Abstract
Anaerobic digestion of animal manure results in the production of renewable energy (biogas) and nutrient-rich biofertilizer. A further benefit of the technology is decreased greenhouse gas emissions that otherwise occur during manure storage. Since animal manure makes anaerobic digestion cost-efficient and further advance the technology for higher methane yields, it is of utmost importance to find strategies to improve bottlenecks such as the degradation of lignocellulose, e.g., in cattle manure, or to circumvent microbial inhibition by ammonia caused by the degradation of nitrogen compounds in, e.g., chicken, duck, or swine manure. This review summarizes the characteristics of different animal manures and provides insight into the underlying microbial mechanisms causing challenging problems with the anaerobic digestion process. A particular focus is put upon the retention time and organic loading rate in high-ammonia processes, which should be designed and optimized to support the microorganisms that tolerate high ammonia conditions, such as the syntrophic acetate oxidizing bacteria and the hydrogenotrophic methanogens. Furthermore, operating managements used to stabilize and increase the methane yield of animal manure, including supporting materials, the addition of trace elements, or the incorporation of ammonia removal technologies, are summarized. The review is finalized with a discussion of the research needed to outline conceivable operational methods for the anaerobic digestion process of animal manure to circumvent process instability and improve the process performance.
1. Introduction
Anaerobic digestion technology combines waste management with the production of renewable energy (biogas). Biogas can replace fossil fuels in producing electricity and heat, reducing greenhouse gas emissions. By removing carbon dioxide to reach a methane content above 90% (i.e., upgrading), biogas can also be used to replace fossil gas (i.e., natural gas) in gas grids and transportation [1,2]. Another valuable product from the anaerobic digestion process is the digestate, which is rich in nutrients such as nitrogen, phosphorus, and potassium and is, therefore, very suitable for utilization as organic fertilizer.
Biogas can be produced from various organic waste streams such as food waste, wastewater sewage sludge, and animal manure. In particular, implementing anaerobic degradation of animal manure as an efficient waste management technology brings many benefits to society. Driven by the increase in global demand for meat, eggs, and dairy products, the animal husbandry industry has expanded rapidly over the last few decades. With that, large volumes of animal manure are constantly generated. According to FAO, the amount of nitrogen in animal manure reached 128 million tons globally in 2019 [3]. This is of concern since the conventional manure storage facilities and the land-spreading of untreated manure significantly contribute to greenhouse gas emissions [4,5]. About 10% of the total CH4 emissions from the agricultural sector have been estimated to derive from improper manure management [6]. By instead treating the animal manure with anaerobic digestion, the methane will be captured and used as renewable energy. Other benefits of the anaerobic treatment of animal manure are land-spreading of the generated digestate, lower nitrogen leachate, and a reduced risk of spreading pathogens [7], antibiotics, and antibiotic resistance [8].
During anaerobic degradation of the organic material, protein, lipids, and carbohydrates are step-wise converted to methane and carbon dioxide through hydrolysis, acidogenesis, acetogenesis, and methanogenesis by anaerobic microorganisms [9]. However, maintaining the process to be stable and well-performing can be challenging since the different active microbial groups have their preferred requirements for optimal metabolism [10]. Hence, bottlenecks in the anaerobic degradation process may appear depending on operating conditions and the characteristic of the animal manure, such as the carbon/nitrogen ratio. For instance, during the anaerobic treatment of animal manure with high lignocellulose content, such as cattle manure, the hydrolysis step is often the rate-limiting step, which obstructs the full exploitation of the methane potential [11] and raises the risk of methane emission during the storage of the digestate [12]. The challenge for the anaerobic degradation of nitrogen-rich manure is often related to the high level of ammonia formed during protein and uric acid degradation, which causes the accumulation of volatile fatty acid (VFA) and reduces methane production [13]. During increasing ammonia levels, the inhibition of acetoclastic methanogens (AM) with low tolerance towards ammonia has shown to be a significant reason for the accumulation of organic acids [14,15]. By allowing the microbial community to adapt to the high ammonia levels, ammonia-tolerant populations can become established in the microbial community. This ammonia adaptation often includes the development of an acetate-degrading pathway involving syntrophic acetate-degrading bacteria (SAOB), which cooperates with hydrogenotrophic methanogens (HM) [16]. While these microorganisms can tolerate ammonia, they can still be stressed by the high ammonia levels, and methane production is generally slower than in the low-ammonia process [17,18]. In addition, reducing the nitrogen content of the substrate by co-digestion and modifying the C/N ratio to a suitable range (20–30) may be the primary strategy to alleviate ammonia inhibition during the anaerobic digestion of nitrogen-rich substrates, and it is easy to implement [13]. For example, co-digestion of cow manure and duck manure [19], swine manure and corn straw [20], and cow manure and chicken manure [21] have achieved good biogas production performance. It is, therefore, essential to design the operating conditions to support ammonia-tolerant syntrophic microorganisms [16].
In addition to the animal manure characteristic, process operating conditions such as hydraulic retention time (HRT), organic loading rate (OLR), and temperature strongly influence the microbial community, the performance, and the stability of biogas production [22]. Due to the complex and interlinked interactions between operating conditions and microbial activities, it can be almost overwhelming for a plant operator to project an efficient process. The optimal goal would be to find simple strategies to improve the animal degradation processes and to circumvent bottlenecks, such as ammonia inhibition. Even though general strategies for alleviating ammonia inhibition can be applied to a broad range of processes, which has been described in the other literature [23,24], the course of action still needs to be guided by the animal manure characteristics and operating conditions. To disentangle these interactions and to present a possible course of action, the present review summarizes the current understanding of links between process operation and the microbial community. It discusses operating management approaches that can improve biogas production from different types of animal manure.
2. Anaerobic Digestion of Animal Manure
2.1. Characteristics of Animal Manure
Animal manure differs in physicochemical properties depending on the animal species, diet and manure management, etc., which impact the methane production potential (Table 1 and Figure 1). Regarding the C/N ratio, cattle manure generally fulfills the recommended value for anaerobic degradation (C/N 15–30) [25]. However, a fiber-rich diet and the inclusion of bedding material, such as straw, often provide cattle manure with a high lignocellulosic content (about 50% based on dry weight). Since lignocellulose is a highly resistant material, it is resistant to anaerobic degradation and restricts the first hydrolytic step [26]. Chicken, duck, and swine manure often have C/N ratios below the recommended value of 3–10, 10–15, and 10–20, respectively (Table 1). For example, chicken manure has been reported to contain a crude protein content of about 25–30% of the dry weight, of which the uric acid content represents about 57–84% of the total nitrogen content [27,28,29]. Uric acid can rapidly hydrolyze to form ammonia nitrogen in anaerobic environments, which inhibits anaerobic microorganisms such as methanogens if formed at high levels [30,31]. Although lower ammonia nitrogen levels (0.05–0.2 g/L) are beneficial to bacteria as a nitrogen-nutrient source for microbial cell synthesis, the anaerobic digestion of nitrogen-rich manure with a higher protein content produces undesirably high concentrations for many anaerobic microorganisms [13]. Consequently, the anaerobic processing of nitrogen-rich animal manure as a mono-substrate has been a longstanding challenge.
In addition to the C/N ratio, water and solid organic content of the animal manure are other vital parameters impacting methane production. The solid content of different manures varies significantly due to the different collection methods. For instance, cattle manure can be collected with or without bedding material, giving a solid content range of 5~12% [32]. However, swine manure is often collected with flushing water, resulting in a low total solid (TS) content (TS < 10%) [10,28]. Chicken manure and duck manure typically have a higher total solid (TS > 20%). Still, this material is often diluted to below TS of 10% before entering the anaerobic digestion process to alleviate severe ammonia inhibition [33,34]. Due to the high water content and the high content of lignocellulose, cattle manure generally has a relatively low biogas-producing potential (0.2–0.3 L/g volatile solid, VS) [10,27]. Chicken manure has a higher organic matter content (VS/TS), especially protein, which results in higher methane production potential. The methane-producing potential of chicken manure can reach 0.25–0.45 L/g VS and 0.20–0.40 L/g VS for pig manure, as summarized in Table 1.
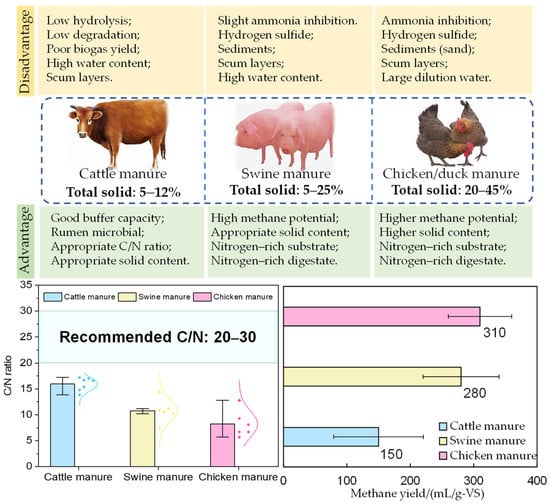

Table 1.
Common characteristics of animal manure and operating parameters frequently used in the anaerobic digestion of these substrates.
Table 1.
Common characteristics of animal manure and operating parameters frequently used in the anaerobic digestion of these substrates.
| Feedstock | Total Solid (TS, %) | Volatile Solid (VS, % of TS) | C/N Ratio | REQUIRED HRT a (d) | Methane Yield Potential (L/g-VS) | CH4 Content of Produced Biogas (%) | Degree of vs. Degradation (%) | Frequent Problems during the Anaerobic Digestion Process | Possible Solutions | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Chicken/Duck manure | 20–45 | 70–85 | 3–12 | >30 | 0.20–0.40 | 60–80 | 40–70% | Ammonia inhibition; hydrogen sulfide; high solids content; sediments (sand); scum layers. | Long-term adaption; Addition of iron and other trace elements; ammonia removal; co-digestion with material with a higher C/N ratio and lower TS. | [35,36,37,38,39,40,41,42,43,44,45] |
| Duck manure | 15–30 | 70–85 | 10–15 | >30 | 0.20–0.40 | 60–80 | 45–70% | Ammonia inhibition; hydrogen sulfide; High solids content; Sediments (sand); scum layers. | Long-term adaption; add iron and other trace elements; Removal ammonia; co-digestion with material with a higher C/N ratio and lower TS. | [46,47] |
| Swine manure | 5–25 | 78–80 | 10–20 | 20–40 | 0.20–0.35 | 60–70 | 30–70% | Ammonia inhibition; Hydrogen sulfide; Scum layers; Sediments. | Long-term adaption; Addition of iron and other trace elements; Removal of ammonia; Co-digestion with material with higher C/N ratio. | [48,49,50,51,52,53] |
| Cattle manure | 5–12 | 75–95 | 15–30 | 20–30 | 0.15–0.30 | 55–65 | 20–35% | Scum layers; Low hydrolysis; Low degradation; Poor biogas yield. | Increased operating temperature; Phase separation pretreatment. | [54,55,56] |
Note: a: recommended hydraulic retention time required for stable operation; TS: Total solid; VS: Volatile solid.
2.2. Common Anaerobic Bioreactors and Operating Parameters for Animal Manure
Anaerobic reactors most commonly used for animal manure include continuously stirred tank reactors (CSTR), but in some places, such as in North America, plug flow reactors (PFR) are frequently used [57]. In CSTR, this technology is suitable for treating animal manure with a relatively low total solid concentration (TS 5–15%). The plug-flow technology often involves a horizontal tank, in which the substrate is pushed or screwed along, which enables handling substrates with high TS (8%~30%) [58,59].
