Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation
Abstract
1. Introduction
2. Materials and Methods
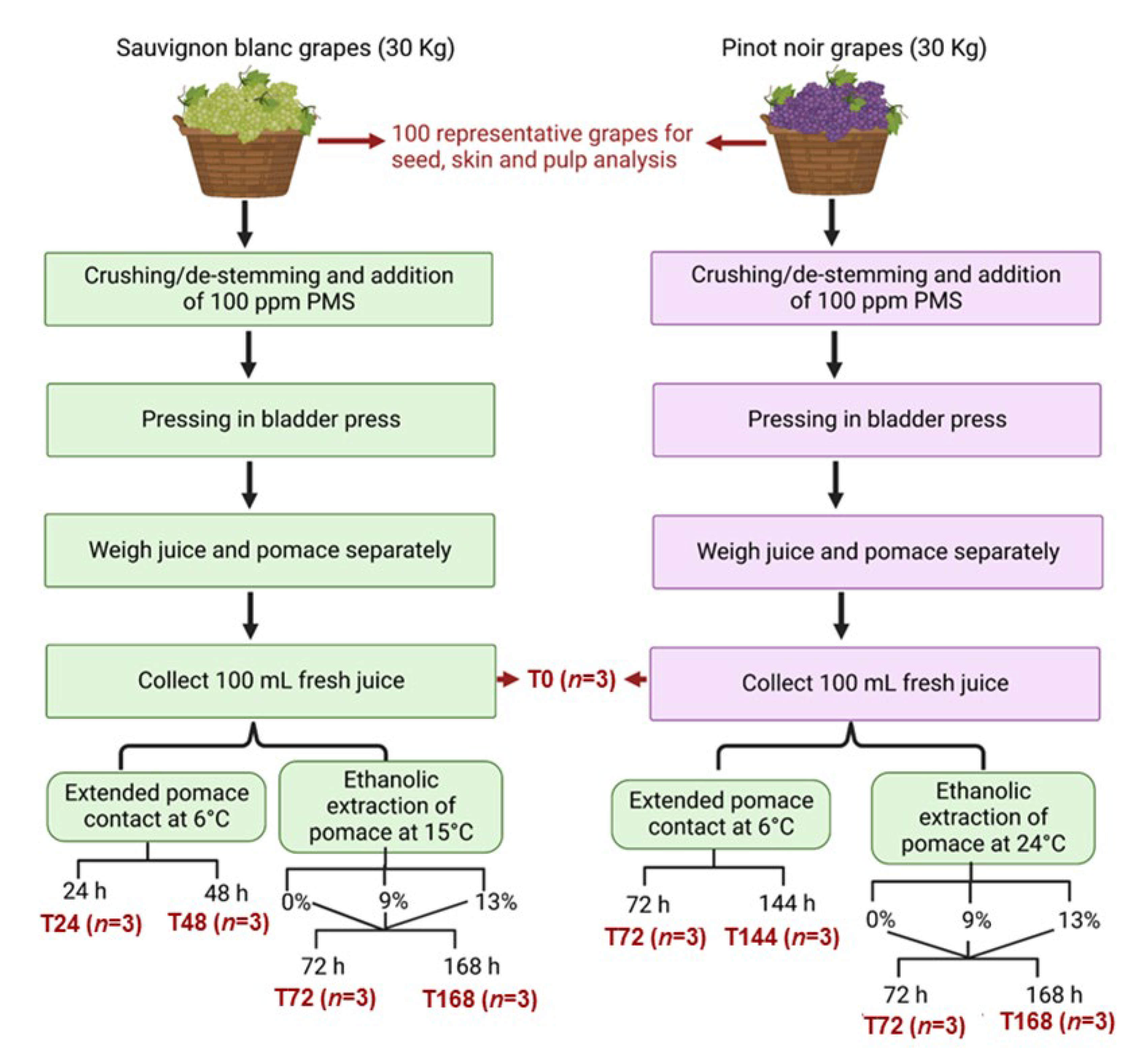
2.1. Experimental Design-Collection of Grapes and Sampling Protocols
2.2. Determination of Major Oenological Parameters
2.3. Quantification of Major Organic and Amino Acids
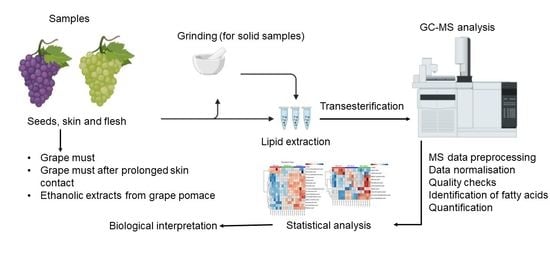
2.4. Lipid Extraction and Transesterification
2.5. GC-MS Analysis
2.6. GC-MS Data Mining
2.7. Statistical Analysis
3. Results and Discussion
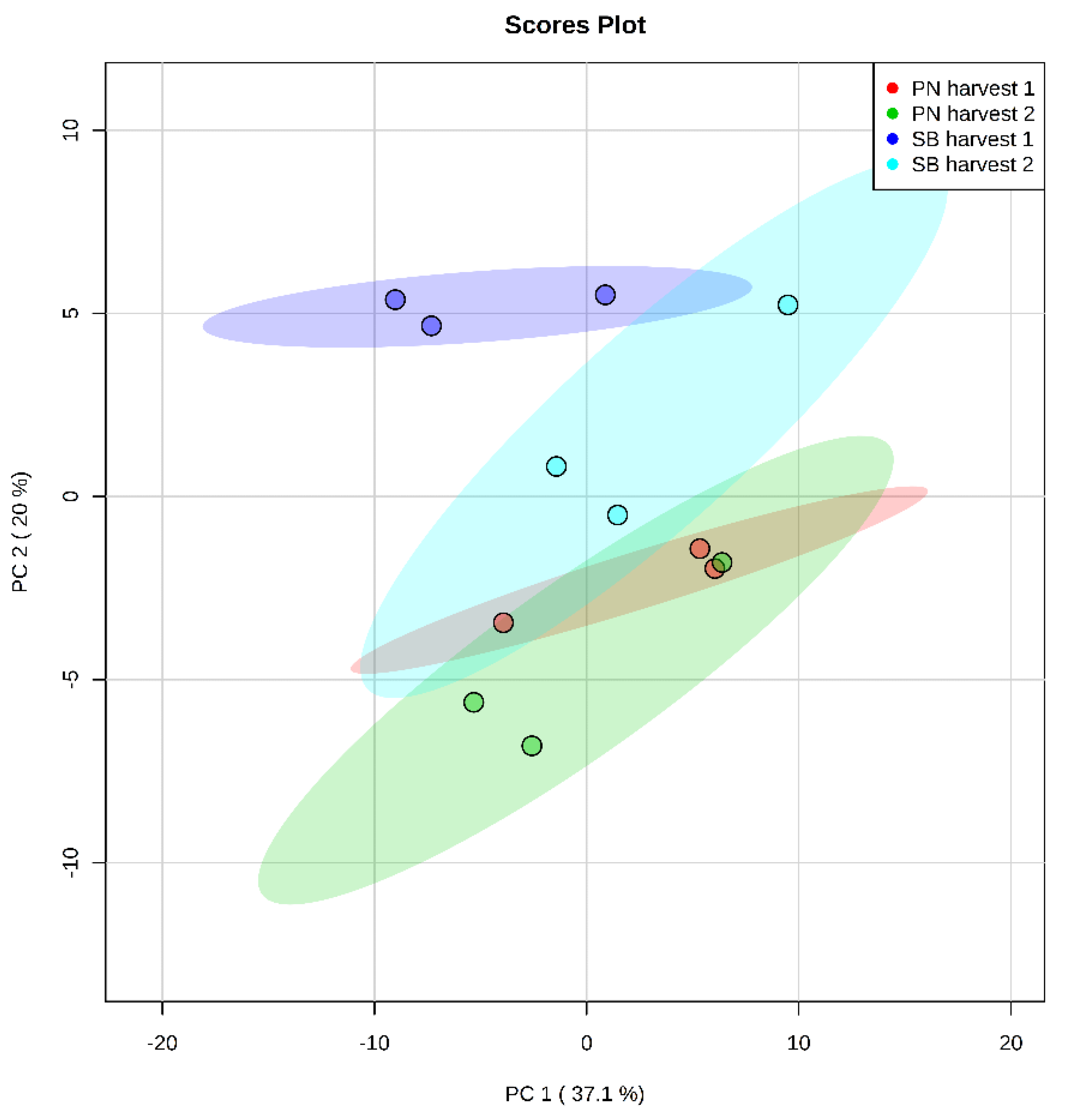
3.1. Chemical Composition of the Sauvignon Blanc (SB) and Pinot Noir (PN) Juices and Alcoholic Extracts
3.2. Overview of Fatty Acids Present in Grapes and Their Extracts Harvested at Different Time Points
3.3. Total Lipids and Fatty Acids in Sauvignon Blanc and Pinot Noir Grape Tissues
3.4. Total Lipid and Fatty Acid Contents in Sauvignon Blanc and Pinot Noir Grape Juices and Extracts
3.4.1. Effect of Harvest Time on Total Lipids and Fatty Acids in Grape Juices
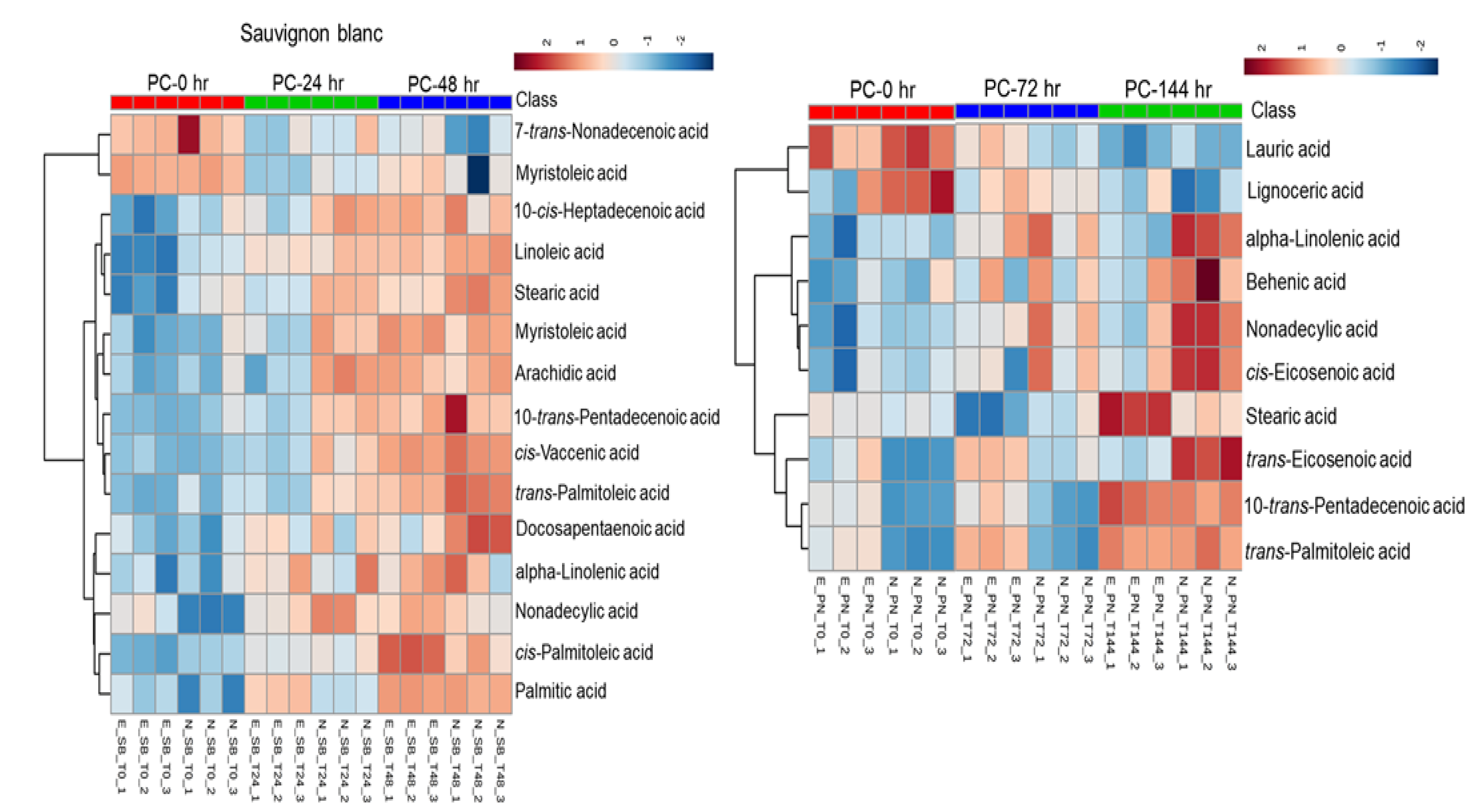
3.4.2. Effect of Pomace Contact Time on Lipid Extraction and Fatty Acids in Grape Must
3.5. Effect of Ethanol Concentration Relevant to Fermentation on Lipid Extraction from Grape Pomace
3.6. Correlation of Lipids and Fatty Acids with Major Oenological Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phan, Q.; Hoffman, S.; Tomasino, E. Contribution of Lipids to Taste and Mouthfeel Perception in a Model Wine Solution. ACS Food Sci. Technol. 2021, 1, 1561–1566. [Google Scholar] [CrossRef]
- Sherman, E. A Flavoromics Approach to the Characterisation of Pinot Noir Wine Sensory Properties. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2017. [Google Scholar]
- Kehelpannala, C.; Rupasinghe, T.; Hennessy, T.; Bradley, D.; Ebert, B.; Roessner, U. The state of the art in plant lipidomics. Mol. Omics 2021, 17, 894–910. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Da Ros, A.; Masuero, D.; Izquierdo-Cañas, P.M.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Mattivi, F.; Vrhovsek, U. LC-MS/MS analysis of free fatty acid composition and other lipids in skins and seeds of Vitis vinifera grape cultivars. Food Res. Int. 2019, 125, 108556. [Google Scholar] [CrossRef]
- Gallander, J.F.; Peng, A.C. Lipid and Fatty Acid Compositions of Different Grape Types. Am. J. Enol. Vitic. 1980, 31, 24. [Google Scholar] [CrossRef]
- Grncarevic, M.; Radler, F. A Review of the Surface Lipids of Grapes and Their Importance in the Drying Process. Am. J. Enol. Vitic. 1971, 22, 80. [Google Scholar]
