Abstract
Sludge management is considered a difficult and challenging task and is a priority of environmental policy. This study evaluates the transformation of the anaerobic sludge of agroindustrial wastes (cow manure, anaerobic sludge mixtures) directly to compost using Eisenia fetida earthworms (vermicomposting) in relation to sludge quality; moreover, it investigates the effects of different sludge compositions on vermicompost physicochemical properties. In particular, the biostabilization of anaerobic sludge (AS1) produced in excess from the wastewater treatment plant (WWTP) of Patras, Greece, and mixed anaerobic sludge (AS2) originating from the effluents of a laboratory anaerobic co-digestion system treating an agroindustrial waste mixture (olive mill wastewater, cheese whey, and liquid cow manure in a ratio of 55: 40: 5 (w/w), respectively) mixed with cow dung (CD) using Eisenia fetida earthworms was examined. Comparing the mixtures of CD-AS1 to CD-AS2, superior results were obtained with the use of AS2 since an increase in N-P-K was observed when either 10% (22%, 51.8%, and 2.4%, respectively) or 15% of AS2 (38.7%, 14.1%, and 8.1% respectively) was used. Although a reduction in earthworms’ growth was observed compared to 100% CD, during the vermicomposting of the CD-AS mixtures, 410 and 250 mg/earthworm was sustained in the mixtures of 85% CD-15% AS2 and 80% CD-20% AS2 after a period of 63 and 70 days of vermicomposting, respectively.
1. Introduction
Contaminated soil is caused by the presence of xenobiotics resulting from human activities (e.g., the use of chemical fertilizers) as well as subsequent modifications in the natural soil environment due to such activities. Excessive landfill deposits and the incineration of biowastes, such as municipal sludges and agricultural and/or industrial organic wastes, often result in land and surface (and subsurface) water pollution, thus having a great environmental and social impact [1]. Therefore, their cost-effective and sustainable management is of great importance and ecological need. Unexploited high organic-loaded municipal sewage sludges and agroindustrial wastes (such as olive mill wastewater, cheese whey, and cow manure) are rich in nutrients that can be of value to plant growth and soil fertility. Prior to their application in agricultural fields, the bio-composting of such wastes ensures the stabilization and hygienization of these organic materials, avoiding the potential risk of pathogens [2]. In particular, the vermicomposting procedure enhances waste composting using epigeic earthworms such as Eisenia fetida. Many research studies have investigated the ability of Eisenia fetida to transform sludges (sewage, paper sludge, etc.) amended with cow dung into an added-value product [3,4,5].
Recent studies have focused on the valorization (not only stabilization) of sewage sludge by vermicomposting using Eisenia fetida earthworms. Numerous parameters for vermicomposts revealed an increase in nutrient elements, i.e., phosphorus, nitrogen, and potassium, among others, demonstrating the potential use of vermicomposts as (superior) fertilizers over conventional composts such as cattle-dung compost [6]. Vermicompost is considered an organic fertilizer and natural biological control agent promoting beneficial aerobic bacteria and protecting plants from plant pathogens and pests [7,8]. Phytotoxicity tests indicated a low, nontoxic effect on plant growth under the use of soil conditioning vermicompost [9]. A pilot-scale vermicomposting of sewage sludge (SS) combined with recycled mature vermicompost proved to be economically efficient in terms of the maturity and stability of the SS, improving the activity of earthworms and, thus, the efficiency of vermicomposting [10]. Overall, vermicomposting is an ecological, environmentally friendly, pollutant-free management of sewage sludge, among others, used as soil amendment and improving plant development [11,12,13].
Limited vermicompost studies have, however, examined the degradation of anaerobic sludges [14,15]. An innovative discipline of vermiculture biotechnology, the breeding and propagation of earthworms, and the use of their castings has become a valuable tool for waste recycling all over the world. Epigeic worms, such as Eisenia fetida, have been used for converting organic wastes (agro waste and domestic refuse) into vermicompost. Vermicomposting has thus been proven as an established low-cost technology for processing organic wastes and agricultural residues into a value-added product, i.e., vermicasts. The main objective of this study was to evaluate the process performance of sludge transformation, using the earthworms Eisenia fetida, in relation to the quality of sludge used (mixtures of cow manure with anaerobic sludge), and to investigate the effects of different sludge compositions on the final product’s physicochemical properties in view of its potential use as a composting material. The tested agroindustrial mixtures contained solid cow manure and anaerobic sludges, namely biological sludge from Patras’ municipal wastewater treatment plant as well as sludge from an anaerobically co-digested mixture of olive mill wastewater, cheese whey, and liquid cow manure. Based on our results, the vermiconversion of anaerobically treated sludges by Eisenia fetida earthworms may successfully produce a good-quality compost if mixed with cow dung in appropriate quantities.
