Abstract
In this study, the effect of solid-state fermentation with Bacillus subtilis GYB6, Saccharomyces cerevisiae NJ1, and Bacillus amyloliquefaciens Y8 on the anti-nutritional factors, nutritional components, bioactive compounds, antioxidant activity, functional properties, and structure of rapeseed meal (RSM) were investigated. Results showed that the action of three strains in the fermentation of RSM caused a significant decline in glucosinolates, phytic acid, crude fiber, and tannins by 99.18%, 42.41%, 27.21%, and 34.17%, respectively. The amount of crude protein, amino acids, and peptides of RSM increased significantly after fermentation. The SDS-PAGE results showed that 12S globulin and 2S albumin protein were almost entirely degraded. Fermentation considerably increased the concentration of total phenolics and flavonoids, and activated antioxidant activity and functional properties. Furthermore, the structural variation was observed by scanning electron microscopy and FTIR spectroscopy. Thus, these results indicated that the solid-state fermentation process in this study was a promising approach to enhance both the nutritional value and bioactivity of RSM, which could be used as value-added functional animal food ingredients.
1. Introduction
The demand for new protein sources for feed has grown dramatically in recent years. It is very necessary to explore non-food protein sources and develop efficient technologies to enhance the quality [,]. The agricultural by-product protein resources are abundant, but underdeveloped. Rapeseed is the third largest source of vegetable oil globally []. Rapeseed meal (RSM) is the by-product after the extraction of oil from rapeseed. RSM has attracted significant attention as feedstuff and become the second most widely traded plant protein after soybean meal because of its high potential nutritional value and rich source []. RSM has high protein levels (~35%, w/w) and a well-balanced amino acid profile [,]. It is also a good source of calcium, iron, manganese, selenium, and vitamin []. Therefore, RSM has been attracted as an essential and potential feedstuff. However, the utilization efficiency of RSM in animal diets is limited due to the presence of anti-nutritional factors such as glucosinolates, phytic acid, tannins, fiber, etc. [,]. Glucosinolates, the major anti-nutritional factors, are catalyzed by myrosinase enzyme presented in the rapeseed or animal gastrointestinal tract into isothiocyanate, thiocyanate, and nitrile which disrupt thyroid, liver, and kidney function of animals [,]. Phytic acid reduces mineral bioavailability and protein digestibility []. Tannins, which are secondary compounds, have been reported to bind proteins and some enzymes, thus affecting the digestion of animals [,]. Moreover, RSM contains a relatively high level of fiber which inhibits the absorption of other nutrients, such as minerals and proteins []. Therefore, the nondetoxified RSM not only affects the digestibility and utilization of nutrients, but also causes adverse influence on animal performance, which greatly lowed the application of RSM as feed.
Various approaches, including biological, chemical, and physical detoxification, have been exploited for detoxifying plant protein sources [,]. Solid state-fermentation has been recognized as an ideal biological method to reduce anti-nutritional factors because microorganisms can secrete complex enzymes to decompose the toxic components of RSM [,]. Solid-state fermentation can also improve the nutrient utilization of RSM, such as the biotransformation of proteins into more readily absorbable and functional peptides, and the production of some bioactive compounds [,]. Notably, the studies showed that solid-state fermentation also owned the advantages of improving the palatability of feed, digestibility, and utilization of nutrients, substantially improving animal performance and health [,,]. Generally, the available literature confirms the feasibility of improving the quality of RSM by solid-state fermentation. Solid-state fermentation with RSM as the substrate using Rhizopus oligosporus [], Bacillus licheniformis, Yeast and Lactobacillus [], Bacillus subtilis YY-1 and Saccharomyces cerevisiae YY-2 [], and S. cerevisiae or Saccharomyces boulardii [] decreased the content of glucosinolates, neutral detergent fiber, and phytic acid, and increased the content of crude protein, lactic acids, and total amino acids. To our knowledge, there are few studies applying Bacillus amyloliquefaciens in the solid-state fermentation of RSM. B. amyloliquefaciens has been reported to be applied in the improvement in the nutritional quality of soybean meal by solid-state fermentation []. B. amyloliquefaciens is characterized by producing various enzymes, including α-amylase, protease, cellulase, xylanase, pectinase, and peroxidase, and antimicrobial compounds inhibiting the growth of pathogens, including non-ribosomal peptides and polyketides [].
Numerous studies have demonstrated that the effect of multi-microbial fermentation is superior to single-strain because multiple microorganisms promisingly produce synergistic effects to improve the quality of the substrates []. Multi-microorganism can produce various enzymes facilitating the biotransformation of anti-nutritional factors and macro-molecular materials [,]. Multiple microorganisms can also produce more abundant metabolites compared to pure culture.
In this study, a novel solid-state fermentation with B. amyloliquefaciens Y8, B. subtilis GYB6, and S. cerevisiae NJ1 was applied to improve the nutritional quality of RSM. The anti-nutritional factors, nutritional components, bioactive compounds, antioxidant activity, functional properties, and structure of RSM and fermented RSM (FRSM) were characterized and compared.
2. Materials and Methods
2.1. Materials
Strain Y8 and GYB6 were isolated from the soil of the rape field. S. cerevisiae NJ1 was from our lab. The capacity of degrading glucosinolates was primarily screened and secondarily screened by the power of producing peptides and degrading phytic acid, tannins, and crude fiber in RSM. Lastly, three strains were preferred for the solid-state fermentation of RSM according to the comprehensive capacity described above. Y8 and GYB6 were identified as B. amyloliquefaciens and B. subtilis by morphological characteristics, physiological and biochemical identification, and 16s rDNA sequence analysis. RSM was provided by Hailong Feed Co. Ltd. (Jiangsu, China). Before the experiment, RSM was grounded and then fractionated with a 40-mesh sieve for further use. All chemicals used in the experiment were of analytical grade.