- Tumanov, S.; Zubenko, Y.; Greven, M.; Greenwood, D.R.; Shmanai, V.; Villas-Boas, S.G. Comprehensive lipidome profiling of Sauvignon blanc grape juice. Food Chem. 2015, 180, 249–256. [Google Scholar] [CrossRef]
- Pinu, F.R.; Tumanov, S.; Grose, C.; Raw, V.; Albright, A.; Stuart, L.; Villas-Boas, S.G.; Martin, D.; Harker, R.; Greven, M. Juice Index: An integrated Sauvignon blanc grape and wine metabolomics database shows mainly seasonal differences. Metabolomics 2019, 15, 3. [Google Scholar] [CrossRef]
- Arita, K.; Honma, T.; Suzuki, S. Comprehensive and comparative lipidome analysis of Vitis vinifera L. cv. Pinot Noir and Japanese indigenous V. vinifera L. cv. Koshu grape berries. PLoS ONE 2017, 12, e0186952. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; Hory, C.; Bard, M.H.; Taisant, C.; Olsson, A.; Le Fur, Y. Effects of skin contact and settling on the level of the C18:2, C18:3 fatty acids and C6 compounds in burgundy chardonnay musts and wines. Food Qual. Prefer. 1995, 6, 35–41. [Google Scholar] [CrossRef]
- Masuero, D.; Škrab, D.; Chitarrini, G.; Garcia-Aloy, M.; Franceschi, P.; Sivilotti, P.; Guella, G.; Vrhovsek, U. Grape Lipidomics: An Extensive Profiling thorough UHPLC-MS/MS Method. Metabolites 2021, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Casu, F.; Pinu, F.R.; Fedrizzi, B.; Greenwood, D.R.; Villas-Boas, S.G. The effect of linoleic acid on the Sauvignon blanc fermentation by different wine yeast strains. FEMS Yeast Res. 2016, 16, fow050. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Torrea, D.; Schmidt, S.A.; Ancin-Azpilicueta, C.; Henschke, P.A. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Deroite, A.; Legras, J.-L.; Rigou, P.; Ortiz-Julien, A.; Dequin, S. Lipids modulate acetic acid and thiol final concentrations in wine during fermentation by Saccharomyces cerevisiae × Saccharomyces kudriavzevii hybrids. AMB Express 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Villas-Boas, S.G.; Martin, D. Pre-fermentative supplementation of fatty acids alters the metabolic activity of wine yeasts. Food Res. Int. 2019, 121, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.B.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Sanchez, I.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. Impact of high lipid contents on the production of fermentative aromas during white wine fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 6435–6449. [Google Scholar] [CrossRef]
- Liu, P.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Effect of Unsaturated Fatty Acids on Intra-Metabolites and Aroma Compounds of Saccharomyces cerevisiae in Wine Fermentation. Foods 2021, 10, 277. [Google Scholar] [CrossRef]