The HRT and solid retention time (SRT) are often the same in CSTR and PFR. These parameters must be managed carefully to avoid the washout of essential microorganisms. For instance, in the anaerobic digestion of nitrogen-rich animal manure, such as chicken manure, a long HRT (>30 days) is often required to avoid microbial loss and reactor failure since the ammonia inhibition slows down the activity and growth rate of the anaerobic microorganisms [38,40]. This is often observed by the accumulation of VFA and low methane yield in processes operating at HRT for less than 30 days (Table 2). On the other hand, in mesophilic anaerobic processes that are fed manure with higher C/N content or when fed material diluted with water and thus exposed to less pressure for ammonia inhibition, a generally shorter HRT of 20–40 days can be applied (Table 2) [34]. It is important to consider that the degradation of lignocellulosic material is a slow process, and implementing a short hydraulic retention time (HRT) can result in the incomplete degradation of less resistant fibers. This can lead to a reduction in methane yield [60,61,62].
Another critical parameter is the organic loading rate (OLR), which depends on the feeding volatile solid (VS) ratio and the HRT and can be expressed as OLR = VSfeed/volume reactor. A low OLR leads to the insufficient processing capacity of the anaerobic reactor. In contrast, high OLR can cause overloading, resulting in the accumulation of VFA and even failure of the anaerobic digestion process [35]. It is noted that the propionate level should be focused on during anaerobic digestion, which is very unfriendly to methane production. In the batch experiment of co-digestion of cow manure and food waste, when the initial propionate concentration was 22–56 mM, the lag phase of biogas production could reach 9–60 days [63]. The inhibitory threshold concentration of propionate varies greatly, in the range of 4.2–43 mM, which may be related to the type of substrate, operating parameters, etc. [64,65]. When propionate/acetate is greater than 1.4, and the concentration of acetate is greater than 0.8 g/L, the fermentation system can experience serious acid inhibition [66]. In anaerobic digestion of chicken manure, OLR around 2.0–3.0 g VS/(L·d)) has been reported in mesophilic full and pilot-scale reactors [34] (Table 2). Still, studies have demonstrated the feasibility of operating high-solid anaerobic digestion of chicken manure with satisfactory methane production performance at an extremely high ammonia concentration (TAN, 6.5–7.5 g/L) when maintaining a low OLR of 1.5–2.5 g VS/(L·d) (HRT 60 d, feeding TS 10–15%) [38,40]. Maintaining operation at a relatively low OLR (<3 g-VS/(L·d)) condition at this point can be one solution to continue the operation at high ammonia (TAN > 5 g/L), where the systems may enter an “inhibited steady-state” and continue to produce methane with a smaller amount of it [67,68]. However, it is crucial to monitor such processes constantly since if the OLR increases, the process can rapidly be overloaded, often leading to synergistic ammonia and fatty acid inhibition [69].
Temperature is a critical parameter for the anaerobic degradation process. As previously reported, based on the summary, in articles published between 2010–2019, a vast majority of anaerobic co-digestion processes of various scales fed animal manure operate under mesophilic conditions (85%). In contrast, operation at thermophilic conditions (50–55 °C) is less common (13%) [70]. The reason is that a lower cost is needed for heating, the microbial community structure is more abundant, and the fermentation performance is more stable in the mesophilic digester [35,71]. Another reason is that high temperature increases the free ammonia nitrogen (FAN, NH3) ratio, resulting in FAN levels above the tolerance threshold of microorganisms, especially for high ammonia level digesters. As free ammonia can diffuse into microorganisms, it is considered a key factor in inhibiting the metabolic activity of anaerobic microorganisms. FAN is in equilibrium with NH4+ (NH3 + H2O⇌HN4OH⇌NH4+ + HO−) and increases with pH and temperature [23]. Therefore, high-temperature anaerobic treatment is usually not used in the methane production stage to avoid ammonia inhibition for nitrogen-rich manure, such as chicken manure, duck manure, and pig manure. In addition, thermophilic anaerobic digestion commonly provides a high biogas production rate and benefits pathogen reduction [7,72,73]. The thermophilic condition is also widely used in the hydrolysis and acidogenesis stage compared to the methane production stage in the two-stage anaerobic digestion technology. For example, hyper-thermophilic (70 °C) pretreatment is used in the hydrolysis and acidogenesis process of chicken manure [74], and thermophilic (55 °C) pretreatment is used in pig manure [7]. Of course, high temperature is more widely used in refractory substrate anaerobic treatment, such as cattle manure.

Table 2.
Operating conditions and methane yields of different lab-, pilot-scale, and full-scale biogas plants fed animal manure as the primary substrate (the following references are listed according to the OLR from high to low).
Table 2.
Operating conditions and methane yields of different lab-, pilot-scale, and full-scale biogas plants fed animal manure as the primary substrate (the following references are listed according to the OLR from high to low).
| Digester Type | Temperature (°C) | Reactor Scale | TS (%) | OLR (g VS/(L·d)) | HRT (d) | TAN (g/L) | VFAs (g/L) | Methane Yield (L/g-VS) | References |
|---|---|---|---|---|---|---|---|---|---|
| Chicken manure | |||||||||
| LBR | 36 | Lab-scale | 14.0–16.0 | / | / | 10.0 | 15.0 | 0.11 | [75] |
| CSTR | 37 | Lab-scale | 20.0 | 7.1 | 20 | 8.5 | 25.2 | 0.02 | [34] |
| CSTR | 35 | Lab-scale | 17.0 | 6.3 | 20 | 8.6 | 12.9 | 0.21 | [76] |
| CSTR | 40 | Lab-scale | 15.0 | 6.0 | 40 | 6.9 | 9.3 | 0.27 | [77] |
| CSTR | 37 | Lab-scale | 15.0 | 5.3 | 20 | 6.9 | 13.6 | 0.19 | [35] |
| CSTR | 37 | Lab-scale | 15.0 | 5.3 | 20 | 6.8 | 21.9 | 0.19 | [78] |
| CSTR | 37 | Lab-scale | 10.0 | 3.6 | 20 | 6.5 | 6.7 | 0.28 | [34] |
| CSTR | 40 | Lab-scale | 15.0 | 3.5 | 35 | 6.9 | 8.2 | 0.19 | [79] |
| CSTR | 35 | Lab-scale | 11.0 | 2.8 | 30 | 8.0 | 5.0 | 0.30 | [80] |
| CSTR | 35 | Full-scale | 8.3 | 2.8 | 20 | 6.0 | ND | ND | [81] |
| CSTR | 35 | Lab-scale | 8.0 | 2.7 | 30 | 5.0 | 2.0 | 0.20 | [44] |
| CSTR | 37 | Lab-scale | 7.5 | 2.7 | 20 | 5.0 | 2.2 | 0.34 | [34] |
| CSTR | 55 | Lab-scale | 8.0 | 2.7 | 30 | 3.7 | ND | 0.13 | [44] |
| CSTR | 55 | Lab-scale | 10.0 | 2.7 | 30 | 3.7 | 4.0–6.0 | 0.14 | [43] |
| CSTR | 37 | Full-scale | 9.0 | 2.3 | 33 | 6.2 | 2.1 | 0.30 | [36] |
| CSTR | 37 | Lab-scale | 5.0 | 1.8 | 20 | 2.3 | 0.4 | 0.36 | [34] |
| CSTR | 35 | Lab-scale | 13.5 | 1.8 | 23 | 5.9 | 7.6 | 0.26 | [82] |
| CSTR | 37 | Lab-scale | 5.0 | 1.7 | 20 | 2.4 | 0.3 | 0.38 | [83] |
| CSTR | 55 | Lab-scale | 5.0 | 1.7 | 20 | 2.4 | 0.3 | 0.33 | [83] |
| CSTR | 37 | Lab-scale | 15.0 | 1.5 | 60 | 7.4 | 0.5 | 0.33 | [40] |
| Swine manure | |||||||||
| SBR | 24 | Farm-scale | 5.6 | 8.0 | 7 | 4.6 | ND | ND | [84] |
| CSTR | 35 | Lab-scale | 23.6 | 5.2 | 41 | 4.0 | 3.5 | 0.20 | [85] |
| CSTR | 37 | Lab-scale | 7.3 | 3.5 | 15 | 1.5 | 0.2 | 0.22 | [20] |
| CSTR | 38 | Lab-scale | 7.6 | 3.0 | 21 | 4.7 | ND | 0.14 | [20] |
| PFR | 38 | Pilot-scale | 20.0 | 2.4 | 60 | 4.3 | ND | ND | [86] |
| USR | 36 | Full-scale | 3.3 | 1.8 | 15 | 1.4 | ND | 0.27 | [87] |
| USR + PFR | 26 | Full-scale | 6.2 | 1.3 | 15 + 22 | 1.6 | ND | ND | [88] |
| CSTR | 35 | Full-scale | 7.4 | 1.2 | 40 | 2.0 | ND | ND | [81] |
| USR | 36 | Full-scale | 3.5 | 1.2 | 22 | 1.5–2.4 | ND | 0.43 | [87] |
| PFR | 25 | Lab-scale | 5.8 | 0.9 | 67 | ND | 0.8 | 0.41 | [89] |
| PFR | 25 | Pilot-scale | 5.4 | 0.6 | 67 | ND | 0.9 | 0.41 | [89] |
| Cattle manure | |||||||||
| CSTR (Cattle manure + energy crops) | 47 | Full-scale | 12.2 | 5.4 | 34 | 4.8 | 0.6 | 0.24 | [90] |
| CSTR | 50 | Pilot-scale | 8.5 | 3.5 | 20 | 2.1 | 0.4 | 0.18 | [91] |
| CSTR | 35 | Pilot-scale | 8.5 | 3.5 | 20 | 1.9 | 0.3 | 0.16 | [91] |
| CSTR | 35 | Full-scale | 7.1 | 3.1 | 20 | 2.3 | 0.4 | 0.15 | [91] |
| CSTR | 37 | Lab-scale | 6.6 | 3.0 | 27 | 5.2 a | 0.9 | 0.28 | [92] |
| CSTR | 35 | Full-scale | 6.4 | 0.8 | 50 | 1.5 | ND | ND | [81] |
Note: CSTR: continuous stirred tank reactor; PFR: plug flow reactor; LBR: leach bed reactor; USR: up-flow solid reactors; SBR: sequencing batch reactors; TS: total solids; VS: volatile solids; OLR: organic loading rate; HRT: hydraulic retention time; TAN: total ammonium nitrogen; VFAs: volatile fatty acids; a: add ammonium chloride to increase ammonia nitrogen concentration; ND: data not involved.
3. The Methane Production Pathways under Different Operating Parameters and Ammonia Stress
3.1. Hydrolysis and Acidogenesis Process
Depending on the characteristic of the animal manure, the rate of the hydrolytic step can vary. For example, in the anaerobic degradation of non-calcitrant carbohydrates and specific proteins, etc., the hydrolytic and acidogenic steps are performed relatively fast [93]. Consequently, in two-stage animal manure degrading processes, the first digester in which these steps are performed can operate with a relatively short HRT of 3–10 days [94,95]. Furthermore, while treating cattle manure containing recalcitrant matter, the two-stage anaerobic digestion technology has also increased methane yield and VS removal at mesophilic and thermophilic conditions [96,97,98].
Acetoclastic methanogens are believed to be the most sensitive microorganisms to ammonia; the associated inhibition is also accounted for in the widely adopted Anaerobic Digestion Model nr 1 (ADM1) [99]. However, hydrolysis and acidogenesis stages have been reported to be inhibited during thermophilic anaerobic digestion of nitrogenous chicken manure in which TAN reached 4.7–4.8 g/L (1.9 g FAN/L) [43]. At mesophilic conditions, hydrolysis and acidogenesis stages were inhibited at 5.5 and 6.5 g/L of TAN (FAN, 0.7 and 0.8 g/L), respectively [35]. Consequently, even though it is vital to consider that the high temperature can enhance the rate of hydrolysis and acidogenesis steps for animal manure [100], care must be taken to avoid ammonia inhibition since the higher temperature increases the ratio of the toxic ammonia. Additionally, in the anaerobic digestion of swine manure, the hydrolytic step has been negatively affected by a higher temperature range of 50–60 °C [55]. Increasing temperature from 55 to 65 °C decreased methane production and increased VFAs in the anaerobic digestion of cattle manure (HRT 15 days) [100]. Therefore, a reasonable increase in temperature may be beneficial to the hydrolysis and acidogenesis process of organic matter. Still, it must be controlled within a specific range below 60 °C.