2.2. Solid-State Fermentation
For inoculation, B. amyloliquefaciens Y8, and B. subtilis GYB6 were precultured in broth medium at 37 °C for 18 h, while S. cerevisiae NJ1 was precultured in yeast extract peptone dextrose medium at 30 °C for 24 h. Then, the biomass was tested and adjusted to 108 CFU/mL for the further solid-state fermentation of RSM. Solid-state fermentation conditions were optimized with the comprehensive index of the decline of glucosinolates and the increase in peptides. The solid-state fermentation experiment was performed under the optimum conditions as follows: inoculation amount of mixed microbial culture 30% (B. subtilis GYB6 10%, S. cerevisiae NJ1 15%, and B. amyloliquefaciens Y8 5%); fermentation temperature 37 °C; fermentation moisture 55%; and fermentation time 96 h. After fermentation, the samples were dried in the oven at 60 °C for related index determination.
2.3. Measurement of Chemical Compositions
The palladium chloride method determined the glucosinolates according to the procedure described previously by [], which was slightly modified. An aliquot of the ground sample (100 mg) mixed with 80% alcohol (10 mL) was well stirred and shaken at 170 r/min at 40 °C for 4 h. Then, the samples were centrifuged at 4000× g for 10 min, and the extract solution was collected. The 1 mL of RSM or FRSM extract solution was added to a tube containing 2 mL of 0.15% sodium carboxymethylcellulose and 1 mL of 4 mM palladium chloride. After incubating at 24 °C for 1.5 h, the absorbance at 540 nm was measured. The mixture with sodium carboxymethylcellulose, palladium chloride, and distilled water was used as a reagent blank. Glucosinolates content was calculated by a sinigrin standard curve. The results were expressed as μmol glucosinolates/g dry sample.
Peptides were analyzed according to the method of Rawdkuen et al. [], slightly modified. A total of 9 mL of 5% TCA was added to 1 g of samples, and the mixture was shaken at the speed of 150 r/min at 30 °C for 1 h and centrifuged at 8000× g for 5 min. TCA-soluble peptides in the supernatant were measured by the method of Lowry.
The phytic acid content in samples was analyzed by the ferric chloride colorimetric method, as described by Sotelo et al. []. Tannins content was determined colorimetrically using the Folin–Denis reagent, according to Sugiharto et al. []. The crude fiber content was determined according to Van Soest et al. []. The crude protein content was analyzed by the Kjeldahl method (984.13, AOAC1990) []. RSM or FRSM samples (1 g) were extracted with 40% ethanol (20 mL), and the mixture was centrifuged at 8000 rpm for 10 min. The resulting supernatant was freeze-dried to test total phenolics and flavonoid content as well as antioxidant activity. Total phenolic content was detected using the Folin–Ciocalteu reagent as described previously by Olukomaiya et al. []. Total flavonoid content was estimated by a colorimetric method []. The amino acids were obtained by hydrolysis of RSM or FRSM at 110 ± 1 °C for 24 h with 6M HCl. The final hydrolysates were analyzed by Sykam automatic amino acid analyzer S 433-D (Sykam GmbH, Eresing, Germany) equipped with cation exchange chromatographic column LCA K06/Na.
2.4. The Molecular Weight Distribution of the Proteins
The molecular weight (MW) distribution of the proteins was determined by SDS-PAGE. The RSM and FRSM were dried at a low temperature of 35 °C and then crushed and screened. The proteins were extracted by NaOH. Briefly, 0.1 g of milled samples were added to 20 mL of 0.1 M NaOH and then shaken at 150 r/min at 37 °C for 3 h. Then, samples were centrifuged at 10,000× g for 10 min at 4 °C to collect the soluble fraction for SDS-PAGE analysis.
2.5. Antioxidant Activity
The DPPH radical scavenging activity assay was conducted following the method of Oskoueian et al. [], with slight modification. Briefly, 1 mL of the DPPH solution (0.1 mM in 95% ethanol) was added to 0.5 mL of sample solutions at different concentrations. The mixtures were incubated at room temperature for 30 min in the dark. The absorbance of the mixtures was detected at a wavelength of 517 nm using a spectrophotometer. The DPPH radical scavenging activity was calculated using the following equation:
where A1 is the absorbance of DPPH in ethanol, A2 is the absorbance of sample mixed DPPH, and A0 is the absorbance of the sample in ethanol.
DPPH radical scavenging activity (%) = [1 − (A2 − A0)/A1)] × 100%
Reducing power was conducted following the method of Liu et al. [] with slight modification. Briefly, 2.5 mL of phosphate buffer (pH 6.6, 0.2 M) and 2.5 mL of 1% potassium ferrocyanide were added to 2.5 mL of samples. The mixture was incubated at 50 °C for 20 min. Then, 2.5 mL of 10% trichloroacetic acid was added to the mixture and centrifuged. Subsequently, 2.5 mL of the supernatant, 2.5 mL of distilled water, and 0.5 mL of 1% FeCl3 were mixed and reacted at room temperature for 10 min. The reaction mixture was centrifuged at 6000 rpm for 10 min. The absorbance of the supernatant was measured at a wavelength of 700 nm using a spectrophotometer.
2.6. Functional Properties
The functional properties containing bulk density, water absorption capacity, swelling index, and swelling capacity were evaluated. Bulk density, water absorption capacity, and swelling index were determined according to the method described by Olukomaiya et al. []. The bulk density was expressed in g dry sample/mL sample volume. Water absorption capacity was expressed as percentage increase in the sample weight after adding distilled water. Swelling index was calculated as the ratio of the swollen volume to the initial volume after adding distilled water. Swelling capacity was measured according to the method described by Chandra et al. [] and expressed as the final volume (mL) occupied by the sample after adding distilled water.