- Landolfo, S.; Zara, G.; Zara, S.; Budroni, M.; Ciani, M.; Mannazzu, I. Oleic acid and ergosterol supplementation mitigates oxidative stress in wine strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2010, 141, 229–235. [Google Scholar] [CrossRef]
- Rupčić, J.; Jurešić, G.C.; Blagović, B. Influence of stressful fermentation conditions on neutral lipids of a Saccharomyces cerevisiae brewing strain. World J. Microbiol. Biotechnol. 2010, 26, 1331–1336. [Google Scholar] [CrossRef]
- Redón, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effect of lipid supplementation upon Saccharomyces cerevisiae lipid composition and fermentation performance at low temperature. Eur. Food Res. Technol. 2009, 228, 833–840. [Google Scholar] [CrossRef]
- Mannazzu, I.; Angelozzi, D.; Belviso, S.; Budroni, M.; Farris, G.A.; Goffrini, P.; Lodi, T.; Marzona, M.; Bardi, L. Behaviour of Saccharomyces cerevisiae wine strains during adaptation to unfavourable conditions of fermentation on synthetic medium: Cell lipid composition, membrane integrity, viability and fermentative activity. Int. J. Food Microbiol. 2008, 121, 84–91. [Google Scholar] [CrossRef]
- Girardi-Piva, G.; Casalta, E.; Legras, J.-L.; Nidelet, T.; Pradal, M.; Macna, F.; Ferreira, D.; Ortiz-Julien, A.; Tesnière, C.; Galeote, V.; et al. Influence of ergosterol and phytosterols on wine alcoholic fermentation with Saccharomyces cerevisiae strains. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Phan, Q.; Tomasino, E. Untargeted lipidomic approach in studying pinot noir wine lipids and predicting wine origin. Food Chem. 2021, 355, 129409. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.; DuBois, A.; Osborne, J.; Tomasino, E. Effects of Yeast Product Addition and Fermentation Temperature on Lipid Composition, Taste and Mouthfeel Characteristics of Pinot Noir Wine. Horticulturae 2022, 8, 52. [Google Scholar] [CrossRef]
- Martin, D.; Grose, C.; Fedrizzi, B.; Stuart, L.; Albright, A.; McLachlan, A. Grape cluster microclimate influences the aroma composition of Sauvignon blanc wine. Food Chem. 2016, 210, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Lindsay, M.; Kilmartin, P.; Dias Araujo, L.; Rutan, T.; Yvon, M.; Stuart, L.; Grab, F.; Moore, T.; Scofield, C.; et al. Grape berry size is a key factor in determining New Zealand Pinot noir wine composition. OENO One 2022, 56, 389–411. [Google Scholar] [CrossRef]
- Shi, S.; Condron, L.; Larsen, S.; Richardson, A.E.; Jones, E.; Jiao, J.; O’Callaghan, M.; Stewart, A. In situ sampling of low molecular weight organic anions from rhizosphere of radiata pine (Pinus radiata) grown in a rhizotron system. Environ. Exp. Bot. 2011, 70, 131–142. [Google Scholar] [CrossRef]
