The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus Acidophilus and Lactobacillus Helveticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture Preparation
2.2. Ultrasonic Treatment
2.3. Kinetic Growth
2.4. Cell Membrane Permeability
2.5. Hydrogen Potential Determination
2.6. β-Galactosidase Activity
2.7. Total Protein Content
2.8. Proteolysis
2.9. Organic Acids Quantification
2.10. Statistical Analysis
3. Results and Discussion
3.1. Kinetic Growth
3.2. Cellular Viability or Cell Membrane Permeability
3.3. pH Kinetics
3.4. β-Galactosidase Activity
3.5. Total Protein Content
3.6. Proteolysis
3.7. Organic Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malashree, L.; Angadi, V.; Yadav, K.S.; Prabha, R. “Postbiotics”—One Step Ahead of Probiotics. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2049–2053. [Google Scholar] [CrossRef]
- Taranto, M.; Médici, M.; Font de Valdez, G. Alimentos funcionales probióticos Dras. María Pía Taranto, Marta Médici y Graciela Font de Valdez * Probiotic functional foods. Aliment. Pharmacol. Ther. 2005, 4, 26–34. [Google Scholar]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.Q.; Cruz, A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef]
- Assimos, D.G. Re: Metabolomic Profiling of Oxalate-Degrading Probiotic Lactobacillus acidophilus and Lactobacillus gasseri. J. Urol. 2020, 203, 247–248. [Google Scholar] [CrossRef]
- Santiago-López, L.; Hernández-Mendoza, A.; Garcia, H.S.; Mata-Haro, V.; Vallejo-Cordoba, B.; González-Córdova, A.F. The effects of consuming probiotic-fermented milk on the immune system: A review of scientific evidence. Int. J. Dairy Technol. 2015, 68, 153–165. [Google Scholar] [CrossRef]
- Bolivar Jacobo, N.A.; Reyes Villagrana, R.A.; Chávez-Martínez, A. Relación entre probióticos—Postbióticos y sus principales efectos bioactivos. Tecnociencia Chihuah. 2021, 15, 124–139. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Hosseini, S.; Moghadaszadeh, M. Postbiotics as Dynamic Biological Molecules and Their Antimicrobial Activity: A Review Literature Review: Postbiotics. Biointerface Res. Appl. Chem. 2021, 10, 317. [Google Scholar]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Gilani, N.; Abbasi, A.; Khani, N. Postbiotics, as dynamic biomolecules, and their promising role in promoting food safety. Biointerface Res. Appl. Chem. 2021, 11, 14529–14544. