓ | ↑/↓ | [19,48] |
| Alkaline | ↑/↓ | ↑/↓ | ↑/↓ | [19,48] |
| Temperature stress | ||||
| High temperature | ↑/↓ | - | ↑/↓ | [49] |
| Low temperature | ↑/↓ | - | ↑/↓ | [49] |
| Light stress | ||||
| Low Light | ↑/↓ | ↑/↓ | ↓ | [50] |
| High Light | ↑/↓ | ↑/↓ | ↑/↓ | [50] |
| Saline stress | ↑ | ↑ | ↑/↓ | [19,51] |
| Microalgae Species | Co-Substrate | Microalgae/ Co-Substrate Ratio | Operating Conditions | Biomethane Yield (mL CH4/g VS) | Improvement in Biomethane Yield with Co-Digestion (%) | References |
|---|---|---|---|---|---|---|
| Mixed microalgae consortium of Chlorella sorokiniana and Scenedesmus sp. | None | NA | Batch; Mesophilic (37 °C) | 331 ± 76 | NA | [111] |
| Waste-activated sludge from the aerobic phase of the wastewater treatment plant | 0.1 g of algae/1 g of sludge (TS) | 400 ± 22 | 21 | |||
| Waste-activated sludge from the anaerobic phase of the wastewater treatment plant | 0.1 g of algae/1 g of sludge (TS) | 560 ± 24 | 69 | |||
| Scenedesmus quadricauda | None | NA | Batch; Mesophilic (35 °C) | 197 | NA | [112] |
| Thickened waste-activated sludge from the wastewater treatment plant | 49 g of algae/51 g of sludge (VS) | 222 | 12 | |||
| Mixed microalgae–bacteria consortium | None | NA | Semi-continuous; 30 d HRT; Mesophilic (37 °C) | 90 ± 2 | NA | [113] |
| Thickened primary sludge from the wastewater treatment plant | 75 g of algae/25 g of sludge (VS) | 133 ± 6 | 48 | |||
| 50 g of algae/50 g of sludge (VS) | 216 ± 1 | 140 | ||||
| 25 g of algae/75 g of sludge (VS) | 291 ± 9 | 223 | ||||
| Mixed microalgae–bacteria consortium, dominated by Dictyosphaerium sp. | None | NA | Batch; Mesophilic (35 °C) | 332 | NA | [114] |
| Synthetic food waste (20% rice, 15% meat, 20% beans, 25% lettuce, 10% carrot, and 10% tomato) | 50 g of algae/50 g of food waste (VS) | 409 | 23 | |||
| 25 g of algae/75 g of food waste (VS) | 514 | 55 | ||||
| Mixed microalgae–bacteria consortium dominated by Chlorella sp., Scenedesmus sp., and pennate diatoms | None | NA | Batch; Mesophilic (25–32 °C) | 274 | NA | [115] |
| Swine wastewater | 50: 50 (v/v) | 326 | 19 | |||
| Mixed microalgae–bacteria consortium | None | NA | Batch; Mesophilic (34.5–35.5) | 174 ± 2 | NA | [117] |
| Deproteinated cheese whey | 17 g algae/83 g of whey (VS) | 302 ± 7 | 74 | |||
| Cellulose | 16 g of algae/84 g of cellulose (VS) | 272 ± 12 | 56 | |||
| None | NA | Semi-continuous; 30 d HRT; Mesophilic (33–35 °C) | 36–81 mL CH4/g COD | NA | ||
| Deproteinated cheese whey | 17 g algae/83 g of whey (VS) | 210–222 mL CH4/g COD | 167–500 | |||
| Cellulose | 16 g of algae/84 g of cellulose (VS) | 97–122 mL CH4/g COD | 51–169 | |||
| Chlorella vulgaris | None | NA | Batch; Mesophilic (35 °C) | 167 | NA | [116] |
| Potato processing waste (discarded parts and peels) | 25 g of algae/75 g of potato waste (VS) | 266 | 59 | |||
| Chlorella vulgaris | Potato processing waste (discarded parts) | 25 g of algae/75 g of potato waste (VS) | Semi-continuous; 20 d HRT; Mesophilic (37 °C) | 300 mL CH4/g COD | NA | [110] |
| Potato processing waste (discarded parts) + glycerol | 25 g of algae/75 g of potato waste (VS) + 1% glycerol (v/v) | 730 mL CH4/g COD | 143 | |||
| Potato processing waste (peels) | 25 g of algae/75 g of potato waste (VS) | 330 mL CH4/g COD | NA | |||
| Potato processing waste (peels) + glycerol | 25 g of algae/75 g of potato waste (VS) + 1% glycerol (v/v) | 550 mL CH4/g COD | 67 | |||
| Mixed microalgae–bacteria consortium | None | NA | Batch; Mesophilic (35 °C) | 140 ± 4 | NA | [118] |
| Thickened primary sludge | 50 g of algae/50 g of sludge (VS) | 207 ± 5 | 48 | |||
| Thickened primary sludge and fat, oil, and grease (FOG) | 50 g of algae/50 g of sludge + 10% FOG (VS) | 259 ± 13 | 85 | |||
| 50 g of algae/50 g of sludge + 20% FOG (VS) | 293 ± 8 | 109 |
| Microalgae Species | Genetic Engineering Approach | Outcome | References |
|---|---|---|---|
| Chlamydomonas reinhardtii hpm91 mutant | Mutant lacking PGR5 (Proton Gradient Regulation 5) | Produced 7287 mL/10 Lreactor biohydrogen in 26 days under sulfur deprivation | [164] |