- Henderson, J.W.J.; Brooks, A. Improved Amino Acid Methods using Agilent ZORBAX Eclipse Plus C18 Columns for a Variety of Agilent LC Instrumentation and Separation Goals; Agilent Application Note 5990–4547EN; Agilent Technologies, Inc.: Wilmington, NC, USA, 2010. [Google Scholar]
- De Vivar, M.E.D.; Zárate, E.V.; Rubilar, T.; Epherra, L.; Avaro, M.G.; Sewell, M.A. Lipid and fatty acid profiles of gametes and spawned gonads of Arbacia dufresnii (Echinodermata: Echinoidea): Sexual differences in the lipids of nutritive phagocytes. Mar. Biol. 2019, 166, 96. [Google Scholar] [CrossRef]
- Kramer, J.K.; Hernandez, M.; Cruz-Hernandez, C.; Kraft, J.; Dugan, M.E. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef]
- Van Wychen, S.; Laurens, L.M.L. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by In Situ Transesterification; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2013. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Sherman, E.; Yvon, M.; Martin, D. Influence of surface area to volume ratio on ethanol yield of commercial scale pinot noir ferments. In A Report from Plant and Food Research Ltd. to New Zealand Winegrowers; Milestone No. 76003. Contract No. 30998 var5. SPTS No. 17017; New Zealand Wine: Auckland, New Zealand, 2018. [Google Scholar]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of winemaking treatment and wine aging on phenolic content in Vranec wines. J. Food Sci. Technol. 2012, 49, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.L.; Quinn, M.; Van Wychen, S.; Templeton, D.W.; Wolfrum, E.J. Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification. Anal. Bioanal. Chem. 2012, 403, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.-H.; Lauvergeat, V.; Gomès, E.; Li, S.-H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2013, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Dal Santo, S.; Fasoli, M.; Negri, S.; D’Inca, E.; Vicenzi, N.; Guzzo, F.; Tornielli, G.B.; Pezzotti, M.; Zenoni, S. Plasticity of the Berry Ripening Program in a White Grape Variety. Front. Plant Sci. 2016, 7, 17. [Google Scholar] [CrossRef]
- Trought, M.C.T.; Naylor, A.P.; Frampton, C. Effect of row orientation, trellis type, shoot and bunch position on the variability of Sauvignon Blanc (Vitis vinifera L.) juice composition. Aust. J. Grape Wine Res. 2017, 23, 240–250. [Google Scholar] [CrossRef]
- Vondras, A.M.; Commisso, M.; Guzzo, F.; Deluc, L.G. Metabolite Profiling Reveals Developmental Inequalities in Pinot Noir Berry Tissues Late in Ripening. Front. Plant Sci. 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef]
