Abstract
This study aimed to implement a microencapsulated form of selected autochthonous lactic-acid bacteria (LAB) isolated from the cheese-production chain and natural rennet obtained from suckling lambs in the traditional production of hard sheep cheese, “Paški sir”, from the island of Pag, Croatia. Two different formulations of microparticles were prepared: (i) microparticles containing the strain of both Lactiplantibacillus plantarum and Lactococcus lactis (S2) and (ii) microparticles containing both strains and natural rennet (S3). These formulations were used in the production of Paški sir cheese simultaneously with standard production using non-encapsulated commercial starter cultures and commercial rennet (S1). The number of Lc. lactis isolates decreased at day 30 and were not isolated during the remaining ripening process, whereas the number of L. plantarum remained stable throughout the ripening process. The level of LAB and the release of the rennet from microsphere formulations at the end allowed for the production of cheese with the same characteristics as the commercial product, indicating no negative interactions of natural rennet, bacterial culture, and chemical components of microparticles. To our knowledge, this is the first report of a microencapsulated L. plantarum (isolated from the abomasum of lambs) coupled with natural lamb’s rennet used in the production of hard sheep cheese. This pilot study showed the great potential for maintaining authenticity in cheese production by combining traditional and sustainable innovative technologies.
1. Introduction
The development and introduction of novel technologies in the food industry have always been a priority to improve existing production and food quality. An example of such a technological process is microencapsulation, in which active ingredients are encapsulated in a suitable polymer material to obtain microparticles capable of retaining the biological activity of the encapsulated ingredient. In the food industry, using microencapsulation to protect, isolate, or control the release of an active ingredient offers numerous advantages [1,2,3] and mainly refers to the use of bioactive molecules and living cells [4]. In the dairy industry, especially in cheese production, the potential is closely related to the encapsulation of starter cultures [5,6,7], which may improve conditions during the ripening process compared to traditional inoculation. Moreover, the added value of such products lies in the bioactive compounds isolated from various natural sources, as they contribute to preserving biodiversity, quality, and recognition of the final product. In this context, the simultaneous encapsulation of more than one ingredient, such as lactic-acid bacteria and rennet, can lead to a product that can easily be incorporated into cheese production and simplify the whole process from a technological point of view [8].
However, there is a lack of research on the development and application of these microparticles in cheese making. Indeed, products containing encapsulated bioactive compounds are still rare in the food industry, in contrast to their widespread use in the pharmaceutical industry. Possible reasons for this are complex requirements for food matrices, as the sensory properties of foods must not be affected by the addition of microparticles, and being orally ingested, they must withstand the adverse conditions of the gastrointestinal tract [2]. This study aimed to produce a microencapsulated form of selected lactic-acid bacteria isolated from the traditional cheese-production chain as potentially the most suitable starter cultures alongside natural rennet obtained from suckling lambs. The formulated microparticles were used in traditional Paški sir (hard cheese from sheep milk, island of Pag, Croatia). When encapsulating lactic-acid bacteria and lamb’s rennet in biopolymeric microparticles the advantages are a smaller opportunity for dosage errors, less time spent in production (i.e., no need to wait 20–30 min after the addition of dairy cultures), their common influence on the ripening process, and the smaller production cost per unit of capsules when they are loaded with more bioactive components. The application of the formulations during the production of traditional Paški sir cheese has the biggest effect in the preservation of biodiversity. Moreover, the gradual release of encapsulated components influences the improvement of the aromatic properties of the final product. There are several studies that showed how the encapsulation process could be a great tool for ensuring the cells’ survival and viability in the intricate food matrix. For example, in the case of fermented sausages, the combination of non-encapsulated and encapsulated bacterial cultures may be particularly effective for the application of functional starter cultures with proven probiotic potential in order to enhance the hygienic and sensory qualities while also delivering probiotics in finished products [9,10].
This paper presents the results of the characterization of microsphere formulations, the isolation of lactic-acid bacteria, their identification, and the confirmation of encapsulation success by molecular analysis. In addition, the physicochemical properties of experimental cheeses during the ripening stages are presented as a conclusion of the effectiveness of microencapsulation as a method, especially considering the use of natural rennet and the selected lactic-acid bacteria based on their specific properties and the potential of using them as a starter culture.
2. Materials and Methods
As previously reported, 150 isolates of lactic-acid bacteria from raw sheep milk, lamb abomasum, cheese curds, and Pag-island cheese were identified [11]. Based on the biochemical properties identified, the Lactiplantibacillus plantarum strain from the lamb abomasum and the Lactococcus lactis strain from raw sheep milk were selected for microencapsulation. Two formulations were created: (i) microparticles containing both strains (S2) and (ii) microparticles containing both strains and natural rennet (S3). These novel formulations were applied in the production technology of Paški sir cheese simultaneously with standard production using non-encapsulated commercial starter cultures (Bioprox, Noyant-Villages, France) and commercial rennet (Bioren, Chr. Hansen, Hørsholm, Denmark) as the control (S1).
2.1. Microencapsulation
To generate sufficient biomass for microencapsulation, a pure culture of each strain was inoculated into 300 mL of de Man, Rogosa and Sharpe broth (MRS, Merck Millipore, Burlington, MA, USA) and incubated at 30 °C for 24 h. The broth containing the propagated culture was then centrifuged at 3800 rpm for five minutes (Eppendorf 5804r, Hamburg, Germany), the supernatant was discarded, and cells from both broths were resuspended in 15 mL of Brain Hearth Infusion broth (BHI, Thermo Fisher Scientific, Waltham, MA, USA). Cleaned and dried lamb’s abomasa were prepared and extracted according to Green [12] with some modifications. Abomasa were cut into 5–10 mm strips and put into a 6% (w/v) NaCl and distilled-water solution. The ratio of abomasa and NaCl solution was 1:8 (w/w). After the first 24 h, the extract was filtered and 50% of the solution amount was added from the day before. The next day (48 h after), the extract was filtered and 50% of the solution amount was added from the day before. After 72 h, the extract was filtered and all aliquots were combined and centrifuged for 45 min at 16,000–20,000 g to remove impurities. After extraction, the zymogen was activated to the active form of the enzyme by lowering the pH of the extract to 2.00 for 30 min with HCl (36.5% (v/v); 25% (v/v)) and then adjusting it to 5.5 with NaOH (2 mol/dm3; 1 mol/dm3; 0.5 mol/dm3). The clotting activity and chymosin/pepsin content were determined according to the relevant ISO standards [13,14]. Cultures and rennet were microencapsulated in the Department of Chemistry, Faculty of Agriculture, using a Büchi-B390 encapsulator (Labortechnik AG, Flawil, Switzerland). Fifteen mL of BHI broth was homogenized with 85 mL of sodium alginate. The final concentration of sodium alginate solution was adjusted to 1.5% (w/v).