2.7. Correlations among Variables Analysis
Principal component (PC) analysis was applied to analyze the correlation between the different variables, including nutritional components, anti-nutritional factors, bioactive compounds, antioxidant activity, and functional properties of RSM and FRSM. Variables with positive correlations cluster together, and variables with negative correlations are distributed at either end of the line passing through the origin. The coordinates of each variable correspond to the correlation and directionality with PC1 and PC2, respectively.
2.8. Structural Analysis
The microstructure of RSM and FRSM was observed using field-emission scanning electron microscopy (SEM; Tescan Mira3, Tescan, Brno, Czech Republic) in different magnifications. FTIR spectra were analyzed with the spectrometer (Nicolet iS5, Thermo Scientific, Waltham, MA, USA). Sample discs were prepared with KBr (1:200, w/w). The FTIR spectra from 500 to 4000 cm−1 were obtained with 32 scans at a resolution of 4 cm−1.
2.9. Statistical Analysis
All experiments were performed in triplicate, and the data were expressed as mean ± standard deviation. The content of phytic acid, tannins, crude fiber, crude protein, peptides, and amino acids was expressed as a percentage of dry sample weight. The content of total phenolics and flavonoids was expressed as mg total phenolics or flavonoids/g dry sample weight. Statistical analyses were performed using OriginPro 8 software (OriginLab Corp, Northampton, MA, USA). Data were analyzed for mean differences by one-way analysis of variance (ANOVA) using the Tukey test at a significance level of p < 0.05 and a highly significant level of p < 0.01.
3. Results and Discussion
3.1. Effects of Fermentation on Anti-Nutritional Factors of RSM
As the goitrogenic properties of glucosinolates, the detoxification of glucosinolates has constantly been a focus of RSM research []. RSM fermented with B. subtilis reduced glucosinolates from 38.70 µmol/g to 19.72 µmol/g []. B. subtilis YY-1 and S. cerevisiae YY-2 were applied to fermenting extruded-pretreatment RSM, and the degradation rate of glucosinolates was 88.62% []. Predictably, fermentation in the presence of B. subtilis, S. cerevisiae, and B. amyloliquefaciens in this study led to the elimination of almost all of the glucosinolate. Compared to unfermented RSM, the glucosinolates content in FRSM was decreased by 99.18% (Table 1). Glucosinolates comprise a glucose molecule and a sulfur-containing group of atoms with various side chains attached []. The glucose and sulphur moieties of the glucosinolate compounds presented in the RSM may be consumed by microbial enzymes, which may lead to the decrease in glucosinolates.

Table 1.
Anti-nutritional factors content in RSM and FRSM.
Except for glucosinolates, the content of three other anti-nutritional factors also declined significantly (p < 0.01) with the fermentation process (Table 1). The phytic acid content decreased significantly from 3.11% to 1.79% after fermentation, consistent with the result reported by Vig et al. [] that found that the RSM processed with R. oligosporus decreased the phytic acid content. The decrease in phytic acid may be attributed to the activity of phytase synthesized by microorganisms [,]. The reduction in crude fiber content was also pronounced when the fermentation process was completed. Vig et al. [] and Hu et al. [] obtained the same result with fermentation; crude fiber declined by 25.5% and 24.68%, respectively. However, an increase of 9.2% in FRSM was observed by Plaipetch et al. []. The difference in the variation of the crude fiber may depend on the fermentation conditions and the capacity of the microorganisms utilized for fermentations to degrade cellulose, hemicellulose, and lignin of RSM []. The tannins content of the FRSM decreased to 8.19%, compared to 11.25% in the RSM (Table 1). The reduction of 36.36% in tannins after fermentation of RSM with B. subtilis, Candida utilis, and Enterococcus faecalis was similar to the result observed in this study []. Such significant elimination of these anti-nutritional factors is essential from a nutritional point of view and will widen the application of RSM in feed.
3.2. Effects of Fermentation on Nutritional Components of RSM
Crude protein was increased from 33.16% in RSM to 38.56% in FRSM by the fermentation process, with an increased rate of 16.28% (Table 2). Likewise, studies reported an increase in the protein content from 34.84% to 40.72% for 72 h of fermentation with A. niger [] and 34.95% to 38.85% for 72 h of fermentation with S. cerevisiae []. Crude protein is a crucial index for feed, so increasing crude protein is crucial. The crude protein concentration of RSM was increased, possibly due to the mycoprotein synthesis by microbial fermentation at the expense of fermentable compositions [,]. Some authors also considered that gas production during fermentation led to the loss of a portion of the sample, and then the crude protein content of RSM was increased.

Table 2.
Crude protein, peptides, and amino acids content in RSM and FRSM.
Table 2 showed a much higher (p < 0.01) peptide content of FRSM, which was 4.00 times RSM. Small peptides have more advantages because peptides are not only easier to absorb but also exhibit bioactive activity such as antioxidant activity, immune activity, and nerve activity [,]. The increase in peptides was consistent with the fermentation of RSM with A. niger from 2.57% to 8.39% [] and B. licheniformis (1.0813), Yeast (ACCC20060), and Lactobacillus (ACCC10637) from 11.9 mg/g to 66.0 mg/g [].