- Bokulich Nicholas, A.; Collins Thomas, S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler Susan, E.; Mills David, A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. Mbio 2016, 7, e00616–e00631. [Google Scholar] [CrossRef]
- Stefanini, I.; Carlin, S.; Tocci, N.; Albanese, D.; Donati, C.; Franceschi, P.; Paris, M.; Zenato, A.; Tempesta, S.; Bronzato, A.; et al. Core Microbiota and Metabolome of Vitis vinifera L. cv. Corvina Grapes and Musts. Front. Microbiol. 2017, 8, 457. [Google Scholar] [CrossRef]
- Bauman, J.A.; Gallander, J.F.; Peng, A.C. Effect of maturation on the lipid content of Concord grapes. Am. J. Enol. Vitic. 1977, 28, 241–244. [Google Scholar] [CrossRef]
- Le Fur, Y.; Hory, C.; Bard, M.-H.; Olsson, A. Evolution of phytosterols in Chardonnay grape berry skins during last stages of ripening. Vitis 1994, 33, 127–131. [Google Scholar]
- Barron, L.; Santa-María, G. A relationship between triglycerides and grape-ripening indices. Food Chem. 1990, 37, 37–45. [Google Scholar] [CrossRef]
- Girardi Piva, G.; Casalta, E.; Legras, J.-L.; Tesnière, C.; Sablayrolles, J.-M.; Ferreira, D.; Ortiz-Julien, A.; Galeote, V.; Mouret, J.-R. Characterization and Role of Sterols in Saccharomyces cerevisiae during White Wine Alcoholic Fermentation. Fermentation 2022, 8, 90. [Google Scholar] [CrossRef]
- Liu, P.-T.; Zhang, B.-Q.; Duan, C.-Q.; Yan, G.-L. Pre-fermentative supplementation of unsaturated fatty acids alters the effect of overexpressing ATF1 and EEB1 on esters biosynthesis in red wine. LWT 2020, 120, 108925. [Google Scholar] [CrossRef]
- Tumanov, S.; Pinu, F.R.; Greenwood, D.R.; Villas-Bôas, S.G. Effect of free fatty acids and lipolysis on Sauvignon Blanc fermentation. Aust. J. Grape Wine Res. 2018, 24, 398–405. [Google Scholar] [CrossRef]
- Rosi, I.; Bertuccioli, M. Influences of lipid addition on fatty acid composition of Saccharomyces cerevisiae and aroma characteristics of experimental wines. J. Inst. Brew. 1992, 98, 305–314. [Google Scholar] [CrossRef]



| Sauvignon Blanc | Pinot Noir | |||||
|---|---|---|---|---|---|---|
| Harvest 1 | Harvest 2 | p-Value | Harvest 1 | Harvest 2 | p-Value | |
| Total soluble solids (°Brix) | 19.9 (0.1) | 21.8 (0.0) | <0.05 | 22.1 (0.1) | 24.7 (0.1) | <0.05 |
| Titratable acidity (g/L) | 11.7 (0.04) | 9.77 (0.02) | <0.05 | 9.09 (0.06) | 6.95 (0.05) | <0.05 |
| pH | 3.02 (0.01) | 3.05 (0.00) | >0.05 | 3.12 (0.01) | 3.22 (0.01) | >0.05 |
| Tartaric acid (g/L) | 7.66 (0.14) | 7.09 (0.02) | >0.05 | 6.30 (0.07) | 3.30 (0.08) | <0.01 |