3.2. Acetogenesis Process
In the acetogenesis stage, acidogenesis products such as lactic acid, butyrate, and propionate are decomposed into acetate and CO2/H2 by acetogenic bacteria [101]. These products are crucial precursors for methane production. Several of these acetogenic bacteria require the hydrogenotrophic methanogens to maintain a low hydrogen partial pressure during the process to make the acetogenic reaction thermodynamically feasible.
In the anaerobic digestion of animal manure, ammonia nitrogen can also influence the acetogenesis step. For instance, by reducing the TAN concentration from 5.6 to 3.8 g/L in a hyper-thermophilic (70 °C) digester treating chicken manure, the acetogenesis efficiency increased by 52%. In addition, an increase in ammonia concentration (TAN, 0.8 to 6.9 g/L) has been shown to cause distinct shifts in the acetogenic population structure in a mesophilic digester [102]. Since various microorganisms cooperate in the anaerobic system, ammonia directly or indirectly affects their activities. Hence, structural changes in the acetogenic population can also be driven by the fluctuation of organic acids and pH caused by high ammonia concentration. As the acetogenic step is not considered the bottleneck step, few studies have looked into the effect of ammonia inhibition on acetogenic bacteria.
3.3. Acetoclastic Methanogenesis and the Impact of the Operating Parameter on Methanogenic Community Structure
The methanogenic pathway mediates methane production and the organic acid concentration in anaerobic digestion. Certain methanogenic groups are known to be highly sensitive to ammonia inhibition. For instance, pure culture inhibits the acetoclastic Methanosaeta sp. at 3 g/L of TAN. In contrast, the mixotrophic Methanosarcina sp. can form methane from acetate and hydrogen during levels of up to 7 g TAN/L [103]. In the anaerobic degradation of cattle manure, Methanosaeta sp. is commonly the dominant species due to the generally low ammonia level (<2.5 g/L) and the common use of slightly low OLR (2.5–3.5 g VS/(L·d)) [91,104]. However, in the anaerobic digestion of swine and chicken manure, Methanosarcina sp. is more often observed [20,44,85] (Table 3). The methanogenic community structure subsequently impacts the acetate level in the digester since acetoclastic Methanosaeta sp. is known to have a high affinity (1–10 mM) for acetate and a relatively low growth rate, whereas Methanosarcina sp. has a lower substrate affinity (20–80 mM) [105]. This implies that Methanosarcina sp. will not reduce the acetate level as low as Methanosaeta sp. Along with Methanosarcina sp., the hydrogenotrophic methanogenic genera Methanoculleus sp. and members of the methanogenic order Methanobacteriales are other methanogens with a high tolerance to ammonia [13]. For example, the growth of hydrogenotrophic Methanobacterium sp. has been observed up to a TAN concentration of 9 g/L in the pure culture [106].
The competitiveness for acetate by Methanosaeta sp. was demonstrated during the lowering of ammonia nitrogen concentration (from 6.6 to 2.9 g/L) by ammonia stripping [107]. Nevertheless, methane production from acetoclastic Methanosaeta sp. under a high ammonia nitrogen concentration has also been reported in chicken manure digesters [36,44]. Consequently, the possibility of steering the process to support the acetoclastic methanogens at a high ammonia level may still exist [36]. In a biogas plant of chicken manure under a TAN of 6.2 g/L (FAN 1.1 g/L), the relative abundance of acetoclastic Methanosaeta sp. reached 5% through adaption for more than 10 years. Interestingly, although acetoclastic Methanosaeta sp. was less abundant, it contributed 42% of methane production [36]. Therefore, the non-negligible role of acetoclastic Methanosaeta sp. under high ammonia nitrogen conditions needs further exploration.
OLR is a factor of importance for the methanogen community. A summary of results from digesters treating chicken manure demonstrated the dominance of Methanoculleus sp., Methanobrevibacter sp., or Methanosarcina sp. in mesophilic conditions and Methanothermobacter sp. at thermophilic conditions during operation with an OLR below 3 g VS/(L·d) (Table 3). It is also noticeable that it can be challenging to obtain high methane yields during operation at OLR above 3 g VS/(L·d) to treat chicken manure under mesophilic digestion (Table 3). This was particularly distinct during mesophilic degradation of chicken manure, where OLR increased from 1.8 to 5.3 g VS/(L·d) at maintained HRT (20 days) [74]. The result showed that Methanosarcina sp. replaced Methanoculleus sp. as the dominant methanogen and the methane yield decreased from 0.36 to 0.19 L/g VS [34].
As methanogens differ in growth rate depending on species and the growth conditions, it is critical to operate at suitable HRT for the prevailing methanogenic community. In laboratory cultivation of representative hydrogenotrophic methanogenic species, a doubling time of around 10–65 h has been reported [108,109,110,111], which is longer than what has been obtained for representative acetoclastic methanogenic species (2–20 h) [112,113,114]. Consequently, a long HRT can be required in a process dominated by hydrogenotrophic methanogens. In cocultures comprising Methanoculleus bourgensis and syntrophic acetate oxidizers (30 °C, pH 7.3), the doubling time of Methanoculleus bourgensis 8–18 d at a TAN of 0.7–2.8 g/L increased to 23–50 d at 4.8 g/L [115]. Therefore, it is necessary to appropriately extend the HRT and decrease OLR at a high ammonia level to avoid the wash-out and maintain the metabolic activity of the ammonia-tolerant methanogens.

Table 3.
The most dominant methanogens detected in anaerobic degradation processes fed animal manure.
Table 3.
The most dominant methanogens detected in anaerobic degradation processes fed animal manure.
| Temperature (°C) | OLR (g VS/(L·d) | HRT (d) | TAN (g/L) | FAN (g/L) | Archaea (Genus) | Relative Abundance (% of Total Archaea) | Methane Yield (L-CH4/g VSadd) | Microbial Community Investigation | References |
|---|---|---|---|---|---|---|---|---|---|
| Chicken manure | |||||||||
| 37 | 1.7 | 20 | 2.4 | 0.7 | Methanoculleus | 94% | 0.38 | 16S rRNA gene amplicon sequencing | [83] |
| 55 | 1.7 | 20 | 2.0 | 1.1 | Methanothermobacter | 96% | 0.33 | 16S rRNA gene amplicon sequencing | [83] |
| 37 | 2.5 | 40 | 6.2 | 1.1 | Methanobrevibacter | 74% | 0.31 | 16S rRNA gene amplicon sequencing | [36] |
| 55 | 2.7 | 30 | 5.2 | 2.4 | Methanothermobacter | 95% | 0.08 | 16S rRNA gene cloning and sequencing | [44] |
| 37 | 2.7 | 20 | 5.0 | 1.4 | Methanoculleus | 99% | 0.34 | 16S rRNA gene amplicon sequencing | [34] |
| 55 | 2.7 | 30 | 5.2 | 1.7 | Methanothermobacter | 95% | 0.28 | 16S rDNA gene cloning and sequencing | [43] |
| 40 | 3.5 | 35 | 6.9 | 0.8 | Methanosarcina | 85% | 0.33 | Illumina sequencing, 454 pyrosequencing and T-RFLP analysis | [79] |
| 37 | 3.6 | 20 | 6.5 | 0.8 | Methanosarcina | 94% | 0.28 | 16S rRNA gene amplicon sequencing | [34] |
| 37 | 5.3 | 20 | 6.8 | 0.5 | Methanosarcina | 73% | 0.19 | 16S rDNA gene cloning and sequencing | [78] |
| 37 | 5.3 | 20 | 5.8 | 0.6 | Methanosarcina | 83% | 0.25 | 16S rDNA gene cloning and sequencing | [78] |
| Swine manure | |||||||||
| 38 | 3 | 21 | 4.7 | 0.3 | Methanoculleus | 3% (percentage of total microbial) | 0.10 a | 16S rRNA gene amplicon sequencing | [20] |
| 37 | 3.5 | 15 | 1.5 | 0.07 | Methanosaeta | 76% | 0.14 b | 16S rDNA gene cloning and sequencing by PCR-DGGE analysis | [51] |
| 35 | 5.2 | 35 | 4.0 | 0.8 | Methanobrevibacter | 45% | 0.42 | 16s RNA amplification and Illumina Hiseq sequencing | [85] |
| 37 | NA | ND | ND | ND | Methanoculleus | 58% | 0.27 | 16s RNA amplification, Illumina sequencing | [116] |
| 35 | NA | ND | ND | ND | Methanosaeta | 23% | 0.54 a | 16S rRNA gene amplicon sequencing | [117] |
| Cattle manure | |||||||||
| 37–40 | 2.7 | 25 | ND | ND | Methanosarcina | 49% | 0.21 | DNA extraction and Illumina sequencing | [104] |
| 37 | 2.8 | 25 | ND | ND | Methanosarcina | 98% | ND | 454 pyrosequencing of bacterial and archaeal 16S rRNA genes | [118] |
| 37 | 2.1 | 27 | 5.2 | 0.3 | Methanosarcina | 99% | 0.28 | 16S rRNA gene amplicon sequencing | [92] |
| 35 | 3.1 | 20 | 1.9 | 0.05 | Methanosarcina | 15% (percentage of the total reads) | 0.15 | 454 pyrosequencing of bacterial and archaeal 16S rRNA genes | [91] |
| 50 | 3.1 | 20 | 2.1 | 0.11 | Methanosarcina | 12% (percentage of the total reads) | 0.18 | 454 pyrosequencing of bacterial and archaeal 16S rRNA genes | [91] |
| 38 | 4.0 | 26 | ND | ND | Methanobacterium Methanosaeta | 28% 26% | 0.24 | 16S rRNA gene amplicon sequencing | [104] |
| 37 (WS: CM = 4:6) | ND | ND | ND | ND | Methanoculleus Methanosphaera | 21% 17% | 0.32 | High-throughput 16S rRNA gene sequencing | [119] |
| 35 (CS:CM = 3:1) | ND | ND | ND | ND | Methanosaeta | 68% | 0.17 | High-throughput 16S rRNA gene sequencing | [120] |
| 35–40 | ND | ND | ND | ND | Methanosarcina | 43% | 0.32 | 16s RNA gene amplification and DNA sequencing using Illumina HiSeq 2500 | [11] |
Note: a: Methane yield based on the amount of VS removed; b: Methane yield based on COD of influent. WS: wheat straw; CM: cattle manure; CS: corn stover; ND: data not involved.
3.4. Microbial-Mediated Methane Production Pathways under Ammonia Stress
As mentioned above, inhibiting acetoclastic methanogens by high ammonia conditions often results in the development of a two-step pathway for acetate degradation. In this pathway, syntrophic acetate-oxidizing bacteria convert acetate to carbon dioxide (CO2) and hydrogen (H2), which is used by hydrogenotrophic methanogens to form methane [16]. However, since hydrogen is a product of acetate oxidation, hydrogen partial pressure must be kept at very low levels to render this process exergonic. It was estimated that the H2 partial pressure required for acetate oxidation can be even lower than the one needed for some of the acetogenic pathways, for example, propionate oxidation [121]. Extensive literature has demonstrated the critical contribution of this pathway, referred to as syntrophic acetate oxidation (SAO), during biogas production under high ammonia levels [16,122,123,124]. This pathway has also been included in modifications of the ADM1 proposed in the literature [121,125]. The characterized SAOB include Thermacetogenium phaeum [126], Pseudothermotoga/Thermotoga lettingae [127], Schnuerera ultunense [128], Syntrophaceticus schinkii [129], and Tepidanaerobacter acetatoxydans [130], where the three latter have been isolated from high-ammonia digesters (NH4+-N, 6–7 g/L) have been shown to tolerate ammonia nitrogen concentrations up to 10 g/L [126,127,130]. The mechanism behind ammonia tolerance has not yet been fully explained. Still, it has been suggested that the lack of ammonium transporters in SAOB can help them to avoid excess ammonia influx [131]. For the ammonia-tolerant SAOB, ammonia levels up to 0.2 M, temperature (up to 45 °C), and acetate concentrations (0.15–0.30 M) have shown a positive effect on the methane yield [16]. In pure culture, the syntrophic bacteria (S. schinkii, S. ultunense, and T. acetatoxydans) can grow at NH4Cl levels up to 7.0 g/L, showing their tolerance for ammonia [132].