2. Materials and Methods
Fresh cow dung (CD) was collected from a local dairy farm in Patras breeding 70 cows in total. Fresh anaerobic sludges were obtained from the municipal wastewater treatment plant (AS1) located near Patras, (Western) Greece, and methanogenic anaerobic sludge (AS2) was produced from a co-digested mixture of 55% olive mill wastewater, 40% cheese whey, and 5% liquid cow manure at our laboratory of Biochemical Engineering and Environmental Technology, University of Patras, Greece [16,17].
Physicochemical analysis of all substrates tested was performed before use. Prior to analysis, a homogenized (via manual mixing) sample was obtained from the (vermi)reactors. Measurements of pH value and electrical conductivity (EC) were measured in 1/10 (w/v) aqueous solution. Earthworms are susceptible to pH, thus the pH of vermicompost may be a factor that limits the distribution and number of earthworms [18]. EC shows the variation of salt and mineral ion concentrations [9]. In practice, EC measurement in vermicompost is to check its safe use in agricultural systems [19]. EC values in vermicomposts increase over time. Total Kjeldahl Nitrogen (TKN) was determined after digesting the sample with concentrated diacid H2SO4 and concentrated HClO4 (9: 1, v/v). For total K (TK) and total phosphorus (TP) determination, the sample was digested in a diacid mixture (concentrated HNO3: concentrated HClO4, 2:1, v/v). The analysis of total available phosphorus (TAP) and total potassium (TK) was performed using spectrophotometry (Cary 50 Varian UV/Vis), namely the vanadomolybdate complex formation method, and emission spectrometry (Perkin-Elmer AAnalyst 300), respectively. All aforementioned determinations were carried out according to Standard Methods [20].
All the chemicals used were analytical reagent grade supplied by Merck. The samples were used on a dry-weight basis for chemical analysis. All the samples were analyzed in duplicate, and results were reproducible within 5–7% error limits.
Prior to experimental testing, all sludges (30% dry matter) were pretreated by manual mixing and aeration once a day for 15 days. Thus, the characteristic smell of putrescible substances was reduced and biotoxic compounds formed under anaerobiosis were decreased [21]. An amount of 200 g (on a dry-weight basis) of sludge mixture was then placed into 5 L plastic conical pots perforated at the bottom (for excess water to drain out) and enriched with Eisenia fetida earthworms (purchased from a worm farm in Corinth, south-central Greece, originating from New Zealand) spread on the cow dung and anaerobic sludge(s) surface in each pot. All substrate quantities are referred to on a dry-weight basis at 105 °C.
Table 1 represents the feed mixtures used for vermicomposting in this study, namely cow dung (CD), anaerobic sludges of municipal sewage sludge (AS1), and anaerobically co-digested methanogenic agroindustrial mixture sludge (i.e., 55% olive mill wastewater, 40% cheese whey, and 5% liquid cow manure, AS2). Cow manure was collected at different time intervals from a local farm (CD1, CD3), thus the initial composition analysis between samples was different in terms of TKN, TP, and TK. Cow dung CD2 was left for aeration for 5 months after the collection of CD1, whereas the latter was left for 15 days of aeration, as was CD3.

Table 1.
The feed mixtures of cow dung (CD), anaerobic sludges of municipal sewage sludge (AS1), and anaerobically co-digested methanogenic agroindustrial mixture sludge (i.e., 55% olive mill wastewater, 40% cheese whey, and 5% liquid cow manure, AS2) tested for vermicomposting.
Initially, five earthworms, including adults, were added to the mixtures, and their performance was monitored for 8 weeks. After their mortality, the vermicompost experiments were continued. Additionally, ten juveniles and clitellated Eisenia fetida earthworms were inoculated in the same mixtures for 18.5 weeks (CD1 *).