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Koleilat, A. Beyond probiotics the Postbiotics. Gastroenterol. Hepatol. Open Access 2019, 10, 324–326. [Google Scholar] [CrossRef]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Zavoshti, H.F.; Abbasi, A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot. Perspect. 2020, 10, 3–4. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Tomasik, P.; Tomasik, P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl. Sci. 2020, 10, 1740. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.Y.; Lee, D.K.; Kim, S.A.; An, H.M.; Kim, J.R.; Kim, M.J.; Cha, M.G.; Lee, S.W.; Kim, K.J.; et al. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Arch. Pharm. Res. 2010, 33, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Del Rocio Ruiz-Briseño, M.; Sánchez-Reyes, K.; Alvarez-Zavala, M.; González-Hernández, L.A.; Ramos-Solano, M.; Andrade-Villanueva, J.F. Homeostasis intestinal: Colaboración del sistema inmune con la microbiota. Rev. Médica MD 2018, 9, 337–340. [Google Scholar]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, Y.; Dai, C.; Sun, L.; Ma, H.; He, R. Stimulation of low intensity ultrasound on fermentation of skim milk medium for yield of yoghurt peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019, 51, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Chemat, F.; Ashokkumar, M. Power Ultrasonics for Food Processing; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420361. [Google Scholar] [CrossRef]
- Humphrey, V.F. Ultrasound and matter—Physical interactions. Prog. Biophys. Mol. Biol. 2007, 93, 195–211. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Lee, J.; Martini, S. Modifying the physical properties of butter using high-intensity ultrasound. J. Dairy Sci. 2019, 102, 1918–1926. [Google Scholar] [CrossRef]