The ammonia level at which the methane production pathway gets dominated by the syntrophic association has been shown to vary between different processes. For instance, in anaerobic co-digestion of swine manure and nitrogen-rich waste, the TAN threshold for dominance of acetate-degrading pathways has been reported to be 1.0 g/L (AM), 3.5 g/L (SAO-HM and AM), and 6.0 g/L (SAO-HM) (Figure 2) [133]. For cattle manure and stillage, a shift from AM to SAO-HM was reported at 0.15 g FAN/L [16,134]. It was reported that the SAO-HM pathway begins to compete with the AM at FAN 0.2–0.5 g/L and outcompetes the AM pathway with a FAN of more than 0.5 g/L with acetate (110 mmol/L) as a substrate in mesophilic conditions [135] (Figure 2). Given the functional importance of syntrophic bacteria in methanogenic systems, an increased understanding of these populations is critical for predicting process failures and developing process optimization strategies.
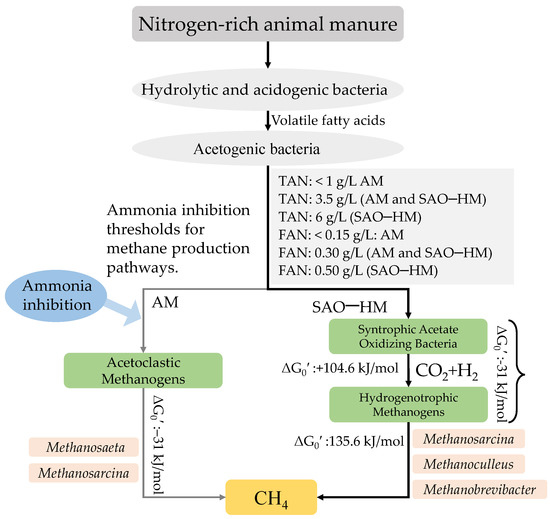
Figure 2.
Methane production path with ammonia pressure in mesophilic anaerobic digestion.
4. Operating Management with the Potential to Improve Anaerobic Treatment of Nitrogen-Rich Animal Manure
4.1. Methods to Remove Ammonia from an Anaerobic System
Physico-chemical methods have been developed to reduce the ammonia nitrogen concentration of the biogas system and thereby avoid the ammonia inhibition of the anaerobic consortia [136]. Such methods include ammonia stripping [137], membrane separation [33], struvite precipitation [138], and ion exchange [139], in which ammonia stripping and membrane separation have been the most investigated in animal manure processes (Table 4). For example, in-situ ammonia stripping has been evaluated in several studies of the anaerobic degradation of chicken manure, showing a higher methane yield and lower VFA levels, and the possibility of reducing TAN by 12–72% (Table 4). Many factors affect ammonia stripping, including temperature, pH, gas flow rate, CO2 composition in biogas, and digestate composition [137]. Still, part of the ammonia removal in a digester can be achieved by controlling parameters such as the stripping frequency so that the ammonia nitrogen concentration can be maintained at an acceptable concentration. The hollow fiber membrane method for ammonia separation has been tested in the anaerobic digestion of chicken manure, where the TAN concentration decreased from 5.8 to 2.4 g/L within 20 days of operation [33]. In another study, the membrane ammonia separation method was used to remove ammonia nitrogen in the anaerobic digestion of chicken manure in the leaching bed reactor. The study found that ammonia can significantly reduce the equilibrium concentration in the digester from 10 g/L to 2 g/L [75]. A benefit of the abovementioned methods is that degrees of ammonia removal can be adjusted by controlling the flow rate, the concentration of the acid-absorbing liquid, and the membrane area [24,137]. However, these methods require energy input, and no engineering case of membrane deamination technology has been found yet. The biogas plant operator needs to make calculations to ensure that the gained energy in the form of biogas exceeds the energy required for the removal method.

Table 4.
Summary of ammonia removal methods during anaerobic degradation and the obtained process performance.
Table 4.
Summary of ammonia removal methods during anaerobic degradation and the obtained process performance.
| Operation Mode | Substrate | OLR (g VS/(L·d)) | HRT (d) | Ammonia Removed Methods | Findings | Reference |
|---|---|---|---|---|---|---|
| CSTR | Chicken manure | 5.3 | 20 | Ammonia stripping | 15% reduction in the TAN (from 6.8 to 5.8 g/L), 30% reduction of volatile fatty acids, and methane yield increased by 34%. | [78] |
| Two stages (CSTR + stripping AnMBR) | Chicken manure | 4.0 | 4 + 15 | Ammonia stripping | 47% reduction in the TAN (from 5.7 to 3.1 g/L), 29% reduction of volatile fatty acids, and methane yield increased by 65%. | [39] |
| CSTR | Chicken manure | 9.0 | 15 | Ammonia stripping | 35% reduction in the TAN, The methane yield reached 0.20 L/g VS which was higher than control digesters (0.03 L/g VS). | [140] |
| CSTR | Chicken manure | 5.3 | 40 | Ammonia stripping | 12–72% reduction in TAN concentration, the specific gas yield was 0.39 L/g VS. | [77] |
| CSTR | Chicken manure | 2.7 | 64 | Activated carbon particles | 19% reduction in the TAN (from 14.2 to 11.5 g/L), 25% reduction of volatile fatty acids, and methane yield increased by 10%. | [141] |
| Leach-bed process | Chicken manure | ND | ND | Membrane separation | Methane production in the membrane-integrated reactor was 2.3 times higher than in the control reactor. | [75] |
| CSTR | Swine manure | 3.7 | 20 | Membrane separation | 23% TAN reduction in the membrane-separation reactor Specific methane yield increased by 17% compared with the control reactor. | [142] |
Note: AnMBR: anaerobic membrane bioreactor; ND: data not involved.
4.2. Supplementation of Trace Elements
Management methods that support and improve the activity of ammonia-tolerant microorganisms are available and enable the operation of the anaerobic process without investing energy to remove the ammonia. Such management approaches include supplementing certain trace elements, especially iron [143,144]. All these management approaches still demand start-up and operation that allow microbial adaption and maintained activity of ammonia-tolerant microorganisms. The anaerobic digestion process requires nutrients such as carbon, nitrogen, and phosphorus, but also trace elements are essential in the growth of anaerobic microorganisms. For example, Fe, Ni, Co, Se, W, Mo, and other metal elements are used by microorganisms to construct critical coenzymes or cofactors needed in various enzymatic reactions [145,146,147]. In the anaerobic digestion of chicken manure, adding Co, Ni, and Se has increased methane yield (Table 5). For instance, adding Ni, Co, Mo, W, Mo, and Se increased the methane production from 0.12 to 0.26 L/g VS compared with a control reactor during anaerobic treatment of chicken manure at a high TAN concentration (7.2 g/L) [148]. In another study, adding Fe, Mo, Ni, Mn, and Co to corn straw and chicken manure, co-digestion increased methane yield by 31–35% compared with the control digester [149]. The level and specific trace elements that benefit the process performance vary with the substrate characteristic and the microbial community composition [150]. Hence, general recommendations regarding the concentration of trace elements that should be added for optimal performance of animal manure processes are challenging to formulate.
An important aspect to highlight in the discussion of trace element addition is the effect of iron addition on removing sulfide. Sulfide is produced during the anaerobic digestion and causes process disturbance due to microbial inhibition and forms hydrogen sulfide (H2S) in the biogas, which subsequently causes a corrosive effect on downstream pipelines. Sulfides also form metal-sulfide complexes with essential trace elements for microorganisms and can thus cause micronutrient deficiency. The addition of iron is a practical approach to removing sulfide through the precipitation of iron sulfide (FeS) [151,152]. It has also been reported that the process of hydrogen evolution from iron corrosion by the addition of iron is beneficial to the metabolic activity of hydrogenotrophic methanogens [153,154], and iron (zero-valence iron and Fe2+) is beneficial in reducing the oxidation-reduction potential of the fermentation system [155]. All of the aforementioned reasons may explain why iron enhances methane production. Many studies have proved the positive role of iron in promoting the performance of the anaerobic digestion process [143,156,157]. The amount of iron added in studies of animal-manure-degrading anaerobic processes varies greatly, ranging from 20 mg/L to 20 g/L (Table 5), so care should be taken when choosing the iron concentration since there is a risk of causing excessive heavy metal content in the digestate, and high levels do not improve methane production.

Table 5.
Impacts of multiple trace element supplementation on anaerobic digestion of animal manure.
Table 5.
Impacts of multiple trace element supplementation on anaerobic digestion of animal manure.
| Operation Mode | Substrate | OLR (g-VS/(L·d)) | HRT (d) | TAN (g/L) | FAN (g/L) | Types and Concentrations (mg/L) | Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Continuous | Chicken manure | 4.8 | 20 | 6.8 | 0.6 | Fe: 280, Ni: 2 | Increased 34% methane production and 29% reduction of VFAs against control. SAMA and SHMA increased by 89% and 40%, respectively. The relative abundance of Methanosarcina sp. in the control group was 75%, significantly lower than in the trace element reactor (95%). | [45] |
| Continuous | Chicken manure | 3.6 | 30 | 6.6 | 0.7 | Ni:1, Co:1, Mo: 0.2, Se: 0.2, W: 0.2, | Methane yield increased by 117%. The relative abundance of hydrogenotrophic Methanoculleus bourgensis was 53%. | [158] |
| Continuous | Chicken manure | 3.7 | 30 | 6.0 | 0.7 | Ni:1, Co:1, Mo: 0.2, Se: 0.2, W: 0.2, | CH4 yield increased five times more than the control (0.05 vs. 0.31 L/g VS). The relative abundance of Methanoculleus bourgensis in the control group was only 3%, significantly lower than the trace element reactor (53%). | [148] |
| Continuous | Chicken manure | 2.8–3.0 | 30 | 5.9 | 0.5 | Ni:1, Co:1, Mo: 0.2, Se: 0.2, W: 0.2, | CH4 yield increased 146% to control (0.32 vs. 0.13 L/g VS). The relative abundance of Methanobrevibacter sp. increased from 20% to 80%. | [150] |
| Continuous | Chicken manure | 2.8 | 30 | 5.0 | 0.4 | Se: 0.2 | CH4 yield increased 107% to control (0.27 vs. 0.13 L/g VS). The relative abundance of Methanoculleus bourgensis increased from 5% to 63%. | [150] |
| Continuous | Chicken manure | 1.3–1.5 | 20 | 5.0 | 0.6 | Ni:8, Co:1, Mo:2, Se: 0.2, W: 0.3, Zn:100, Mn: 150, Fe:500 | Improved methane production efficiency by 38% and decreased the H2S content. | [159] |
| Batch | Chicken manure | / | / | 6.0 | / | Ni:1, Co:1, Mo: 0.2, Se: 0.2, W: 0.2, Fe: 5 | CH4 production and production rate improved by 7–8% and 5–6%. | [147] |
| Batch | Chicken manure | / | / | 4.0 | / | Ni:1, Co:1, Mo: 0.2, Se: 0.2, W: 0.2, Fe: 5 | CH4 production and production rate were increased by 20% and 40%. | [147] |
| Batch | Chicken manure and corn stover | / | / | ND | / | Fe: 5, Ni: 1.0, Mn: 0.5, Co: 0.5, Mo: 0.1 | The relative abundance of Methanosarcina sp. in the control group was 86%; it was higher than that of RFe (71%), R-Mo (54%), R-Ni (54%), R-Mn (53%), and R-Co (56.7%). Methanosarcina sp., Methanobacterium sp., and Methanospirillum sp. were enriched in all trace element reactors. | [149] |
| Batch | Livestock manure | / | / | ND | / | Fe: 20 | Increased the biogas and methane volume by 1.45 and 1.59 times by the control, respectively. | [143] |
| Batch | Livestock manure | / | / | ND | / | Fe3O4: 20 | Increased the biogas and methane volume by 1.66 and 1.96 times by the control, respectively. | [143] |
| Batch | Pig manure | / | / | ND | / | ZVI: 20 (g/L) | Increased 20–26% of CH4 yields. | [160] |
| Batch | Swine manure | / | / | ND | / | ZVI: 5 (g/L) | Increased the CH4 yield by 17.6%. ZVI significantly increased the relative abundances of Methanothrix sp. and Methanolinea sp. to 37.5% and 8.6%, corresponding to an improvement of 19.8% and 16.2%. | [161] |
| Batch | Cattle manure | / | / | ND | / | ZVI: 80 | Increased the CH4 yield by 6.56%. | [144] |
| Batch | Cattle Manure | / | / | ND | / | Fe3O4: 18 | Increased the biogas and methane production by 27.6% and 25.4% compared to the control, respectively. | [162] |
Note: ZVI: microscale zero-valence iron; ND: data not involved.