The moisture of the treated material was maintained at about 75% (±5%) by water spraying the sludge surface every 2 days, securing appropriate moisture levels for earthworm growth. The vermistabilization of CD and sludge mixtures was estimated by evaluating the vermicompost in terms of its fertilizer quality. The parameters that were monitored during vermicomposting were EC, pH, volatile solids, and important plant nutrients such as N, P, and K. Additionally, the growth and reproduction of Eisenia fetida earthworms were also determined in vermireactors of different waste mixtures weekly within 30 to 75 days depending on the tested mixtures. The growth, maturation, and mortality of earthworms were monitored in a range of different feed mixtures in the laboratory under controlled environmental conditions (room constant temperature at 20 ± 2 °C). Thus, the biomass production by E. fetida in different vermireactors was measured as mean individual biomass (mg per number of earthworms) during vermicomposting. The time of the initial mixing of cow dung with anaerobic sludges before the inoculation of earthworms was set to zero (t = 0) days after the aeration of the sludge mixtures. The time of final vermicomposting refers to the time that the mortality of all E. fetida earthworms was recorded.
3. Results and Discussion
Table 2 presents the physicochemical analysis of the initial feed mixtures (after mixing different compositions of cow dung and anaerobic sludges) and vermicompost obtained at the termination of the experiments. The vermicomposted mixtures were compared with the physicochemical analysis of the same-time-collected composted cow manure (CD3, reference). The total potassium analysis for CD1 was as high as 18 mg/g compared to 36 mg/g for CD3 collected at a different time of year. Elevated concentration levels of N, P, and K for CD2 were measured compared to CD1 and CD3. N, P, and K concentration analysis for CD1 * was lower compared to that for CD2 (aerated for 150 days compared to 15 (pre-aeration) + 54 days vermicomposting), implying that the addition of Eisenia fetida earthworms accelerated the composting process.

Table 2.
Physicochemical analysis of the initial feeds and vermicompost obtained at the termination of experiments in different feed mixtures of cow dung with anaerobic sludges. t refers to the experimental sampling time following the earthworms’ addition (mean ± SD, n = 2).
Considering the difference in the characteristics of anaerobic sludges of municipal sewage sludge and co-digested methanogenic agroindustrial mixture (i.e., 55% olive mill wastewater, 40% cheese whey, and 5% liquid cow manure) sludge, all the feed mixtures were analyzed in terms of physicochemical quality parameters.
3.1. Vermicompost with Cow Dung
For vermireactor CD1, the humidity was constant at 75% during the course of vermicomposting and VS showed a relative reduction of 3.5% of the initial measured volatile solids. Moisture generally remained at the same level except for some deviations due to abrupt changes in ambient temperature conditions. pH showed an increase of 5.8%, whereas electrical conductivity showed a 31% increase compared to the initial value of 0.94 dS/m. The pH of the worm bed should be maintained within 6.8–8 and decreases over the time of vermicomposting [13]. Since pH decrease is usually attributed to organic acid accumulation from microbial metabolism or from the production of fulvic and humic acids during decomposition [22], no such metabolic products can be anticipated in the case of CD1. The increase in EC might have been due to the loss of the weight of organic matter (indicated by the VS decrease) and the release of different mineral salts in ionic form (such as phosphate, ammonium, and potassium) that can increase the sample conductivity [23]. A previous study revealed that the survival of the worms was affected at values of 0.92 dS/m [13]. In this study, both concentration levels of nitrogen and potassium were increased (54% and 39%, respectively), whereas phosphorus followed a relative decrease (39%) during the experiment.
Following the vermicomposting of CD1 using 5 adult Eisenia fetida earthworms, 10 mixed juvenile and adult earthworms were added to the same vermicomposted cow dung (vermireactor CD1 *). A decrease of 7% of VS and a reduction in pH (22%) was observed in this case, whereas an increase in conductivity from 1.07 to 3.3 dS/m was monitored, as a result of active vermicomposting. All N, P, and K values were augmented (17%, 70%, and 36%, respectively).
For vermireactor CD2, 20 juvenile and adult earthworms were added. A decrease in volatile solids and pH value was seen (4 and 16%, respectively), whereas an increase in conductivity was observed (80%). An increase in total nitrogen (17%) was seen in contrast to the decrease in phosphorus and potassium (39 and 17%, respectively).