The ionic-gelation technique was used to prepare the calcium-alginate microspheres containing the strains. A 100 mL sodium-alginate solution was dripped into 100 mL of 1 mol/dm3 calcium-chloride solution through a nozzle (450 µm) and continuously stirred using a magnetic stirrer. In the case of the S3 formulation, lyophilized lamb rennet in a concentration of 10% (w/v) was additionally homogenized with cultures and sodium-alginate solution and added to the calcium-chloride solution, as explained above. After the procedure, the microspheres were separated by filtration, washed with sterile water, and freeze-dried in an Alpha 1–2 LD Plus freeze-dryer (Christ, Osterode am Harz, Germany). Aseptic conditions were provided throughout the process of encapsulation.
2.1.1. Microscopic Observations
The mixture of natural rennet/mixtures of bacterial culture/2% CaCl2, microsphere size, shape, and morphology were examined by optical microscopy (OM) (Olympus BX 60, Center Valley, PA, USA) and a scanning electron microscope (SEM) (Axia ChemiSEM Scanning, Thermo FisherAbout, Waltham, MA, USA). One hundred microspheres were randomly selected from three batches to determine the size distribution. Mean diameters of wet and dry microspheres were determined by optical microscopy using Olympus Soft Imaging Solutions GmbH, version E_LCmicro_09Oct2009 (Münster, Germany).
2.1.2. Swelling Degree and Release of Rennet from Microsphere Formulation
A detailed method for determining the degree of swelling (Sw) has been previously described [15], and Sw was calculated using the following equation:
where wt is the weight of the swollen microspheres, and w0 is their initial weight.
In-vitro-release studies from microparticles were performed at room temperature. A system was formed by placing 0.01 g of dry microspheres in 50 mL of distilled water. The amount of rennet released was determined by UV/Vis spectroscopy (UVIVIS 1900i, Shimadzu, Kyoto, Japan). Results are presented as the fraction of rennet using the following equation:
where f represents the fraction of cumulatively released rennet, Rt is the rennet released at time t, and Rtot is the total amount of rennet loaded in the microsphere formulation. The rennet-release fraction (fRe) can be described by the following equation:
According to the Korsmeyer–Peppas model [16], the release constant k is related to the microspheres’ overall diffusive-solubility coefficient and geometric properties, and the exponent n is characteristic of the mechanism controlling the release rate.
2.1.3. Determination of Electrostatic Charge, Zeta Potential, and Size of Aggregates in Mixtures of Natural Rennet/Bacterial Cultures and Calcium-Chloride Solutions
Electrostatic charge and zeta potential (ζ/mV) of rennet suspensions were measured with a Zetasizer Nano ZS (Malvern, UK), which measures electrophoretic mobility based on laser Doppler particle electrophoresis. Zeta potential was estimated from electrophoretic measurements using Henry’s equation.
where ζ is the zeta potential, ε is the dielectric constant, Ue is the electrophoretic mobility, and η is the viscosity. In this case, fκa is 1.5 and is called the Smoluchowski approximation. The deviation was within ±1 mV. All measurements were performed in triplicate at room temperature. The hydrodynamic diameter (d/nm) was estimated by the Einstein–Stokes equation, assuming a spherical aggregate. Results are presented as the mean value of at least three to six measurements.
2.2. Cheese Production
Prepared microcapsules were used in the experimental production of traditional Paški sir cheese, with six cheese wheels made from each formulation (18 wheels in total). Every day, one 60 L milk batch was divided into three 20 L batches, with each group represented by one batch. Experimental production was conducted for six consecutive days, resulting in 6 cheese wheels for each group. Activated microparticles in distilled water were added to the pasteurized milk at 63–65 °C for 30 min and cooled to 33–34 °C, then stirred for 1 min. In S1 and S2, the cultures were added 25 min before the rennet, whereas in S3, they were added together. Other production stages were the same. Figure 1 displays the experimental-production process and its stages.
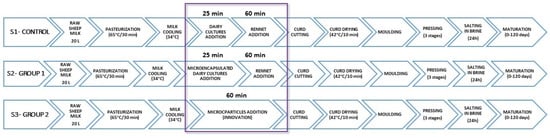
Figure 1.
Experimental-production scheme; S1—non-encapsulated commercial starter cultures and rennet; S2—microparticles containing Lactiplantibacillus plantarum and Lactococcus lactis and non-encapsulated natural rennet; S3—microparticles containing both strains and natural rennet.
In formulation S2, the microparticles (with L. plantarum and Lc. lactis) were added at a concentration of 0.20 g/20 L and rennet at a concentration of 0.43 g/20 L of milk. The concentration of microparticles in S3 was higher (2.15 g/20 L) due to the additional presence of rennet. The number of added particles was determined during preliminary tests combining payload release and curd firmness reached in 60 min. The rennet was added at 600 IMCU per 20 L of milk in both cases to make an equivalent of 3000 IMCU per 100 L of sheep milk, which is the average used in Paški sir cheese production. According to the manufacturer’s recommendations, commercial starter cultures in cheese group S1 were added at a concentration of 0.17 g/20 L. The firmness of the curd was tested after 60 min of coagulation, then cut and heated at 42 °C for 10 min. After shaping and pressing the curd, it was kept in brine for 24 h, then dried and ripened for 120 days (17 °C; 70–80% RH).