5. Challenges and Perspectives
5.1. Feedstock Pretreatment and Reactor Innovation
Cellulose, hemicellulose, and indigestible lignin compounds in manure are firmly bound to form a complex structure. High contents of lignocellulose limit the hydrolysis of anaerobic digestion, which is usually the rate-limiting step in the whole process. Therefore, a pretreatment process is required to break the dense structure of lignocellulose and improve the accessibility of microorganisms to organic substances [163]. Currently, the pretreatment of lignocellulose includes mechanical, thermal, biological, chemical, and combined methods [164]. Each of the aforementioned methods can be effective for pretreatment, but combining multiple methods can provide additional benefits. One example is the use of a combination of heat and chemical treatments to process cow manure [165]; steam explosion is combined with ultra-fine grinding to treat cow manure and straw [166,167]. For nitrogen-rich manure, ammonia nitrogen removal became more critical, like stripping technology [78,168]. However, pretreatment sometimes induces extra chemical and energy costs and contaminants and requires complex operation [164]. There are still significant challenges in developing low-cost and high-efficiency animal manure pretreatment technology.
Given the differences in suitable conditions for various microorganisms in different fermentation steps of anaerobic digestion, a multi-step reactor would be helpful. The reactor can provide the desired fermentation conditions for hydrolysis, acidogenesis, and methanogenesis [97,98]. For example, CSTR can be combined with AnMBR to treat chicken manure, which realizes the treatment of chicken manure (TS 10%) by a membrane bioreactor. In addition, reactors combine thermophilic and mesophilic to treat swine manure [7], cattle manure [62], etc. Additionally, part of the hydrogen sulfide, mainly in protein-rich animal manure, is produced and emission in the hydrolysis and acidogenesis stage. Compared with the single-step reactor, the hydrogen sulfide concentration in the methanogenesis process is reduced, alleviating the toxic effects of hydrogen sulfide on methanogens. Therefore, advanced anaerobic reactors and technology for animal manure treatment still need deliberation.
5.2. Identifying the Functional Microbial Community
The microbial composition and the networks formed in anaerobic systems vary widely depending on the operating factors and substrate composition. Studies reported the changes in microbial community structure that may be linked to process operation and performance using 16S rRNA gene amplicon sequencing. The results helped to take the first step toward understanding the link between process and microbiology. However, the abundance of microbial species might not be directly linked to microbial activity and its importance for the degradation process [169]. One example is the methanogenic group, which is often detected with low relative abundance but significantly contributes to methane production [170]. When analyzing the microbial community, both viable and dead cells were detected through a response on DNA [169]. Consequently, the results may not reflect the actual functioning bacteria. RNA-based assays should become mainstream, which can represent an active community. At present, fluorescence in situ hybridization (FISH) analysis has become the primary tool, which mainly uses genetic probes and fluorochrome for labeling to distinguish between viable and dead cells, and it is widely used to identify active methanogens in anaerobic digestion [171,172]. In addition, propidium monoazide (PMA) polymerase chain reaction (PCR) or quantitative PCR (qPCR) can analyze viable cells in samples qualitatively or quantitatively [173]. The functional microbial community structure was accurately identified by viable cells, thus providing accurate information for adjusting operating parameters and realizing the efficient fermentation process.
5.3. Quantitative Supplementation of Trace Elements
Positive effects on process performance and stability by adding trace elements have been demonstrated during the anaerobic treatment of various animal manure. However, there are significant differences in the metals species and the dosage depending on feedstock and operating conditions. For example, Fe, Co, and Ni can effectively optimize hydrolysis and acidogenesis, as adding trace elements increases the dissolution and production of organic acids [152,174]. Fe, Co, Ni, Se, W, Mo, etc., especially Se, can rapidly increase the activity of methanogens [147,148]. The interlinkage between chemical and microbiological interactions makes trace elements supplementation technology complex. A critical aspect regarding supplementation in processes’ nitrogen-rich animal manure is the degradation of protein-rich substrates, which can also lead to high sulfide concentration in addition to the high ammonia, which in turn can lead to the formation of metal sulfide precipitates that reduce the bioavailability of essential trace elements [151,175]. Although adding biological chelating agents (soluble microbial products, extracellular polymeric substances, yeast extract, etc.) can improve the bioavailability of trace elements, the effect of chelating agents on the anaerobic consortia and whether it improves the utilization efficiency of trace elements remains uncertain [151]. The goal is that a few trace elements can significantly improve the performance and not introduce unnecessary metals in the digestate. Therefore, improving trace elements’ bioavailability is a challenge. Further research is needed to provide a complete picture of the effects of adding trace elements to animal manure digesters.
5.4. Solid-Liquid Separation of Digestate
Solid-liquid separation is usually carried out by press filtration and centrifugal dehydration [176]. The solid-liquid separation process reduces the organic content of the biogas slurry and methane emissions from the uncovered digestate storage tank [176]. Efficient separation requires serious consideration. However, various factors affect the effectiveness of digestate solid-liquid separation [177]. Suppose the solid and liquid components cannot be sufficiently separated. In that case, the suspended solids will enter the liquid phase that needs further treatment by both biological and chemical processes. Flocculants that can enhance the solid-liquid separation have been extensively studied but more for sewage sludge than animal manure digestate [178]. In addition, the digestate as inoculum for other anaerobic digestion processes and interconnected different types of anaerobic digestion after solid-liquid separation can increase the diversity of microorganisms and enhance the stability of the anaerobic system. Examples include cow dung biogas for pig manure anaerobic digestion inoculation, and cow manure and sludge for municipal solid waste anaerobic treatment [179,180]. Current commercial technologies can handle high flow rates and efficient dewatering of solid components but still cannot achieve deep dewatering at high loads. Then, the more efficient and convenient solid-liquid separation technology of the digestate needs further exploration.
5.5. High-Value Products from Methane
Methane in biogas is mainly used for power generation. However, the low price of methane makes anaerobic digestion projects challenging to operate without a subsidy. High-price products generated from methane that can extend the value of methane are an excellent way for animal anaerobic treatment. For example, methane can produce hydrogen through chemical looping and reforming technology [181,182,183]. Furthermore, producing single-cell protein or microbial protein from methane has high economic value. It can be based on a circular economy, thereby alleviating environmental and socioeconomic pressures due to the limitations of traditional agriculture [184,185]. Microbial protein is a biomass of microorganisms, such as fungi, yeast, microalgae, bacteria, etc., that contains essential amino acids and can replace conventional protein food or feed sources [185]. Currently, there are commercial applications for producing microbial protein using hydrogen-oxidizing bacteria (HOB), mainly for food and feed ingredients [186]. However, it is noted that the anaerobic digestion of animal manure will produce hydrogen sulfide (H2S), especially poultry manure. Therefore, it is necessary to purify the biogas to remove harmful gases such as H2S before methane valorization. For example, in situ biological desulfurization significantly reduces the cost and complexity of subsequent biogas purification in the anaerobic digestion of chicken manure [187]. The high-value utilization of methane is a new concept for the biogas industry, and more efforts should be made to demonstrate the benefits and the possibilities for wide application.
6. Conclusions
Even though anaerobic digestion processes are widely applied and commercialized, some processes still face challenges depending on the animal manure composition and operating conditions. Pre-treating animal manure to enhance hydrolysis can be dependent on the energy efficiency of the process as well as the complexity of the pre-treatment unit. For cattle manure, pretreatment can improve the biodegradability of the content of lignocellulose matter. Ammonia inhibition is a longstanding challenge for nitrogen-rich manure. It requires more profound insights into the functional microbial community, the importance of syntrophic metabolism, how that can be increased, and the impact of adding trace elements. Hence, several potentials exist to overcome the inhibitory effects of ammonia, such as the design of innovative reactors and process technologies that support high activities of essential microorganisms, which are crucial for the development of the next generation of anaerobic digestion of animal manure for biogas production and nutrient recovery.
Author Contributions
Y.S.: investigation, writing of original draft. W.Q.: writing—review and editing, supervision, project administration, funding acquisition, conceptualization. M.W.: writing—review and editing. G.H.: Resources. M.J.T.: Writing—review and editing. R.D.: Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Beijing, China (No.6222029) and the Key Research and Development Program of Hainan Province of China (ZDYF2021SHFZ065).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This work is a review paper and does not have human and animal studies.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This study was carried out as part of the Natural Science Foundation of Beijing and the Key Research and Development Program of the Hainan Province of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patterson, T.; Esteves, S.; Dinsdale, R.; Guwy, A. An evaluation of the policy and techno-economic factors affecting the potential for biogas upgrading for transport fuel use in the UK. Energy Policy 2011, 39, 1806–1816. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.-V.N.; Anjum, H.; Chang, C.-K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO [EB/OL]. Available online: http://www.fao.org/home/en/ (accessed on 5 May 2022).