The amino acids profile of RSM and FRSM is presented in Table 2. The total amino acids (TAAs) content in RSM and FRSM was increased from 30.90% to 36.48%. The content of non-essential amino acids (NEAAs) of the FRSM was 1.25 times RSM. Essential amino acids (EAAs) changed slightly with the fermentation (p > 0.05). The increase in TAAs was in harmony with the results of Wang et al. [] and in partial agreement with the results of Wang et al. [], in which the content of most of EAAs improved. Among the amino acids, the magnitude of change in the content of Asp, Leu, Thr, Ser, Glu, Gly, His, and Arg was apparent, and the content was increased by 13.57%, 20.08%, 25.00%, 32.32%, 33.86%, 22.66, 44.96%, and 54.20% (p < 0.01), respectively. The concentration of Ala and Pro was also upgraded by 12.13% and 7.44% (p < 0.05), respectively. Li et al. reported that RSM fermented with Bacillus subtilis only increases the Arg, Leu, and Met []. Glu is the natural umami amino acid that can enhance the flavor of low-salt foods. Asp, Leu, Thr, Ala, and Pro are hydrophobic amino acids that may relate to antioxidant activity []. Leu, Glu, Gly, and Arg are considered to be the functional amino acids to improve growth and health in mammals and fish []. The increases in the concentration of certain AAs and the changes in the AA profile of FRSM could be attributed to the rise of protein by fermentation and microbial metabolism during the fermentation [].
3.3. Effects of Fermentation on Protein Molecular Distribution of RSM
The SDS-PAGE patterns of the RSM and FRSM are presented in Figure 1a. Studies reported that RSM proteins mainly included cruciferin (12S globulin, α subunit, and β subunit) with six subunit pairs, napin (2S albumin), and some small proteins, such as insulin inhibitors and lipoproteins [,,]. As shown in Figure 1a, 12S globulin and 2S albumin approximately disappeared in FRSM (lanes 2, 3, and 4). It indicated that protease secreted by B. amyloliquefaciens, B. subtilis, and S. cerevisiae successfully hydrolyzed 2S globulin and 2S albumin. Many small-sized proteins or peptides (less than 14.4 kDa) emerged. The result was consistent with the increased content of peptides in this study. Similarity, Fermentation with B. licheniformis (1.0813), Yeast (ACCC20060) and Lactobacillus (ACCC10637) [], B. subtilis YY-1 and S. cerevisiae YY-2 [], A. sojae and A. ficuum [], and A. niger [] changed the protein molecular weight distribution of RSM.
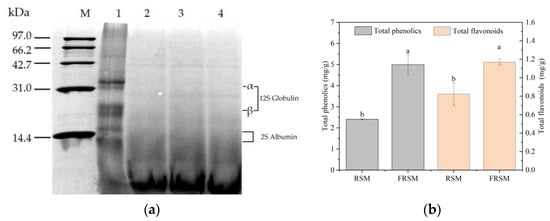
Figure 1.
(a) MW distribution presented by SDS-PAGE; M: protein marker; 1: protein sample of unfermented rapeseed meal (RSM); 2, 3, and 4: protein samples of fermented rapeseed meal (FRSM). (b) Total phenolic and total flavonoid content. a, b mean values which do not share a common superscript letter are significantly different (p < 0.05).
3.4. Effects of Fermentation on Bioactive Compounds of RSM
Phenolics and flavonoids are bioactive compounds, and their content is an important factor in determining the antioxidant capacity of RSM []. The effect of fermentation on the content of total phenolics and flavonoids was studied (Figure 1b). The results showed that the fermentation process significantly improved the total phenolic and flavonoid content of RSM (p < 0.01). The total phenolic content of FRSM (4.99 ± 0.47 mg/g) was significantly higher than that of RSM (2.41 ± 0.03 mg/g) (p < 0.01). The flavonoid content of FRSM (1.17 ± 0.03 mg/g) was also significantly higher than that of RSM (0.82 ± 0.12 mg/g) (p < 0.01). During the fermentation, a variety of enzymes, such as cellulase and hemicellulose, were secreted by the microorganism, which could break down the structure of RSM [] and may liberate phenolic and flavonoid compounds from the RSM. The increase in phenolic and flavonoid content may also be due to more or new phenolics and flavonoids synthesized by the microorganism during fermentation [,]. A similar result was reported by Wang et al. [], that the fermentation enhanced the total phenolic content of extruded-pretreatment RSM. Liu et al. reported that the total flavonoids content of dandelion increased significantly after fermentation []. However, reversely, the content of total phenolics declined by 21.58–23.55% using the yeast to ferment RSM []. It indicated that the microorganism would influence the metabolism of the substrate.
3.5. Effects of Fermentation on Antioxidant Activity of RSM
The DPPH radical scavenging activities of the RSM and FRSM is presented in Figure 2a. DPPH radical scavenging activities of RSM and FRSM were different in the concentration of 1.5 mg/mL (p < 0.05) and significantly different in the concentration of 0.3–1.2 mg/mL (p < 0.01). DPPH radical scavenging activity of FRSM was 91.63 ± 3.17%, 81.13 ± 3.52%, 54.08 ± 1.35%, and 39.72 ± 2.56%, respectively, at the concentration of 1.2, 0.9, 0.6, 0.3 mg/mL, while that of the RSM reached 77.90 ± 2.89%, 65.23 ± 4.51%, 47.79 ± 1.47%, and 29.32 ± 1.42%, respectively, at the same concentration. The EC50 of FRSM was 0.72 ± 0.01 mg/mL, while the EC50 of RSM was 0.91 ± 0.07 mg/mL, which indicated that the FRSM displayed a higher DPPH scavenging activity compared to the RSM.
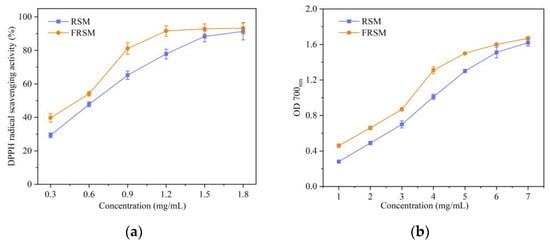
Figure 2.
Antioxidant activity of RSM and FRSM. (a) DPPH radical scavenging activity; (b) Reducing power; RSM = rapeseed meal; and FRSM = fermented rapeseed meal.