2.3. Microbiological Analysis
During the 120 days of ripening, cheese samples were taken every 30 days (90 samples in total), starting from day 0, and microbiologically analyzed at the Department of Hygiene, Technology, and Food Safety at the Faculty of Veterinary Medicine, University of Zagreb. Whey samples were collected with the cheese on day 0 and analyzed microbiologically (N = 18). For isolation and determination of the number of lactic-acid bacteria, 10 g of the sample were aseptically collected, diluted in 90 mL of saline, and homogenized for two minutes (Stomacher 400 Circulator, Seward, Worthing, UK). From the selected decimal dilutions, 0.1 mL was collected, surface inoculated onto MRS agar (BioMerieux, Marcy l’Etoile, France), and incubated for 48 h under anaerobic conditions (AnaeroGen, Thermo Fisher Scientific, Waltham, MA, USA) at 30 °C. After incubation, the number of bacteria per gram of sample was calculated using an automatic colony counter (Scan 1200, Interscience, Cantal, France) and expressed as log10 CFU/g. To gain insight into the microbial population and the proportion of bacterial species, at least ten morphologically distinct colonies were selected from each analysis and identified by MALDI-TOF mass spectrometry using the MBT Compass 5.0 software package (Bruker Daltonik, Bremen, Germany) at the Physical Chemistry Division of the Ruđer Bošković Institute, Zagreb, Croatia. Since the output of the MALDI Biotyper is a logarithmic-score value in the range of 0–3.0, indicating the probability of correct identification of the isolate, a score of 2000 to 3000 was used as the identification criterion, indicating a high-confidence species identification. After identification, the microencapsulated species isolated from each sample were stored in a microbiological-culture-preservation system at −80 °C (Deltalab, Barcelona, Spain) until further molecular analysis.
2.4. Molecular Analysis
A RAPD-PCR analysis was performed at the Veneto Agricoltura Institute of Food Quality and Technology (Thiene, Italy) to confirm the authenticity of the isolated L. plantarum strain with the original microencapsulated one. A total of 15 strains isolated during the ripening process was used for analysis, along with a reference strain and positive control L. plantarum ATCC 14917. After DNA extraction by thermal lysis with MicroLysis-Plus solution and Lysis Profile 1 according to the manufacturer’s instructions (Microzone, Stourbridge, UK), the RAPD-PCR protocol was performed with two specific primers (Sigma Aldrich, St. Louis, MO, USA) and an amplification program in the thermal cycler (PTC-200, MJ Research, Waltham, MA, USA) according to the primer used (Table 1). Identification of the RAPD-PCR products was performed by agarose-gel electrophoresis. In contrast, the similarity between the obtained RAPD-PCR profiles of the different isolates was compared and analyzed using a laboratory database and Gelcompare software (Applied Maths Software, Sint-Martens-Latem, Belgium) with a dendrogram as the final result.

Table 1.
Primers and corresponding amplification cycles used for RAPD-PCR analysis.
2.5. Physicochemical Cheese Analysis
Cheese samples (N = 90) were taken every 30 days during the 120 day ripening period. The samples were taken on days 0, 30, 60, 90, and 120 and transported to the Department of Dairy Science at the Faculty of Agriculture, University of Zagreb. Analyses were done in the Reference laboratory for milk and Dairy products accredited according to HRN EN ISO/IEC 17025 [17]. Standard analyzed parameters were (1) total solids content [18], (2) protein content per the Kjeldahl principle [19], (3) milk-fat content [20], (4) NaCl content [21], and (5) pH values measured with the “Seven Multi” (Mettler Toledo, Greifensee, Switzerland) pH meter per the manufacturer’s instructions.
2.6. Statistical Analysis
Results were analyzed using descriptive statistics methods (Statistica 13.5, TIBCO Software, Palo Alto, CA, USA; PROC GLM, SAS 9.4, Cary, NC: SAS Institute Inc., Greenwood Village, CO, USA) by determining the arithmetic mean with standard deviation (x ± SD) and the minimum (min) and maximum (max) values. A one-way ANOVA was used with a probability level of 0.05 to determine statistically significant differences between the number of lactic-acid bacteria in the experimental cheese groups by sampling days. In contrast, differences between individual isolates were determined by post-hoc analysis. The same test was used to determine differences between observations regarding experimental cheese groups (N = 3) and maturation stages (N = 5) at a probability level of 0.001. The proportion of bacterial species within the different cheese groups and sampling days was visualized by heat-map analysis.
3. Results
3.1. Microsphere Morphology, Shape, and Size
Before the simultaneous encapsulation of sheep rennet and bacterial cultures (L. plantarum, Lc. lactis) in microparticles, lyophilization of rennet was performed, which converts rennet from liquid to powder (solid). The solid form of rennet was mixed with prepared bacterial cultures. The mixture was homogenized with sodium alginate to obtain microspheres through ionic gelation with a 2% solution of CaCl2. The microphotographs obtained by optical microscope (OM) and scanning electron microscope (SEM) of the set-up mixtures are presented in Figure 2.
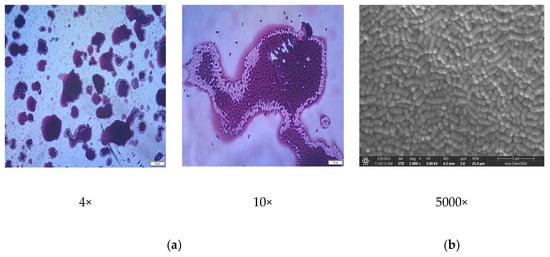
Figure 2.
Microphotographs of (a) rennet and mixture of bacterial cultures obtained by optical microscope (OM) and (b) 2% solution of calcium-chloride solution obtained by scanning electron microscope (SEM). Bars and magnifications are indicated.
Spherical microparticles were formed by rennet encapsulation in a biopolymer matrix of sodium alginate under the action of calcium ions as gelling cations. Figure 3 shows microphotographs of calcium-alginate microspheres, rennet-loaded calcium-alginate microspheres, and calcium-alginate formulations containing both rennet and bacterial strains (S3) obtained via the ionic-gelation process (wet) and after the drying process to a constant mass via the lyophilization process (dry).
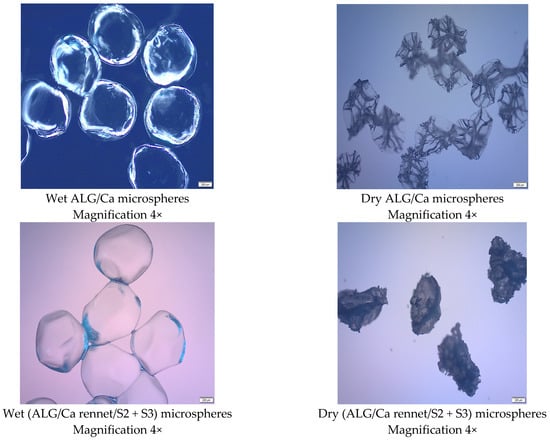
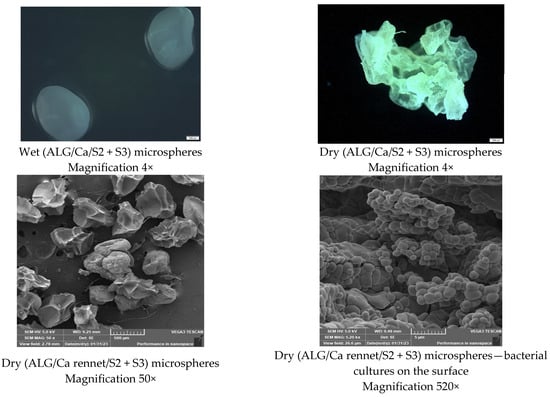
Figure 3.