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed. Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Feng, L.; Bonne Guldberg, L.; Jorgen Hansen, M.; Ma, C.; Vinther Ohrt, R.; Bjarne Moller, H. Impact of slurry removal frequency on CH4 emission and subsequent biogas production; a one-year case study. Waste Manag. 2022, 149, 199–206. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Sarria-GuzmÁN, Y.; Dendooven, L.; Luna-Guido, M. Methanogenesis and Methanotrophy in Soil: A Review. Pedosphere 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Lin, M.; Ren, L.; Mdondo Wandera, S.; Liu, Y.; Dong, R.; Qiao, W. Enhancing pathogen inactivation in pig manure by introducing thermophilic and hyperthermophilic hygienization in a two-stage anaerobic digestion process. Waste Manag. 2022, 144, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Ileleji, K.E.; Martin, C.; Jones, D. Chapter 17: Basics of Energy Production through Anaerobic Digestion of Livestock Manure. In Bioenergy; Academic Press: Cambridge, MA, USA, 2015; pp. 287–295. [Google Scholar] [CrossRef]
- Nasir, I.M.; Ghazi, T.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Abid, M.; Wu, J.; Seyedsalehi, M.; Hu, Y.-Y.; Tian, G. Novel insights of impacts of solid content on high solid anaerobic digestion of cow manure: Kinetics and microbial community dynamics. Bioresour. Technol. 2021, 333, 125205. [Google Scholar] [CrossRef]
- Ahlberg-Eliasson, K.; Westerholm, M.; Isaksson, S.; Schnürer, A. Anaerobic Digestion of Animal Manure and Influence of Organic Loading Rate and Temperature on Process Performance, Microbiology, and Methane Emission from Digestates. Front. Energy Res. 2021, 9, 740314. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Hashimoto, A. Ammonia inhibition of methanogenesis from cattle wastes. Agric. Wastes 1986, 17, 241–261. [Google Scholar] [CrossRef]
- Westerholm, M.; Dolfing, J.; Sherry, A.; Gray, N.D.; Head, I.M.; Schnurer, A. Quantification of syntrophic acetate-oxidizing microbial communities in biogas processes. Environ. Microbiol. Rep. 2011, 3, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Moestedt, J.; Schnurer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]
- Schnürer, A.; Nordberg, A. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef]
- Moestedt, J.; Bettina, M.; Westerholm, M.; Anna, S. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2015, 9, 180–194. [Google Scholar] [CrossRef]
- Wan, S.; Xi, B.; Xia, X.; Li, M.; Lv, D.; Wang, L.; Song, C. Using fluorescence excitation-emission matrix spectroscopy to monitor the conversion of organic matter during anaerobic co-digestion of cattle dung and duck manure. Bioresour. Technol. 2012, 123, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lim, T.T.; Duong, C.; Zhang, W.; Xu, C.; Yan, L.; Mei, Z.; Wang, W. Long-Term Mesophilic Anaerobic Co-Digestion of Swine Manure with Corn Stover and Microbial Community Analysis. Microorganisms 2020, 8, 188. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Orhan, Y.; Burak, D. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Walker, M.; Iyer, K.; Heaven, S.; Banks, C.J. Ammonia removal in anaerobic digestion by biogas stripping: An evaluation of process alternatives using a first order rate model based on experimental findings. Chem. Eng. J. 2011, 178, 138–145. [Google Scholar] [CrossRef]
- Sakar, S.; Yetilmezsoy, K.; Kocak, E. Anaerobic digestion technology in poultry and livestock waste treatment—A literature review. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Fan, R.; Song, Y.; Mahdy, A.; Dong, R.; Qiao, W. Enhancing Anaerobic Degradation of Lignocellulose-Rich Reed Straw by Adopting Grinding Pretreatment and High Temperature. Waste Biomass Valorization 2021, 12, 6067–6079. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional characteristics and energy potential of Chinese animal manure by type and as a whole. Appl. Energy 2015, 160, 108–119. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Cai, Y. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Sürmeli, R.n.; Alli, B. Dry anaerobic digestion of chicken manure coupled with membrane separation of ammonia. Bioresour. Technol. 2017, 244, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic performance of anaerobic digestion of chicken manure under wet, high solid, and dry conditions. Bioresour. Technol. 2020, 296, 122342. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F.; Dong, R. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Hu, W.; Mahdy, A.; Dong, T.; Sun, Y.; Qiao, W.; Dong, R. The metabolic performance and microbial communities of anaerobic digestion of chicken manure under stressed ammonia condition: A case study of a 10-year successful biogas plant. Renew. Energy 2021, 167, 644–651. [Google Scholar] [CrossRef]
- Yin, D.M.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Mahdy, A.; Bi, S.; Song, Y.; Qiao, W.; Dong, R. Overcome inhibition of anaerobic digestion of chicken manure under ammonia-stressed condition by lowering the organic loading rate. Bioresour. Technol. Rep. 2020, 9, 100359. [Google Scholar] [CrossRef]
- Yin, D.M.; Taherzadeh, M.J.; Lin, M.; Jiang, M.M.; Dong, R.J. Upgrading the anaerobic membrane bioreactor treatment of chicken manure by introducing in-situ ammonia stripping and hyper-thermophilic pretreatment. Bioresour. Technol. 2020, 310, 123470. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hu, W.; Qiao, W.; Westerholm, M.; Wandera, S.M.; Dong, R. Upgrading the performance of high solids feeding anaerobic digestion of chicken manure under extremely high ammonia level. Renew. Energy 2022, 194, 13–20. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, W.; Luo, W.; Fang, H.; Lv, H.; Liu, R.; Niu, Q. Anaerobic co-digestion of chicken manure and cardboard waste: Focusing on methane production, microbial community analysis and energy evaluation. Bioresour. Technol. 2021, 321, 124429. [Google Scholar] [CrossRef]
- Dalkilic, K.; Ugurlu, A. Biogas production from chicken manure at different organic loading rates in a mesophilic-thermopilic two stage anaerobic system. J. Biosci. Bioeng. 2015, 120, 315–322. [Google Scholar] [CrossRef]
- Niu, Q.; Hojo, T.; Qiao, W.; Qiang, H.; Li, Y.-Y. Characterization of methanogenesis, acidogenesis and hydrolysis in thermophilic methane fermentation of chicken manure. Chem. Eng. J. 2014, 244, 587–596. [Google Scholar] [CrossRef]
- Niu, Q.; Takemura, Y.; Kubota, K.; Li, Y.Y. Comparing mesophilic and thermophilic anaerobic digestion of chicken manure: Microbial community dynamics and process resilience. Waste Manag. 2015, 43, 114–122. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Mandy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe 2+ and Ni 2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef]
- Choi, H.L.; Sudiarto, S.; Renggaman, A. Prediction of livestock manure and mixture higher heating value based on fundamental analysis. Fuel 2014, 116, 772–780. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Xu, X.; Jiang, X.; Zheng, B.; Liu, X.; Pan, X.; Kardol, P. Emissions of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag. 2014, 34, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, Y.Y.; Wang, S.F.; Cao, Z.P.; Li, H.Z.; Fu, X.M.; Wang, K.J.; Zuo, J.E. Effects of thermal treatment on high solid anaerobic digestion of swine manure: Enhancement assessment and kinetic analysis. Waste Manag. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Dennehy, C.; Lawlor, P.G.; McCabe, M.S.; Cormican, P.; Sheahan, J.; Jiang, Y.; Zhan, X.; Gardiner, G.E. Anaerobic co-digestion of pig manure and food waste; effects on digestate biosafety, dewaterability, and microbial community dynamics. Waste Manag 2018, 71, 532–541. [Google Scholar] [CrossRef]
- Duan, N.; Zhang, D.; Lin, C.; Zhang, Y.; Zhao, L.; Liu, H.; Liu, Z. Effect of organic loading rate on anaerobic digestion of pig manure: Methane production, mass flow, reactor scale and heating scenarios. J. Environ. Manag. 2019, 231, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Panichnumsin, P.; Ahring, B.; Nopharatana, A.; Chaiprasert, P. Microbial community structure and performance of an anaerobic reactor digesting cassava pulp and pig manure. Water Sci. Technol. 2012, 66, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Batch anaerobic co-digestion of Kimchi factory waste silage and swine manure under mesophilic conditions. Bioresour. Technol. 2012, 124, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Wu, J.; Chen, X.; Peng, Y. Improving two-stage thermophilic-mesophilic anaerobic co-digestion of swine manure and rice straw by digestate recirculation. Chemosphere 2021, 274, 129787. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and ternary trace elements to enhance anaerobic digestion of cattle manure: Focusing on kinetic models for biogas production and digestate utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef] [PubMed]
- El-Mashad, H.M.; Zeeman, G.; van Loon, W.K.; Bot, G.P.; Lettinga, G. Effect of temperature and temperature fluctuation on thermophilic anaerobic digestion of cattle manure. Bioresour. Technol. 2004, 95, 191–201. [Google Scholar] [CrossRef]
- Negral, L.; Castrillon, L.; Maranon, E.; Fernandez-Nava, Y.; Ormaechea, P. Inverted phase fermentation as a pretreatment for anaerobic digestion of cattle manure and sewage sludge. J. Environ. Manag. 2017, 203, 741–744. [Google Scholar] [CrossRef]
- Plug Flow Reactor Market—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2019–2027. Available online: https://www.transparencymarketresearch.com/plug-flow-reactor-market.html (accessed on 10 August 2022).
- Batstone, D.J.; Puyol, D.; Alsina, X.F.; Rodríguez, J. Mathematical modelling of anaerobic digestion processes: Applications and future needs. Rev. Environ. Sci. Biotechnol. 2015, 14, 595–613. [Google Scholar] [CrossRef]
- Sharma, V.K.; Testa, C.; Lastella, G.; Cornacchia, G. Inclined-plug-flow type reactor for anaerobic digestion of semi-solid waste. Appl. Energy 2000, 65, 173–185. [Google Scholar] [CrossRef]
- Linke, B.; Muha, I.; Wittum, G.; Plogsties, V. Mesophilic anaerobic co-digestion of cow manure and biogas crops in full scale German biogas plants: A model for calculating the effect of hydraulic retention time and VS crop proportion in the mixture on methane yield from digester and from digestate storage at different temperatures. Bioresour. Technol. 2013, 130, 689–695. [Google Scholar] [CrossRef]
- Gomez-Quiroga, X.; Aboudi, K.; Alvarez-Gallego, C.J.; Romero-Garcia, L.I. Successful and stable operation of anaerobic thermophilic co-digestion of sun-dried sugar beet pulp and cow manure under short hydraulic retention time. Chemosphere 2022, 293, 133484. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef]
- Han, Y.; Green, H.; Tao, W. Reversibility of propionic acid inhibition to anaerobic digestion: Inhibition kinetics and microbial mechanism. Chemosphere 2020, 255, 126840. [Google Scholar] [CrossRef]
- Qiao, W.; Takayanagi, K.; Niu, Q.; Shofie, M.; Li, Y.Y. Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Bioresour. Technol. 2013, 149, 92–102. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Wagner, A.O.; Reitschuler, C.; Illmer, P. Effect of different acetate:propionate ratios on the methanogenic community during thermophilic anaerobic digestion in batch experiments. Biochem. Eng. J. 2014, 90, 154–161. [Google Scholar] [CrossRef]
- Hao, T.; Xiao, Y.; Varjani, S. Transiting from the inhibited steady-state to the steady-state through the ammonium bicarbonate mediation in the anaerobic digestion of low-C/N-ratio food wastes. Bioresour. Technol. 2022, 351, 127046. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.S.; Jalalah, M.; Li, X.; Salama, E.S. Synergistic ammonia and fatty acids inhibition of microbial communities during slaughterhouse waste digestion for biogas production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Khoshnevisan, B.; Duan, N. Meta-analysis of anaerobic co-digestion of livestock manure in last decade: Identification of synergistic effect and optimization synergy range. Appl. Energy 2021, 282, 116128. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Xing, Z.; Ma, Y.; Nan, F.; Pan, L.; Chen, J. Anaerobic co-digestion of Chinese cabbage waste and cow manure at mesophilic and thermophilic temperatures: Digestion performance, microbial community, and biogas slurry fertility. Bioresour. Technol. 2022, 363, 127976. [Google Scholar] [CrossRef]