- Delalande, A.; Kotopoulis, S.; Postema, M.; Midoux, P.; Pichon, C. Sonoporation: Mechanistic insights and ongoing challenges for gene transfer. Gene 2013, 525, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.G.; Mann, J.A. Fourier Acoustics: Sound Radiation and Nearfield Acoustical Holography. J. Acoust. Soc. Am. 2000, 108, 1373. [Google Scholar] [CrossRef]
- Kooiman, K.; Vos, H.J.; Versluis, M.; De Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef] [PubMed]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on Their Ability to Modify Biological Responses, Inactivation Methods and Perspectives on Their Application in Foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Cabeza, E.A. Bacterias ácido-lácticas (BAL): Aplicaciones como cultivos estárter para la industria láctea y cárnica. In Proceedings of the Simposio Regional de Microbiología: Microorganismos Eficientes en el Sector Productivo, Barranquilla, Colombia, 15–16 September 2006; Volume 14, pp. 549–566. [Google Scholar] [CrossRef]
- Gholamhosseinpour, A.; Hashemi, S.M.B. Ultrasound pretreatment of fermented milk containing probiotic Lactobacillus plantarum AF1: Carbohydrate metabolism and antioxidant activity. J. Food Process Eng. 2019, 42, e12930. [Google Scholar] [CrossRef]
- Gotoh, M.; Sashihara, T.; Ikegami, S.; Yamaji, T.; Kino, K.; Orii, N.; Taketomo, N.; Okubo, K. Efficacy of oral administration of a heat-killed Lactobacillus gasseri OLL2809 on patients of japanese cedar pollinosis with high japanese-cedar pollen-specific IgE. Biosci. Biotechnol. Biochem. 2009, 73, 1971–1977. [Google Scholar] [CrossRef]
- Chen, W. Lactic Acid Bacteria Omics and Functional Evaluation; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783319600215. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. Health-promoting properties of Lactobacillus helveticus. Front. Microbiol. 2012, 3, 392. [Google Scholar] [CrossRef]
- Ianni, F.; Altomare, A.A.; Cenci-Goga, B.T.; Blasi, F.; Grispoldi, L.; Regazzoni, L.; Cossignani, L. Chromatographic characterization and in vitro bioactivity evaluation of lactobacillus helveticus hydrolysates upon fermentation of different substrates. Appl. Sci. 2021, 11, 811. [Google Scholar] [CrossRef]