Microphotographs of wet and dry microsphere formulations obtained by optical microscope (OM) and scanning electron microscope (SEM). Bars and magnifications are indicated.
3.2. Effect of Calcium-Ion Concentration on Bacterial Cultures and Rennet Mixtures in Solutions
The influence of calcium-cation concentration used for microsphere production on the mixture of bacterial-cell cultures and rennet solutions was examined microscopically and with size/zeta-potential measurements. The values of size and zeta-potential measurements of natural rennet, mixtures of bacterial cultures, and rennet, as well as mixtures of natural rennet, bacterial cultures, and 2% calcium ions, are presented in Table 2.

Table 2.
The values of size and zeta-potential measurements of natural rennet, mixtures of bacterial cultures L. plantarum and Lc. lactis, mixtures of natural rennet and 2% calcium ions, mixtures of bacterial cultures L. plantarum and Lc. lactis with rennet, and mixtures of natural rennet, bacterial cultures, and 2% calcium ions.
The shape and size of the formulation of microspheres, as well as the charge of the encapsulated rennet and mixture of bacterial cultures, has a significant influence on their release mechanism and kinetics, which is in accordance with research on their release in the production process of traditional Pag cheese. There is already evidence that the most basic function of particles, degradation to release bioactive components, depends on particle shape [22].
3.3. Kinetic and Mechanism of Natural Rennet Release from Microspheres
When microsphere formulations are dispersed in water, the activation and releasing process occur over a particular period of time, which is associated with a change in specific properties of microparticle formulations, such as stability, mechanical strength, the permeability of the aqueous medium, and disintegration time [23]. Previous knowledge about the release of bioactive components from biodegradable microspheres has enabled the selection of optimal parameters for the simulated encapsulation of the mixture of bacterial cultures and rennet in order to achieve optimal release [24].
The release profile of natural rennet and bacterial strains from prepared formulations is shown in Figure 4.
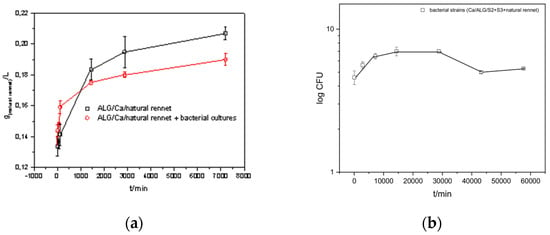
Figure 4.
(a) The amount of rennet released in time t from the prepared ALG/Ca/natural rennet microsphere formulations (black line) and ALG/Ca/natural rennet + bacterial cultures (red line), (b) fraction of released average (log10 CFU/mL) of released bacterial strains with time from ALG/Ca/natural rennet + bacterial culture microspheres at the optimal concentration of calcium chloride, c(CaCl2), and sodium alginate. The figure also shows the standard deviations.
The values of the release constant k and exponent n are shown in Table 3.

Table 3.
Variation of release constant (k), exponent (n), and correlation coefficient (R2) of natural rennet and bacterial cultures released from (ALG/Ca/natural rennet) microsphere formulations.
The swelling degree of the ALG/Ca/natural rennet was 53.88 ± 3.71% and that of ALG/Ca/natural rennet + bacterial cultures was 208.63 ± 18.95, whereas the swelling degree of microspheres without rennet was 48.07 ± 12.21%. Knowledge of kinetics and the mechanism controlling the release of components is essential for creating optimal microparticle formulations based on biopolymer materials. The design of controlled-distribution systems involves the optimization of many parameters. The most important parameters are the biopolymer type and concentration of biopolymer and gelling cations.
3.4. Isolation and Identification of Lactic-Acid Bacteria
The initial number of bacterial cultures before encapsulation was 9.39 ± 0.11 log10 CFU/mL, which provided sufficient biomass for encapsulation. After lyophilization, the average number of bacteria in both formulations was 10.69 ± 0.27 log10 CFU/g microparticles, the weight of which was adjusted so that the final number of bacteria reached approximately eight log10 CFU/L milk, comparable to the recommended dosage of commercial starter cultures used in the control group. The results of the microbiological analysis of the cheeses during the ripening process are shown in Table 4.

Table 4.
Lactic-acid bacteria count (log10 CFU/g ± SD) in cheeses produced with different (non)encapsulated formulations.
The average number of lactic-acid bacteria was highest on day 0 (8.36 ± 0.49 log10 CFU/g) and gradually decreased toward the end of the ripening process (Table 4). After every 30 days, the number varied uniformly among the three cheese groups but without statistically significant difference (p > 0.05). The average number of lactic-acid bacteria in whey was 3.53 ± 0.54 log10 CFU/mL. As expected, the highest number was in whey made from cheese produced with commercial starter cultures and commercial rennet (3.78 ± 0.55 log10 CFU/mL) but without significant difference compared to groups with encapsulated indigenous cultures and natural rennet (p > 0.05).
MALDI-TOF analysis after day 0 showed that Lc. lactis accounted for most of the bacterial population (>80%), followed by Leuconostoc mesenteroides and L. plantarum in all three groups. However, the number of Lc. lactis isolates decreased at day 30 and were not isolated later during the ripening process in all three groups. On the other hand, the percentage of isolated L. plantarum strains increased at day 30 and was most abundant at day 60 and 90 in groups S2 and S3, respectively, whereas their proportion was significantly lower in the cheese batch with commercial starters, which could be an initial indicator of successful encapsulation. In addition to the above species, Enterococcus faecium and Micrococcus luteus were also frequently isolated.