- Cieslik, M.; Dach, J.; Lewicki, A.; Smurzynska, A.; Janczak, D.; Pawlicka-Kaczorowska, J.; Boniecki, P.; Cyplik, P.; Czekala, W.; Jozwiakowski, K. Methane fermentation of the maize straw silage under meso- and thermophilic conditions. Energy 2016, 115, 1495–1502. [Google Scholar] [CrossRef]
- Lin, M.; Wang, A.; Ren, L.; Qiao, W.; Wandera, S.M.; Dong, R. Challenges of pathogen inactivation in animal manure through anaerobic digestion: A short review. Bioengineered 2022, 13, 1149–1161. [Google Scholar] [CrossRef]
- Yin, D.M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.-J. Enhancing hyper-thermophilic hydrolysis pre-treatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure by a leach-bed process coupled with side-stream membrane ammonia separation. Bioresour. Technol. 2018, 258, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, J.; Zhai, L.; Liu, H. Enhanced methane production and syntrophic connection between microorganisms during semi-continuous anaerobic digestion of chicken manure by adding biochar. J. Clean. Prod. 2019, 240, 118178. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Mahdy, A.; Wandera, S.M.; Yin, D.; Dong, R. Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ibragimov, E.M.; Vankov, P.Y.; Miluykov, V.A.; Ziganshin, A.M. Comparison of anaerobic digestion strategies of nitrogen-rich substrates: Performance of anaerobic reactors and microbial community diversity. Waste Manag. 2016, 59, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.Y. Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Liu, Y.; Dach, J.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Molaey, R.; Sürmeli, R.Ö.; Sahinkaya, E.; Calli, B. Biogas production from chicken manure: Co-digestion with spent poppy straw. Int. Biodeterior. Biodegrad. 2016, 119, 205–210. [Google Scholar] [CrossRef]
- Yin, D.M.; Westerhol, M.; Qiao, W.; Bi, S.J.; Wandera, S.M.; Fan, R.; Jiang, M.M.; Dong, R.J. An explanation of the methanogenic pathway for methane production in anaerobic digestion of nitrogen-rich materials under mesophilic and thermophilic conditions. Bioresour. Technol. 2018, 264, 42–50. [Google Scholar] [CrossRef]
- Daniel, M.; Gilbert, Y.; Topp, E. Pathogen removal in farm-scale psychrophilic anaerobic digesters processing swine manure. Bioresour. Technol. 2011, 102, 641–646. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Wu, J.; Li, H.Z.; Poncin, S.; Wang, K.J.; Zuo, J.E. Study of an enhanced dry anaerobic digestion of swine manure: Performance and microbial community property. Bioresour. Technol. 2019, 282, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, M.; Li, K.; Li, C.; Wang, K. Optimizing dry anaerobic digestion at pilot scale for start-up strategy and long-term operation: Organic loading rate, temperature and co-digestion. Bioresour. Technol. 2020, 316, 123828. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Dong, H.; Zhu, Z.; Li, B. Performance Evaluation of a Large-Scale Swine Manure Mesophilic Biogas Plant in China. Trans. ASABE 2017, 60, 1713–1720. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, W.; Han, Z.; Wen, X.; Yang, M.; Zhang, Y. Effect of temperature on the persistence of fecal bacteria in ambient anaerobic digestion systems treating swine manure. Sci. Total Environ. 2021, 791, 148302. [Google Scholar] [CrossRef]
- Masse, D.I.; Yan, G.; Saady, N.; Liu, C. Low-temperature anaerobic digestion of swine manure in a plug-flow reactor. Environ. Technol. 2013, 34, 2617–2624. [Google Scholar] [CrossRef]
- Cavinato, C.; Fatone, F.; Bolzonella, D.; Pavan, P. Thermophilic anaerobic co-digestion of cattle manure with agro-wastes and energy crops: Comparison of pilot and full scale experiences. Bioresour. Technol. 2010, 101, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Moset, V.; Poulsen, M.; Wahid, R.; Hojberg, O.; Moller, H.B. Mesophilic versus thermophilic anaerobic digestion of cattle manure: Methane productivity and microbial ecology. Microb. Biotechnol. 2015, 8, 787–800. [Google Scholar] [CrossRef]
- Christou, M.L.; Vasileiadis, S.; Karpouzas, D.G.; Angelidaki, I.; Kotsopoulos, T.A. Effects of organic loading rate and hydraulic retention time on bioaugmentation performance to tackle ammonia inhibition in anaerobic digestion. Bioresour. Technol. 2021, 334, 125246. [Google Scholar] [CrossRef]
- Chen, S.; Dong, X. Proteiniphilum acetatigenes gen. nov., sp. nov., from a UASB reactor treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 2005, 55, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Eugenio, G.; Fardeau, M.L.; Cayol, J.L.; Patel, B.; Ollivier, B. Sporanaerobacter acetigenes gen. nov., sp. nov., a novel acetogenic, facultatively sulfur-reducing bacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 1217–1223. [Google Scholar] [CrossRef]
- Menes, R.J.; Mux, L.A. Anaerobaculum mobile sp. nov., a novel anaerobic, moderately thermophilic, peptide-fermenting bacterium that uses crotonate as an electron acceptor, and emended description of the genus Anaerobaculum. Int. J. Syst. Evol. Microbiol. 2002, 52, 157–164. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Mladenovska, Z.; Westermann, P.; Ahring, B.K. Comparison of two-stage thermophilic (68 degrees C/55 degrees C) anaerobic digestion with one-stage thermophilic (55 degrees C) digestion of cattle manure. Biotechnol. Bioeng. 2004, 86, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chai, W.S.; Lay, C.-H.; Chen, C.-C.; Lee, C.-Y.; Show, P.L. Optimization of Hydrolysis-Acidogenesis Phase of Swine Manure for Biogas Production Using Two-Stage Anaerobic Fermentation. Processes 2021, 9, 1324. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Ibrahim, A.A.; Mladenovska, Z. Effect of temperature increase from 55 to 65 °C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res. 2001, 35, 2446–2452. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Westerholm, M.; Muller, B.; Arthurson, V.; Schnurer, A. Changes in the acetogenic population in a mesophilic anaerobic digester in response to increasing ammonia concentration. Microbes Environ. 2011, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Na, D.; Zhang, Y.; Liu, Z.; Li, B.; Zhang, D.; Dong, T. Anaerobic co-digestion of chicken manure and microalgae Chlorella sp.: Methane potential, microbial diversity and synergistic impact evaluation. Waste Manag. 2017, 68, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cao, G.; Guo, X.; Liu, T.; Wu, J.; Ren, N. Efficient biogas production from cattle manure in a plug flow reactor: A large scale long term study. Bioresour. Technol. 2019, 278, 450–455. [Google Scholar] [CrossRef]
- Conklin, A.; Stensel, H.D.; Ferguson, J. Growth Kinetics and Competition between Methanosarcina and Methanosaeta in Mesophilic Anaerobic Digestion. Water Environ. Res. 2006, 78, 486–496. [Google Scholar] [CrossRef]
- Hendriksen, H.V.; Ahring, B.K. Effects of ammonia on growth and morphology of thermophilic hydrogen-oxidizing methanogenic bacteria. FEMS Microbiol. Lett. 1991, 85, 241–246. [Google Scholar] [CrossRef]
- Lauterböck, B.; Nikolausz, M.; Lv, Z.; Baumgartner, M.; Liebhard, G.; Fuchs, W. Improvement of anaerobic digestion performance by continuous nitrogen removal with a membrane contactor treating a substrate rich in ammonia and sulfide. Bioresour. Technol. 2014, 158, 209–216. [Google Scholar] [CrossRef]
- Cuzin, N.; Ouattara, A.S.; Labat, M.; Garcia, J.L. Methanobacterium congolense sp. nov., from a methanogenic fermentation of cassava peel. Int. J. Syst. Evol. Microbiol. 2001, 51, 489–493. [Google Scholar] [CrossRef]
- Savant, D.V.; Shouche, Y.S.; Prakash, S.; Ranade, D.R. Methanobrevibacter acididurans sp. nov., a novel methanogen from a sour anaerobic digester. Int. J. Syst. Evol. Microbiol. 2002, 52, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhilina, T.N.; Zavarzina, D.G.; Kevbrin, V.V.; Kolganova, T.V. Methanocalculus natronophilus sp. nov., a new alkaliphilic hydrogenotrophic methanogenic archaeon from a soda lake, and proposal of the new family Methanocalculaceae. Microbiology 2014, 82, 698–706. [Google Scholar] [CrossRef]
- Zellner, G.; Messner, P.; Winter, J.; Stackebrandt, E. Methanoculleus palmolei sp. nov., an irregularly coccoid methanogen from an anaerobic digester treating wastewater of a palm oil plant in North-Sumatra, Indonesia. Int. J. Syst. Bacteriol. 1998, 48, 1111–1117. [Google Scholar] [CrossRef]
- Patel, G.B.; Sprott, G.D. Methanosaeta concilii gen. nov. sp. nov. (“Methanothrix concilii”) and Methanosaeta thermoacetophila nom. rev., comb. nov. Int. J. Syst. Bacteriol. 1990, 40, 79–82. [Google Scholar] [CrossRef]
- Kai, M.; Liu, X.; Dong, X. Methanosaeta harundinacea sp. nov., a novel acetate-scavenging methanogen isolated from a UASB reactor. Int. J. Syst. Evol. Microbiol. 2006, 56, 127–131. [Google Scholar] [CrossRef]
- Kern, T.; Fischer, M.A.; Deppenmeier, U.; Schmitz, R.A.; Rother, M. Methanosarcina flavescens sp. nov., a methanogenic archaeon isolated from a full-scale anaerobic digester. Int. J. Syst. Evol. Microbiol. 2016, 66, 1533–1538. [Google Scholar] [CrossRef]
- Westerholm, M.; Dolfing, J.; Schnurer, A. Growth Characteristics and Thermodynamics of Syntrophic Acetate Oxidizers. Environ. Sci. Technol. 2019, 53, 5512–5520. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, J.; Wei, Y.; Shen, P. Effects of ferric oxide on the microbial community and functioning during anaerobic digestion of swine manure. Bioresour. Technol. 2019, 287, 121393. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, S.; Kwon, O.S. Effect of applied voltage and temperature on methane production and microbial community in microbial electrochemical anaerobic digestion systems treating swine manure. J. Ind. Microbiol. Biotechnol. 2019, 46, 911–923. [Google Scholar] [CrossRef]
- Sun, L.; Pope, P.B.; Eijsink, V.G.; Schnurer, A. Characterization of microbial community structure during continuous anaerobic digestion of straw and cow manure. Microb. Biotechnol. 2015, 8, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, C. Anaerobic codigestion of pretreated wheat straw with cattle manure and analysis of the microbial community. Bioresour. Technol. 2015, 186, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yuan, H.; Wachemo, A.C.; Li, X. Anaerobic co-digestion of cattle manure and liquid fraction of digestate (LFD) pretreated corn stover: Pretreatment process optimization and evolution of microbial community structure. Bioresour. Technol. 2020, 296, 122282. [Google Scholar] [CrossRef] [PubMed]
- Montecchio, D.; Esposito, G.; Gagliano, M.C.; Gallipoli, A.; Gianico, A.; Braguglia, C.M. Syntrophic acetate oxidation during the two-phase anaerobic digestion of waste activated sludge: Microbial population, Gibbs free energy and kinetic modelling. Int. Biodeterior. Biodegrad. 2017, 125, 177–188. [Google Scholar] [CrossRef]
- Dyksma, S.; Jansen, L.; Gallert, C. Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome 2020, 8, 105. [Google Scholar] [CrossRef]
- Lue, F.; Hao, L.; Guan, D.; Qi, Y.; Shao, L.; He, P. Synergetic stress of acids and ammonium on the shift in the methanogenic pathways during thermophilic anaerobic digestion of organics. Water Res. 2013, 47, 2297–2306. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Kissas, K.; Angelidaki, I. Effect of different ammonia sources on aceticlastic and hydrogenotrophic methanogens. Bioresour. Technol. 2018, 250, 390–397. [Google Scholar] [CrossRef]
- Rivera-Salvador, V.; Lopez-Cruz, I.L.; Espinosa-Solares, T.; Aranda-Barradas, J.S.; Huber, D.H.; Sharma, D.; Toledo, J.U. Application of Anaerobic Digestion Model No. 1 to describe the syntrophic acetate oxidation of poultry litter in thermophilic anaerobic digestion. Bioresour. Technol. 2014, 167, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Kamagata, Y.; Hanada, S.; Shoun, H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Weijma, J.; Stams, A.J.M. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int. J. Syst. Evol. Microbiol. 2002, 52, 1361–1368. [Google Scholar] [CrossRef]