| Malic acid (g/L) | 5.74 (0.11) | 4.70 (0.01) | <0.05 | 4.68 (0.05) | 3.62 (0.01) | <0.05 |
| YAN (mg N/L) | 155 (14) | 179 (4) | <0.05 | 191 (3) | 219 (0) | <0.05 |
| Ammonium (mg N/L) | 61 (14) | 67 (2) | >0.05 | 82 (0) | 81 (1) | >0.05 |
| PAA (mg N/L) | 94 (0) | 112 (2) | <0.05 | 109 (2) | 139 (1) | <0.03 |
| Total reducing sugar (g/L) | 221 (19) | 237 (4) | >0.05 | 231 (1) | 293 (11) | <0.05 |
| Glucose (g/L) | 122 (7) | 129 (3) | >0.05 | 121 (2) | 155 (7) | >0.05 |
| Fructose (g/L) | 99 (11) | 108 (7) | <0.05 | 110 (3) | 138 (4) | <0.01 |
| Total Phenolics (mg gallic acid/L) | * | 252 (2) | ND | 424 (5) | 1517 (69) | <0.01 |
| Aspartic acid (µmol/L) | 237 (7) | 397 (3) | <0.01 | 254 (3) | 161 (4) | <0.01 |
| Glutamic acid (µmol/L) | 827 (14) | 861 (14) | <0.05 | 751 (6) | 786 (15) | >0.05 |
| Serine (µmol/L) | 281 (5) | 357 (6) | >0.05 | 377 (5) | 504 (12) | <0.01 |
| Arginine (µmol/L) | 1243 (22) | 1476 (37) | <0.05 | 1473 (20) | 2143 (51) | <0.01 |
| Alanine (µmol/L) | 682 (11) | 806 (7) | <0.01 | 807 (8) | 1181 (21) | <0.01 |
| Histidine (µmol/L) | 810 (12) | 901 (17) | <0.05 | 767 (32) | 1102 (29) | <0.01 |
| Threonine (µmol/L) | 454 (6) | 446 (4) | >0.05 | 551 (9) | 682 (10) | <0.05 |
| Valine (µmol/L) | 143 (4) | 158 (6) | >0.05 | 153 (8) | 171 (4) | >0.05 |
| Proline (µmol/L) | 873 (107) | 1032 (93) | <0.05 | 681 (92) | 636 (54) | >0.05 |
| Methionine (µmol/L) | 77 (6) | 106 (2) | <0.05 | 142 (2) | 213 (4) | <0.01 |
| Isoleucine (µmol/L) | 48 (2) | 59 (1) | >0.05 | 89 (1) | 107 (2) | >0.05 |
| Leucine (µmol/L) | 51 (6) | 72 (1) | >0.05 | 124 (0) | 168 (3) | <0.05 |
| Phenylalanine (µmol/L) | 39 (0) | 64 (1) | <0.05 | 45 (0) | 59 (1) | >0.05 |
| Fatty Acids | Other Known Names | No of Carbons and Double Bonds | Type of Fatty Acid | |
|---|---|---|---|---|
| 1 | Octanoic acid | Caprylic acid | C8:0 | Saturated |
| 2 | Decanoic acid | Capric acid | C10:0 | Saturated |
| 3 | Undecanoic acid * | Undecylic acid | C11:0 | Saturated |
| 4 | Dodecanoic acid | Lauric acid | C12:0 | Saturated |
| 5 | Tridecanoic acid * | Tridecylic acid | C13:0 | Saturated |
| 6 | Tetradecanoic acid | Myristic acid | C14:0 | Saturated |
| 7 | Pentadecanoic acid | C15:0 | Saturated | |
| 8 | Hexadecanoic acid | Palmitic acid | C16:0 | Saturated |
| 9 | Heptadecanoic acid | Margaric acid | C17:0 | Saturated |
| 10 | Octadecanoic acid | Stearic acid | C18:0 | Saturated |
| 11 | Eicosanoic acid | Arachidic acid | C20:0 | Saturated |
| 12 | Heneicosanoic acid | Heneicosylic acid | C21:0 | Saturated |
| 13 | Docosanoic acid | Behenic acid | C22:0 | Saturated |
| 14 | Tetracosanoic acid | Lignoceric acid | C24:0 | Saturated |