3.5. Molecular Analysis of the L. plantarum Isolates
Of the 15 L. plantarum isolates tested from the S3 cheese, RAPD-PCR analysis showed that 12 isolates (80%) had more than 93% similarity to the encapsulated reference strain, with the highest degree of similarity (97%) to a strain isolated on day 0 (Figure 5). This percentage was lower (83%) for one isolate from day 90, whereas the other two had a different profile and showed more similarity to Leuconostoc mesenteroides and Enterococcus durans in the database.
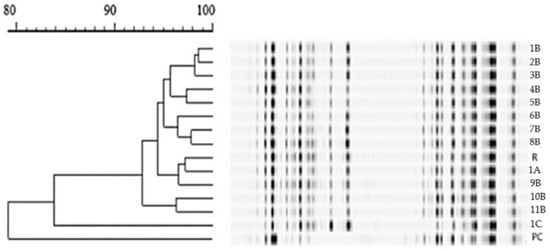
Figure 5.
Obtained RAPD-PCR profiles of L. plantarum isolates. R—referent strain; PC—positive control; A—strain with 97% of similarity; B—strains with more than 93% of similarity; C—strain with 83% of similarity.
3.6. Physicochemical Cheese Analysis
Produced natural rennet, as described in the previous section, had total milk-clotting activity of 1800 IMCU/g [13] and a chymosin: pepsin ratio of 80:20% [14]. During cheese production, there was no significant difference between the S1 group of cheese and those produced with natural rennet and indigenous lactic-acid bacteria regarding curd firmness, whey separation, and the overall production process. The one-way ANOVA showed differences in the determined parameters regarding sampling days (p < 0.001). No significant differences were found between experimental cheese groups (p > 0.05). Table 5 shows the mean values of the measured parameters regarding groups and sampling days (ripening stages).

Table 5.
Physicochemical properties of experimental cheeses by groups and ripening stages.
4. Discussion
In recent years, encapsulation technology has been extensively used to improve the viability of bacterial species and the associated functional properties of the final product [25,26,27]. In the group of lactic-acid bacteria, Lactiplantibacillus plantarum and Lactococcus lactis are the two species most commonly used for lactic-acid fermentation [28,29]. For this reason, and based on our preliminary results [30,31], these two species were characterized and selected for the encapsulation experiment in the production of Paški sir cheese.
From the microphotographs shown in Figure 2, it is easy to see the change in shape, morphology, and color of the rennet-filled microspheres. The plain microspheres are spherical, transparent in color, and wrinkled on the surface because of sodium alginate’s ionic-gelation process and calcium-cation gelation. The wrinkled surface structure of the rennet-free microspheres can be explained by the difference in the concentration of sodium alginate on the surface and inside the microspheres, which is consistent with previous studies [23].
The microparticle formulations loaded with rennet and bacterial cultures have smooth, white, irregular spherical structures. The observed changes result from rennet/bacterial-cell interactions incorporated into the calcium-alginate biopolymer matrix. In general, the size and size uniformity of the microparticle formulations are mainly determined by the experimental fabrication conditions (viscosity of the alginate solution, diameter of the tip of the needle (nozzle) adding the solution, calcium-chloride concentration, and flow rate) and the velocity of the alginate solution and distance from the syringe (nozzle) to the gelling-cation bath [32,33].
The microsphere formulations produced were approximately millimeters in size. The average sizes of the microparticle formulations loaded with rennet were 1386.51 ± 0.231 mm and 1181.21 ± 0.153 mm without a mixture of rennet and bacterial cultures. The slightly larger value of the rennet-filled microsphere size can be explained by electrostatic interactions between the negatively charged rennet (−14.38 ± 1.402 mV) and negatively charged mixtures of bacterial cells (−22.27 ± 0.4727 mV) and the positively charged calcium ions. Adding calcium ions to the rennet solution improved rennet flocculation and coagulation and changed the zeta potential of the rennet through the process of electrostatic interactions (Figure 2; Table 2) because calcium ions hold the protein structure together and change the size, color, and morphology of the microparticle formulations [34,35]. Lactic-acid bacteria are Gram-positive bacteria; their cell walls consist of a complex structure of macromolecules. The cytoplasmic membrane is surrounded by a peptidoglycan sacculus embellished with proteins, teichoic acids, and other glycopolymers such as polysaccharides [36]. Precisely because of this structure, they can bind metal ions to the membrane. The binding of calcium ions to the membrane of Gram-positive bacteria leads to partial neutralization of the cell membrane, which also causes a reduction in repulsion between bacterial cells and their grouping into larger aggregates. Because of that, such formulations of microspheres have a white color [23]. Moreover, the addition of calcium ions increases the adhesion of bacterial cultures, which is also one of the leading forces in their grouping at the surfaces [37].
After freeze-drying, the calcium-alginate microspheres (ALG/Ca) and microspheres were loaded with natural rennet and the bacterial mixtures (ALG/Ca/natural rennet + bacterial mixtures) lost their spherical character, and these particles acquired an irregular structure indicative of a porous structure. Changes in shape, size, and morphology were caused by complete water loss.
After water loss, the microspheres shrank significantly, reducing their size. The average size of the lyophilized ALG/Ca microparticles was 542.31 ± 0.69 µm and of the ALG/Ca/natural rennet + bacterial mixtures was 861.61 ± 43.21 µm, respectively. Complete moisture loss resulted in an average size reduction of over 60%. The size and morphology of wet and dry microspheres with bacterial strains were similar to the other types of microparticle formulation. The morphology and surface changed dramatically, becoming rough, porous, and layered. Additionally, the color of the lyophilized ALG/Ca/microparticle formulations was transparent, whereas the color of the lyophilized ALG/Ca/natural rennet + bacterial mixture microparticles was white because of natural-rennet and bacterial-mixture encapsulation.
The kinetics and mechanism of rennet release were investigated for microparticles prepared with a fixed concentration of calcium chloride and a fixed concentration of sodium alginate. It is important to emphasize that natural rennet is characterized by an initial rapid release followed by a slow release. The mechanism controlling natural rennet from both types of microsphere-formulation release is a diffusion mechanism. The gelation rate of alginate is a crucial factor in controlling the uniformity and strength of the microspheres. Slow gelation produces a homogeneous structure with better mechanical properties. Homogeneous microparticles improve the diffusion rate due to the uniform pore size, whereas heterogeneous microparticles diffuse slowly in a gel-network structure. In the case of formulations loaded with natural rennet and a mixture of bacterial cultures, smaller amounts of rennet are released, which occurs because of complex interactions between rennet, bacterial cultures, and calcium ions. In the formulation of microparticles loaded with natural rennet and a mixture of bacterial cultures, larger aggregates are formed, which causes a decrease in the amount of natural rennet released. The release curve of the bacterial strains indicates their long-term survival and activity even after 40 days, which indicates that the formulations can be stored for a long time without losing their activity.