The reducing powers of the different concentrations of RSM and FRSM is presented in Figure 2b. The reducing powers of RSM and FRSM were significantly different in the concentration of 1–5 mg/mL (p < 0.01). OD700 nm of the FRSM was 1.50 ± 0.05, 1.31 ± 0.04, 0.87 ± 0.02, 0.66 ± 0.02, and 0.46 ± 0.01, respectively, at the concentration of 5, 4, 3, 2, and 1 mg/mL, while that of the RSM reached 1.30 ± 0.02, 1.01 ± 0.03, 0.70 ± 0.04, 0.49 ± 0.02, and 0.28 ± 0.01, respectively, at the same concentration. The EC50 of FRSM was 3.53 ± 0.18 mg/mL, while the EC50 of RSM was 4.78 ± 0.65 mg/mL, which indicated that the FRSM owned the better reducing power compared to the RSM.
According to the reaction mechanisms, methods commonly used to determine the total antioxidant capacity can be divided into two major groups: assays based on a single electron transfer reaction (SET) and assays based on a hydrogen atom transfer reaction (HAT) []. SET monitors through a change in color as the oxidant is reduced, while HAT detects the competition between the antioxidant and the substrate for the free radical. The results of two SET reaction-based methods, DPPH radical scavenging activity and reducing power, all confirmed that antioxidant activity of the FRSM was improved compared to that of the RSM, indicating that more bioactive compounds were produced by fermentation. Likewise, RSM inoculated with selected protease-assisting B. subtilis and L. plantarum showed stronger antioxidant activity than unfermented RSM [].
3.6. Effects of Fermentation on Functional Properties of RSM
The functional properties of RSM and FRSM are presented in Table 3. The functional properties were all enhanced significantly (p < 0.01) by fermentation, except for bulk density. Enhancing the ability of the meal to absorb and retain water is a valuable functional property because it may improve the binding of the structure, strengthen flavor retention, improve mouth feel, and reduce moisture and fat losses of food products []. FRSM exhibited a significant increase (p < 0.01) in water absorption capacity, the percentage increase (p < 0.05) being 24.09% compared to the RSM. This is comparable to that reported canola meal fermented by A. sojae and A. ficuum []. The value of the swelling index and swelling capacity of FRSM was higher than RSM’s. The swelling index of FRSM was 1.27 times RSM. The swelling capacity of 14.5 mL of RSM significantly increased (p < 0.05) to 24.8 mL of FRSM. The swelling index and swelling capacity values increase consistently with previous reports on fermented cassava flour [] and fermented canola meal [].

Table 3.
Functional properties of RSM and FRSM.
3.7. Correlations among Variables of RSM
The PC analysis of the chemical characteristics, metabolite production, antioxidant activity, and functional properties of the RSM and FRSM samples was shown as a loading plot (Figure 3). Total phenolics, flavonoids, and peptides were closely associated with a cluster that was positively related to DPPH radical scavenging activity andreducing power, indicating the increment of total phenolics, flavonoids, and peptides might be significant contributors to the upgrade of antioxidant activity of FRSM. Oskoueian et al. reported that phenolics and flavonoids were highly correlated to the field of antioxidant activities []. Wang et al. illustrated that the change trends of DPPH radical scavenging activity agreed with those of total phenolic and small peptides []. Crude protein and peptides were positively related to water absorption capacity, which agreed with the literature stating that increasing low molecular weight proteins and the number of polar protein groups could increase water absorption capacity []. Crude protein, peptides, and total amino acids were closely associated with a cluster. These parameters were negatively associated with glucosinolates, phytic acid, crude fiber, and tannins, highlighting solid-state fermentation’s importance for the improvement in nutritional values and degradation of anti-nutritional factors.
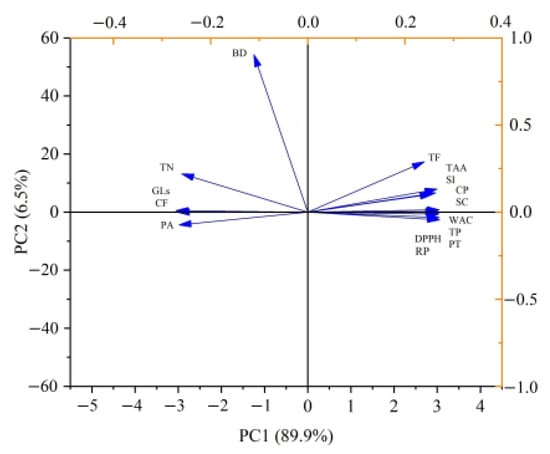
Figure 3.
Principal component analysis of the correlation between the different variables during solid-sate fermentation. GLs: glucosinolates; phytic acid: PA; crude fiber: CF; TN: tannins; TF: total flavonoids; TP: total phenolics; CP: crude protein; PT: peptide; TAA: total amino acids; DPPH: DPPH radical scavenging activity; RP: reducing power; BD: bulk density; WAC: water absorption capacity; SI: swelling index; and SC: swelling capacity.