| 15 | 9-cis-Tetradecenoic acid | Myristoleic acid | C14:1 | Unsaturated |
| 16 | 9-trans-Tetradecenoic acid | Myristelaidic acid | C14:1 | Unsaturated |
| 17 | 10-cis-Pentadecenoic acid | C15:1 | Unsaturated | |
| 18 | 10-trans-Pentadecenoic acid | C15:1 | Unsaturated | |
| 19 | 9-cis-Hexadecenoic acid | Palmitoleic acid (cis) | C16:1 | Unsaturated |
| 20 | (E)-9-hexadecenoic acid | Palmitoleic acid (trans) | C16:1 | Unsaturated |
| 21 | 10-cis-Heptadecenoic acid | C17:1 | Unsaturated | |
| 22 | 10-trans-Heptadecenoic acid | C17:1 | Unsaturated | |
| 23 | 9-trans-Octadecenoic acid+ | Elaidic acid (trans) | C18:1 | Unsaturated |
| 24 | 9-cis-Octadecenoic acid+ | Oleic acid | C18:1 | Unsaturated |
| 25 | 11-trans-Octadecenoic acid+ | trans-Vaccenic acid | C18:1 | Unsaturated |
| 26 | 11-cis-Octadecenoic acid | cis-Vaccenic acid | C18:1 | Unsaturated |
| 27 | 9,12,15-cis-Octadecatrienoic acid | alpha-Linolenic acid | C18:3 | Unsaturated |
| 28 | 9,12-cis-Octadecadienoic acid | Linoleic acid | C18:2 | Unsaturated |
| 29 | 9,12-trans-Octadecadienoic acid | Linolelaidic acid | C18:2 | Unsaturated |
| 30 | cis-6,9,12-octadecatrienoic acid | gamma-Linolenic acid | C18:3 | Unsaturated |
| 31 | 10-trans-Nonadecenoic acid | Nonadecylic acid | C19:1 | Unsaturated |
| 32 | 7-trans-Nonadecenoic acid | C19:1 | Unsaturated | |
| 33 | 11,14,17-cis-Eicosatrienoic acid | Eicosatrienoic acid | C20:3 | Unsaturated |
| 34 | 11,14-cis-Eicosadienoic acid | Eicosadienoic acid | C20:2 | Unsaturated |
| 35 | 11-trans-Eicosenoic acid | Eicosenoic acid | C20:1 | Unsaturated |
| 36 | 11-cis-Eicosenoic acid+ | C20:1 | Unsaturated | |
| 37 | 8,11,14-cis-Eicosatrienoic acid | C20:3 | Unsaturated | |
| 38 | 5,8,11,14,17-cis-Eicosapentaenoic acid | C20:5 | Unsaturated | |
| 39 | 5,8,11,14-cis-Eicosatetraenoic acid | C20:4 | Unsaturated | |
| 40 | 13,16-cis-Docosadienoic acid | C22:2 | Unsaturated | |
| 41 | 13-cis-Docosenoic acid | C22:1 | Unsaturated | |
| 42 | 13-trans-Docosenoic acid | C22:1 | Unsaturated | |
| 43 | 7,10,13,16,19-docosapentaenoaic acid | C22:5 | Unsaturated | |
| 44 | 4,7,10,13,16,19-Docosahexaenoic acid | C22:6 | Unsaturated | |
| 45 | 7,10,13,16-cis-Docosatetraenoic acid | Docosapentaenoic acid | C22:4 | Unsaturated |
| 46 | 4,7,10,13,16-docosapentaenoaic acid | C22:5 | Unsaturated | |
| 47 | 15-cis-Tetracosenoic acid | C24:1 | Unsaturated |
| Pulp (g/g) | Seeds (g/g) | Skin (g/g) | |
|---|---|---|---|
| SB harvest 1 | 0.009 (0.001) | 0.311 (0.075) | 0.013 (0.004) |
| SB harvest 2 | 0.016 (0.008) | 0.486 (0.089) | 0.023 (0.001) |
| Comparison (fold-change, SB harvest 1 vs. harvest 2) | 1.84 | 1.83 | 1.56 |