Total Kjeldahl Nitrogen (TKN) analysis in all vermireactors treating cow manure feed showed increased values. TKN analysis measures both organic nitrogen and ammonia nitrogen. Similar findings showed that the nitrogen content was increased by E. fetida’s vermiconversion of cow dung slurry, probably because of the mineralization of organic matter [24]. A decrease in pH may also be an important factor contributing to nitrogen retention and eventual increase, as this element (N) is lost as volatile ammonia at higher pH values [25]. Indeed, in all vermicomposting treatments with cow dung, a decrease in pH values was observed. Earthworms are also considered to affect nitrogen transformations in manure, enhancing nitrogen mineralization to the point of retaining mineral nitrogen in nitrate form [26].
The amount of TP in the mixtures increased with the incubation period (after 75 days of vermicomposting with cow dung, CD1 *, or 129 days from the initial feed, CD1). Comparable results were found in the literature [4,27,28,29]. TP analysis of cow dung sampling feeds showed an increase of nearly 50% [4,27]. Garg and co-authors [28] underlined that the increase in TP during vermicomposting may be attributed to the mineralization and mobilization of phosphorus considering the bacterial and fecal phosphatase activity of earthworms. The activity of earthworms may encompass the direct action of worm gut enzymes and indirectly by the stimulation of the microflora. In contrast to CD1 * feed, TP analysis after CD1 and CD2 vermicomposting after 54 and 36 days of sampling showed comparable low values, possibly due to the small number of earthworms used (five in CD1) and/or the short incubation time (in CD2). The addition of adult earthworms and also a large number of adult and juvenile worms (in this case 20) is a threshold for phosphorus augmentation. Parveresh and co-authors [29] reported a decrease in phosphorus levels after the experiment, which was attributed to the adsorption of inorganic phosphorus released from the earthworms’ tissues. The presence of earthworms and their activities in feed accelerates cow dung decomposition, resulting in the mineralization of organic compounds. Volatile solids’ measurements indicate the stabilization of the feed tested (i.e., cow dung). In our case, VS was reduced by 4%, 7%, and 4% by the end of 54, 75, and 36 days, respectively, thus, the resulting vermicompost was not yet stabilized enough, even after such a long time. The differences in the VS reduction were within experimental error. Further stabilization or a complementary process to obtain desirable results may thus be required.
TK concentrations were also higher in the final product compared to the initial CD1 and CD1 * feeds; an increase of 39% and 36%, respectively. However, TK analysis of the final CD2 showed a decrease of 17%. Elvira et al. [3] reported a decrease in TK for the vermicomposting of paper-pulp mill sludge, attributing this decrease to the leaching of soluble elements by the excess water that drained through the mass. In this study, no measurements of leachates were performed.
3.2. Vermicompost with Cow Dung Mixed with Anaerobic Sludges
The vermiconversion of anaerobic sludges in different vermireactors was monitored after physicochemical quality parameters analysis. The feed mixtures varied from 90% to 80% of cow dung with 10% to 20% of anaerobic sludges, respectively (namely, obtained from the municipal wastewater treatment plant (AS1) and/or methanogenic anaerobic sludge (AS2) produced from a co-digested mixture of 55% olive mill wastewater, 40% cheese whey, and 5% liquid cow manure). Following the vermicomposting of cow dung CD1 including five adult E. fetida earthworms, anaerobic sludges were added forming mixtures (namely 90% CD1 and 10% AS1 * and 90% CD1 and 10% AS2 *) with the addition of 10 earthworms. Figure 1 compares the initial to the final measurements of pH, EC, TKN, TP, and TN in cow dung after vermicomposting.
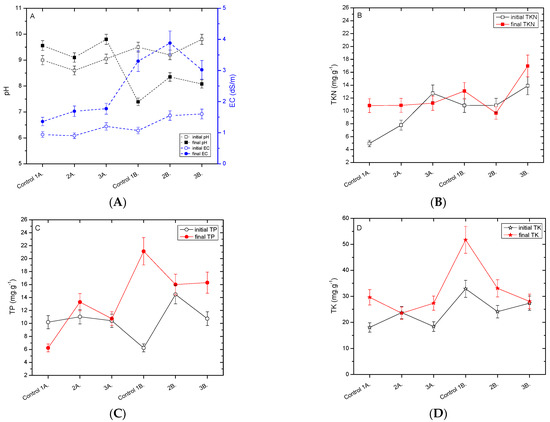
Figure 1.