According to the Korsmeyer–Peppas model [16], the release constant k includes the total-dissolution-diffusion coefficient and the geometric characteristics of the particles, and the exponent n is characteristic of the mechanism that controls the release rate. The value for n < 0.45 indicates that the controlling mechanism of release was the Fickian diffusion mechanism, indicating a reduced concentration gradient over time (Table 3). The K value was higher for the formulations loaded with both natural rennet and mixed bacterial cultures, suggesting that at the beginning, there was a faster release of natural rennet from those types of microparticles. On the other hand, the bacterial cultures that were located on the surface of the microparticle formulations (ALG/Ca/bacterial cultures) were released relatively quickly (K value was high) by the diffusion process, which achieved a pH value that is optimal for cheese production.
The release profile of rennet from the prepared formulations of ALG/Ca/natural rennet microparticles is related to the degree of swelling Sw. The gel swells under external pressure (swelling pressure) due to the action of the solvent. At equilibrium, the swelling pressure is zero due to the balance of two opposing trends. Generally, the increase in entropy is due to the mixing of polymer, solvent, and network [38].
Swelling values for microparticles loaded with rennet (ALG/Ca/natural rennet + bacterial cultures) were relatively high because calcium promotes increased coagulation of natural rennet and bacterial cells. The calcium ions, bacterial cells, and natural rennet caused an increase in gel-network-density cross-linking and, thus, the degree of swelling. Similar results have been previously reported for the encapsulation of cells [23]. This may be due to electrostatic repulsion between the negatively charged natural rennet enzyme, bacterial cells, and the free portion of the alginate chain (the zeta potential of calcium-alginate matrices is approximately −10 V) [39] and the mechanical interaction.
Regarding the survival rate of the culture in microparticles, preliminary studies were performed with the L. plantarum strain. It was demonstrated that the dynamics of the culture’s survival in microparticles follow the dynamics of its release, leading to the conclusion that microspheres create favorable conditions for the growth of the strain [11]. These results agree with other studies that proved that the microencapsulation process has a positive effect on the survival and activity of bacteria [40,41,42,43]. However, their release may depend on various factors, such as the encapsulation-material type, the polymer concentration [15], and the ambient temperature [44]. However, our results [11] show that the strain L. plantarum was released immediately when in contact with the aqueous medium on the day of encapsulation and reached its highest value on the fifth day, after which its concentration gradually decreased. Similar results were obtained for the strain Lactobacillus sakei by Jurić et al. [23].
As expected, the highest number of lactic-acid bacteria was at day 0 in all three groups but without significant differences in commercial starter cultures and rennet, indicating that the microparticles largely facilitated the release of bacterial cells in a short period of time. There were no differences in further sampling either, as their numbers varied evenly between the three groups throughout the ripening process, with a final number of 6.83 ± 0.41 log10 CFU/g at day 120. These results contrast with those of other studies [27,45,46], in which the authors found significant differences between the number of encapsulated cultures, slightly higher than those used as free cells. As Özer et al. [45] explained, a possible reason for the limited decline in the number of encapsulated bacteria could be the slow decay of the microparticles during the ripening process or the delayed release of the cells in the deeper layer of the polymer [23].
In both cases, this information may also indicate the success of encapsulation, i.e., the fact that only a tiny portion of the microparticles was released from the whey. Accordingly, in our study, higher CFU losses in the whey were observed in the cheese group with commercial cultures but without any significant difference between the other two groups. However, Fortin et al. [25] demonstrated the same with encapsulated cultures, which could indicate lower CFU levels in the curd. However, as the author noted, the fraction that ended up in the whey was only a small portion of the added cells, so this ultimately did not affect the number of viable cells in the curd.
Among encapsulated cultures, Lc. lactis accounted for the majority of the isolated population in all groups on day 0 (>80%), in contrast to L. plantarum, which was less than 10%. Since no molecular analysis of Lc. lactis was performed, we cannot know whether it originates from microparticles or milk due to its abundance in the microflora of sheep milk and other animal species [47,48]. Since there are no data in the literature on the release kinetics of two different bacterial species and they are contained in the same concentration in the microparticles, we can hypothesize that the size of the bacterial cells, in this case, could be a reason for the increased number of Lc. lactis. However, these hypotheses are verified by SEM microscopic analyses of the microsphere-microparticle formulation surface, which showed that Lc. lactis cells were on the surface (Figure 3).
Nevertheless, already at day 30, the percentage of Lc. lactis isolates decreased, whereas at the same time, the population of L. plantarum increased due to its strong competitive ability [31], which is probably the reason for the inhibition of Lc. lactis growth since it was no longer isolated at the end of the ripening. In a similar study by Salazar-Montoya et al. [46], Lc. lactis was encapsulated in a separate form and the microorganisms remained viable throughout the ripening process, with no decrease in the population by the end of the ripening period.
The percentage of isolated L. plantarum strains in both experimental cheese groups gradually increased from day 30, peaked at days 60 and 90, and then decreased toward the end of ripening. In contrast to Lc. lactis, L. plantarum was isolated in the S1 group only on day 0, where it represented less than 10% of the population, adding to the evidence of RAPD-PCR analysis confirming the authenticity of the isolated strains with the encapsulated one.
Together with indigenous lactic-acid bacteria, the production of natural rennet from lamb abomasa has the potential to preserve biodiversity and utilize animal by-products. Some authors suggest that lamb rennet is best for clotting that species’ milk [49], and thus, the produced rennet has higher overall clotting activity than other natural rennets on the market, and the chymosin: pepsin ratio of 80:20% is optimal for producing ripened cheeses [8]. The physicochemical results show no significant differences in the determined parameters regarding experimental cheese groups (p > 0.05, N = 3) but show significant differences between ripening stages (p < 0.001, N = 5). Differences in ripening stages or sampling days were expected. During the ripening stages, water evaporated, significantly increasing other parameters. Total solids differed in every stage, whereas milk fat and protein content had no significant differences between days 30 and 60. Chloride content and pH values were only significantly different between days 0 and 30 (p < 0.001), when the majority of water and whey evaporated. The aforementioned differences are expected for Paški sir cheese while it is ripening [50]. The absence of differences between experimental groups can be noted as the successful production of natural rennet and dairy cultures from the indigenous territory and its implementation into a traditional cheese, Paški sir, which is PDO (Protected Designation of Origin). It can be an excellent way to introduce new technologies into conventional cheese production, emphasizing the local in contrast to the global.