| PN harvest 1 | 0.016 (0.005) | 0.221 (0.025) | 0.025(0.007) |
| PN harvest 2 | 0.029 (0.008) | 0.438 (0.077) | 0.072 (0.009) |
| Comparison (fold-change, PN harvest 1 vs. harvest 2) | 1.78 | 1.99 | 2.92 |
| Sample | Pomace Contact Time (h) | Total Lipids-Harvest 1 (g/L) | Total Lipids-Harvest 2 (g/L) | Change in Lipid Level (%) |
|---|---|---|---|---|
| Sauvignon blanc | 0 | 0.14 (0.01) | 0.22 (0.03) | 36.13 |
| 24 | 0.41 (0.03) | 0.61 (0.05) | 32.55 | |
| 48 | 0.72 (0.04) | 1.12 (0.08) | 35.84 | |
| Pinot noir | 0 | 0.27 (0.03) | 0.33 (0.01) | 16.24 |
| 72 | 0.23 (0.02) | 0.59 (0.04) | 60.95 | |
| 144 | 0.39 (0.05) | 0.93 (0.06) | 58.02 |
| Variety | Sauvignon Blanc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ethanol (% v/v) | 0 | 0 | Increase (%) | 9 | 9 | Increase (%) | 13 | 13 | Increase (%) |
| Pomace contact time (h) | 72 | 168 | 72 | 168 | 72 | 168 | |||
| Total lipids-harvest 1 (g/L) | 0.70 (0.04) | 1.09 (0.05) | 35.51 | 0.86 (0.16) | 1.80 (0.19) | 52.02 | 1.04 (0.18) | 1.75 (0.14) | 40.43 |
| Total lipids-harvest 2 (g/L) | 0.69 (0.04) | 1.14 (0.10) | 39.45 | 0.99 (0.10) | 1.84 (0.12) | 45.96 | 1.53 (0.09) | 3.11 (0.30) | 50.93 |
| Variety | Pinot Noir | ||||||||
| Ethanol (% v/v) | 0 | 0 | Increase (%) | 9 | 9 | Increase (%) | 13 | 13 | Increase (%) |
| Pomace contact time (h) | 72 | 168 | 72 | 168 | 72 | 168 | |||
| Total lipids-harvest 1 (g/L) | 0.38 (0.02) | 0.85 (0.06) | 54.62 | 0.48 (0.08) | 1.06 (0.13) | 54.15 | 1.05 (0.08) | 1.60 (0.17) | 34.65 |
| Total lipids-harvest 2 (g/L) | 0.54 (0.03) | 0.89 (0.07) | 38.60 | 0.89 (0.05) | 1.24 (0.04) | 28.51 | 1.44 (0.02) | 1.85 (0.14) | 22.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherman, E.; Yvon, M.; Grab, F.; Zarate, E.; Green, S.; Bang, K.W.; Pinu, F.R. Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation. Fermentation 2023, 9, 357. https://doi.org/10.3390/fermentation9040357
Sherman E, Yvon M, Grab F, Zarate E, Green S, Bang KW, Pinu FR. Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation. Fermentation. 2023; 9(4):357. https://doi.org/10.3390/fermentation9040357
Chicago/Turabian StyleSherman, Emma, Muriel Yvon, Franzi Grab, Erica Zarate, Saras Green, Kyung Whan Bang, and Farhana R. Pinu. 2023. "Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation" Fermentation 9, no. 4: 357. https://doi.org/10.3390/fermentation9040357
APA StyleSherman, E., Yvon, M., Grab, F., Zarate, E., Green, S., Bang, K. W., & Pinu, F. R. (2023). Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation. Fermentation, 9(4), 357. https://doi.org/10.3390/fermentation9040357