Comparison of (A) pH and EC; (B) TKN; (C) TP; and (D) TK in cow dung (Control 1A, 1B) and anaerobic sludge mixtures after vermicomposting by E. fetida; Control 1A: CD1, 2A: 90%CD1–10% AS1, 3A: 90%CD1–10% AS2, Control 1B: CD1 *, 2B: 90%CD1–10% AS1 *, 3B: 90%CD1–10% AS2 *. (Values are mean, n = 2, error bars indicate SD).
The measurements of pH values and volatile solids of all vermicomposts tested with cow dung mixed with anaerobic sludges are comparable (Figure 1A). However, for longer durations of composting, pH values had a tendency to decrease (10 to 20%). The pH shift is directly related to the reduction in volatile solids and to the growth of earthworm biomass [5]. A pH increase was observed initially for 90% CD1 with both anaerobic sludges (namely AS1 and AS2) (6–8%) compared to the control (of 100% CD1). The increase in phosphorus, calcium, and potassium concentrations in the vermicomposted sludge sample may be attributed to the action of the earthworms during vermicomposting [13,30]. However, in mixture feeds with a high amount of 15 to 20% of anaerobic sludge, AS1 and AS2, and also with the addition of an extra 10 earthworms (namely 90% CD1 and 10% AS1 * and/or AS2 *, respectively), the pH values decreased, indicating that the initial small number of earthworms used (five adults in vermireactors 5 and 6) did not result in effective vermicomposting. The mineralization of nitrogen and phosphorus into nitrites/nitrates and orthophosphates, respectively, typically occurs during vermicomposting, accompanied by the bioconversion of organic material into intermediate metabolites such as organic acids. The pH shift behavior is also related to the specific type of substrate used in the vermicomposting; thus, different intermediate molecular species may appear that can affect the pH value [31].
A decrease in volatile solids (~4–8%) was measured in all tested mixtures. The electrical conductivity (EC) was increased (Figure 1A) in all mixtures of CD with AS (AS1 or AS2) after vermicomposting due to the fact that organic matter was degraded by earthworms and mineral salts are released, i.e., ammonium, phosphate, potassium, etc. [23]. Samples from the control vermireactors (100% CD2) gave the highest increase in electrical conductivity measurements (390% after 36 days of vermicompost duration), comparable to the feed mixture of 85% CD2–15% AS1 and 15% AS2.
The vermiconversion of anaerobic sludges in different vermireactors was also monitored after nutrient analysis for nitrogen, phosphorus, and potassium (Figure 1B–D). Contradictory results were obtained for the feed mixtures with AS1 and AS2. TKN analysis showed an increase of up to 40% compared to the initial feed mixture analysis for the feed mixture of 90% CD1–10% AS1, 90% CD1–10% AS2 *, and 85% CD2–15% AS2. At a low content of anaerobic sludge AS2, the observed earthworm tolerance to the feed mixture was higher compared to anaerobic sludge AS1. This is illustrated by the mortality of E. fetida earthworms. For the feed mixture of 90% CD1–10% AS1, 90% CD1–10% AS2 *, and 85% CD2–15% AS2, the mortality was 80% (after 47 days), 80% (after 40 days), and 85% (after 56 days), respectively. In each case, thus, in the feed mixture of 90% CD1 and 10% AS1 and 85% CD1 and 15% AS2, a 40% increase in TKN was observed for a vermicomposting duration of 55 and 63 days, respectively. Bohlen and co-authors [32] suggested that the decrease in total soil N was possibly due to the stimulation of denitrification by earthworms. Total nitrogen variations in the vermicomposting of different wastes are mainly attributed to the quality of the physicochemical composition of substrates tested in feeding earthworms, which strongly affects the mineralization of nitrogenous organic compounds and the amount of nitrogen released from these compounds.
The TP content in the composted mixtures of vermireactors was increased by up to 50% for the feed mixture of 90% CD1 and 10% AS2 * after 73 days (Figure 1C). The increase in TP is associated directly with the action of worm gut enzymes and indirectly with the stimulation of the microflora [28]. The physical breakdown of the material is attributed to the activity of earthworm ingestion. However, feed mixtures of 85% CD2 and 15% AS1 and 80% CD1 and 20% AS1 showed a decrease in TP content, which may be due to the limited time of vermicomposting (32 and 48 days, respectively) and also to the high amount (15 and 20%) of anaerobic sludge AS1 used.