- Bolivar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Santellano-Estrada, E.; Tirado-Gallegos, J.M.; Chávez-Martínez, A. Culture Age, Growth Medium, Ultrasound Amplitude, and Time of Exposure Influence the Kinetic Growth of Lactobacillus acidophilus. Fermentation 2023, 9, 63. [Google Scholar] [CrossRef]
- Molecular Probes Inc. LIVE/DEAD® BacLightTM Bacterial Viability and Counting Kit; Product Information; Molecular Probes Inc.: Eugene, OR, USA, 2004; pp. 1–5. [Google Scholar]
- Ibrahim, A.H. Enhancement of β-galactosidase activity of lactic acid bacteria in fermented camel milk. Emir. J. Food Agric. 2018, 30, 256–267. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Probiotic Strains as Starter Cultures Improve Angiotensin-Converting Enzyme Inhibitory Activity in Soy Yogurt. J. Food Sci. 2005, 70, m375–m381. [Google Scholar] [CrossRef]
- Dahroud, B.D.; Mokarram, R.R.; Khiabani, M.S.; Hamishehkar, H.; Bialvaei, A.Z.; Yousefi, M.; Kafil, H.S. Low Intensity Ultrasound Increases the Fermentation Efficiency of Lactobacillus Casei Subsp.Casei ATTC 39392. Int. J. Biol. Macromol. 2016, 86, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Shokri, S.; Shekarforoush, S.S.; Hosseinzadeh, S. Efficacy of Low Intensity Ultrasound on Fermentative Activity Intensification and Growth Kinetic of Leuconostoc Mesenteroides. Chem. Eng. Process.—Process Intensif. 2020, 153, 107955. [Google Scholar] [CrossRef]
- Shokri, S.; Terefe, N.S.; Shekarforoush, S.S.; Hosseinzadeh, S. Ultrasound-Assisted Fermentation for Enhancing Metabolic and Probiotic Activities of LactoBacillus Brevis. Chem. Eng. Process.—Process Intensif. 2021, 166, 108470. [Google Scholar] [CrossRef]
- Abesinghe, A.M.N.L.; Islam, N.; Vidanarachchi, J.K.; Prakash, S.; Silva, K.F.S.T.; Karim, M.A. Effects of Ultrasound on the Fermentation Profile of Fermented Milk Products Incorporated with Lactic Acid Bacteria. Int. Dairy J. 2019, 90, 1–14. [Google Scholar] [CrossRef]
- Potoroko, I.; Kalinina, I.; Botvinnikova, V.; Krasulya, O.; Fatkullin, R.; Bagale, U.; Sonawane, S.H. Ultrasound Effects Based on Simulation of Milk Processing Properties. Ultrason. Sonochem. 2018, 48, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Ge, S. Sonoporation: Underlying Mechanisms and Applications in Cellular Regulation. BIO Integr. 