5. Conclusions
The present study results show that the number of microencapsulated indigenous L. plantarum reached the required level of free bacterial cells compared to commercial cultures, especially in the S3 cheese batch, where cultures and natural rennet were applied together in microencapsulated form. It indicates no negative interactions of natural rennet, bacterial culture, and chemical components of the microparticles. The number of encapsulated indigenous L. plantarum remained stable throughout the ripening process, and in the end, a cheese with the same characteristics as the commercial product was produced. The physicochemical properties and release of rennet from microsphere formulations indicate that encapsulation of rennet with calcium alginate has excellent potential for application in the hard-sheep-cheese manufacturing process. To our knowledge, this is the first report of using an encapsulated strain of lactic-acid bacteria isolated from the abomasum of lambs and natural rennet in the production of hard sheep cheese. This approach can benefit traditional and boutique cheese varieties by making them safer with pasteurization and unique by adding indigenous dairy cultures. The preservation of biodiversity can have a great added value through the diversification of cheeses of the same type (i.e., semi-hard, hard cheese) on a local and global scale.
Author Contributions
Conceptualization, M.K. and N.Z.; methodology, N.Z. and M.K.; software, M.K.; validation, N.Z., N.M., S.J. and M.V.; formal analysis, M.K., S.K. and N.Z.; investigation, M.K., S.J., I.M. and F.O.; resources, S.K., M.V. and F.O.; writing—original draft preparation, M.K.; writing—review and editing, N.Z., M.V., V.D. and N.M.; visualization, M.K.; supervision, N.Z.; project administration, N.M.; funding acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Potential of microencapsulation in cheese production” KK.01.1.1.04.0058 from the Operational Program Competitiveness and Cohesion 2014–2020 and supported by the “Food Safety and Quality Center” KK.01.1.1.02.0004 project, funded by the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful to the Veneto Agricoltura Institute of Food Quality and Technology (Thiene, Italy) for their support in performing RAPD-PCR analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A review of applications in the food and pharmaceutical industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Do Amaral, P.H.R.; Lopes Andrade, P.; Costa De Conto, L. Microencapsulation and Its Uses in Food Science and Technology: A review. In Microencapsulation-Processes, Technologies and Industrial Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Yang, M.; Liang, Z.; Wang, L.; Qi, M.; Luo, Z.; LI, L. Microencapsulation Delivery System in Food Industry-Challenge and the Way Forward. Adv. Polym. Technol. 2020, 2020, 7531810. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Masondole, L. Survival of free and microencapsulated Lactobacillus acidophilus and Bifidobacterium lactis and their effect on texture of feta cheese. Aust. J. Dairy Technol. 2005, 60, 252–258. [Google Scholar]
- Özer, B.; Uzun, Y.S.; Kirmaci, H.A. Effect of microencapsulation on viability of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-12 during Kasar cheese ripening. Int. J. Dairy Technol. 2008, 61, 237–244. [Google Scholar] [CrossRef]
- De Prisco, A.; Van Valenberg, H.J.F.; Fogliano, V.; Mauriello, G. Microencapsulated Starter Culture During Yoghurt Manufacturing, Effect on Technological Features. Food Bioprocess Technol. 2017, 10, 1767–1777. [Google Scholar] [CrossRef]
- Oštarić, F.; Antunac, N.; Cubric-Curik, V.; Curik, I.; Jurić, S.; Kazazić, S.; Kiš, M.; Vinceković, M.; Zdolec, N.; Špoljarić, J.; et al. Challenging Sustainable and Innovative Technologies in Cheese Production: A Review. Processes 2022, 10, 529. [Google Scholar] [CrossRef]
- Mrkonjić Fuka, M.; Žgomba Maksimović, A.; Hulak, N.; Kos, I.; Marušić Radovčić, N.; Jurić, S.; Tanuwidjaja, I.; Karolyi, D.; Vinceković, M. The survival rate and efficiency of non- encapsulated and encapsulated native starter cultures to improve the quality of artisanal game meat sausages. J. Food Sci. Technol. 2021, 58, 710–719. [Google Scholar] [CrossRef]
- Ribeiro, L.L.S.M.; Araújo, G.P.; de Oliveira Ribeiro, K.; Torres, I.M.S.; De Martinis, E.C.P.; Marreto, R.N.; Alves, V.F. Use of encapsulated lactic acid bacteria as bioprotective cultures in fresh Brazilian cheese. Braz. J. Microbiol. 2021, 52, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Kiš, M.; Kazazić, S.; Vinceković, M.; Dobranić, V.; Oštarić, F.; Mikulec, N.; Zdolec, N. Potential of microencapsulation in cheese production: Selection of indigenous dairy culture for Pag cheese. In Proceedings of the Veterinarski dani 2021, Vodice, Croatia, 26–29 September 2021; pp. 363–370. [Google Scholar]
- Green, M.L. Preparation and properties of rennets from lamb’s and kid’s abomasa. J. Dairy Res. 1980, 47, 221–230. [Google Scholar] [CrossRef]
- ISO 23058:2006; Milk and Milk Products—Ovine and Caprine Rennets—Determination of Total Milk-Clotting Activity. ISO: Geneva, Switzerland, 2006.
- ISO 15163:2012; Milk and Milk Products—Calf Rennet and Adult Bovine Rennet—Determination by Chromatography of Chymosin and Bovine Pepsin Contents. ISO: Geneva, Switzerland, 2006.
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- HRN EN ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. Croatian Standard Institute: Zagreb, Croatia, 2017.
- HRN EN ISO 5534:2008; Cheese and Processed Cheese—Determination of the Total Solids Content (Reference Method). Croatian Standard Institute: Zagreb, Croatia, 2008.
- HRN EN ISO 8968-1:2014; Milk and Milk Products—Determination of Nitrogen Content, Part 1: Kjeldahl Principle and Crude Protein Calculation. Croatian Standard Institute: Zagreb, Croatia, 2014.
- HRN ISO ISO 3433:2008; Cheese—Determination of Fat Content—Van Gulik Method. Croatian Standard Institute: Zagreb, Croatia, 2008.