Total potassium concentration values in all vermireactors were also higher in the final product of vermicasts compared to the initial feed mixtures (Figure 1D). However, the decrease in the TK content of the feed mixture of 85% CD2 and 15% AS1 was attributed to the limited duration of the vermicomposting time of 32 days. Elvira and co-authors [3] explained the decrease in potassium in the leaching of soluble elements, i.e., potassium, by excess water draining through the mass. However, no excess water was observed in the studied vermicasts, thus avoiding the leaching of minerals by runoff water.
3.3. Growth and Reproduction of E. fetida in Different Vermireactors
During vermicomposting, the biomass production by E. fetida in different vermireactors was measured and the worm growth rate was recorded in terms of mean individual biomass (mg per earthworm) over the observation period (Figure 2). The earthworms reared in different waste mixtures had differences in growth rate. The highest mean worm biomass attained was observed in vermireactor no.1, indicated as Control 1A (100% cow dung; 1275 mg·earthworm−1) after 51 days. The weight per earthworm continuously increased during all experimentation periods. After 46 days, there was a reduction in the number of earthworms by one; however, the weight per earthworm increased. On the 54th day, the experiment ended. Comparable results were obtained in vermireactor no. 2, indicated as Control 1B (CD1 *) in Figure 2. An increase in the weight of earthworms was observed in the first two weeks, and reproduction started in the third week. The mean earthworm biomass was 970.6 mg·earthworm−1 after 33 days.
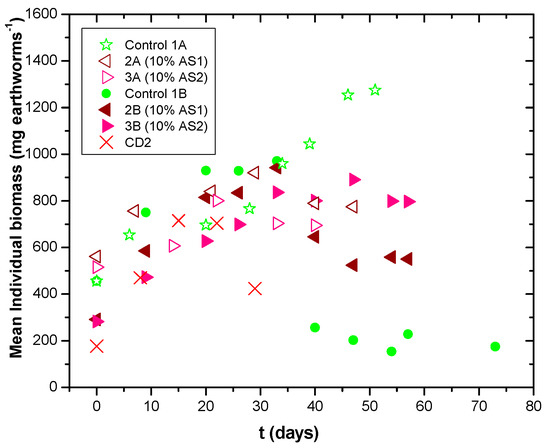
Figure 2.
Biomass production by E. fetida (mg·earthworms−1) in different feed mixtures with time. Control 1A: CD1, 2A: 90%CD1–10% AS1, 3A: 90%CD1–10% AS2, Control 1B: CD1 *, 2B: 90%CD1–10% AS1 *, 3B: 90%CD1–10% AS2 *; and CD2: cow dung, left for aeration for 5 months after the collection of CD1.
For cow dung CD2, an increase in the average weight of the earthworms was observed up to the 29th day, when the number of earthworms was reduced to eight. This vertical decrease was due to a sudden temperature increase which resulted in a sharp decrease in the humidity of the mixture and, finally, to the mortality of the last eight earthworms on the 36th day of the experiment. This experiment was considered in the comparative results (as a control) since the mortality of the earthworms was detected as early as 36 days.
The tested mixtures of anaerobic sludges in the vermireactors promoted a decrease in biomass gain by E. fetida. The range of worm biomass attained in anaerobic sludge mixtures was 600 to 800 mg·earthworm−1 after 33 days. The initial increase in biomass was followed by stabilization and then a biomass decrease was observed in all the vermireactors. Such a decrease in earthworm biomass can be attributed to the exhaustion of food. The lost weight depended upon the quantity and nature of the ingestible sludge substrates [5]. However, as the data revealed, the final mean earthworm biomass was higher in the case of AS2 (approx. 800 mg·earthworm−1 in 3B) compared to AS1, ca. 550 mg·earthworm−1 in 2B, indicating differences in the components of AS2 vs. AS1 that can support higher earthworm biomass.
Comparing mixtures of 85% CD2–15% AS1 and 85% CD2–15% AS2, the latter showed a larger increase in the values of N-P-K concentrations (39%, 14%, and 8%, respectively), as the duration of the vermicomposting experiment was longer (63 and 32 days for 85% CD2–15% AS2 and 85% CD2-15% AS1, respectively). Comparing mixtures of 80% CD2–20% AS1 and 80% CD2–20% AS2, again, the latter showed a larger increase in the values of P-K concentrations (14% and 95%, respectively) as the duration of the vermicomposting experiment was longer (70 and 48 days for 80% CD2–20% AS2 and 80% CD2–20% AS1, respectively). Overall, the growth of earthworms was higher (410 and 250 mg·earthworm−1) for the mixtures of 85% CD2–15% AS2 and 80% CD2–20% AS2, respectively, with a longer duration of the vermicomposting experiment (63 and 70 days for 85% CD2–15% AS2 and 80% CD2–20% AS2, respectively). These results again support the superiority of AS2 compared to AS1, as noted in the case of 10% AS test runs.