2021, 2, 29–36. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Stimulating Fermentative Activities of Bifidobacteria in Milk by Highintensity Ultrasound. Int. Dairy J. 2009, 19, 410–416. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus Helveticus: The Proteolytic System. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed]

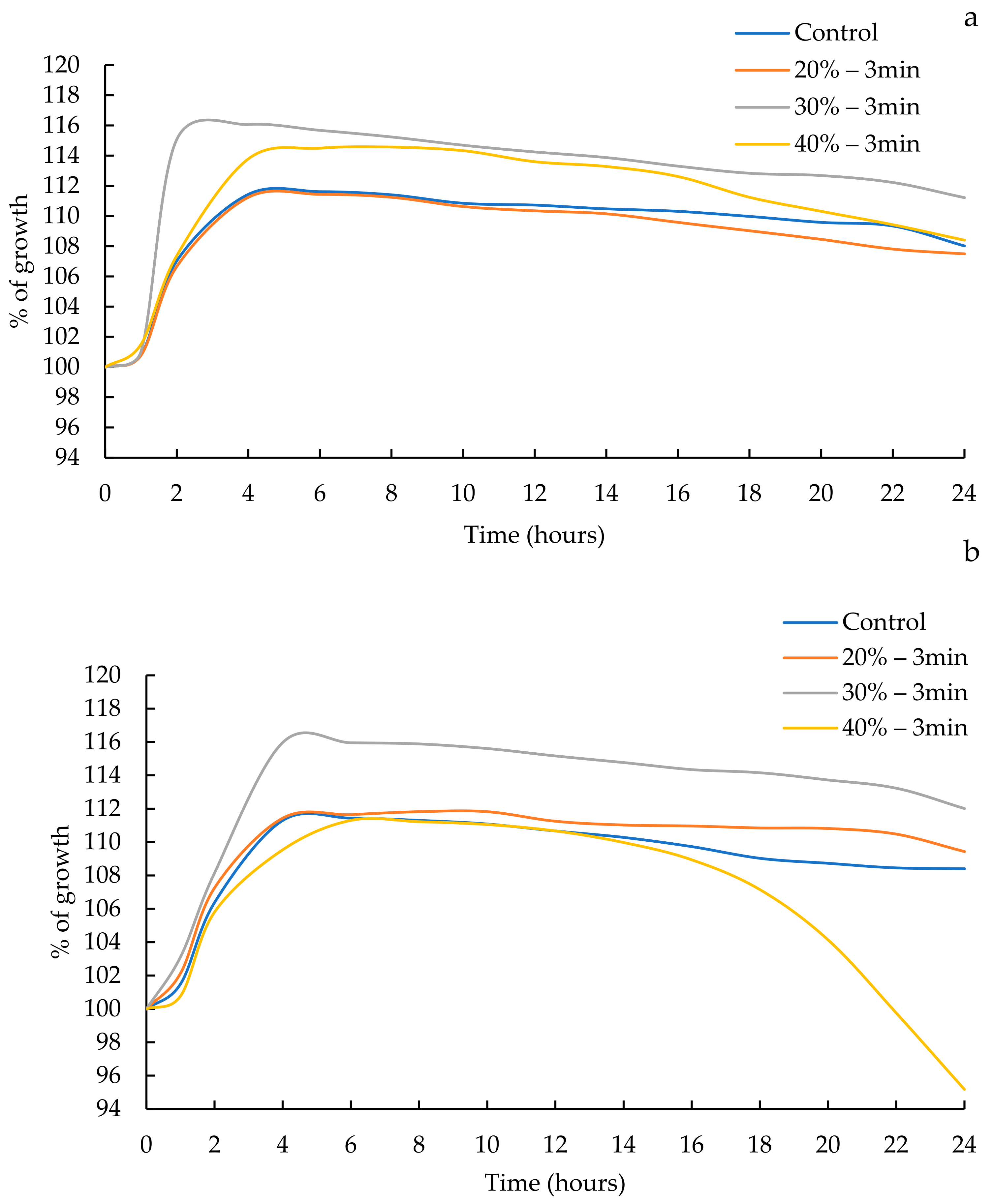




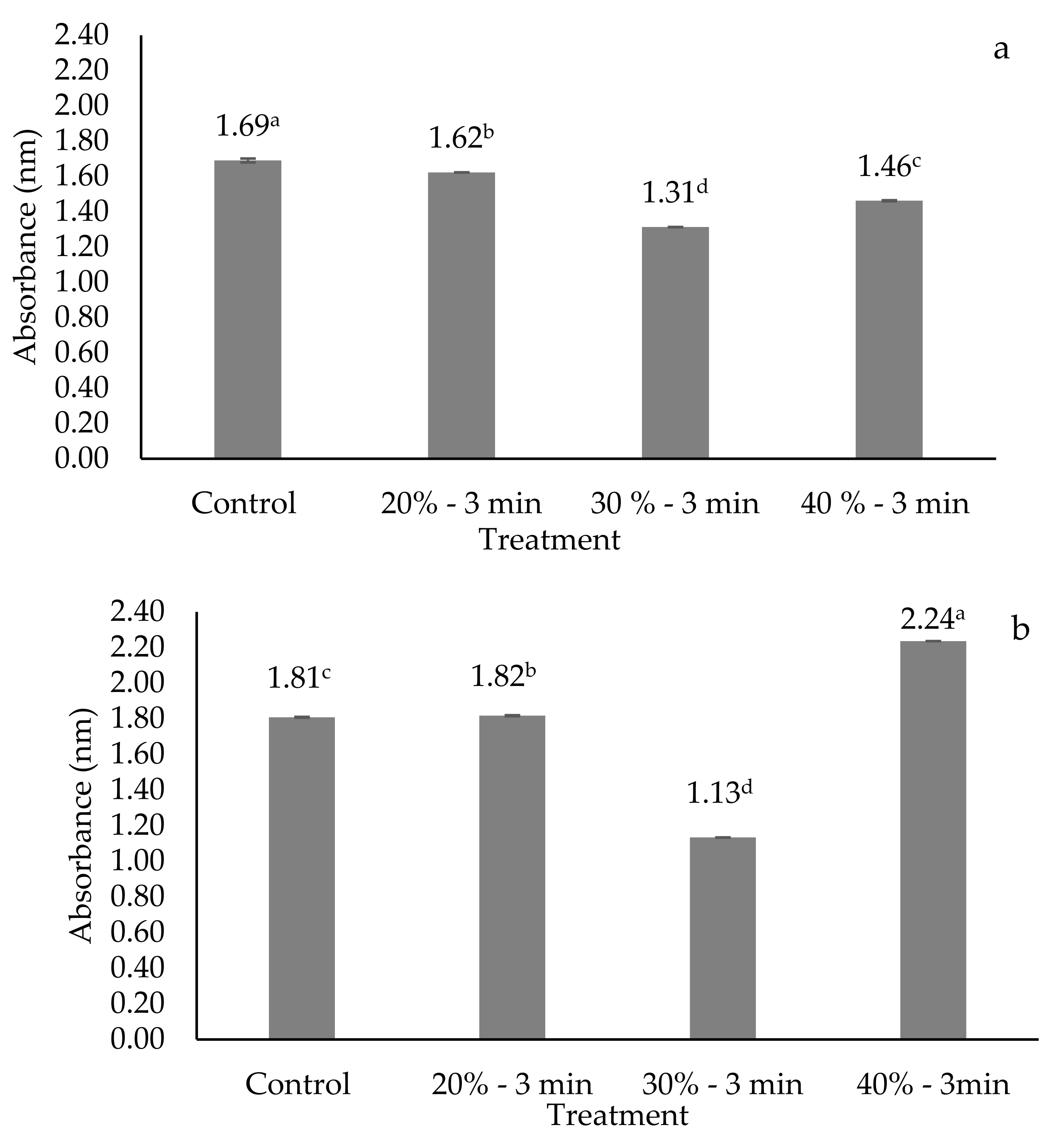
| Treatment | Yo | Lag (hours) | Rate (μmax) (1/Hours) | Tmax (Hours) | R2 |
|---|---|---|---|---|---|
| L. acidophilus | |||||
| Control | 8.08± 0.000 a | 0.870 ± 0.017 b | 0.500 ± 0.014 b | 2.578 ± 0.024 b | 0.96 |
| 20%—3 min | 8.08± 0.000 a | 0.861 ± 0.019 b | 0.470 ± 0.014 b | 2.622 ± 0.031 b | 0.93 |
| 30%—3 min | 8.08± 0.000 a | 0.923 ± 0.000 a | 1.065 ± 0.000 a | 1.998 ± 0.000 c | 0.93 |
| 40%—3 min | 8.08± 0.000 a | 0.748 ± 0.003 c | 0.475 ± 0.007 b | 2.985 ± 0.031 a | 0.91 |
| L. helveticus | |||||
| Control | 8.08± 0.000 a | 0.696 ± 0.006 a | 0.395 ± 0.007 b | 2.886 ± 0.003 c | 0.95 |
| 20%—3 min | 8.08± 0.000 a | 0.573 ± 0.017 b | 0.410 ± 0.000 a | 2.720 ± 0.018 d | 0.96 |
| 30%—3 min | 8.08± 0.000 a | 0.391 ± 0.035 c | 0.410 ± 0.000 a | 3.272 ± 0.026 b | 0.94 |
| 40%—3 min | 8.08± 0.000 a | 0.390 ± 0.046 c | 0.225 ± 0.001 c | 4.174 ± 0.006 a | 0.94 |
| Treatment | Probiotic | |
|---|---|---|
| L. acidophilus | L. helveticus | |
| Control | 268.66 ± 0.00 c | 87.54 ± 0.00 c |
| 20%—3 min | 318.23 ± 0.00 b | 137.11 ± 0.00 b |
| 30%—3 min | 452.8 ± 0.00 a | 159.78 ± 0.00 a |
| 40%—3 min | 77.43 ± 0.00 d | 70.54 ± 0.00 d |
| Treatment | Oxalic Acid | Citric Acid | Malic Acid | Succinic Acid | Acetic Acid | Lactic Acid |
|---|---|---|---|---|---|---|
| L. acidophilus | ||||||
| Control | 0.053 ± 0.013 b | 6.115 ± 0.118 a | 0.792 ± 0.020 a | 1.283 ± 0.075 a | 6.546 ± 0.082 a | 716.45 ± 0.970 b |
| 20%—3 min | 0.102 ± 0.007 a | 4.297 ± 0.034 b | 0.153 ± 0.013 a | 1.126 ± 0.080 a | 5.773 ± 0.082 b | 637.20 ± 8.170 b |
| 30%—3 min | 0.053 ± 0.003 b | 4.757 ± 0.780 ab | 0.371 ± 0.257 a | 1.174 ± 0.019 a | 5.972 ± 0.028 b | 681.60 ± 26.30 b |
| 40%—3 min | 0.045 ± 0.001 b | 4.206 ± 0.342 b | 0.547 ± 0.186 a | 1.610 ± 0.346 a | 4.145 ± 0.044 c | 859.40 ± 30.90 a |