3.8. Effects of Fermentation on Functional Groups and Chemical Bonds of RSM
The alterations in the functional groups and chemical bonds of the RSM and FRSM were observed with FTIR (Figure 4). Broad and robust peaks were evident at 3293 cm−1 because of the stretching and bending vibrations of the –OH groups, especially in cellulose and hemicellulose []; FRSM showed higher absorbance values as compared to RSM, which may be due to the structural changes in cellulose and hemicellulose in the RSM. Absorption bands at 2800–3000 cm−1 were attributable to the C-H stretching of methylene in the polysaccharides []. The peaks at 2926 and 2854 cm−1 were intensified after fermentation, suggesting the release of shorter-chain polysaccharides. RSM had the characteristic peaks at 1743 cm−1 (carbonyl group stretching of ester group) (C–O stretching of COOH in uronic acid). Still, FRSM had no such peak, possibly due to the degradation of uronic acids and pectin []. The band at 1448 cm−1 to the C–H bending vibration of cellulose and the band at 1068 cm−1 to the C–O stretching vibrations of polysaccharides or polysaccharide-like substances [,] were shifted to 1447 cm−1 and 1071 cm−1, and peak shape was changed, indicating the polysaccharides could be hydrolyzed during the fermentation. The absorption peak at 1658 cm−1, 1538 cm−1, and 1241 cm−1 is the characteristic peak of protein amide I, amide II, and amide III, respectively [,]. Compared to RSM, the peak was intensified, and peak positions of amide I, II, and III in FRSM were shifted. These results indicated that the protein structure was changed, and the amount of protein was increased. The present results indicate that the composition of RSM was changed substantially by fermentation. The cellulose, amino acids, proteins, and polyphenolic compounds contain functional groups, including carboxyl, hydroxyl, and methyl groups, which may offer therapeutic effects such as antimicrobial, anti-inflammatory, anticancer, and antioxidant activities [].
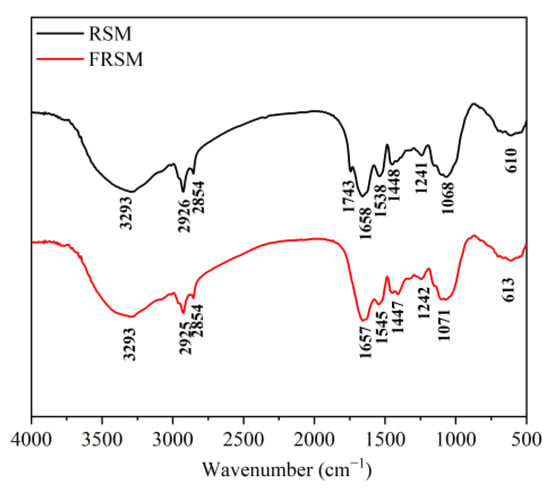
Figure 4.
FTIR spectra of RSM and FRSM; RSM = rapeseed meal; and FRSM = fermented rapeseed meal.
3.9. Effects of Fermentation on Microstructures of RSM
The microstructures of RSM and FRSM were observed by SEM (Figure 5). Microstructure was clearly different between RSM and FRSM. The surface structure of the RSM was dense. After solid-sate fermentation, the surface structure was changed substantially to be rough, loose, and porous for FRSM. It demonstrated that the three strains changed and decomposed the structure of RSM during the fermentation, which is consistent with the result of FTIR spectra. The loose and porous nature may contribute to the enhancement of the water absorption of FRSM in this study. Furthermore, RSM had smaller particles than FRSM. Extracellular hydrolases (especially lignocellulolytic enzymes) secreted during solid-state fermentation by microorganisms might have hydrolyzed the fiber and other components of RSM, and the products of hydrolysis were used by the microorganism, which led to the disruption of RSM []. Correspondingly, the decomposition may be beneficial in increasing the contact area between microbial enzymes and RSM, and then promoting the effective release of active substances. On the surface of FRSM, a large number of granules existed. This could be due to the production and release of more protein aggregated to become small granules during fermentation [].
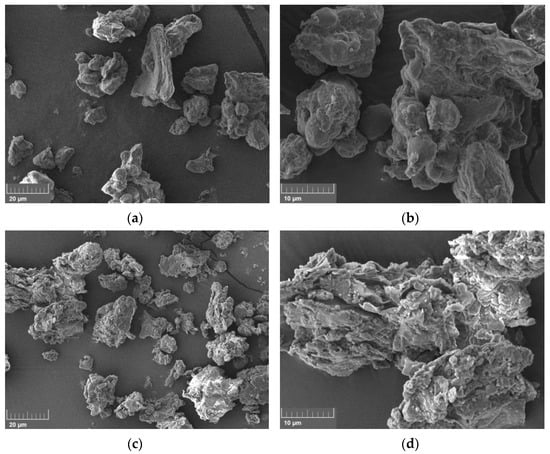
Figure 5.
Microstructure of unfermented and fermented rapeseed meal. (a,b): unfermented. Rapeseed meal (RSM); (c,d): fermented rapeseed meal (FRSM); Magnifications of SEM photographs (a,c) and (b,d) are 2000× and 5000×, respectively.
4. Conclusions
A novel solid-state fermentation conducted by B. subtilis GYB6, S. cerevisiae NJ1 and B. amyloliquefaciens Y8 was developed to transform the agricultural by-product RSM into high-value functional food ingredients rich in nutritional components and bioactive compounds. Concerning the anti-nutritional factors, glucosinolates, phytic acid, crude fiber, and tannins content in FRSM significantly decreased compared to RSM. The nutritional components (crude protein, amino acids, and peptides) and bioactive compounds (phenolics and flavonoids) all increased considerably after fermentation. Fermented RSM revealed better antioxidant activity and functional properties. These results indicated that FRSM could be a promising nutritional food ingredient in animal feed formulations.