4. Conclusions
The aim of this study was to investigate the stabilization of the anaerobic sludges of municipal sewage sludge and a co-digested methanogenic agroindustrial mixture (i.e., 55% olive mill wastewater, 40% cheese whey, and 5% liquid cow manure) mixed with cow dung, employing the epigeic earthworms E. fetida. Physicochemical analysis in different substrate mixtures was performed and the growth of E. fetida earthworms was monitored. Based on our results, the vermiconversion of anaerobically treated sludges by E. fetida worms may successfully produce a vermicompost with increased N-P-K concentrations if mixed with cow dung in appropriate quantities. However, the vermicomposting with cow manure endured (73 days) longer than the vermicomposting with anaerobic sludge mixtures. The vermicomposting of the methanogenic anaerobic sludge of co-digested agroindustrial wastes (55% OMW, 40% cheese whey, and 5% liquid cow manure) with cow dung (in a ratio of 10:90 or 15:85) resulted in pH and volatile acid decreases, whereas electrical conductivity measurements were increased. Nutrient analysis of TKN, TP, and TK showed increases of 22%, 51.8%, and 2.4%, respectively, in the case of the 10:90 mixture, while 38.7%, 14.1%, and 8.1% increases were measured in the case of the 15:85 mixture (AS2:CD). Finally, the earthworm biomass growth increased up to 600 to 800 mg earthworm−1 up to day 33 and then decreased till the end of the vermicompost testing, indicating the exhaustion of feed and a decrease in earthworm and microbial activity.
Author Contributions
Conceptualization, A.I.V. and M.K.; writing—original draft preparation, A.I.V.; writing—review and editing, M.K.; supervision, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from the European Commission project LIFE08/ENV/GR578-INTEGRASTE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be given upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. Applications of the anaerobic digestion process. Adv. Biochem. Eng. Biotechnol. 2003, 82, 1–33. [Google Scholar] [PubMed]
- Ahmad, R.; Jilani, G.; Arshad, M.; Zahir, Z.A.; Khalid, A. Bio-conversion of organic wastes for their recycling in agriculture: An overview of perspectives and prospects. Ann. Microbiol. 2007, 57, 471–479. [Google Scholar] [CrossRef]
- Elvira, C.; Sampedro, L.; Benitez, E.; Nogales, R. Vermicomposting of sludges from paper mill and dairy industries with Eisenia andrei: A pilot-scale study. Bioresour. Technol. 1998, 63, 205–211. [Google Scholar] [CrossRef]
- Kaushik, P.; Garg, V.K. Dynamics of biological and chemical parameters during vermicomposting of solid textile mill sludge mixed with cow dung and agricultural residues. Bioresour. Technol. 2004, 94, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Garg, V.K. Stabilization of primary sewage sludge during vermicomposting. J. Hazard. Mater. 2008, 153, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Georgi, K.; Ekaterina, S.; Alexander, P.; Alexander, R.; Kirill, Y.; Andrey, V. Sewage sludge as an object of vermicomposting. Bioresour. Technol. Rep. 2022, 20, 101281. [Google Scholar] [CrossRef]
- Soobhany, N.; Mohee, R.; Garg, V.K. Inactivation of bacterial pathogenic load in compost against vermicompost of organic solid waste aiming to achieve sanitation goals: A review. Waste Manag. 2017, 64, 51–62. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kumar, C.V.; Kiran, B.S.; Kumar, S.; Athokpam, V. A review on vermicomposting: By-products and its importance. Plant. Cell Biotechnol. Mol. Biol 2021, 22, 156–164. [Google Scholar]