| Chlamydomonas reinhardtii C3 mutant | Altered the ratio between PSI and PSII from 0.85 to 0.33 | Produced biohydrogen with a rate of 3 mL/L.d for 42 days | [165] |
| Chlorella sp. DT mutant | Modified amino acid residues A105I, V265W, G113I, or V273I around the hydrogenase gas tunnel to prevent oxygen from accessing the enzyme active site via site-directed mutagenesis | Produced 7 times more biohydrogen than the wild type in the presence of 5% oxygen | [166] |
| Chlamydomonas reinhardtii FACHB-265 | Randomly mutated by atmospheric and room temperature plasma (ARTP) | Produced 2.7–3.1 times higher biohydrogen than the wild type | [167] |
| Chlamydomonas reinhardtii | Used artificial miRNA (amiRNA) to regulate the function of D1-encoded gene, psbA | Produced 60% more biohydrogen content than the wild type | [168] |
| Microalgae Species | Process | Product | Energy/Product Yield or Recovery | References |
|---|---|---|---|---|
| Chlorella sp. | Dark fermentation | Biohydrogen | 0.5 kJ/g VS | [171] |
| Dark fermentation and photo fermentation | Biohydrogen | 1.9 kJ/g VS | ||
| Dark fermentation and anaerobic digestion | Biohydrogen and Biomethane | 6 kJ/g VS | ||
| Chlorella sp. | Dark fermentation | Biohydrogen | 0.4%/g VS | [175] |
| Dark fermentation and anaerobic digestion | Biohydrogen and Biomethane | 57%/g VS | ||
| Algal bloom dominated by Microcystis sp. | Dark fermentation | Biohydrogen | 0.4%/g VS | [179] |
| Dark fermentation and anaerobic digestion | Biohydrogen and Biomethane | 39–44%/g VS | ||
| Scenedesmus obliquus UTEX 393 | Lipid extraction/transesterification | Biodiesel | 13%/g VS | [177] |
| Lipid extraction/transesterification and dark fermentation | Biodiesel and Biohydrogen | 19%/g VS | ||
| Lipid extraction/transesterification, dark fermentation, and anaerobic digestion | Biodiesel, Biohydrogen, and Biomethane | 30%/g VS | ||
| Arthrospira platensis | Dark fermentation | Biohydrogen | 0.5–1 kJ/g VS | [172] |
| Dark fermentation and photo fermentation | Biohydrogen | 2.4–4.6 kJ/g VS | ||
| Dark fermentation, photo fermentation, and anaerobic digestion | Biohydrogen and biomethane | 9.9–10.5 kJ/g VS | ||
| Chromochloris zofingiensis SAG 211-14 | Anaerobic digestion | Biomethane | 287 L/kg TS (10,343 kJ) | [206] |
| Carotenoid extraction and anaerobic digestion | Carotenoids (Mainly astaxanthin) | 10 g/kg TS | ||
| Biomethane | 198 L/kg TS (7153 kJ) | |||
| Carotenoid extraction, yeast-based fermentation, and anaerobic digestion | Carotenoids (Mainly astaxanthin) | 10 g/kg TS | ||
| Bioethanol | 143 g/kg TS (3832 kJ) | |||
| Biomethane | 123 L/kg TS (4428 kJ) | |||
| Haematococcus pluvialis SAG 192.80 | Carotenoid extraction and anaerobic digestion | Astaxanthin | 39 g/kg TS | [207] |
| Biomethane | 192 L/kg TS (6939 kJ) | |||
| Carotenoid extraction, yeast-based fermentation, and anaerobic digestion | Astaxanthin | 39 g/kg TS | ||
| Bioethanol | 170 g/kg TS (4666 kJ) | |||
| Biomethane | 67 L/kg TS (2430 kJ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parakh, S.K.; Tian, Z.; Wong, J.Z.E.; Tong, Y.W. From Microalgae to Bioenergy: Recent Advances in Biochemical Conversion Processes. Fermentation 2023, 9, 529. https://doi.org/10.3390/fermentation9060529
Parakh SK, Tian Z, Wong JZE, Tong YW. From Microalgae to Bioenergy: Recent Advances in Biochemical Conversion Processes. Fermentation. 2023; 9(6):529. https://doi.org/10.3390/fermentation9060529
Chicago/Turabian StyleParakh, Sheetal Kishor, Zinong Tian, Jonathan Zhi En Wong, and Yen Wah Tong. 2023. "From Microalgae to Bioenergy: Recent Advances in Biochemical Conversion Processes" Fermentation 9, no. 6: 529. https://doi.org/10.3390/fermentation9060529
APA StyleParakh, S. K., Tian, Z., Wong, J. Z. E., & Tong, Y. W. (2023). From Microalgae to Bioenergy: Recent Advances in Biochemical Conversion Processes. Fermentation, 9(6), 529. https://doi.org/10.3390/fermentation9060529