- HRN EN ISO 5943:2007; Cheese and Processed Cheese—Determination of Chloride Content—Potentiometric Titration Method. Croatian Standard Institute: Zagreb, Croatia, 2007.
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Tanuwidjaja, I.; Mrkonjić Fuka, M.; Vlahoviček Kahlina, K.; Marijan, M.; Boras, A.; Udiković Kolić, N.; Vinceković, M. Encapsulation of two fermentation agents, Lactobacillus sakei and calcium ions in microspheres. Colloids Surf. B Biointerfaces 2020, 197, 111387. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Kapadia, J.R. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.H.; Champagne, C.P.; St-Gelais, D.; Britten, M.; Fustier, P.; Lacroix, M. Viability of Bifidobacterium longum in cheddar cheese curd during manufacture and storage: Effect of microencapsulation and point of inoculation. Dairy Sci. Technol. 2011, 91, 599–614. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. The viability of probiotic Lactobacillus rhamnosus (non-encapsulated and encapsulated) in functional reduced-fat cream cheese and its textural properties during storage. Food Control. 2019, 100, 8–16. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G.; Kinik, Ö.; Ates, M.; Kaplan, M.; Satir, G. Symbiotic microencapsulation to enhance Bifidobacterium longum and Lactobacillus paracasei survival in goat cheese. Food Sci. Technol. 2020, 42, e55620. [Google Scholar] [CrossRef]
- Song, A.A.L.; In, L.L.A.; Lim, S.H.E.; Rahim, R.A. A review on Lactococcus lactis: From food to factory. Microb. Cell Factories 2017, 16, 55. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasević, I.; Lorenzo, J.M.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef]
- Pajač, L.; Kiš, M.; Kazazić, S.; Zdolec, N. Characterization and selection of Lactococcus lactis strains from ewe’s milk as potential cheese starter culture. In Proceedings of the International Congress Veterinary Science and Profession 2021, Zagreb, Croatia, 9 October 2021; Available online: https://www.bib.irb.hr/1149224 (accessed on 1 April 2023).
- Dujmović, H. Functional Properties of Lactiplantibacillus plantarum S3 from Lamb’s Abomasum—Potential Application in Dairy. Master Thesis, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia, 2022. (In Croatian). Available online: https://repozitorij.vef.unizg.hr/islandora/object/vef:1039 (accessed on 1 April 2023).
- Blandino, A.; Macias, M.; Cantero, D. Glucose oxidase release from calcium alginate gel capsules. Enzym. Microb. Technol. 2000, 27, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Ouwerx, C.; Velings, N.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Sandra, S.; Cooper, C.; Alexander, M.; Corredig, M. Coagulation properties of ultrafiltered milk retentates measured using rheology and diffusing wave spectroscopy. Food Res. Int. 2011, 44, 951–956. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Szajnar, K. Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels. Molecules 2021, 26, 5563. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014, 29, 13. [Google Scholar] [CrossRef]
- Larsen, N.; Nissen, P.; Willats, W.G.T. The effect of calcium ions on adhesion and competitive exclusion of Lactobacillus ssp. and E. coli O138. Int. J. Food Microbiol. 2007, 114, 113–119. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A quantitative analysis of alginate swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Rokstad, A.M.A.; Lacík, I.; de Vos, P.; Strand, B.L. Advances in biocompatibility and physicochemical characterization of microspheres for cell encapsulation. Adv. Drug Deliv. Rev. 2014, 67–68, 111–130. [Google Scholar] [CrossRef]
- Cui, J.H.; Goh, J.S.; Kim, P.H.; Choi, S.H.; Lee, B.J. Survival and stability of bifidobacteria loaded in alginate poly-l-lisine microparticles. Int. J. Pharm. 2000, 210, 51–59. [Google Scholar] [CrossRef]
- Iyer, C.; Kailasapathy, K. Effect of co-encapsulation of probiotics with prebiotics on increasing the viability of encapsulated bacteria under in vitro acidic and bile salt conditions and in yogurt. J. Food Sci. 2005, 70, 18–23. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Sinigaglia, M. Shelf life of alginate beads containing lactobacilli and bifidobacteria: Characterisation of microspheres containing Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Food Sci. Technol. 2011, 46, 2212–2217. [Google Scholar] [CrossRef]
- Goderska, K.; Zybała, M.; Czarnecki, Z. Characterisation of Microencapsulated Lactobacillus rhamnosus LR7 Strain. Pol. J. Food Nutr. Sci. 2003, 53, 21–24. [Google Scholar]
- Corbo, M.R.; Bevilacqua, A.; Gallo, M.; Speranza, B.; Sinigaglia, M. Immobilization and microencapsulation of Lactobacillus plantarum: Performances and in vivo applications. Innov. Food Sci. Emerg. Technol. 2013, 18, 196–201. [Google Scholar] [CrossRef]
- Özer, B.; Kirmaci, H.A.; Shenel, E.; Atamer, M.; Hayaloglu, A. Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int. Dairy J. 2009, 19, 22–29. [Google Scholar] [CrossRef]
- Salazar-Montoya, J.A.; Gonzales-Cuello, R.; Flores-Giron, E.; Ramos-Ramirez, E.G. Effect of free and microencapsulated Lactococcus lactis on composition and rheological properties of Manchego-type cheeses during ripening. Food Res. Int. 2017, 105, 59–64. [Google Scholar] [CrossRef]
- Karakas-Sen, A.; Kararakas, E. Isolation, identification and technological properties of lactic acid bacteria from raw cow milk. Biosci. J. 2018, 34, 385–399. [Google Scholar] [CrossRef]
- Tormo, H.; Ali Haimoud Lekhal, D.; Roques, C. Phenotypic and genotypic characterization of lactic acid bacteria isolated from raw goat milk and effect on farming practices on the dominant species of lactic acid bacteria. Int. J. Food Microbiol. 2019, 210, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.K.; Broe, M.L.; Qvist, K.B. The Production, Action and Application of Rennet and Coagulants. In Technology of Cheesemaking, 2nd ed.; Law, B.A., Tamime, A.Y., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 98–129. [Google Scholar]
- Oštarić, F.; Antunac, N.; Prpić, Z.; Mikulec, N. Influence of the Rennet Type on the Pag Island Cheese (Croatian: Paški sir). Mljekarstvo 2015, 65, 101–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).