- Yilmaz Cincin, R.G.; Agdag, O.N. Co-vermicomposting of Wastewater Treatment Plant Sludge and Yard Waste: Investigation of Operation Parameters. Waste Biomass Valorization 2020, 11, 6211–6222. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, T.; Tian, G.; Zhang, L.; Bian, B. Pilot-scale vermicomposting of sewage sludge mixed with mature vermicompost using earthworm reactor of frame composite structure. Sci. Total Environ. 2021, 767, 144217. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Domínguez, J. Recycling of Solid Organic Wastes Through Vermicomposting: Microbial Community Changes Throughout the Process and Use of Vermicompost as a Soil Amendment. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1289–1312. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Rusănescu, M.; Voicu, G.; Paraschiv, G.; Biriș, S.Ș.; Popescu, I.N. The Recovery of Vermicompost Sewage Sludge in Agriculture. Agronomy 2022, 12, 2653. [Google Scholar] [CrossRef]
- Hanc, A.; Vasak, F. Processing separated digestate by vermicomposting technology using earthworms of the genus Eisenia. Int. J. Environ. Sci. Technol. 2014, 12, 1183–1190. [Google Scholar] [CrossRef]
- Hanc, A.; Dreslova, M. Effect of composting and vermicomposting on properties of particle size fractions. Bioresour. Technol. 2016, 217, 186–189. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Effect of hydraulic retention time (HRT) on the anaerobic co-digestion of agro-industrial wastes in a two-stage CSTR system. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Khare, N.; BHARGAVA, D.; Bhattacharya, S. Effect of initial substrate pH on vermicomposting using Perionyx excavatus (Perrier, 1872). Appl. Ecol. Environ. Res. 2005, 4, 85–97. [Google Scholar]
- Arumugam, K.; Renganathan, S.; Babalola, O.O.; Muthunarayanan, V. Investigation on paper cup waste degradation by bacterial consortium and Eudrillus eugeinea through vermicomposting. Waste Manag. 2018, 74, 185–193. [Google Scholar] [CrossRef]
- Carranzo, I.V. Standard Methods for examination of water and wastewater. In Anales de Hidrología Médica; Universidad Complutense de Madrid: Madrid, Spain, 2012; p. 185. [Google Scholar]
- Masciandaro, G.; Ceccanti, B.; Garcia, C. “In situ” vermicomposting of biological sludges and impacts on soil quality. Soil. Biol. Biochem. 2000, 32, 1015–1024. [Google Scholar] [CrossRef]
- Albanell, E.; Plaixats, J.; Cabrero, T. Chemical changes during vermicomposting (Eisenia fetida) of sheep manure mixed with cotton industrial wastes. Biol. Fertil. Soils 1988, 6, 266–269. [Google Scholar] [CrossRef]
- Sharma, S. Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour. Technol. 2003, 90, 169–173. [Google Scholar]
- Loh, T.C.; Lee, Y.C.; Liang, J.B.; Tan, D. Vermicomposting of cattle and goat manures by Eisenia foetida and their growth and reproduction performance. Bioresour. Technol. 2005, 96, 111–114. [Google Scholar] [CrossRef]
- Hartenstein, R.; Hartenstein, F. Physicochemical Changes Effected in Activated Sludge by the Earthworm Eisenia Foetida; Wiley Online Library: Hoboken, NJ, USA, 1981; pp. 377–381. [Google Scholar]
- Atiyeh, R.M.; Domínguez, J.; Subler, S.; Edwards, C.A. Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei, Bouché) and the effects on seedling growth. Pedobiologia 2000, 44, 709–724. [Google Scholar] [CrossRef]
- Vig, A.P.; Singh, J.; Wani, S.H.; Singh Dhaliwal, S. Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour. Technol. 2011, 102, 7941–7945. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.K.; Kaushik, P.; Dilbaghi, N. Vermiconversion of wastewater sludge from textile mill mixed with anaerobically digested biogas plant slurry employing Eisenia foetida. Ecotoxicol. Environ Saf. 2006, 65, 412–419. [Google Scholar] [CrossRef]
- Parvaresh, A.; Movahedian, H.; Hamidian, L. Vermistabilization of municipal wastewater sludge with Eisenia fetida. J. Environ. Health Sci. Eng. 2004, 1, 43–50. [Google Scholar]
- Malińska, K.; Zabochnicka-Świątek, M.; Cáceres, R.; Marfà, O. The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol. Eng. 2016, 90, 35–41. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S.; Das, K. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12. [Google Scholar] [CrossRef]
- Bohlen, P.J.; Parmelee, R.W.; Allen, M.F.; Ketterings, Q.M. Differential Effects of Earthworms on Nitrogen Cycling from Various Nitrogen-15-Labeled Substrates. Soil Sci. Soc. Am. J. 1999, 63, 882–890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).