| L. helveticus | ||||||
| Control | 0.064 ± 0.003 a | 3.786 ± 0.688 a | 0.313 ± 0.123 b | 1.567 ± 0.168 a | 4.643 ± 0.032 b | 841.00 ± 15.40 b |
| 20%—3 min | 0.056 ± 0.001 ab | 4.702 ± 0.214 a | 0.587 ± 0.016 ab | 1.850 ± 0.165 a | 4.953 ± 0.075 a | 927.51 ± 3.540 a |
| 30%—3 min | 0.050 ± 0.004 b | 4.537 ± 0.098 a | 0.594 ± 0.053 a | 1.676 ± 0.073 a | 4.457 ± 0.008 b | 824.45 ± 3.690 b |
| 40%—3 min | 0.013 ± 0.001 c | 1.051 ± 0.055 b | ND | ND | 0.074 + 0.104 c | 15.710 ± 3.250 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolívar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Espino-Solís, G.P.; Rentería-Monterrubio, A.L.; Arévalos-Sánchez, M.M.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Flores, D.; Chávez-Martínez, A. The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus Acidophilus and Lactobacillus Helveticus. Fermentation 2023, 9, 356. https://doi.org/10.3390/fermentation9040356
Bolívar-Jacobo NA, Reyes-Villagrana RA, Espino-Solís GP, Rentería-Monterrubio AL, Arévalos-Sánchez MM, Sánchez-Vega R, Santellano-Estrada E, Chávez-Flores D, Chávez-Martínez A. The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus Acidophilus and Lactobacillus Helveticus. Fermentation. 2023; 9(4):356. https://doi.org/10.3390/fermentation9040356
Chicago/Turabian StyleBolívar-Jacobo, Norma Angélica, Raúl Alberto Reyes-Villagrana, Gerardo Pavel Espino-Solís, Ana Luisa Rentería-Monterrubio, Martha María Arévalos-Sánchez, Rogelio Sánchez-Vega, Eduardo Santellano-Estrada, David Chávez-Flores, and América Chávez-Martínez. 2023. "The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus Acidophilus and Lactobacillus Helveticus" Fermentation 9, no. 4: 356. https://doi.org/10.3390/fermentation9040356
APA StyleBolívar-Jacobo, N. A., Reyes-Villagrana, R. A., Espino-Solís, G. P., Rentería-Monterrubio, A. L., Arévalos-Sánchez, M. M., Sánchez-Vega, R., Santellano-Estrada, E., Chávez-Flores, D., & Chávez-Martínez, A. (2023). The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus Acidophilus and Lactobacillus Helveticus. Fermentation, 9(4), 356. https://doi.org/10.3390/fermentation9040356