Author Contributions
Conceptualization, X.Z. and X.L.; methodology, X.Z. and S.H.; software, Y.C. and S.H.; validation, S.J.; formal analysis, X.Z.; investigation, X.Z. and X.L.; resources, S.J.; data curation, S.J.; writing—original draft preparation, S.H. and Y.C.; writing—review and editing, X.Z., S.J. and X.L.; visualization, Y.C. and S.H.; supervision, X.Z. and X.L.; project administration, X.Z. and X.L.; funding acquisition, X.Z. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 22278171 and 32002408, and by the Provincial Key Research and Development Program of Jiangsu, grant number BE2020392.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Enhancing the Quality of Total Mixed Ration Containing Cottonseed or Rapeseed Meal by Optimization of Fermentation Conditions. Fermentation 2021, 7, 234. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; El-Hais, A.M.; Olivier, A. Effect of Partial Replacement of Fish Meal by Fermented Rapeseed Meal on Growth, Immune Response and Oxidative Condition of Red Sea Bream Juvenile, Pagrus major. Aquaculture 2018, 490, 228–235. [Google Scholar] [CrossRef]
- Kaur, H.; Wang, L.; Stawniak, N.; Sloan, R.; van Erp, H.; Eastmond, P.; Bancroft, I. The Impact of Reducing Fatty Acid Desaturation on the Composition and Thermal Stability of Rapeseed oil. Plant. Biotechnol. J. 2020, 18, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, X.; Xiao, Q.; Zhang, F.; Liu, N.; Tang, L.; Wang, J.; Ma, X.; Tan, B.; Chen, J.; et al. Rapeseed Meal and Its Application in Pig Diet: A Review. Agriculture 2022, 12, 849. [Google Scholar] [CrossRef]
- Wongsirichot, P.; Gonzalez-Miquel, M.; Winterburn, J. Recent Advances in Rapeseed Meal as Alternative Feedstock for Industrial Biotechnology. Biochem. Eng. J. 2022, 180, 108373. [Google Scholar] [CrossRef]
- Xie, C.; Li, W.; Gao, R.; Yan, L.; Wang, P.; Gu, Z.; Yang, R. Determination of Glucosinolates in Rapeseed Meal and Their Degradation by Myrosinase from Rapeseed Sprouts. Food Chem. 2022, 382, 132316. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.; Cao, L. Biotransformation Technology and High-value Application of Rapeseed Meal: A Review. Bioresour. Bioprocess. 2022, 9, 103. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Zou, Y.; He, R.; Ju, X.; Yuan, J. Effect of Static-state Fermentation on Volatile Composition in Rapeseed Meal. J. Sci. Food Agric. 2020, 100, 2145–2152. [Google Scholar] [CrossRef]
- Konkol, D.; Szmigiel, I.; Domzal-Kedzia, M.; Kulazynski, M.; Krasowska, A.; Opalinski, S.; Korczynski, M.; Lukaszewicz, M. Biotransformation of Rapeseed Meal Leading to Production of Polymers, Biosurfactants, and Fodder. Bioorg. Chem. 2019, 93, 102865. [Google Scholar] [CrossRef] [PubMed]
- Khajali, F.; Slominski, B.A. Factors that Affect the Nutritive Value of Canola Meal for Poultry. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef]
- Yao, J.; Chen, P.; Ringo, E.; Zhang, G.; Huang, Z.; Hua, X. Effect of Diet Supplemented with Rapeseed Meal or Hydrolysable Tannins on the Growth, Nutrition, and Intestinal Microbiota in Grass Carp (Ctenopharyngodon idellus). Front. Nutr. 2019, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.O.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state Fermentation of Canola Meal with Aspergillus sojae, Aspergillus ficuum and Their co-cultures: Effects on Physicochemical, Microbiological and Functional properties. LWT 2020, 127, 109362. [Google Scholar] [CrossRef]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state Fermented Plant Protein Sources in the Diets of Broiler Chickens: A Review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.C.; Fine, F. Rapeseed and Sunflower Meal: A Review on Biotechnology Status and Challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Stępniowska, A.; Kiesz, M. Effect of Fermented Rapeseed Meal as a Feed Component on the Redox and Immune System of Pregnant Sows and Their Offspring. Ann. Anim. Sci. 2022, 22, 201–219. [Google Scholar] [CrossRef]
- Bu, X.; Wang, Y.; Chen, F.; Tang, B.; Luo, C.; Wang, Y.; Ge, X.; Yang, Y. An Evaluation of Replacing Fishmeal with Rapeseed Meal in the Diet of Pseudobagrus ussuriensis: Growth, Feed Utilization, Nonspecific Immunity, and Growth-related Gene Expression. J. World Aquac. Soc. 2018, 49, 1068–1080. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of Fermentation Strategy in Aquafeed for Sustainable Aquaculture. Rev. Aquac. 2019, 12, 987–1002. [Google Scholar] [CrossRef]
- Vig, A.P.; Walia, A. Beneficial Effects of Rhizopus oligosporus Fermentation on Reduction of Glucosinolates, Fibre and Phytic acid in Rapeseed (Brassica napus) Meal. Bioresour. Technol. 2001, 78, 309–312. [Google Scholar]
- Wang, Y.; Liu, J.; Wei, F.; Liu, X.; Yi, C.; Zhang, Y. Improvement of the Nutritional Value, Sensory Properties and Bioavailability of Rapeseed Meal Fermented with Mixed Microorganisms. LWT 2019, 112, 108238. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Han, B.; Li, H.Y.; Liu, X.L. Improvement of Nutritional Value, Molecular Weight Patterns (soluble peptides), Free Amino Acid Patterns, Total Phenolics and Antioxidant Activity of Fermented Extrusion Pretreatment Rapeseed meal with Bacillus subtilis YY-1 and Saccharomyces cerevisiae YY-2. LWT 2022, 160, 113280. [Google Scholar] [CrossRef]