- Schnurer, A.; Schink, B.; Svensson, B.H. Clostridium ultunense sp. nov., a Mesophilic Bacterium Oxidizing Acetate in Syntrophic Association with a Hydrogenotrophic Methanogenic Bacterium. Int. J. Syst. Bacteriol. 1996, 46, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Roos, S.; Schnürer, A. Syntrophaceticus schinkii gen. nov., sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter. Fems Microbiol. Lett. 2010, 309, 100–104. [Google Scholar] [CrossRef]
- Westerholm, M.; Roos, S.; Schnürer, A. Tepidanaerobacter acetatoxydans sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from two ammonium-enriched mesophilic methanogenic processes. Syst. Appl. Microbiol. 2011, 34, 260–266. [Google Scholar] [CrossRef]
- Manzoor, S.; Bongcam-Rudloff, E.; Schnürer, A.; Müller, B. Genome-Guided Analysis and Whole Transcriptome Profiling of the Mesophilic Syntrophic Acetate Oxidising Bacterium Syntrophaceticus schinkii. PLoS ONE 2016, 11, e0166520. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fotidis, I.A.; Irini, A. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol. Ecol. 2015, 91, fiv130. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, J.; Guivernau, M.; Fernandez, B.; Vila, J.; Vinas, M.; Riau, V.; Prenafeta-Boldu, F.X. Functional biodiversity and plasticity of methanogenic biomass from a full-scale mesophilic anaerobic digester treating nitrogen-rich agricultural wastes. Sci. Total Environ. 2019, 649, 760–769. [Google Scholar] [CrossRef]
- Westerholm, M.; Leven, L.; Schnurer, A. Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Appl. Environ. Microbiol. 2012, 78, 7619–7625. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Fan, L.; Chapleur, O.; Guenne, A.e.; Bize, A.; Bureau, C.; Lü, F.; He, P.; Bouchez, T.; Maz’eas, L. Gradual development of ammonia-induced syntrophic acetate-oxidizing activities under mesophilic and thermophilic conditions quantitatively tracked using multiple isotopic approaches. Water Res. 2021, 204, 117586. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A critical review of ammonia recovery from anaerobic digestate of organic wastes via stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous recovery of ammonium, potassium and magnesium from produced water by struvite precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Ham, K.; Beom, S.K.; Kwon, Y.C. Enhanced ammonium removal efficiency by ion exchange process of synthetic zeolite after Na+ and heat pretreatment. Water Sci. Technol. 2018, 78, 1417–1425. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Yu, Q.; Ma, R. Removal of nitrogen from chicken manure anaerobic digestion for enhanced biomethanization. Fuel 2018, 232, 395–404. [Google Scholar] [CrossRef]
- Zhou, M.; Li, C.; Ni, F.; Chen, A.; Li, M.; Shen, G.; Deng, Y.; Deng, L. Packed activated carbon particles triggered a more robust syntrophic pathway for acetate oxidation-hydrogenotrophic methanogenesis at extremely high ammonia concentrations. Renew. Energy 2022, 191, 305–317. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, I.; Riano, B.; Molinuevo-Salces, B.; Vanotti, M.B.; Garcia-Gonzalez, M.C. Improved anaerobic digestion of swine manure by simultaneous ammonia recovery using gas-permeable membranes. Water Res. 2021, 190, 116789. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Ma, J.; Gu, J.; Wang, X.; Peng, H.; Bao, J. Effects of nano-zerovalent iron on antibiotic resistance genes during the anaerobic digestion of cattle manure. Bioresour. Technol. 2019, 289, 121688. [Google Scholar] [CrossRef]
- Feng, X.M.; Karlsson, A.; Svensson, B.H.; Bertilsson, S. Impact of trace element addition on biogas production from food industrial waste--linking process to microbial communities. FEMS Microbiol. Ecol. 2010, 74, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Einarsson, P.; Schnürer, A.; Sundberg, C.; Ejlertsson, J.; Svensson, B.H. Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J. Biosci. Bioeng. 2012, 114, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure: Influence of trace element supplementation. Eng. Life Sci. 2019, 19, 143–150. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Calli, B. Long-term inuence of trace element deciency on anaerobic mono-digestion of chicken manure. J. Environ. Manag. 2018, 223, 743–748. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Z.; Ran, W.; Yuan, H.; Li, X. Performance and microbial community dynamics in anaerobic co-digestion of chicken manure and corn stover with different modification methods and trace element supplementation strategy. Bioresour. Technol. 2021, 325, 124713. [Google Scholar] [CrossRef] [PubMed]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.O.; Çalli, B. Influence of trace element supplementation on anaerobic digestion of chicken manure: Linking process stability to methanogenic population dynamics. J. Clean. Prod. 2018, 181, 794–800. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Ismail, N.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Qilin, Y.; Xu, Z.; Quan, X. Enhanced high-solids anaerobic digestion of waste activated sludge by the addition of scrap iron. Bioresour. Technol. 2014, 159, 297–304. [Google Scholar] [CrossRef]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.M.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sustain. Energy Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Xin, K.; Yu, S.; Shuang, X.; Wen, F.; Liu, J.; Li, H. Effect of Fe0 addition on volatile fatty acids evolution on anaerobic digestion at high organic loading rates. Waste Manag. 2017, 71, 719–727. [Google Scholar] [CrossRef]
- Hao, X.; Wei, J.; Van Loosdrecht, M.C.M.; Cao, D. Analysing the mechanisms of sludge digestion enhanced by iron. Water Res. 2017, 117, 58–67. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Recep, S.; Alli, B. Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation. Biomass Bioenergy 2018, 108, 439–446. [Google Scholar] [CrossRef]
- Cai, Y.; Janke, L.; Meng, X.; Zheng, Z.; Zhao, X.; Pr¨oter, J.; Sch¨afer, F. The absolute concentration and bioavailability of trace elements: Two vital parameters affecting anaerobic digestion performance of chicken manure leachate. Bioresour. Technol. 2022, 350, 126909. [Google Scholar] [CrossRef]
- Liang, Y.G.; Li, X.J.; Zhang, J.; Zhang, L.G.; Cheng, B. Effect of microscale ZVI/magnetite on methane production and bioavailability of heavy metals during anaerobic digestion of diluted pig manure. Environ. Sci. Pollut. Res. 2017, 24, 12328–12337. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, F.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Combined effect of zero valent iron and magnetite on semi-dry anaerobic digestion of swine manure. Bioresour. Technol. 2022, 346, 126438. [Google Scholar] [CrossRef]
- Singh, D.; Malik, K.; Sindhu, M.; Kumari, N.; Rani, V.; Mehta, S.; Malik, K.; Ranga, P.; Sharma, K.; Dhull, N.; et al. Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials 2022, 12, 497. [Google Scholar] [CrossRef]
- Bolado-Rodriguez, S.; Toquero, C.; Martin-Juarez, J.; Travaini, R.; Garcia-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Hendriks, A.T.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Usman Khan, M.; Kiaer Ahring, B. Improving the biogas yield of manure: Effect of pretreatment on anaerobic digestion of the recalcitrant fraction of manure. Bioresour. Technol. 2021, 321, 124427. [Google Scholar] [CrossRef]
- Gaballah, E.S.; Abomohra, A.E.; Xu, C.; Elsayed, M.; Abdelkader, T.K.; Lin, J.; Yuan, Q. Enhancement of biogas production from rape straw using different co-pretreatment techniques and anaerobic co-digestion with cattle manure. Bioresour. Technol. 2020, 309, 123311. [Google Scholar] [CrossRef]
- Lizasoain, J.; Trulea, A.; Gittinger, J.; Kral, I.; Piringer, G.; Schedl, A.; Nilsen, P.J.; Potthast, A.; Gronauer, A.; Bauer, A. Corn stover for biogas production: Effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 2017, 244, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Verde, I.; Regueiro, L.; Lema, J.M.; Carballa, M. Blending based optimisation and pretreatment strategies to enhance anaerobic digestion of poultry manure. Waste Manag. 2018, 71, 521–531. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Regueiro, L.; Props, R.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Lema, J.M.; Carballa, M. Presence does not imply activity: DNA and RNA patterns differ in response to salt perturbation in anaerobic digestion. Biotechnol. Biofuels 2016, 9, 244. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Goesmann, A.; Jaenicke, S.; Junemann, S.; Eikmeyer, F.; Szczepanowski, R.; Al-Soud, W.A.; Sorensen, S.; Puhler, A.; Schluter, A. Profiling of the metabolically active community from a production-scale biogas plant by means of high-throughput metatranscriptome sequencing. J. Biotechnol. 2012, 158, 248–258. [Google Scholar] [CrossRef]
- Huber, D.; Voith von Voithenberg, L.; Kaigala, G.V. Fluorescence in situ hybridization (FISH): History, limitations and what to expect from micro-scale FISH? Micro Nano Eng. 2018, 1, 15–24. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef]
- Nocker, A.; Sossa, K.E.; Camper, A.K. Molecular monitoring of disinfection efficacy using propidium monoazide in combination with quantitative PCR. J. Microbiol. Methods 2007, 70, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gomec, C.Y.; Ahn, Y.; Speece, R.E. Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ. Technol. 2003, 24, 1183–1190. [Google Scholar] [CrossRef]
- Aquino, S.F.; Stuckey, D.C. Bioavailability and Toxicity of Metal Nutrients during Anaerobic Digestion. J. Environ. Eng. 2007, 133, 28–35. [Google Scholar] [CrossRef]
- Grell, T.; Marchuk, S.; Williams, I.; McCabe, B.K.; Tait, S. Resource recovery for environmental management of dilute livestock manure using a solid-liquid separation approach. J. Environ. Manag. 2022, 325, 116254. [Google Scholar] [CrossRef]
- Liu, X.-M.; Sheng, G.-P.; Luo, H.-W.; Feng, Z. Contribution of Extracellular Polymeric Substances (EPS) to the Sludge Aggregation. Environ. Sci. Technol. 2010, 44, 4355–4360. [Google Scholar] [CrossRef]
- Vaxelaire, J.; Cezac, P. Moisture distribution in activated sludges: A review. Water Res. 2004, 38, 2214–2229. [Google Scholar] [CrossRef]
- Wi, J.; Lee, S.; Ahn, H. Influence of Dairy Manure as Inoculum Source on Anaerobic Digestion of Swine Manure. Bioengineering 2023, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, F.; Tommasi, T.; Deorsola, F.A.; Marchisio, D.; Fino, D. Effect of inoculum origin and substrate-inoculum ratio to enhance the anaerobic digestion of organic fraction municipal solid waste (OFMSW). J. Clean. Prod. 2022, 351, 131539. [Google Scholar] [CrossRef]
- Fan, Z.; Xiao, W. Electrochemical Splitting of Methane in Molten Salts To Produce Hydrogen. Angew. Chem. Int. Ed. Engl. 2021, 60, 7664–7668. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbæk, J.S.; Vendelbo, S.B.; Bendixen, F.B. Electrified methane reforming A compact approach to greener industrial hydrogen production. Science 2019, 364, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.; Eid, K.; El-Naas, M.H.; Kumar, D.; Kumar, A. Catalytic Methane Decomposition to Carbon Nanostructures and COx-Free Hydrogen: A Mini-Review. Nanomaterials 2021, 11, 1226. [Google Scholar] [CrossRef]
- Kerckhof, F.M.; Sakarika, M.; Van Giel, M.; Muys, M.; Vermeir, P.; De Vrieze, J.; Vlaeminck, S.E.; Rabaey, K.; Boon, N. From Biogas and Hydrogen to Microbial Protein Through Co-Cultivation of Methane and Hydrogen Oxidizing Bacteria. Front. Bioeng. Biotechnol. 2021, 9, 733753. [Google Scholar] [CrossRef] [PubMed]
- Anupamaa; Ravindra, P. Value-added food: Single cell protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef]
- Pander, B.; Mortimer, Z.; Woods, C.; McGregor, C.; Dempster, A.; Thomas, L.; Maliepaard, J.; Mansfield, R.; Rowe, P.; Krabben, P. Hydrogen oxidising bacteria for production of single-cell protein and other food and feed ingredients. Eng. Biol. 2020, 4, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Mahdy, A.; Hou, Z.; Lin, M.; Stinner, W.; Qiao, W.; Dong, R. Air Supplement as a Stimulation Approach for the In Situ Desulfurization and Methanization Enhancement of Anaerobic Digestion of Chicken Manure. Energy Fuels 2020, 34, 12606–12615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).