- Vlassa, M.; Filip, M.; Taranu, I.; Marin, D.; Untea, A.E.; Ropota, M.; Dragomir, C.; Saracila, M. The Yeast Fermentation Effect on Content of Bioactive, Nutritional and Anti-Nutritional Factors in Rapeseed Meal. Foods 2022, 11, 2972. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, X.B.; Chen, H.L.; Sun-Waterhouse, D.; Zhong, H.B.; Cui, C. A Value-added Approach to Improve the Nutritional Quality of Soybean Meal Byproduct: Enhancing Its Antioxidant Activity Through Fermentation by Bacillus amyloliquefaciens SWJS22. Food Chem. 2019, 272, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in Research on Solid-state Fermented Feed and Its Utilization: The Pioneer of Private Customization for Intestinal Microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Liu, X. A Novel Fermented Rapeseed Meal, Inoculated with Selected Protease-Assisting Screened B. subtilis YY-4 and L. plantarum 6026, Showed High Availability and Strong Antioxidant and Immunomodulation Potential Capacity. Foods 2022, 11, 2118. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Physicochemical Properties Analysis and Secretome of Aspergillus niger in Fermented Rapeseed Meal. PLoS ONE 2016, 11, e0153230. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Sai-Ut, S.; Khamsorn, S.; Chaijan, M.; Benjakul, S. Biochemical and Gelling Properties of Tilapia Surimi and Protein Recovered Using an Acid-alkaline Process. Food Chem. 2009, 112, 112–119. [Google Scholar] [CrossRef]
- Sotelo, A.; Gonzalez-Osnaya, L.; Sanchez-Chinchillas, A.; Trejo, A. Role of Oxate, Phytate, Tannins and Cooking on Iron Bioavailability from Foods Commonly Consumed in Mexico. Int. J. Food Sci. Nutr. 2010, 61, 29–39. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I. Assay of Antioxidant Potential of Two Filamentous Fungi Isolated from the Indonesian Fermented Dried Cassava. Antioxidants 2016, 5, 6. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Hendra, R.; Karimi, E. Bioactive compounds, antioxidant, xanthine oxidase inhibitory, tyrosinase inhibitory and anti-inflammatory activities of selected agro-industrial by-products. Int. J. Mol. Sci. 2011, 12, 8610–8625. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The Effects of Solid-state Fermentation on the Content, Composition and in Vitro Antioxidant Activity of Flavonoids from Dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of Functional Properties of Composite Flours and Sensorial Attributes of Composite Flour Biscuits. J. Food Sci. Technol. 2015, 52, 3681–3688. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ji, X.; Deng, X.; Hu, S.; Wang, J.; Ding, K.; Liu, N. Effect of Rapeseed Meal Degraded by Enzymolysis and Fermentation on the Growth Performance, Nutrient Digestibility and Health Status of Broilers. Arch. Anim. Nutr. 2023, 76, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Li, A.; Wang, Z.; Zhang, X.; Yun, T.; Qiu, L.; Yin, Y. Effects of Fermented Rapeseed Meal on Antioxidant Functions, Serum Biochemical Parameters and Intestinal Morphology in Broilers. Food Agric. Immunol. 2015, 27, 182–193. [Google Scholar] [CrossRef]
- Plaipetch, P.; Yakupitiyage, A. Effect of Replacing Soybean Meal with Yeast-fermented Canola Meal on Growth and Nutrient Retention of Nile tilapia, Oreochromis niloticus (Linnaeus 1758). Aquac. Res. 2014, 45, 1744–1753. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Q.; Huang, Z.; Wang, Y.; Roubik, H.; Yang, K.; Cai, M.; Sun, P. Solid-State Fermentation of Soybean Meal with Edible Mushroom Mycelium to Improve Its Nutritional, Antioxidant Capacities and Physicochemical Properties. Fermentation 2023, 9, 322. [Google Scholar] [CrossRef]
- Pereira, R.T.; Rosa, P.V.; Gatlin, D.M. Glutamine and arginine in diets for Nile tilapia: Effects on growth, innate immune responses, plasma amino acid profiles and whole-body composition. Aquaculture 2017, 473, 135–144. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Ballus, C.A.; Meinhart, A.D.; de Souza Campos, F.A.; Godoy, H.T. Total Phenolics of Virgin Olive Oils Highly Correlate with the Hydrogen Atom Transfer Mechanism of Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Sreerama, Y.N.; Sasikala, V.B.; Pratape, V.M. Nutritional Implications and Flour Functionality of Popped/Expanded Horse Gram. Food Chem. 2008, 108, 891–899. [Google Scholar] [CrossRef]
- Odey, G.N.; Lee, W.Y. Evaluation of the Quality Characteristics of Flour and Pasta from Fermented Cassava Roots. Int. J. Food Sci. Technol. 2019, 55, 813–822. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Impact of Germination on Flour, Protein and Starch Characteristics of Lentil (Lens culinari) and Horsegram (Macrotyloma uniflorum L.) Lines. LWT 2016, 65, 137–144. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, X.; Yue, Y.; Yue, T.; Yuan, Y. Improved flavonoid content in mulberry leaves by solid-state fermentation: Metabolic profile, activity, and mechanism. Innov. Food Sci. Emerg. Technol. 2023, 84, 103308. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohydr. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Alokika; Singh, B. Enhanced production of bacterial xylanase and its utility in saccharification of sugarcane bagasse. Bioprocess. Biosyst. Eng. 2020, 43, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Shen, Q.R.; Yu, G.H.; Ran, W.; Xu, Y.C. Fate of biopolymers during rapeseed meal and wheat bran composting as studied by two-dimensional correlation spectroscopy in combination with multiple fluorescence labeling techniques. Bioresour. Technol. 2012, 105, 88–94. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Dai, C.; Wang, Y.; Chen, W.; Ju, X.; Yuan, J.; He, R. Physical stability and microstructure of rapeseed protein isolate/gum Arabic stabilized emulsions at alkaline pH. Food Hydrocoll. 2019, 88, 50–57. [Google Scholar] [CrossRef]
- Yasar, S.; Tosun, R.; Sonmez, Z. Fungal fermentation inducing improved nutritional qualities associated with altered secondary protein structure of soybean meal determined by FTIR spectroscopy. Measurement 2020, 161, 107895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).